Abstract
Objectives
To determine the relationship between nutritional status and nevirapine exposure by comparing the pharmacokinetics of nevirapine in HIV-infected children of different ages with and without malnutrition receiving divided tablets of Triomune®30 (stavudine + lamivudine + nevirapine) in accordance with Malawi National Guidelines.
Methods
Children were recruited in weight-based dosage bands and nutritional status classified according to weight for height. Total and unbound plasma nevirapine concentrations were measured over a full dosing interval. Multivariate linear and logistic regression analyses were performed to determine the effects of malnutrition, age, dose and other factors on nevirapine exposure and likelihood of achieving therapeutic nevirapine trough concentrations.
Results
Forty-three children were recruited (37 included for analysis). Mild to moderate malnutrition was present in 12 (32%) children; 25 (68%) were of normal nutritional status. There was no effect of malnutrition on any measure of total drug exposure or on the unbound fraction of nevirapine. Nevirapine exposure was strongly related to dose administered (P = 0.039) and to age (for every yearly increase in age there was an ∼88% increase in the odds of achieving a therapeutic nevirapine concentration; P = 0.056, 95% confidence interval 0.983–3.585).
Conclusions
Use of divided adult Triomune®30 tablets in treating young children results in significant underdosing. No independent effect of malnutrition on total and unbound nevirapine exposures was observed. These data support the use of bespoke paediatric antiretroviral formulations.
Keywords: Africa, paediatric, Triomune
Introduction
Children in resource-poor settings are under-represented in antiretroviral therapy (ART) programmes.1 A major obstacle is lack of affordable, accessible and appropriate paediatric antiretroviral drug formulations.2 In order to provide ART, several countries, including Malawi, have prescribed divided adult fixed-dose combination (FDC) antiretroviral tablets of 30 mg of stavudine, 150 mg of lamivudine and 200 mg of nevirapine (Triomune®30) to children.3 A problem with this approach is that the proportions of drugs in divided adult formulations may not be appropriate for children.
Dosage recommendations of antiretroviral drugs in children may be based on weight or surface area. Prescriptions based on surface area are complex and prone to error.4 In recognition of this the WHO HIV Working Group produced standardized dosing tables for the use of fractions of FDC tablets in children.5 Recommended doses aimed for an ‘optimal’ dose for weight bands determined by calculating surface area values from median heights for weight of international growth charts. The Malawian national ART guidelines for children are adapted from these standardized tables.3
Initial studies of Triomune®30 have shown good tolerability and a satisfactory clinical response in children.6–8 A pharmacokinetic study looking at a small cohort of children given fractions of a nevirapine-, lamivudine- and stavudine-containing FDC in an adult formulation showed nevirapine levels within the therapeutic range after 2 months of treatment.9 This study did not measure total drug exposure and younger age groups (<3 years) were under-represented. Uniformity of drug distribution within the tablet has been demonstrated.10
Concerns remain that both the fixed proportions of drugs in adult formulations and the weight-band-based dosing tables may not be appropriate for all children, particularly younger children and those with malnutrition. Younger children have more rapid nevirapine clearance than adults and consequently a higher dose of nevirapine/surface area has been recommended in children <9 years.11 Both the use of adult FDC tablets and the higher surface area to weight ratio of younger children could place them at risk of under-dosing by weight-band-based dosing tables. Dosing of children with wasting secondary to malnutrition is complex. The greater surface area to weight in these children could lead to under-dosing in this group. Reduced drug absorption could also contribute to lower drug levels.12 Conversely the pathophysiological profile in malnutrition may alter drug metabolism. Studies have demonstrated reduced protein binding of several drugs and reduced hepatic metabolism13 in malnourished children, which may contribute to higher drug levels and risk of toxicity. Both younger (<3 years) and malnourished children have been under-represented in previous data-rich pharmacokinetic studies of adult FDC tablets in children.14
We wished to determine the relationship between nutritional status and nevirapine exposure by comparing the pharmacokinetics of nevirapine in HIV-1-infected children of different ages with and without mild or moderate malnutrition after ∼6 weeks of treatment with divided tablets of Triomune®30.
Methods
Study population
This pharmacokinetic study took place at Queen Elizabeth Central Hospital (QECH) and affiliated local clinics in Blantyre, Malawi between December 2006 and May 2007. HIV-1-infected children (treatment naive), aged 0–16 years, who had received Triomune®30 (30 mg of stavudine, 150 mg of lamivudine and 200 mg of nevirapine) formulations for >4 weeks were enrolled after providing written informed consent from parents and assent by older children. All children had completed a 2 week ‘lead in’ phase of nevirapine dosing, with Triomune®30 once daily and stavudine and lamivudine once daily, prior to starting regular Triomune®30 treatment. ART was provided free by the Ministry of Health. The dosage of Triomune®30 was based on weight-band dosage tables in accordance with national guidelines achieved by dividing the adult FDC tablets into one-quarter, half or three-quarters or leaving as whole tablets. Triomune®30 tablets are not scored but carers were provided with pill cutters and trained in their use. If children were unable to swallow tablets carers were instructed to crush the tablet and mix into a teaspoon of water.
Exclusion criteria were non-adherence to treatment (defined as <95% adherence rate when assessed by pill counts and guardian report), gastrointestinal illness during the previous 2 weeks, severe anaemia and current anti-tuberculosis therapy (lowering nevirapine exposure).
Eligible children were then grouped by nutritional status according to weight for height, with weight for height >85% of median15 classed as ‘normal’ nutrition (representative of the Malawian population), weight for height 70%–85% as mild to moderate malnutrition and weight for height <70% as severe malnutrition.16 Children were weighed on Tanita (M1) digital scales, calibrated daily, accurate to 20 g. Heights were measured to the nearest 1 mm using locally built height boards. Anthropometry protocols followed research standards.17 All children identified as malnourished received nutritional support according to Malawian national guidelines.16
Children were recruited in weight-based dosage bands (<8, 8–<14, 14–<26 and ≥26 kg) to ensure an equal distribution of weights and ages.
Ethics approval was granted by the University of Malawi College of Medicine Research and Ethics Committee.
Study design
To evaluate the pharmacokinetics of total and unbound nevirapine concentrations blood samples at steady state were collected pre-dose and 2, 4, 8 and 12 h post-dose. Hepatitis B core total antibodies, hepatitis C antibodies, liver function tests [alanine transaminase (ALT) and gamma-glutamyl transferase (GGT)], CD4% and viral loads were determined on the day of sampling. Tests for aspartate transferase (AST) and hepatitis antigen/PCR were not locally available.
The primary endpoint was nevirapine exposure [area under the concentration–time curve (AUC)]. We judged that a difference in mean AUC of >25% (the upper cut-off used by the FDA18/EMEA19 for ‘bioequivalence’) would be indicative of a clinically significant difference between nutritional groups. Using adult data20 for geometric mean AUC we estimated that a sample size of 9 children with malnutrition and 18 children without malnutrition would allow us to detect a 25% difference with 80% power (2-sided alpha +0.05). The secondary endpoints were bound and unbound nevirapine exposure and nevirapine maximum (Cmax) and trough (Ctrough) concentrations.
Drug analysis
Total nevirapine from plasma was measured using a validated HPLC methodology with UV detection (HPLC-UV).21 Bound and unbound concentrations were separated using ultrafiltration. Patient plasma was injected into Centrifree® micro-partition devices and centrifuged, and the resultant ultrafiltrate (∼170 µL per sample) was retained for drug analysis. Intra-assay and inter-assay variability (CV%, where CV stands for coefficient of variation) at 790, 3193 and 8197 ng/mL (low, medium and high quality controls) was <5%, respectively. The lower limit of quantification was 101 ng/mL. The therapeutic range for nevirapine Ctrough was defined as 3000–8000 ng/mL.22
Data analysis
Pharmacokinetic parameters AUC0–12 and Cmax and Ctrough at 12 h were calculated using WinNonlin Professional™ software (version 5.0; Pharsight Corp., Mountain View, CA, USA). The percentage of unbound drug exposure in plasma (%AUCunbound) was determined by: %AUCunbound = (unbound AUC0–12/AUC0–12) × 100.
Demographic and drug concentration data were analysed by non-parametric Mann–Whitney U-test when comparing nutritional status for continuous variables, and by χ2 or Fisher's exact test when comparing nutritional status for categorical variables. Differences in nevirapine AUC0–12 between weight bands (<8, 8–<14, 14–<26 and ≥26 kg) were assessed using a Kruskal–Wallis non-parametric ANOVA with Dunn's correction for multiple comparisons.
Drug concentration data were log-transformed and univariate and multivariate linear and logistic regression analysis was performed to determine the effects of weight for height, age, sex, albumin concentration, hepatitis B/C co-infection, CD4%, time between starting ART and sampling and daily nevirapine dose/m2 on nevirapine exposure [AUC (ng·h/mL)] and on achieving therapeutic nevirapine trough concentrations (defined as ≥3000 ng/mL). Variables were included into a full multivariate regression model if P < 0.1, and backwards elimination (P < 0.1) was used to identify the most important predictors (SPSS version 15.0). Tests for non-linear effects were performed using fractional polynomials (Stata 9; StataCorp, TX, USA).
Results
Study population
Baseline and pharmacokinetic data were available for 43 patients. Of these, six were excluded from analysis; four children received the wrong dose according to weight-based guidelines, one was suspected of non-adherence and one commenced anti-tuberculosis therapy between enrolment and day of testing and samples were taken in error.
Of the remaining 37 children, 11, 8, 14 and 4 were <8, 8–<14, 14–<26 and ≥26 kg, respectively. Thirteen (35%) were under 2 years of age and 24 (65%) were aged between 2 and 16 years. The majority of children were moderately to severely immunocompromised, with a median (range) CD4% of 14.7% (1.3%–33.3%). Twenty (54%) children had a suppressed viral load of <400 copies/mL, and 17 (46%) had viral loads of >400 copies/mL (range 437 to >750 000). There was no significant difference in mean time on ART between children with unsuppressed and suppressed viral loads (P = 0.55). Children with unsuppressed viral loads were significantly younger (median age 1.8 years (range 0.7–14.7 years) than those with viral loads of <400 copies/mL (median age 9.5 years: range 0.7–16 years; P = 0.03). However, no baseline viral loads were available for comparison.
Twelve (32%) children were classed as having mild to moderate malnutrition (weight for height 70%–85%) and 25 (68%) were of normal nutritional status (weight for height >85%). Patient baseline demographics for the two nutritional groups are given in Table 1. No child presented with severe malnutrition (weight for height <70%). Stunting was prevalent in both groups with 21 (84%) children in the ‘normal’ group and 10 (83%) in the ‘malnourished’ group presenting with height-for-age z-score of less than −2SD. Two (8%) children of normal nutritional status were co-infected with hepatitis B and 2 (17%) from the malnourished group were co-infected with hepatitis C. ALT/GGT levels in these children were not significantly raised. None of these children had been previously tested for hepatitis. The malnourished children were on average [median (range)] younger and significantly lighter compared with children of normal nutritional status [2.6 (0.7–14.7) versus 6.3 (0.8–16.0) years; P = 0.257 and 7.8 (3.9–24.0) versus 15.9 (6.3–38.9) kg; P = 0.033].
Table 1.
Baseline patient demographics and ART dosing by nutritional status
| All, n = 37 | Normal (wt/ht >85%), n = 25 | Malnourished (wt/ht 75%–85%), n = 12 | P value | |
|---|---|---|---|---|
| Sex, female | 16 (43) | 8 (32) | 8 (67) | 0.049 |
| Age, years | 4.4 (0.7, 16.0) | 6.3 (0.8, 16.0) | 2.6 (0.7, 14.7) | 0.257 |
| Age <2 years (%) | 13 (35) | 7 (28) | 6 (50) | 0.274 |
| Weight, kg | 12.3 (3.9, 38.9) | 15.9 (6.3, 38.9) | 7.8 (3.9, 24.0) | 0.033 |
| Weight <14 kg (%) | 19 (51) | 11 (44) | 8 (67) | 0.295 |
| Height, cm | 84.3 (59.1, 144.0) | 99.4 (65.0, 144.0) | 76.5 (59.1, 133.5) | 0.200 |
| BSA, m2 | 0.52 (0.25, 1.23) | 0.62 (0.32, 1.23) | 0.40 (0.25, 0.96) | 0.095 |
| ALT, U/L | 33 (14, 282) | 33 (16, 282) | 34 (14, 59) | 0.772 |
| GGT, U/L | 71 (18, 449) | 69 (18, 334) | 98 (35, 449) | 0.348 |
| Weight-for-age z-score | −2.55 (−5.08, −0.81) | −2.10 (−4.29, −0.81) | −3.51 (−5.08, −2.05) | 0.002 |
| Height-for-age z-score | −3.10 (−5.85, −0.80) | −3.03 (−5.85, −1.09) | −3.73 (−5.13, −0.80) | 0.299 |
| Viral load, <400 copies/mL (%) | 20 (54) | 15 (60) | 5 (42) | 0.295 |
| CD4% | 14.7 (1.3, 33.3) | 15.8 (2.3, 33.0) | 13.1 (1.3, 33.3) | 0.175 |
| Fractions of Triomune tablets prescribed (mg of nevirapine) | ||||
| quarter OD (50) | 1 (3) | 0 (0) | 1 (8) | 0.297 |
| quarter BD (100) | 10 (27) | 5 (20) | 5 (42) | 0.125 |
| half OD + quarter OD (150) | 7 (19) | 5 (20) | 2 (17) | 1.000 |
| half BD (200) | 1 (3) | 1 (4) | 0 (0) | 1.000 |
| three-quarters OD + half OD (250) | 4 (11) | 3 (12) | 1 (8) | 1.000 |
| three-quarters BD (300) | 10 (27) | 7 (28) | 3 (25) | 0.688 |
| whole OD + three-quarters OD (350) | 1 (3) | 1 (4) | 0 (0) | 1.000 |
| whole BD (400) | 3 (8) | 3 (12) | 0 (0) | 0.540 |
| Total daily nevirapine dose | ||||
| mg | 200 (50, 400) | 250 (100, 400) | 125 (50, 300) | 0.058 |
| mg/m2 | 326.4 (203.2, 431.3) | 336.7 (273.4, 431.3) | 307.4 (203.2, 348.8) | 0.020 |
| <300 mg/m2 | 8 (22) | 3 (12) | 5 (42) | 0.083 |
| Total daily lamivudine dose, mg/kg | 11.0 (7.7, 13.4) | 11.0 (7.7, 13.4) | 11.1 (9.4, 12.8) | 0.537 |
| Total daily stavudine dose, mg/kg | 2.2 (1.5, 2.7) | 2.2 (1.5, 2.7) | 2.2 (1.9, 2.6) | 0.555 |
| Time between starting ART and sampling, weeks | 7.0 (5.9, 24.6) | 7.0 (6.0, 24.6) | 8.0 (5.9, 14.3) | 0.923 |
wt/ht, weight for height; BSA, body surface area; OD, once a day; BD, twice a day.
Values are given as median (range) with non-parametric P values (Mann–Whitney U-test) when comparing nutritional status for continuous variables; and as n (%) with P values (χ2 or Fisher's exact test) when comparing nutritional status for categorical variables.
BSA (m2) = 71.84 × weight (kg)0.425 × height (cm)0.725/10 000.
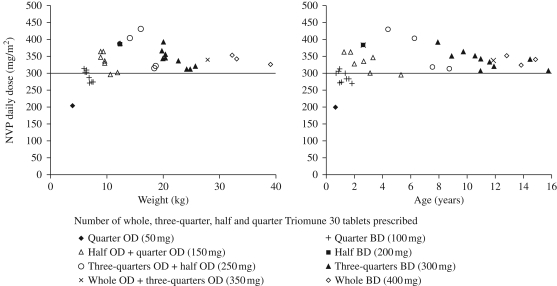
The recommended daily nevirapine dose in children is 300–400 mg/m2.23 Although the majority of children were dosed adequately, suboptimal dosing (<300 mg/m2/day) was observed in eight children, all of whom were within lighter weight bands receiving quartered tablets as part of their regimen. One child prescribed 50 mg of nevirapine/day (quarter tablet once daily: 203 mg/m2), 6 of the 10 children prescribed 100 mg of nevirapine/day (quarter tablet twice daily: median dose 275.3 mg/m2: range 270.9–287.3 mg/m2) and 1 of the 7 children prescribed 150 mg of nevirapine/day (half and quarter tablet once daily: 298.0 mg/m2) were underdosed. Of these 8 children, 7 were under 2 years and weighed <10 kg (range 0.6–1.8 years, weight 3.88–7.46 kg). One child was 5.3 years and weighed 10.6 kg. Fractional polynomial regression suggested a non-linear effect of age and weight upon nevirapine dose/m2 (P < 0.025); whereby, through visual inspection (Figure 1), a deviation from linearity occurred at between 2 and 4 years and a weight <10 kg, respectively. This relationship held true for analyses both including and excluding an outlying child receiving a nevirapine dose of 203 mg/m2 (age 0.7 years and weight 3.88 kg). There was no additional effect of malnutrition on the number of children underdosed (P = 0.908, after adjusting for age and weight).
Figure 1.
Nevirapine (NVP) daily dose (mg/m2) by (a) weight and (b) age; and according to the number of prescribed whole, three-quarter, half and quarter Triomune 30 tablets (n = 37). The continuous line indicates the recommended NVP paediatric dose of 300 mg/m2. Quarter tablet = 50 mg of nevirapine, half tablet = 100 mg of nevirapine, three-quarter tablet = 150 mg of nevirapine and whole tablet = 200 mg of nevirapine. OD, once a day; BD, twice a day.
The recommended daily paediatric doses of lamivudine and stavudine are 8 mg/kg and 2 mg/kg, respectively.23 Children in both study groups received adequate doses of lamivudine [malnourished: 11.1 (9.4–12.8) versus normal: 11.0 (7.7–13.4) mg/kg] and stavudine [malnourished: 2.2 (1.9–2.6) versus normal: 2.2 (1.5–2.7) mg/kg]. Unlike nevirapine, the total daily lamivudine and stavudine dose received was independent of a child's age and weight.
All but one of the children included in the study were receiving co-trimoxazole prophylaxis. Five (14%) children received additional medication (one received amoxicillin, one griseofulvin, two pyrimethamine/sulfadoxine and one received both chloramphenicol and flucloxacillin). The median (range) time from starting ART to the day of pharmacokinetic sampling was 7.0 (5.9–24.6) weeks and was not significantly different between the two nutritional groups [malnourished: 8.0 (5.9–14.3) versus normal: 7.0 (6.0–24.6) weeks; (P = 0.923)].
Nevirapine pharmacokinetics
Total nevirapine AUC0–12 [median (range) ng·h/mL] was not significantly different in the malnourished group compared with the normal group [malnourished: 60 082 (35 328–166 629) versus normal: 79 861 (43 922–146 376) ng·h/mL (P = 0.17)]. There was a trend for lower total nevirapine Cmax (29%) in malnourished (6719 ng/mL; 3401–18 947) versus normal (9483 ng/mL; 5099–22 453) children (P = 0.089). Median (range) total nevirapine Ctrough was 5881 (2305–9097) ng/mL in normal children and 5084 (1645–8489) ng/mL in malnourished children (P = 0.327). Nevirapine exposure was shown to be significantly different across the specified weight bands (P = 0.036) and when correcting for multiple comparisons, this difference appeared driven by low exposure in children <8 kg compared with children 14–<26 kg and ≥26 kg (P < 0.024).
In a univariate analysis increased age, higher nevirapine dose/m2 and increased time on ART were found to be associated with increased nevirapine exposure (Table 2). There was no evidence of an effect of nutritional status (weight for height), sex or hepatitis co-infection. In a multivariate model, however, age and daily dose/m2 were confounding, and following backwards elimination (P < 0.1) only daily dose/m2 and time on ART were significant predictors, where an increase in the daily dose by 50 mg/m2 was independently associated with a 17.8% increase in nevirapine exposure [P = 0.039; 95% confidence interval (CI) 0.004–0.139]. Equally, a one unit (weekly) increase in the time on treatment resulted in a 4.0% increase in nevirapine exposure (P = 0.014; 95% CI 0.004–0.031). This model did not, however, account for the fact that children on unequal dosing bands may have slightly higher nevirapine AUC0–12 than expected for their total daily nevirapine dose/m2 since they receive a greater proportion of this total daily dose in the morning. In order to account for asymmetrical dosing, a binary factor was introduced into the final multivariate model (not shown), but this was shown to have no significant effect on the overall outcome.
Table 2.
Predictors of nevirapine exposure [AUC0–12 (ng·h/mL)] and achieving a therapeutic nevirapine Ctrough ≥3000 ng/mL
| Nevirapine AUC0–12 |
Univariate models |
Simultaneous multivariate modela |
Final multivariate modelb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | beta | 95% CI | % effect | P value | beta | 95% CI | % effect | P value | beta | 95% CI | % effect | P value |
| Wt/ht, malnourished vs normal | −0.090 | −0.212–0.032 | −18.7 | 0.145 | — | — | — | — | — | — | — | — |
| Age, years | 0.015 | 0.005–0.026 | 3.5 | 0.005 | 0.008 | −0.003–0.019 | 2.0 | 0.166 | — | — | — | — |
| Sex, female vs male | −0.008 | −0.132–0.116 | −1.8 | 0.896 | — | — | — | — | — | — | — | — |
| Albumin, mg/mL | −0.004 | −0.014–0.006 | −0.9 | 0.429 | — | — | — | — | — | — | — | — |
| Hepatitis B/C, +ve vs –ve | −0.134 | −0.281–0.014 | −26.5 | 0.074 | −0.062 | −0.203–0.078 | −13.0 | — | — | — | — | — |
| CD4, % | 0.002 | −0.005–0.009 | 0.5 | 0.475 | — | — | — | — | — | — | — | — |
| Time on ART, weeks | 0.016 | 0.002–0.031 | 3.8 | 0.026 | 0.013 | −0.002–0.027 | 3.0 | 0.079 | 0.017 | 0.004–0.031 | 4.0 | 0.014 |
| Daily dose per 50 mg/m2 | 0.082 | 0.018–0.146 | 20.8 | 0.013 | 0.052 | −0.019–0.122 | 12.7 | 0.145 | 0.071 | 0.004–0.139 | 17.8 | 0.039 |
| Centre, QECH vs other | 0.068 | −0.049–0.185 | 16.9 | 0.246 | — | — | — | — | — | — | — | — |
| Therapeutic Ctrough |
Univariate models |
Simultaneous multivariate modela |
Final multivariate modelb |
|||||||||
| Factor | odds ratio | 95% CI | P value | odds ratio | 95% CI | P value | odds ratio | 95% CI | P value | |||
| Wt/ht, malnourished vs normal | 0.500 | 0.106–2.335 | 0.381 | — | — | — | — | — | — | |||
| Age, years | 1.877 | 0.983–3.585 | 0.056 | 1.571 | 0.905–2.728 | 0.108 | 1.877 | 0.983–3.585 | 0.056 | |||
| Sex, female vs male | 0.471 | 0.092–2.418 | 0.367 | — | — | — | — | — | — | |||
| Albumin, mg/mL | 0.908 | 0.792–1.042 | 0.169 | — | — | — | — | — | — | |||
| Hepatitis B/C, +ve vs –ve | 0.500 | 0.073–3.406 | 0.479 | — | — | — | — | — | — | |||
| CD4, % | 1.000 | 0.908–1.101 | 0.994 | — | — | — | — | — | — | |||
| Time on ART, weeks | 1.086 | 0.831–1.420 | 0.546 | — | — | — | — | — | — | |||
| Daily dose per 50 mg/m2 | 6.484 | 1.409–29.837 | 0.016 | 2.566 | 0.625–10.537 | 0.191 | — | — | — | |||
| Centre, QECH vs other | 1.067 | 0.235–4.843 | 0.933 | — | — | — | — | — | — | |||
wt/ht, weight for height.
aIncorporation into multivariate model based on P < 0.1.
bFinal multivariate model based on backwards elimination (P < 0.1).
Median unbound AUC0–12 was 27 840 ng·h/mL (15 416–85 992) in malnourished children versus 43 211 ng·h/mL (19 300–62 361) in normal children (P = 0.215). There was no difference in terms of the percentage unbound exposure of nevirapine (%AUCunbound) [malnourished: 48.4% (42.2%–63.2%) versus normal: 49.5% (41.6%–64.7%)] with a large degree of inter-subject variability. Within-subject variability in percentage of unbound nevirapine remained relatively constant across the 12 h dosing interval, ranging from 44.7% to 50.6% (CV% = 5.9%) in the normal nutritional group and from 48.0% to 50.7% (CV% = 2.2%) in the malnourished group.
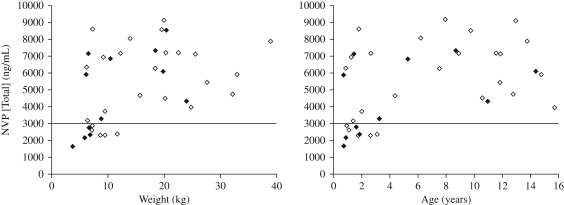
Figure 2 illustrates that mild to moderate malnutrition had no impact on the proportion of children achieving sub-therapeutic nevirapine Ctrough (defined as <3000 ng/mL). Five (20%) children from the normal group and 4 (33%) children from the malnourished group were sub-therapeutic (P = 0.432). The median (range) dose/m2 and age of these children were 302.4 (274.1–363.6) mg/m2 and 1.8 (0.9–3.2) years in the normal children and 279.1 (203.2–313.6) mg/m2 and 1.3 (0.7–1.8) years in the malnourished children. Five (63%) of the eight children receiving <300 mg/m2 (median dose 274.1 mg/m2; range 203.2–287.2 mg/m2; median nevirapine concentration 2617 ng/mL; range 1645–2875 ng/mL) had sub-therapeutic nevirapine Ctrough compared with only four (14%) of the 29 children receiving ≥300 mg/m2 (median dose 325.2 mg/m2; range 302.4–363.6 mg/m2; median nevirapine concentration 2307 ng/mL; range 2158–2371 ng/mL; P = 0.012).
Figure 2.
Nevirapine (NVP) trough concentrations by (a) weight and (b) age; and according to nutritional status [normal, weight for height >85% (n = 25); mild to moderate malnutrition, weight for height 70%–85% (n = 12)]. The continuous line indicates the recommended nevirapine minimum effective concentration [MEC (3000 ng/mL)]. Normal nutritional status (weight for height >85%), open diamonds; mild to moderate malnutrition (weight for height 70%–85%), filled diamonds.
Age and daily dose per 50 mg/m2 were significant univariate predictors of achieving a therapeutic Ctrough (Table 2). In the final multivariate model the effect of age (approaching significance) was found to be independent of dose; where for every yearly increase in age there was an ∼88% increase in the odds of achieving a therapeutic nevirapine concentration (P = 0.056, 95% CI 0.983–3.585).
Discussion
FDC tablets specifically designed for children including two paediatric Triomune® formulations [Triomune® Baby (6 mg of stavudine, 30 mg of lamivudine and 50 mg of nevirapine) and Triomune® Junior (twice the Baby dose)] have recently become available. However, many developing countries have not yet incorporated these new paediatric formulations into national programmes or have only just begun to do so. Since our study, Malawi has incorporated Triomune® Baby into its national programme, although currently only 15 ART treatment centres have access to this formulation. The use of divided adult FDC tablets in children therefore persists in many settings. In this context our study remains clinically important since it adds new data to previous studies of adult FDC tablets in children, particularly as it includes a larger number of children <3 years, and specifically examines the effect of malnutrition.
Persistent moderate and severe malnutrition are the most common entry criteria for children starting ART in Malawi.24 Once started on ART most children show rapid improvement in nutritional status; however, there is an extremely high early mortality rate associated with severe malnutrition. This is reflected in our study population in that few children presented at 6 weeks on ART with moderate malnutrition and no child presented with severe malnutrition. Recruitment of malnourished children, particularly in heavier weight bands, was slow. We continued to recruit in other groups until we had achieved our required sample number, which led to a larger total number of children recruited, with some imbalance across weight bands.
One limitation was our use of weight for height, which identified wasted, but not stunted or both wasted and stunted children reliably. We chose to focus on wasting as the most likely nutritional measure to affect pharmacokinetics and used weight for height as a proxy measure for this. Our ‘normal’ nutrition control group had very low weight-for-age and height-for-age z-scores; however, in the context of Malawi, where up to 50% of all children are stunted,25 we considered our ‘normal’ population to be representative. One previous study14 suggested that stunted children were at higher risk of under-dosing. Our study was not designed to examine this, however, since 31 (84%) of our 37 patients had height-for-age of less than −2SD, if stunting was a significant factor we might have expected a higher rate of under-dosing in the group as a whole. The effect of stunting remains a possible confounding factor and merits further study.
Our data suggest that the greatest driver of total drug exposure was dose/m2. Use of weight-based dosing tables and adult FDC tablets resulted in under-dosing of younger and lighter children. This is consistent with data from a growing number of studies in African children.14 Furthermore, after adjustment in multivariate analyses we observed no effect of malnutrition on any measure (AUC, Cmax, Ctrough) of total drug exposure. Therefore, the apparent trend towards lower AUC0–12 and Cmax in the malnourished group appears to be related to dose/m2 rather than malnutrition per se. Interestingly time on ART was also an independent predictor of nevirapine exposure (P = 0.014). This is potentially because in the current setting time on ART is a confounder of both age and dose (i.e. children receiving ART for longer are older and receiving higher doses/m2).
The prediction that there could be reduced protein binding in the malnourished group (potentially risking toxicity) proved unfounded. No significant differences were observed in the unbound fraction of nevirapine, although large variability was observed between subjects. This is consistent with adult studies,20,26 but we believe our study is the first to specifically determine unbound nevirapine levels in children with and without malnutrition.
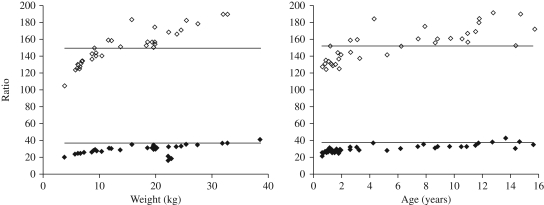
Due to differences in the paediatric dosing recommendations for nevirapine, lamivudine and stavudine it was difficult to achieve the recommended paediatric dose for all three components across the broad range of ages in this study using adult FDC tablets. This is illustrated in Figure 3, where it is evident that the youngest and lightest children require a higher ratio of nevirapine to both lamivudine and stavudine. Practical difficulties in dividing adult tablets, particularly into quarters, may also contribute to underdosing. Increasing the dose of nevirapine by increasing the FDC fraction given would increase the stavudine and lamivudine components and would carry an unacceptable risk of drug toxicity.
Figure 3.
Ratio of nevirapine daily dose (mg/m2) to daily dose (mg/kg) of lamivudine (filled diamonds) and stavudine (open diamonds) by (a) weight and (b) age (n = 37). Continuous lines represent the ratio of the recommended daily paediatric nevirapine dose (300 mg/m2) to the recommended daily paediatric dose of lamivudine (8 mg/kg; bottom line) and stavudine (2 mg/kg; top line).
Our data strongly support the use of paediatric formulations for younger and lighter children.
For simplicity, we would suggest that Triomune® Baby should be given to children <10 kg. Preliminary pharmacokinetic data from 71 Zambian children suggest that ratios of nevirapine, stavudine and lamivudine in the paediatric FDC Triomune® Baby or Junior are appropriate in children weighing >6 kg,27 but recent pharmacokinetic data suggest lower nevirapine exposure in infants weighing 3–6 kg (particularly those aged <5 months).28 Therefore, additional data are required in this group.
For older and heavier children some benefit may accrue from the use of Triomune® Junior but our data suggest that dose/m2 and total drug exposure were appropriate in the vast majority of this group. Any benefit of a change from adult to Triomune® Junior in this group must be balanced against the additional complexity and risk of medication error through having three different formulations of Triomune® in stock. This is against a background of widening access to ART by extending provision of ART from tertiary hospital referral centres towards community and district-based treatment programmes. The new Malawian National Guidelines reflect these considerations, opting to introduce Triomune® Baby in infants <10 kg but continuing the use of divided adult FDC tablets in heavier children.29
Funding
This study was funded in part by a grant from AVERT. The National Institute of Health Research (NIHR – Department of Health) and the Northwest Development Agency (NWDA) provided infrastructural and project support.
Transparency declarations
S. K. and D. B. have received research grants and travel bursaries from Merck, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Abbott and Tibotec. All other authors: none to declare.
Acknowledgements
We gratefully acknowledge the contribution of children, their carers and staff at the QECH.
References
- 1.UNICEF/WHO Technical Consultation: Improving Access to Appropriate Paediatric ARV Formulations. Geneva: WHO; 2004. November. Available at: http://www.who.int/3by5/en/finalreport.pdf. (15 August 2009, date last accessed) [Google Scholar]
- 2.Committee on Pediatric AIDS, Section on International Child Health. Increasing antiretroviral drug access for children with HIV infection. Pediatrics. 2007;119:838–45. doi: 10.1542/peds.2007-0273. [DOI] [PubMed] [Google Scholar]
- 3.Ministry of Health and Population Malawi: National AIDS Commission. Treatment of AIDS. Guidelines for the Use of Antiretroviral Therapy in Malawi, First Edition. 2003. [Google Scholar]
- 4.Menson EN, Walker AS, Sharland M, et al. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997-2005: cohort study. BMJ. 2006;332:1183–7. doi: 10.1136/bmj.332.7551.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Antiretroviral Therapy of HIV Infection in Infants and Children in Resource-Limited Settings, Towards Universal Access: Recommendations for a Public Health Approach. February 2006. Available at: http://www.unicef.org/aids/files/towards_universal_access_report_2008.pdf. (15 August 2009, date last accessed) [PubMed] [Google Scholar]
- 6.Lodha R, Upadhyay A, Kabra SK. Antiretroviral therapy in HIV-1 infected children. Indian Pediatr. 2005;42:789–96. [PubMed] [Google Scholar]
- 7.Puthanakit T, Oberdorfer A, Akarathum N, et al. Efficacy of highly active antiretroviral therapy in HIV-infected children participating in Thailand's National Access to Antiretroviral Program. Clin Infect Dis. 2005;41:100–7. doi: 10.1086/430714. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien DP, Sauvageot D, Zachariah R, et al. In resource-limited settings good early outcomes can be achieved in children using adult fixed-dose combination antiretroviral therapy. AIDS. 2006;20:1955–60. doi: 10.1097/01.aids.0000247117.66585.ce. [DOI] [PubMed] [Google Scholar]
- 9.Chokephaibulkit K, Plipat N, Cressey TR, et al. Pharmacokinetics of nevirapine in HIV-infected children receiving an adult fixed-dose combination of stavudine, lamivudine and nevirapine. AIDS. 2005;19:1495–9. doi: 10.1097/01.aids.0000183625.97170.59. [DOI] [PubMed] [Google Scholar]
- 10.Corbett A, Hosseinipour M, Nyirenda J, et al. Abstracts of the Forty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2005. Washington, DC, USA: American Society for Microbiology; Pharmacokinetics between trade and generic liquid and split tablet formulations of lamivudine, stavudine, and nevirapine in HIV-infected Malawian children. Abstract H-1106, p. 280. [Google Scholar]
- 11.Luzuriaga K, Bryson Y, McSherry G, et al. Pharmacokinetics, safety, and activity of nevirapine in human immunodeficiency virus type 1-infected children. J Infect Dis. 1996;174:713–21. doi: 10.1093/infdis/174.4.713. [DOI] [PubMed] [Google Scholar]
- 12.Krishnaswamy K. Drug metabolism and pharmacokinetics in malnourished children. Clin Pharmacokinet. 1989;17(Suppl 1):68–88. doi: 10.2165/00003088-198900171-00006. [DOI] [PubMed] [Google Scholar]
- 13.Jorquera F, Culebras JM, Gonzalez-Gallego J. Influence of nutrition on liver oxidative metabolism. Nutrition. 1996;12:442–7. doi: 10.1016/s0899-9007(96)00101-3. [DOI] [PubMed] [Google Scholar]
- 14.Ellis JC, L'Homme RF, Ewings FM, et al. Nevirapine concentrations in HIV-infected children treated with divided fixed-dose combination antiretroviral tablets in Malawi and Zambia. Antivir Ther. 2007;12:253–60. [PubMed] [Google Scholar]
- 15.NCHS/WHO. 1982. Combined sex references. As per Malawi National Guidelines for the management of severe acute malnutrition. [Google Scholar]
- 16.Government of Malawi. Interim Guidelines for the Management of Acute Malnutrition Through Community Based Therapeutic Care. 2007. [Google Scholar]
- 17.de Onis M, Onyango AW, Van den Broeck J, et al. Measurement and standardization protocols for anthropometry used in the construction of a new international growth reference. Food Nutr Bull. 2004;25:S27–36. doi: 10.1177/15648265040251S104. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. Food and Drug Administration. Centre for Drug Evaluation and Research. Guidance for Industry. Bioavailability and Bioequivalence Studies for Orally Administered Products - General Considerations. March 2003. Available at: http://www.fda.gov/downloads/Drugs/GuidanceCompliance RegulatoryInformation/Guidances/ucm070124.pdf. (15 August 2009, date last accessed. [Google Scholar]
- 19.European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products. Note for Guidance on the Investigation of Bioavailability and Bioequivalence. July 2001. Available at: http://apps.who.int/prequal/info_applicants/BE/emea_bioequiv.pdf. (15 August 2009, date last accessed) [Google Scholar]
- 20.Almond LM, Edirisinghe D, Dalton M, et al. Intracellular and plasma pharmacokinetics of nevirapine in human immunodeficiency virus-infected individuals. Clin Pharmacol Ther. 2005;78:132–42. doi: 10.1016/j.clpt.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Hollanders RM, van Ewijk-Beneken Kolmer EW, Burger DM, et al. Determination of nevirapine, an HIV-1 non-nucleoside reverse transcriptase inhibitor, in human plasma by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2000;744:65–71. doi: 10.1016/s0378-4347(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 22.La Porte CJL, Back DJ, Blaschke T, et al. Updated guidelines to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:5–14. [Google Scholar]
- 23.Sharland M, Blanche S, Castelli G, et al. PENTA guidelines for the use of antiretroviral therapy, 2004. HIV Med. 2004;5(Suppl 2):61–86. doi: 10.1111/j.1468-1293.2004.00227.x. [DOI] [PubMed] [Google Scholar]
- 24.Poerksen G, Nyirenda M, Pollock L, et al. Comparison of previous and present World Health Organization clinical staging criteria in HIV-infected Malawian children. AIDS. 2009;23:1913–6. doi: 10.1097/QAD.0b013e32832f7b39. [DOI] [PubMed] [Google Scholar]
- 25.National Statistical Office (NSO), Malawi. Malawi Demographic and Health Survey 2004. December 2005. Available at: http://www.measuredhs.com/pubs/pdf/FR175/FR-175-MW04.pdf. (15 August 2009, date last accessed) [Google Scholar]
- 26.Fayet A, Beguin A, de Tejada BM, et al. Determination of unbound antiretroviral drug concentrations by a modified ultrafiltration method reveals high variability in the free fraction. Ther Drug Monit. 2008;30:511–22. doi: 10.1097/FTD.0b013e3181817318. [DOI] [PubMed] [Google Scholar]
- 27.L'Homme RF, Kabamba D, Ewings FM, et al. Nevirapine, stavudine and lamivudine pharmacokinetics in African children on paediatric fixed-dose combination tablets. AIDS. 2008;22:557–65. doi: 10.1097/QAD.0b013e3282f4a208. [DOI] [PubMed] [Google Scholar]
- 28.Mulenga V, Fillekes Q, Kabamba D, et al. Abstracts of the Sixteenth Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, 2009. Alexandria, VA, USA: Foundation for Retrovirology and Human Health; Pharmacokinetics of nevirapine in HIV-infected infants with body weight 3–6 kg taking paediatric fixed dose combination tablets. Abstract 881. [Google Scholar]
- 29.Ministry of Health and Population Malawi: National AIDS Commission. Treatment of AIDS. Guidelines for the Use of Antiretroviral Therapy in Malawi, Third Edition. 2008. [Google Scholar]