Abstract
Objectives
First-line therapy for Pneumocystis jirovecii pneumonia (PCP) is trimethoprim/sulfamethoxazole. Few data exist to guide the choice of second-line therapy for patients failing or developing toxicity to first-line therapy.
Methods
A case note review of 1122 patients with 1188 episodes of HIV-associated PCP from three observational cohorts in Copenhagen, London and Milan, between 1989 and 2004, was conducted.
Results
Trimethoprim/sulfamethoxazole (962 PCP episodes, 81%) was the most frequently used first-line therapy, followed by intravenous pentamidine (87 episodes, 7%), clindamycin/primaquine (72 episodes, 6%) and ‘other’ (atovaquone, dapsone/pyrimethamine, trimetrexate or inhaled pentamidine; 67 episodes, 6%). Rates of unchanged therapy were trimethoprim/sulfamethoxazole = 79%, clindamycin/primaquine = 65% and pentamidine = 60% (P < 0.001). First-line therapy was changed because of failure in 82 (7%) episodes and because of toxicity in 198 (17%) episodes. Three month survival rates were trimethoprim/sulfamethoxazole = 85%, clindamycin/primaquine = 81% and pentamidine = 76% (P = 0.09). After adjustment for possible confounders, pentamidine was associated with a significantly greater risk of death at 3 months [hazard ratio (HR) = 2.0, 95% confidence interval (CI) = 1.2–3.4]. Second-line therapy survival rates differed: trimethoprim/sulfamethoxazole = 85%; clindamycin/primaquine = 87%; and pentamidine = 60% (P = 0.01). Multivariable time-updated Cox regression analysis showed a greater risk of death associated with pentamidine (HR = 3.3, 95% CI = 2.2–5.0), but not for clindamycin/primaquine, when both were compared with trimethoprim/sulfamethoxazole.
Conclusions
Pentamidine was associated with a greater risk of death when used as first- and second-line therapy for HIV-associated PCP, and was associated with more treatment changes. Clindamycin/primaquine appeared superior to pentamidine as second-line therapy for PCP in patients failing or developing toxicity with trimethoprim/sulfamethoxazole. In patients failing first-line treatment with non-trimethoprim/sulfamethoxazole regimens, second-line therapy should be trimethoprim/sulfamethoxazole.
Keywords: PCP, pneumocystis, therapy, adverse drug reactions, HIV-1
Introduction
Pneumocystis jirovecii is the cause of P. jirovecii pneumonia (PCP). Early in the AIDS pandemic, PCP was a major cause of morbidity and mortality. The use of PCP prophylaxis and combination antiretroviral therapy has dramatically reduced the risk of PCP, but it remains a significant problem among individuals without access to these therapies, among individuals who are intolerant or non-adherent and among individuals unaware of their HIV infection.1
The recommended treatment of PCP has remained unchanged for many years. Several options, but few data, exist to guide first- and second-line treatment for PCP. In two randomized clinical trials (RCTs) of HIV-associated PCP, trimethoprim/sulfamethoxazole and pentamidine were equally effective.2,3 However, interpretation of these trials remains limited by a high rate of treatment switches due to toxicity or treatment failure and a limited sample size. In a small non-crossover RCT, treatment with trimethoprim/sulfamethoxazole was associated with fewer adverse events and improved survival.4 Subsequent trials have demonstrated comparable efficacy of oral trimethoprim/sulfamethoxazole, clindamycin/primaquine and trimethoprim/dapsone, but patients with severe PCP were excluded.5,6 Use of primaquine, dapsone and trimethoprim may be restricted, because they can only be administered orally. Thus, intravenous pentamidine has been recommended as the main alternative to trimethoprim/sulfamethoxazole for moderate to severe PCP in spite of its toxicity.7 RCTs of second-line treatment of PCP have not been performed. A meta-analysis suggested that second-line treatment with clindamycin/primaquine is associated with a more favourable outcome compared with pentamidine.8 In an updated systematic review, which included data from patients in the present study who switched treatment due to failure, this finding was confirmed.9 In the present study, we provide a more detailed analysis of the efficacy and toxicity of first- and second-line PCP treatment, based on 1188 episodes of HIV-associated PCP between 1989 and 2004.
Methods
Patients
Data were collected from three cohorts of HIV-associated PCP in Copenhagen (Denmark), London (UK) and Milan (Italy). In Copenhagen, all cases of HIV-associated PCP admitted to the Department of Infectious Diseases or to the Intensive Care Unit (ICU) of Hvidovre University Hospital or Copenhagen University Hospital, from January 1989 through June 2004, were included. Patients were identified from a prospective study of HIV-associated PCP and a manual search of the case records of all HIV-infected patients during the study period.10 In London, all cases of HIV-associated PCP admitted to the specialist HIV/AIDS inpatient facility and to the ICU of University College London Hospitals, from January 1989 through June 2004, were included. Patients were identified from a prospective study of HIV-associated PCP, and from a manual and electronic search of the case records of all HIV-infected patients receiving care at these centres during the study period. In Milan, all cases of HIV-associated PCP admitted to the II Department of Infectious Diseases and to the ICU of Luigi Sacco Hospital from January 1994 through June 2004 were included. All patients had PCP diagnosed by analysis of bronchoscopic alveolar lavage (BAL) fluid, induced sputum, sputum/oral rinse or at autopsy.
Data collection
Treatment data were collected through chart review. Patient demographics, laboratory data, mode of diagnosis of PCP, presence of pulmonary co-pathology, prescription of PCP prophylaxis, dates of treatment start and cessation, treatment switches and reasons for treatment changes were recorded. Additionally, development of pneumothorax, need for ICU and mechanical ventilation (MV), and outcome were recorded for each patient.
Treatment
During the study period, choice of PCP treatment was consistent across all three centres, using similar protocols for drug dosage and administration as recommended in international guidelines:11 a 21 day course of treatment was standard. Additionally, nebulized pentamidine (8 mg/kg/day once daily) was used in a few patients at the beginning of the study period. From 1990 onwards, adjuvant glucocorticoids (methylprednisolone or prednisone in dosages as recommended12) were used for patients with moderate to severe PCP (based on a PaO2 of <9.3 kPa, breathing room air at admission) at all sites. Use of PCP prophylaxis was defined as a patient's receipt of specific PCP prophylaxis during the 3 months prior to admission.
Definitions of endpoints
The reason for a switch of drug treatment was classified as either caused by toxicity or caused by suspected failure of treatment.
Toxicity was determined by the attending physician and frequently was a composite of the following criteria: dermatological—severe rash not present prior to starting PCP therapy; haematological—fall in neutrophil count, methaemoglobinaemia >5%, anaemia (fall in haemoglobin by >3 g/dL) or fall in platelet count (if normal at baseline, fall to <100 × 109/L, or if low at baseline, fall by >25 × 109/L); renal—rise in serum creatinine to >2× the upper limit of normal; gastrointestinal—severe nausea with or without vomiting on ≥2 days, diarrhoea, or a rise in alanine or aspartate aminotransferase or alkaline phosphatase enzyme levels by >2× the upper limit of normal; drug fever—onset of fever to >38°C, after the patient had been afebrile on therapy for >48 h; or other—hypotension, hypoglycaemia, symptomatic hypocalcaemia or elevated amylase level by >5× the upper limit of normal.
Treatment failure was defined as persistent fever and worsening hypoxia, and/or radiographic deterioration, occurring after ≥4 full days of first-line or second-line therapy. Survival was defined as being alive 3 months after therapy was initiated. Treatment response was defined as survival or definitive clinical improvement (defervescence, improvements in dyspnoea and chest radiographs, and hospital discharge).
Statistical analysis
Data were analysed using Stata, version 9.1 (StataCorp). The main endpoint was 3 month mortality after the start of initial PCP treatment. Patients were left-truncated with regard to episode of PCP (i.e. only the last episode was included in analyses). Time to mortality was analysed with multivariable Cox regression models. In all analyses, PCP treatment, arterial PaO2 on admission, age, latest CD4 count, year of diagnosis and country were placed into the model. Additional predictors assessed were use of PCP prophylaxis, sex, HIV risk transmission group and detection of bacterial co-infection or cytomegalovirus (CMV) in BAL fluid. From these predictor variables, a final parsimonious model was defined by backward selection using minimization of the Aikaike Information Criterion, which has been shown to be an appropriate method for selecting the degree to which a model should be simplified.13,14 The proportionality of hazards assumptions was tested using the Schoenfeld residual test and overall model fit by Cox–Snell residuals. Detection of CMV was dropped from the final model, because of many missing variables. In all analyses, robust (Huber–White) variance estimates were used.
Hazard ratios (HRs) are presented with robust 95% confidence intervals (CIs). Since changes of treatment were frequent, many patients died while receiving second- or third-line drugs. In an attempt to analyse the risk of death according to the drug received, time-updated Cox regression analysis was done by splitting exposure time according to each treatment received, which allowed a time-dependent analysis of exposure to each drug. Prognostic variables included were PaO2 at admission, age, CD4 cell count, year of diagnosis, bacterial co-infections, use of previous PCP prophylaxis and country. Optimally, time-updated regression analysis would have included PaO2 at each treatment change; however, since PaO2 measurements during the course of treatment were either unavailable or unreliable, admission PaO2 was used as a fixed value for each patient in the analysis.
Results
In total, 1188 episodes of HIV-associated PCP in 1122 patients from January 1989 through June 2004 were analysed. There were 555 episodes of PCP from Copenhagen, 418 episodes from London and 215 episodes from Milan. Key data for each centre are shown in Table 1.
Table 1.
Clinical characteristics of study subjects by country
| Variable | Copenhagen, Denmark | London, UK | Milan, Italy | Total |
|---|---|---|---|---|
| No. of PCP episodes/patients | 555/531 | 418/376 | 215/215 | 1188/1122 |
| Period, n (%) | ||||
| 1989–92 | 264 (48%) | 156 (37%) | NA | 420 (35%) |
| 1993–96 | 179 (32%) | 172 (41%) | 54 (25%) | 405 (34%) |
| 1997–2000 | 63 (11%) | 57 (14%) | 67 (31%) | 187 (16%) |
| 2001–04 | 49 (9%) | 33 (8%) | 94 (44%) | 176 (15%) |
| Male sex, n (%) | 485 (91%) | 346 (92%) | 170 (79%) | 1001 (89%) |
| Median age, years | 39 (17–76) | 37 (21–72) | 37 (20–75) | 39 (17–76) |
| Method of diagnosis, n (%) | ||||
| bronchoalveolar lavage | 354 (63%) | 385 (92%) | 196 (91%) | 935 (79%) |
| induced sputum | 181 (33%) | 20 (5%) | 15 (7%) | 216 (18%) |
| sputum/oral wash | 18 (4%) | 11 (3%) | 4 (2%) | 33 (3%) |
| autopsy | 2 (0.4%) | 2 (0.5%) | 4 (0.3%) | |
| CD4 T cell count | 6 (0–349) | 40 (0–400) | 30 (0–378) | 21 (0–400) |
| HIV transmission group, n (%) | ||||
| homosexual | 334 (63%) | 309 (82%) | 42 (20%) | 685 (61%) |
| heterosexual | 107 (20%) | 46 (12%) | 79 (37%) | 233 (21%) |
| IDU | 30 (6%) | 8 (2%) | 94 (44%) | 132 (13%) |
| transfusion | 9 (2%) | 9 (1%) | ||
| unknown | 50 (9%) | 13 (3%) | 63 (6%) | |
| PaO2 at admission, kPa | 8.6 (4.1–13.9) | 9.4 (3.9–13.6) | 8.4 (3.5–14.0) | 8.8 (2.9–14.0) |
| Adjunctive steroid, n (%) | 338 (61%) | 193 (46%) | 118 (55%) | 649 (55%) |
| PCP as first AIDS diagnosis, n (%) | 486 (88%) | 368 (88%) | NA | 854 (88%) |
| First-line treatment regimen, n (%) | ||||
| SXT | 507 (91%) | 263 (63%) | 192 (89%) | 962 (81%) |
| pentamidine | 40 (7%) | 44 (11%) | 3 (1%) | 87 (7%) |
| clindamycin/primaquine | 4 (1%) | 53 (13%) | 15 (7%) | 72 (6%) |
| othera | 4 (1%) | 58 (14%)b | 5 (2%) | 67 (6%) |
| Switch of first-line treatment, n (%) | ||||
| no switch | 466 (84%) | 245 (59%) | 166 (77%) | 877 (74%) |
| toxicity switch | 50 (9%) | 120 (29%) | 37 (17%) | 207 (17%) |
| failure switch, >4 days of treatment | 31 (6%) | 43 (10%) | 8 (4%) | 82 (7%) |
| failure switch, <5 days of treatment | 8 (1%) | 10 (2%) | 4 (2%) | 22 (2%) |
| Second-line treatment regimen, n (%) | ||||
| SXT | 8 (9%) | 26 (15%) | 7 (14%) | 41 (13%) |
| pentamidine | 62 (70%) | 32 (18%) | 9 (18%) | 103 (33%) |
| clindamycin/primaquine | 16 (18%) | 83 (48%) | 27 (55%) | 126 (41%) |
| othera | 3 (3%) | 32 (18%) | 6 (12%) | 41 (13%) |
| Switch to third-line regimen, n (%) | 7 (1%) | 43 (10%) | 15 (7%) | 65 (5%) |
| ICU admission, n (%) | 66 (12%) | 46 (11%) | 11 (6%) | 123 (10%) |
| MV, n (%) | 57 (10%) | 28 (7%) | 10 (5%) | 95 (8%) |
| 3 month mortality, % | 18.2% | 13.1% | 7.9% | 14.5% |
IDU, intravenous drug use; NA, not available; SXT, trimethoprim/sulfamethoxazole.
All values are medians and ranges unless otherwise stated.
aOther treatment: atovaquone; dapsone/trimethoprim; or inhaled pentamidine.
bForty-nine patients received inhaled pentamidine.
Following treatment of PCP, 17 (1.5%) patients were lost to follow-up before 3 months.
Throughout the study period, the age of patients and the severity of PCP, as judged by the median PaO2 or the need for admission to the ICU, remained unchanged. Only minor variation in age or severity of disease, as measured by PaO2 at admission, was observed between the three countries, but patients from Milan were more often female (21%) and more often had a history of intravenous drug use (44%) compared with patients from Copenhagen (8% female, 2% intravenous drug users) and London (8% female, 6% intravenous drug users). During the study period, an increasing number of patients presented with PCP as their first AIDS-defining event (in London and Copenhagen, data unavailable for Milan: 1989–92, 87%; 1993–96, 85%; 1997–2000, 93%; and 2001–04, 95%; χ2 for trend P = 0.03) and had never received PCP prophylaxis (1989–92, 88%; 1993–96, 86%; 1997–2000, 93%; and 2001–04, 96%; χ2 for trend P = 0.0072).
Treatment
First-line treatment regimens, reasons for switch and outcome are shown in Tables 2 and 3.
Table 2.
First-line treatment for PCP and reasons for switching treatment
| First-line treatment regimen | Toxicity switch, n (%) | Failure switch, n (%) |
|---|---|---|
| SXT, n = 962 | 159 (17) | 46 (5) |
| Pentamidine, n = 87 | 22 (25) | 13 (15) |
| Clindamycin/primaquine, n = 72 | 18 (25) | 7 (10) |
| Other treatment,an = 67 | 8 (12) | 16 (24) |
| Total | 207 (17) | 82 (7) |
SXT, trimethoprim/sulfamethoxazole.
Data from all episodes of PCP.
Failure switch: switch after >4 full days of treatment because of treatment failure.
Episodes in which treatment was changed before 5 days (n = 22), because of suspected toxicity, are not listed.
Part of these data was published previously.9
aOther treatment: inhaled pentamidine (n = 49); dapsone (n = 8); atovaquone (n = 7); and trimetrexate (n = 3).
Table 3.
Univariate and multivariable analysis of 3 month survival from PCP according to first-line treatment regimen
| Characteristics | Subjects, n (%) | Univariate analysis, HR (95% CI) | P | Adjusted HR (95% CI) | P |
|---|---|---|---|---|---|
| First-line treatment | |||||
| SXT | 929 (83%) | 1.0 (reference group) | |||
| pentamidine | 74 (7%) | 1.70 (1.04–2.77) | 0.035 | 2.0 (1.2–3.4) | 0.013 |
| clindamycin/primaquine | 63 (6%) | 1.25 (0.70–2.20) | 0.465 | 1.6 (0.8–3.2) | 0.145 |
| other treatment | 56 (5%) | 0.22 (0.05–0.90) | 0.035 | 0.7 (0.1–3.5) | 0.682 |
| PaO2 at diagnosis (kPa) | |||||
| >10.2 | 274 (24%) | 1.0 (reference group) | |||
| 8.8–10.2 | 247 (22%) | 1.40 (0.83–2.37) | <0.001a | 1.2 (0.7–2.1) | 0.45 |
| 7.3–8.7 | 276 (25%) | 1.47 (0.88–2.45) | 1.3 (0.8–2.3) | 0.281 | |
| <7.3 | 266 (24%) | 3.12 (1.97–4.93) | 3.2 (1.9–5.4) | <0.001 | |
| missing data | 59 (5%) | ||||
| Age (years) | |||||
| 17–29 | 167 (15%) | 1.0 (reference group) | |||
| 30–39 | 502 (45%) | 0.93 (0.56–1.55) | <0.001a | 0.7 (0.4–1.3) | 0.29 |
| 40–49 | 293 (27%) | 1.39 (0.83–2.35) | 1.3 (0.7–2.2) | 0.411 | |
| ≥50 | 160 (14%) | 2.75 (1.63–4.63) | 2.5 (1.4–4.3) | 0.001 | |
| CD4 cell count (cells/mm3) | |||||
| >100 | 158 (14%) | 1.0 (reference group) | |||
| 50–99 | 168 (15%) | 0.87 (0.42–1.80) | 0.002a | 0.7 (0.3–1.6) | 0.406 |
| 25–49 | 155 (14%) | 1.28 (0.65–2.52) | 1.4 (0.7–2.9) | 0.338 | |
| <25 | 537 (48%) | 2.24 (1.31–3.85) | 1.9 (1.1–3.5) | 0.027 | |
| missing data | 104 (9%) | ||||
| Year of diagnosis | |||||
| 1989–92 | 389 (35%) | 1.0 (reference group) | |||
| 1993–96 | 376 (34%) | 1.36 (0.97–1.91) | 0.014a | 1.3 (0.9–1.9) | 0.22 |
| 1997–2000 | 185 (16%) | 0.81 (0.50–1.31) | 0.7 (0.4–1.3) | 0.321 | |
| 2001–04 | 172 (15%) | 0.56 (0.32–0.99) | 0.6 (0.3–1.2) | 0.211 | |
| Bacterial co-infection detected at admission | |||||
| no | 1014 (90%) | 1.0 (reference group) | |||
| yes | 108 (10%) | 1.89 (1.25–2.85) | 0.002 | 1.48 (0.9–2.4) | 0.119 |
| Prescribed PCP prophylaxis | |||||
| no | 929 (83%) | 1.0 | |||
| yes | 193 (17%) | 1.53 (1.07–2.18) | 0.017 | 1.7 (1.1–2.6) | 0.106 |
| Country | |||||
| Denmark | 531 (47%) | 1.0 (reference group) | |||
| UK | 376 (34%) | 0.7 (0.5–1.1) | 0.07 | 1.2 (0.8–1.9) | 0.368 |
| Italy | 215 (19%) | 0.4 (0.2–0.7) | <0.001 | 0.6 (0.3–1.1) | 0.119 |
SXT, trimethoprim/sulfamethoxazole.
Analysis of last episode of PCP.
Multivariable analysis adjusted for PaO2 at admission, age, year of admission, CD4 cell count, PCP prophylaxis, bacterial co-infection and country.
Goodness of fit: log likelihood = –944.8, χ2 = 102.8, P < 0.00001.
aχ2 for trend.
Trimethoprim/sulfamethoxazole was used as the first-line treatment regimen in 81% of episodes (n = 962). Intravenous pentamidine (n = 87, 7%) and clindamycin/primaquine (n = 72, 6%) were the most frequently used alternative drugs. In London prior to 1992, 49 patients (4%) were treated with nebulized pentamidine. Overall, 18 patients (2%) received dapsone/trimethoprim, atovaquone or trimetrexate as their first-line treatment regimen (Table 1).
In 311 (26%) of episodes, first-line treatment was changed to another drug; in 207 because of toxicity and in 104 because of treatment failure. Among patients in who first-line treatment changed due to suspected failure, 22 patients were changed before 4 full days of therapy and so were excluded from analysis. The majority of such early ‘failure’ changes occurred before 1992. The median time to change was 10 days, regardless of reason. In 65 (6%) of episodes, a third-line regimen was chosen because of toxicity (n = 48) or failure (n = 17). Commonly observed adverse effects leading to a change of treatment were dermatological and haematological toxicity (trimethoprim/sulfamethoxazole), renal toxicity (intravenous pentamidine) and haematological toxicity (clindamycin/primaquine).
Change in first-line treatment occurred more frequently in London (41%) compared with Milan (23%) and Copenhagen (16%). The higher rate of treatment change in London was only partly explained by the use of nebulized pentamidine; 22 patients were changed from nebulized pentamidine (19 failure and 3 toxicity). Excluding these cases, the rate of treatment change in London was 36%. In all three cohorts, there was overall similar availability and access to alternative PCP treatments during the study period. The observed difference was largely explained by less frequent switching due to toxicity; only 9% of patients were switched due to toxicity in Copenhagen compared with 29% in London and 17% in Milan. However, across the study period, only relatively minor changes in the rate and the reasons for change of treatment were observed (23% of patients had their first-line treatment changed in 1989–92 compared with 18% in 2001–04, P = 0.23).
Response to first-line treatment regimen
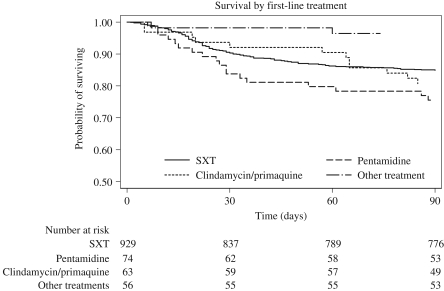
Overall response rates were initially analysed as a function of first-line treatment without adjustment for change of treatment (intention-to-treat) (Table 3 and Figure 1). Three month mortality rates according to first-line treatment were trimethoprim/sulfamethoxazole = 15%, clindamycin/primaquine = 19% and pentamidine = 24%. Mortality was significantly lower (3%) for the few patients who received first-line treatment with ‘other’ treatment (nebulized pentamidine, atovaquone, dapsone/trimethoprim or trimetrexate); however, the majority had mild disease at admission (median PaO2 = 10.7 kPa, compared with PaO2 = 8.6 kPa for patients receiving trimethoprim/sulfamethoxazole, P = 0.0001). In contrast, use of pentamidine was associated with a significantly increased risk of death at 3 months in multivariable analysis, after adjustment for possible confounders [HR = 2.0 (95% CI = 1.2–3.4)]. There was no indication that the efficacy of trimethoprim/sulfamethoxazole declined over the study period. In intention-to-treat analysis of trimethoprim/sulfamethoxazole as first treatment, 3 month mortality fell from 15% in 1989–92 and 18% in 1993–96 to 12% in 1997–2000 and 10% in 2001–04.
Figure 1.
Kaplan–Meier plot of 3 month mortality according to first-line treatment by intention-to-treat. SXT, trimethoprim/sulfamethoxazole.
Response to second-line therapy
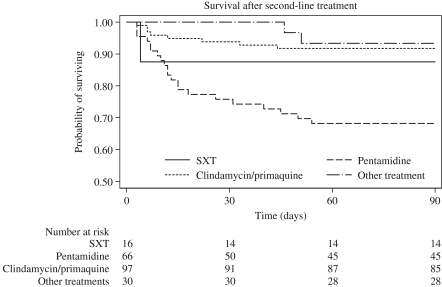
Outcome, defined as survival 3 months after admission, was analysed according to choice of second-line therapy, in which treatment was changed due to toxicity or because of failure (Figure 2). In patients switched because of toxicity, 3 month survival rates were numerically, but non-significantly, lower than for patients switched due to treatment failure: trimethoprim/sulfamethoxazole, 0/9 versus 4/24; pentamidine, 18/63 versus 17/32; clindamycin/primaquine, 7/101 versus 7/20; and ‘other’, 2/33 versus 1/6. The 3 month mortality was significantly higher for patients receiving pentamidine (53%) compared with trimethoprim/sulfamethoxazole (17%) and clindamycin/primaquine (29%) when used because of treatment failure [odds ratio = 3.5 (95% CI = 2.4–4.9)]. In time-updated Cox regression analysis, in which exposure time to individual drugs was taken into account, use of pentamidine conferred an adjusted HR of 3.3 (95% CI = 2.2–5.0) of risk of death before 3 months compared with trimethoprim/sulfamethoxazole (Table 4).
Figure 2.
Kaplan–Meier survival plot of time to death according to second-line treatment after switch of treatment. Only patients in which secondary treatment due to toxicity or suspected treatment failure after 4 days of primary treatment are shown. SXT, trimethoprim/sulfamethoxazole.
Table 4.
Risk of mortality at 3 months according to treatment by time-updated Cox regression analysis
| Treatment | Unadjusted HR (95% CI) | Adjusted HRa (95% CI) |
|---|---|---|
| SXT (reference group) | 1 | 1 |
| Pentamidine | 3.5 (2.4–4.9) | 3.3 (2.2–5.0) |
| Clindamycin/primaquine | 1.2 (0.8–2.0) | 1.6 (0.9–2.8) |
| Other drugs | 0.7 (0.3–1.5) | 1.4 (0.6–3.6) |
SXT, trimethoprim/sulfamethoxazole.
Analysis of last episode of PCP.
Goodness of fit: log likelihood = –932.8, χ2 = 133.3, P < 0.00001.
aAdjusted for PaO2 at admission, age, CD4 cell count, year of diagnosis, bacterial co-infection, previous PCP prophylaxis and country.
The majority of patients initiated treatment with trimethoprim/sulfamethoxazole (n = 962). Overall, a proportionally higher rate of patients who experienced trimethoprim/sulfamethoxazole toxicity were switched to clindamycin/primaquine (n = 88, 56%) rather than pentamidine (n = 54, 34%), whereas more patients received pentamidine (n = 27, 59%) compared with clindamycin/primaquine (n = 16, 35%) for treatment failure. Restricting the analysis to patients initiating treatment with trimethoprim/sulfamethoxazole and who switched therapy due to toxicity (n = 159), 3 month mortality rates were pentamidine = 33% and clindamycin/primaquine = 7% (P = 0.0001). Among patients who failed a first-line regimen of trimethoprim/sulfamethoxazole (n = 46), the 3 month mortality rate for pentamidine was 60% (n = 27) compared with 38% for clindamycin/primaquine (n = 16) (P = 0.17).
Rates of ICU admission and MV were similar among patients failing first-line treatment who were switched to pentamidine (ICU = 50%, MV = 43%) and to clindamycin/primaquine (ICU = 45%, MV = 30%) (P = 0.32 and 0.73, respectively). Among patients who were switched because of toxicity, 14% of patients who received pentamidine as second-line treatment were already in the ICU at the time of the switch, compared with 6% of patients receiving clindamycin/primaquine and none receiving trimethoprim/sulfamethoxazole (P = 0.112). After switch of treatment to pentamidine (regardless of reason), 9% of patients had progressive disease necessitating subsequent ICU admission, compared with 5% of patients switched to clindamycin/primaquine and 5% of trimethoprim/sulfamethoxazole-switched patients (P = 0.156).
Bacterial co-infection
The impact of concomitant infection on outcome of drug treatment was assessed. According to first-line PCP therapy, rates of concomitant bacterial lower respiratory tract infection were pentamidine = 15%, trimethoprim/sulfamethoxazole = 10% and clindamycin/primaquine = 5% (P = 0.031). Among patients switching due to toxicity, a higher rate of concomitant bacterial co-infection was seen in patients switching to pentamidine = 13% compared with other second-line treatment = 3% (P = 0.025).
Discussion
A recent systematic review of second-line therapy for HIV-related PCP, which included some second-line treatment outcome data from the current cohort study, examined the efficacy of different second-line treatments for HIV-associated PCP.9 Analysis was limited to patients failing first-line treatment; adjustments for potential confounding by baseline factors or toxicity were not possible due to the study design (a pooled analysis with an average odds ratio of survival). In this study, we have expanded our analysis, and describe in detail the outcome and toxicity for use of both first- and second-line treatment of PCP.
Our data show that both first- and second-line treatment with intravenous pentamidine was associated with a significantly worse outcome compared with other drug regimens. The increased risk of death associated with pentamidine was primarily related to inferior efficacy when used as second-line treatment. Importantly, the greater risk of death was observed for patients switched to pentamidine because of suspected treatment failure as well as patients who changed to pentamidine because of toxicity. Overall, we observed a better outcome from PCP treated with trimethoprim/sulfamethoxazole compared with other treatment options, for both first- and second-line treatment.
Several explanations may be offered for the apparent inferiority of intravenous pentamidine. First, toxicity caused by pentamidine may have contributed. Several studies have described serious and fatal adverse effects from pentamidine.15,16 Second, the inferior efficacy of pentamidine could result from the absence of an antibacterial effect (in contrast to trimethoprim/sulfamethoxazole or clindamycin/primaquine). This latter hypothesis is difficult to test, but is in part supported by the significantly higher rate of concomitant bacterial co-infection among patients treated with pentamidine, when compared with other agents. Finally, the lower response rate for patients who were switched to pentamidine because of toxicity could be explained by more severe disease rather than lower efficacy, as patients failing their first-line treatment regimen preferentially were switched to pentamidine. However, disease severity, as assessed by need for ICU admission and MV at the time of switch, was similar among patients switched to pentamidine and to clindamycin/primaquine as a result of treatment failure. Additionally, the increased risk of death associated with pentamidine as second-line treatment remained statistically significant in the time-updated model that accounted for individual drug exposure throughout the episode of PCP; albeit, the HR fell from 12- to 3-fold.
The finding of an apparent excess mortality associated with intravenous pentamidine must be interpreted with caution. Since our study was retrospective and observational, inferences about causation are speculative. Our study includes patients from four hospitals in three countries and spans a considerable time period during which several changes in the management of HIV-related PCP were introduced, including adjunctive corticosteroids in 1990 and improved ICU management of all-cause acute respiratory failure from 1996 onwards.12,17 During the study period, the incidence of and mortality from PCP declined. The introduction of combination antiretroviral therapy (cART) after 1996 has led to markedly improved immune function and, thus, explains the decreased incidence of PCP.18 In our study, very few patients were receiving cART at the time of PCP diagnosis, which for the majority was the manifestation of HIV. Until recently, there had not been randomized controlled trials to support or discourage early initiation of cART in the setting of an acute opportunistic infection.19 In our hands, we have deferred cART until the completion of antimicrobial treatment in order to minimize drug toxicity and interactions. A recent study from the AIDS Clinical Trials Group, however, indicates that the early use of cART resulted in less AIDS progression and death.20 Thus, it is likely that the use of cART after diagnosis of PCP may have contributed to improvements in outcome over time.
In clinical practice it may be difficult to distinguish between progressive PCP, drug toxicity or concomitant infection as the cause of clinical deterioration. Previously reported failure rates with trimethoprim/sulfamethoxazole as first-line treatment in HIV-associated PCP have varied considerably, ranging from 10% to 40%.21–23 We observed pronounced differences between the three centres in frequency of treatment changes. More patients were switched due to toxicity in London compared with Copenhagen. It is likely that the observed difference between centres in management of toxicity are explained by different treatment practices. In Denmark, patients experiencing minor toxicity from trimethoprim/sulfamethoxazole are managed by dose reduction. In contrast, in London and Milan toxicity is usually managed by switching treatment to a different drug regimen. Overall, a proportionally higher rate of patients who experienced toxicity from trimethoprim/sulfamethoxazole was switched to clindamycin/primaquine rather than pentamidine compared with those switched because of failure. Although we aimed to correct for this differential choice of second-line agents, residual confounding may be present.
The implications of our study help inform the choice of second-line treatment in patients intolerant of or unresponsive to first-line regimens. Based on our findings, we suggest that clindamycin/primaquine should be preferred to pentamidine for patients who develop treatment-limiting toxicity from trimethoprim/sulfamethoxazole. Although, in theory, recently described sulpha resistance in P. jirovecii may limit the efficacy of trimethoprim/sulfamethoxazole, we observed consistent efficacy of first-line treatment with trimethoprim/sulfamethoxazole over time.24,25 We found equivalent efficacy from trimethoprim/sulfamethoxazole and clindamycin/primaquine when used for patients failing first-line therapy with non-trimethoprim/sulfamethoxazole treatment first-line regimens. Our results suggest that trimethoprim/sulfamethoxazole should remain the second-line treatment of choice, based on greater clinical data and experience with trimethoprim/sulfamethoxazole. For patients failing trimethoprim/sulfamethoxazole therapy as first-line therapy, our findings suggest that clindamycin/primaquine should be used in preference to intravenous pentamidine. However, this recommendation is based on observational data with a high risk of confounding by indication.
Funding
R. F. M. is supported by The National Institutes of Health, USA (Grant R01 HL-090335) and The Dr Hadwen Trust for Humane Research. In Copenhagen, Milan and London, data were generated as part of routine clinical care of patients.
Transparency declarations
None to declare.
Acknowledgements
Part of this work was presented at the American Thoracic Society Meeting, San Diego, CA, USA, 2006 (Abstract A203) and the Ninth International Workshop on Opportunistic Protists, Lisbon, Portugal, 2006 (Abstract PL102).
References
- 1.Morris A, Lundgren JD, Masur H, et al. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004;10:1713–20. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim–sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A prospective randomized trial. Ann Intern Med. 1986;105:37–44. doi: 10.7326/0003-4819-105-1-37. [DOI] [PubMed] [Google Scholar]
- 3.Klein NC, Duncanson FP, Lenox TH, et al. Trimethoprim–sulfamethoxazole versus pentamidine for Pneumocystis carinii pneumonia in AIDS patients: results of a large prospective randomized treatment trial. AIDS. 1992;6:301–5. doi: 10.1097/00002030-199203000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Sattler FR, Cowan R, Nielsen DM, et al. Trimethoprim–sulfamethoxazole compared with pentamidine for treatment of Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a prospective, noncrossover study. Ann Intern Med. 1988;109:280–7. doi: 10.7326/0003-4819-109-4-280. [DOI] [PubMed] [Google Scholar]
- 5.Safrin S, Finkelstein DM, Feinberg J, et al. Comparison of three regimens for treatment of mild to moderate Pneumocystis carinii pneumonia in patients with AIDS: a double- blind, randomized trial of oral trimethoprim–sulfamethoxazole, dapsone–trimethoprim, and clindamycin–primaquine. Ann Intern Med. 1996;124:792–802. doi: 10.7326/0003-4819-124-9-199605010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Toma E, Thorne A, Singer J, et al. Clindamycin with primaquine vs. trimethoprim–sulfamethoxazole therapy for mild and moderately severe Pneumocystis carinii pneumonia in patients with AIDS: a multicenter, double-blind, randomized trial (CTN 004). CTN-PCP Study Group. Clin Infect Dis. 1998;27:524–30. doi: 10.1086/514696. [DOI] [PubMed] [Google Scholar]
- 7.Walzer PD. In: Pneumocystis carinii. Mandell GL, Bennett JE, Dolin R, editors. Philadelphia: Churchill Livingstone; 2000. pp. 2781–95. Principles and Practice of Infectious Diseases. [Google Scholar]
- 8.Smego RA, Jr, Nagar S, Maloba B, et al. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med. 2001;161:1529–33. doi: 10.1001/archinte.161.12.1529. [DOI] [PubMed] [Google Scholar]
- 9.Benfield T, Atzori C, Miller RF, et al. Second-line salvage treatment of AIDS-associated Pneumocystis jirovecii pneumonia: a case series and systematic review. J Acquir Immune Defic Syndr. 2008;48:63–7. doi: 10.1097/QAI.0b013e31816de84d. [DOI] [PubMed] [Google Scholar]
- 10.Benfield TL, Helweg-Larsen J, Bang D, et al. Prognostic markers of short-term mortality in AIDS-associated Pneumocystis carinii pneumonia. Chest. 2001;119:844–51. doi: 10.1378/chest.119.3.844. [DOI] [PubMed] [Google Scholar]
- 11.Helweg-Larsen J, Lundgren JD, Benfield T. Pneumocystis carinii pneumonia. Current Treatment Options in Infectious Diseases. 2002;4:363–75. [Google Scholar]
- 12.The National Institutes of Health–University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. Consensus statement on the use of corticosteroids as adjunctive therapy for pneumocystis pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323:1500–4. doi: 10.1056/NEJM199011223232131. [DOI] [PubMed] [Google Scholar]
- 13.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974;6:716–23. [Google Scholar]
- 14.Ambler G, Brady AR, Royston P. Simplifying a prognostic model: a simulation study based on clinical data. Stat Med. 2002;21:3803–22. doi: 10.1002/sim.1422. [DOI] [PubMed] [Google Scholar]
- 15.Balslev U, Nielsen TL. Adverse effects associated with intravenous pentamidine isethionate as treatment of Pneumocystis carinii pneumonia in AIDS patients. Dan Med Bull. 1992;39:366–8. [PubMed] [Google Scholar]
- 16.O'Brien JG, Dong BJ, Coleman RL, et al. A 5-year retrospective review of adverse drug reactions and their risk factors in human immunodeficiency virus-infected patients who were receiving intravenous pentamidine therapy for Pneumocystis carinii pneumonia. Clin Infect Dis. 1997;24:854–9. doi: 10.1093/clinids/24.5.854. [DOI] [PubMed] [Google Scholar]
- 17.Davis JL, Morris A, Kallet RH, et al. Low tidal volume ventilation is associated with reduced mortality in HIV-infected patients with acute lung injury. Thorax. 2008;63:988–93. doi: 10.1136/thx.2008.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mocroft A, Katlama C, Johnson AM, et al. AIDS across Europe, 1994–98: the EuroSIDA study. Lancet. 2000;356:291–6. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 19.Benson CA, Kaplan JE, Masur H, et al. Treating opportunistic infections among HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association/Infectious Diseases Society of America. MMWR Recomm Rep. 2004;53:1–112. [PubMed] [Google Scholar]
- 20.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selik RM, Starcher ET, Curran JW. Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS. 1987;1:175–82. [PubMed] [Google Scholar]
- 22.Ewig S, Bauer T, Schneider C, et al. Clinical characteristics and outcome of Pneumocystis carinii pneumonia in HIV-infected and otherwise immunosuppressed patients. Eur Respir J. 1995;8:1548–53. [PubMed] [Google Scholar]
- 23.Cohn SE, Klein JD, Weinstein RA, et al. Geographic variation in the management and outcome of patients with AIDS-related Pneumocystis carinii pneumonia. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:408–15. doi: 10.1097/00042560-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Helweg-Larsen J, Benfield TL, Eugen-Olsen J, et al. Effects of mutations in Pneumocystis carinii dihydropteroate synthase gene on outcome of AIDS-associated P. carinii pneumonia. Lancet. 1999;354:1347–51. doi: 10.1016/S0140-6736(99)03320-6. [DOI] [PubMed] [Google Scholar]
- 25.Crothers K, Beard CB, Turner J, et al. Severity and outcome of HIV-associated Pneumocystis pneumonia containing Pneumocystis jirovecii dihydropteroate synthase gene mutations. AIDS. 2005;19:801–5. doi: 10.1097/01.aids.0000168974.67090.70. [DOI] [PubMed] [Google Scholar]