Abstract
This study aims to evaluate the multidrug resistance (MDR) reversal activity by magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) and 5-bromotetrandrine (BrTet) MDR cell line K562/A02 solitarily or symphysially. The proliferation of K562 and K562/A02 cells and the cytotoxicity on peripheral blood mononuclear cells (PMBCs) were evaluated by MTT assay. Cellular accumulation of daunorubicin (DNR) was analyzed by flow cytometry. Real-time polymerase chain reaction and Western blotting analyses were performed to examine the mRNA and protein levels of mdr1, respectively. The results showed that the combination of MNPs-Fe3O4 and BrTet with effective concentrations significantly increased cytotoxicity against MDR cell line K562/A02. Both BrTet and MNPs-Fe3O4 increased the intracellular DNR accumulation in the K562/A02 cell line, and downregulated the level of mdr1 gene and expression of P-glycoprotein. Furthermore, the combination did not have significant cytotoxicity in PMBCs. We propose that MNPs-Fe3O4 conjugated with DNR and BrTet probably have synergetic effects on MDR reversal.
Keywords: magnetic nanoparticles of Fe3O4, 5-bromotetrandrine, multidrug resistance K562/A02
Introduction
Multidrug resistance (MDR), defined as being induced by one kind of drug which generates cross-tolerance to various anticancer drugs with different structures and mechanisms. This cross-tolerance was noted to be the most important mechanism of self-dependence in cytotoxic injury of chemotherapeutics, as well as a major reason for relapse or failure of chemotherapy during treatment for hematological malignancies. The mechanism which generates the MDR phenotype is complex.1 Mechanisms include drug excretion, antiapoptosis, activity changes in drug-metabolizing enzymes such as serum glutathione S epoxide transferase (GST), topoisomerase II (TOPO II), protein kinase C (PKC), and reinforcement of recovery from DNA injury. However the major mechanism in the mediation of MDR was P-glycoprotein (P-gp, P170), a kind of transmembrane glycoprotein.2 P-gp, a member of ATP-binding cassette (ABC) transporter superfamily, encoded by mdr1 gene, mediated drug resistance to anthracycline, vinca alkaloids, etc, in the function of the lipophilic drug excretion pump and leading to a decrease in cytotoxic drug accumulation. Several patients with hematological malignancies, such as acute leukemia,3 non-Hodgkin’s lymphoma,4 which were sensitive to chemotherapy at the initial stage of morbidity, were detected showing a non- or hypo-expression of P-gp pretherapy, but an over expression of P-gp after relapse. Some studies indicated that mdr1/P-gp had a close correlation in the prognosis of the outcome of chemotherapy. Patients with mdr1 overexpression had shorter life span, and a higher recurrence rate. P-gp inhibitors can improve the curative effect of myelodysplastic syndrome with overexpressed P-gp.5 Therefore, the analysis of mdr1/P-gp could be considered an anticipation index for the outcome of hematological malignancies.
At present, only the first and second generation of P-gp inhibitors have been tested in clinic trials, but the therapeutic effects and adverse reactions experienced were not ideal. The unity of the reversal mechanism was an important factor that interfered in clinic utilization. In recent years, drugs packaged by liposome or multimer have been developed. This could change the character of such drugs by affecting tumor cells selectively, decreasing toxicity, and augmenting the MDR reversal effect.6–8 Nanotechnology displayed feasible application perspectives initially through improving administration routes of chemotherapeutics, which rivaled neutralization excretion drugs by tumor cells in order to increase the intracellular accumulation of drugs. Magnetite, possessing a magnetic positive charge group, was the essential component of the magnetic particle. The present understanding is that the magnetic compound of Fe3O4 could self-synthesize into tela, suggesting that Fe3O4 has an existence physically. This characteristic of Fe3O4 was the safeguard for using it as a safe hypotoxic carrier.9
Tetrandrine (Tet), a type of hypotoxic calcium channel antagonist,10 could inhibit P-gp overexpresssion through competing activation of PKC and mdr1, and had a better reversal effect for leukemia in vivo and in vitro. The Tet may reverse MDR through downregulating the expression of mdr1mRNA or P-gp, increasing drug accumulation intracellularly, as well as reinforcing apoptosis induced by anticancer drugs. 5-bromotetrandrine (BrTet), a bromized derivative of Tet, has been shown to be more potent than Tet in the modulation of MDR.11 In the prophase trial, we applied magnetic nanoparticles of Fe3O4 (MNPs-Fe3O4) and BrTet connected with adriamycin (ADM) respectively for MDR reversal, and indicated that both of the drugs could increase the reversal effect. However both of the drugs had different targets, and the complexity of MDR mechanism determined the effect of the synergy in MDR reversal.
Materials and methods
Main reagents
Adriamycin (Hisun Phamaceutical Co., Zhejiang, China) stock solution 3.68 mM and daunorubicin (DNR) (Main Luck Phamaceuticals Inc., Shenzheng, China) stock solution 3.55 mM were prepared with 0.01 M phosphate-buffered saline (PBS, pH =7.4). BrTet (Kanghong Phamaceuticals Inc., Chengdu, China) was also diluted with 0.01 M PBS (pH =7.4). All reagents used in this trial were of analytical grade.
Cell culture
Human chronic myeloid leukemia in blast crisis cell line, K562, and its resistant cell line to ADM, K562/A02, were both cultured in a RPMI 1640 medium (Gibco/BRL, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (FCS) (Sijiqing, Hangzhou, China), 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2, and passaged every two days. The resistant cell line was incubated in the presence of ADM (1 μg/mL) until at least three days before starting the experiments. Peripheral blood mononuclear cells (PBMCs) were assembled and separated from healthy cells, and then cultured in a RPMI 1640 medium supplemented with 15% FCS, 100 ng/mL granulocyte colony-stimulating factor (G-CSF)12 at 37 °C in a humidified atmosphere of 5% CO2 for one week before the commencement of the experiments.
Cytotoxicity assay
The in vitro chemosensitivity was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (Sigma Aldrich, St. Louis, MO, USA) assay. Tumor cells (1.5 ×10 6/mL) were suspended in 100 μL of culture medium on 96-well culture plate (Costar; Fisher Scientific, Hampton, NH, USA) per well. For determining the reversal effect of MNPs-Fe3O4, BrTet was used alone or symphysially in graded concentrations of DNR with or without the reversal agents added. The concentration of BrTet was 0.5 μM, which is half of the recommended reversal concentration according to Chen and colleagues.13 MNPs-Fe3O4, 0.1 (V/V), was conjugated with graded concentrations of DNR and kept at 4 °C for 48 hours before being applied to the experiment.14 PBMCs (2.0 ×10 5/mL) were also suspended in 100 μL of culture medium in 96-well culture plate per well using the same concentration. To determine the antiproliferative effect of BrTet or MNPs-Fe3O4, various concentrations of these two reagents in 100 μL dilute of the culture medium were added into every well. Meanwhile, RPMI 1640 medium was regarded as the bank control and cells without reagents were the negative control. The cells were then incubated for 48 hours at 37 °C, following which, MTT (0.5 mg/mL) 20μL were added to each well and cultured for an additional four hours. The formazan was dissolved with 150 μL dimethyl sulfoxide (Sigma Aldrich) after blotting the culture medium. The plates were shaken lightly for 10 minutes, and the reduction of MTT was quantified by absorbance at a wavelength of 490 nm using a microplate reader (Model-550; Bio-Rad Laboratories, Hercules, CA, USA). The relative growth rates (RGR) of PMBCs, evaluating the antiproliferative effect of BrTet or MNPs-Fe3O4, were transformed into six bands according to Table 1.
Table 1.
The RGR and cytotoxicity gradation of PMBCs incubated with BrTet or MNPs-Fe3O4 for 48 hours
| Cytotoxicity gradation | RGR (%) |
|---|---|
| Band 0 | ≥100 |
| Band 1 | 75∼99 |
| Band 2 | 50∼74 |
| Band 3 | 25∼49 |
| Band 4 | 1∼24 |
| Band 5 | 0 |
Notes: The RGR was calculated as follow: ODtest cells/ODnegative control × 100%. The band 0 or 1 was qualified, band 2 should be evaluated with morphology, and band 3∼5 was unqualified.23
Abbreviations: OD, optical density; PMBCs, peripheral blood mononuclear cells; RGR, relative growth rate.
Cellular accumulation of DNR
Cellular accumulation of DNR was analyzed by flow cytometry (FCM) assay. Briefly, both K562/A02 cells (1.5 × 106/mL) and PMBCs (2.0 × 105 mL) were exposed to 2 μM DNR in the absence or presence of BrTet at 0.5 μM or MNPs-Fe3O4 at 0.1 (V/V) for 24 hours at 37 °C to determine the cytotoxicity of BrTet and MNPs-Fe3O4. The PMBCs were sieved and incubated with CD34-FITC for 15 minutes. After being washed by PBS three times, the cells were suspended by 400 μL PBS and determined by a FACS Calibur flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA) assay at excitation and emission wavelengths of 488 nm and 575 nm, respectively.
Western blotting of P-gp
As described before, K562/A02 cells (1.5 × 106/mL) were treated, harvested, and then lysed in a lysis buffer containing 25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 5 mM EGTA, 10 mM NaF, 1 mM PMSF, 0.5% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM pepstatin for 15 minutes. The 150 μg of total protein was electrophoresesed on 10% SDS-polyacrylamide gels, and transferred onto a nitrocellulose membrane. Nonspecific binding sites were blocked by 5% milk powder dissolved in Tris-buffered saline (TBS) at room temperature for one hour. The primary P-gp antibody was mouse monoclonal anti-human antibody (Neomarkers, Fremont, CA, USA). The secondary antibody was alkaline phosphatase-labeled rabbit-mouse IgG. Membranes were exposed in nitroblue tetrazolium chloride (NBT)/5-bromo-4-chloro-3-indoxylphosphate (BCIP) were used as color detection reagents. After normalization by the corresponding expression of β-actin, the levels of P-gp protein expression were determined by densitometry scans (ECL system, Amersham, UK). Meanwhile, K562 cells without any reagents were negative controls.
Real time PCR analysis of mdr1 mRNA expression
Total cellular RNA was extracted from K562/A02 cells with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and quantified by 1.5% agarose gel. Each group contained RNA from the cells incubated with DNR (2 μ M), MNPs-Fe3O4 (0.1 v/v) loaded with DNR (2 μM), DNR (2 μM) connected with BrTet (0.5 μ M); MNPs-Fe3O4 (0.1 v/v) loaded with DNR (2 μM) and conjugated with BrTet (0.5 μM), respectively. K562/A02 cells and K562 cells without reagents were regarded as positive and negative controls. The reverse transcription reactions were performed using TaKaRa RNA PCR Kit (avian myeloblastosis virus, version. 3.0; Dalian, Liaoning, China). The newly synthetic cDNA was amplified by SYBR Premix Ex TaqTm real-time polymerase chain reaction (PCR) (TaKaRa) at ABI 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Primers involved were the mdr1 primers (forwards 5′-TTGTTTGCCACCACGATA-3′, reverse 5′-GAACCACTGCTTCGCTTT-3′) and the GAPDH primers (forwards 5′-TGAACGGGAAGCTCACTGG-3′, reverse 5′-TCCACCACCCTGTTGCTGTA-3′). The amplified PCR products were 274bp and 205bp for mdr1 and GAPDH, respectively. The conditions for real-time PCR were 50 °C for 20 minutes and 45 cycles of 95 °C for 10 minutes, 94 °C for 15 seconds, 59 °C for 20 seconds, and 72 °C for 30 seconds. The Ct of both mdr1 and GAPDH were detected after each cycle. The expression of mdr1 was given as follows: Ct = Ct mdr1 − CtGAPDH.
Statistical analysis
Data was analyzed using the Statistical Package for Social Science (v. 15.0; SPSS Inc., Chicago, IL, USA). The significance of differences in the mean value between groups was analyzed using one-way ANOVA; P values <0.05 were considered statistically significant.
Results
Effect of cytotoxicity of BrTet or Fe3O4
The cytotoxicity of BrTet or MNPs-Fe3O4 in PBMCs was assayed by the MTT assay. The transformation of RGR and the cytotoxicity gradation were evaluated according to Table 1. The data in Table 2 clearly indicates that BrTet at 0.25∼2 μ M and MNPs-Fe3O4 at 0.025∼0.1 (V/V) did not generate significant cytotoxicity.
Table 2.
The cytotoxicity of BrTet or MNPs-Fe3O4 on PBMCs for 48 hours determined by MTT assay
| Groups | OD (mean ± SD) | RGR (%) | Cytotoxicity gradation |
|---|---|---|---|
| BrTet (μM) | |||
| Control | 1.218 ± 0.154 | – | – |
| 0.25 | 1.247 ± 0.111* | 102.38 | Band 0 |
| 0.5 | 1.261 ± 0.079* | 103.50 | Band 0 |
| 1 | 1.222 ± 0.104* | 100.27 | Band 0 |
| 2 | 1.221 ± 0.195* | 100.22 | Band 0 |
| MNPs-Fe3O4 (V/V) | |||
| Control | 0.763 ± 0.023 | – | – |
| 0.025 | 0.741 ± 0.033** | 97.16 | Band 1 |
| 0.05 | 0.737 ± 0.045** | 96.63 | Band 1 |
| 0.1 | 0.697 ± 0.075** | 91.39 | Band 1 |
| 0.2 | 0.552 ± 0.028** | 72.30 | Band 2 |
Notes:
P > 0.05, compared with control group;
P > 0.05, compared with control group.
Abbreviations: BrTet, 5-bromotetrandrine; MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; OD, optical density; PBMCs, peripheral blood mononuclear cells; RGR, relative growth rate; SD, standard deviation.
Cell survival
According to the MTT assay, the ability of BrTet or MNPs-Fe3O4 used alone or in conjunction to reverse DNR resistance was compared in K562/A02 cell line. BrTet and MNPs-Fe3O4 symphysially showed significant reversal effect on DNR resistance in the K562/A02 cell line, and its potency was greater than using BrTet and Fe3O4 alone. The inhibitory concentration at 50% (IC50) of DNR decreased from 32.33 ± 8.40 μM to 1.80 ± 0.30 μM (P < 0.001) at the combination of BrTet 0.5 μM and MNPs-Fe3O4 0.1 (V/V), while the values were down to 7.49 ± 0.85 μM and 4.25 ± 2.16 μM for Fe3O4 and BrTet, respectively (P < 0.001). The fold reversals were 17.96 of the synergia compared with the 4.32 of MNPs-Fe3O4 and 7.61 of BrTet alone. In contrast, there were no significant differences between those in K562 cell line (Table 3).
Table 3.
The cytotoxicity of BrTet or MNPs-Fe3O4 on K562/A02 and K562 cells for 48 hours determined by MTT assay (mean ± sD)
| Groups |
IC50of DNR (μM) |
|
|---|---|---|
| K562/A02 | K562 | |
| DNR | 32.33 ± 8.40 | 2.74 ± 0.19 |
| DNR – Fe3O4 (0.1 V/V) | 7.49 ± 0.85 (4.32)* | 2.31 ± 0.27 (1.19) |
| DNR + BrTet (0.5 μM) | 4.25 ± 2.16 (7.61)* | 2.80 ± 0.27 (0.98) |
| DNR – Fe3O4 + BrTet | 1.80 ± 0.30 (17.96)* | 2.39 ± 0.20 (1.15) |
Notes: The values in parentheses were FR calculated as follows: FR =IC50 DNR alone/IC50 DNR + agent.
P < 0.05, compared with DNR group.
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; FR, fold reversal; IC50, inhibitory concentration at 50%; MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; OD, optical density; PBMCs, peripheral blood mononuclear cells; RGR, relative growth rate; SD, standard deviation.
Fluorescence intensity of endocellular DNR
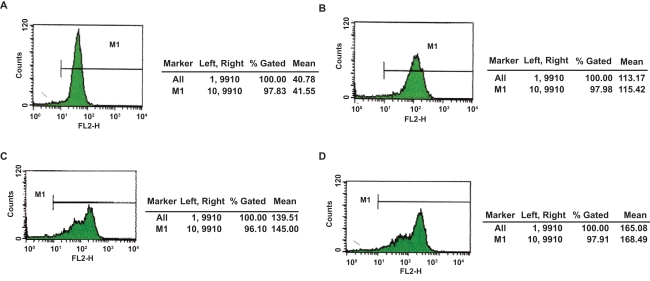
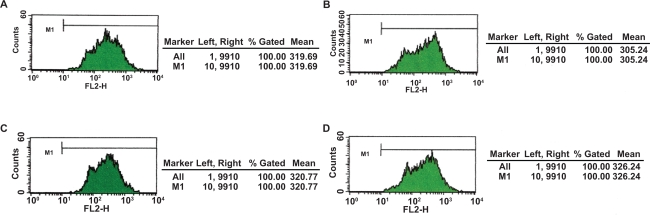
After repeating the trial three times, at a wavelength of 488 nm, DNR was excited to emit at 575 nm wavelength spontaneously where fluorescence intensity (FI) of intracellular DNR could be recorded by FCM. The mean fluorescence intensity of K562/A02 cells preincubated with 2 μM DNR for 48 hours was 44.49 ± 2.57; with DNR-Fe3O4, 117.54 ± 2.53; with DNR-BrTet, 140.61 ± 4.32; and with DNR-Fe3O4-BrTet, 117.34 ± 3.54. The differences were significant when compared with control group (P < 0.001). Furthermore, the fluorescence intensity of intracellular DNR of PMBCs had no dramatic variations (Figures 1, 2).
Figure 1.
Cellular accumulation of DNR in K562/A02 cells after treatment with different reversal agents for 24 hours. A) Incubated with 2 μM DNR; B) Incubated with 2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v); C) Incubated with 2 μM DNR in conjunction with BrTet (0.5 μM); D) Incubated with 2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v) synergia with BrTet (0.5 μM).
Abbreviations: DNR, daunorubicin; MNPs-Fe3O4, magnetic nanoparticles of Fe3O4.
Figure 2.
Intracellular accumulation of DNR in PMBCs after treated with different reversal agents for 24 hours. A) Incubated with 2 μM DNR; B) Incubated with 2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v); C) Incubated with 2 μM DNR in conjunction with BrTet (0.5 μM); D) Incubated with 2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v), synergia with BrTet (0.5 μM).
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; PBMCs, peripheral blood mononuclear cells.
Expression of P-gp
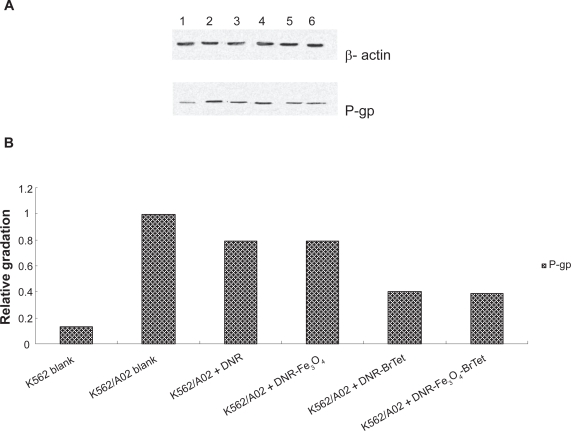
The expression of P-gp determined by western blotting analysis was downregulated to some extent after treatment with DNR in the presence of MNPs-Fe3O4 or BrTet alone or in combination for 48 hours, compared with K562/A02 blank group (P < 0.05). But there were no significant differences between the DNR in company with BrTet group and the group of DNR loaded with MNPs-Fe3O4 and BrTet (P > 0.05). The relative expression was calculated as follows: gradationP-gp/gradationβ-actin (Figure 3).
Figure 3.
P-gp expression of K562/A02 cell line and K562 cell line after treatment with different reagents for 48 hours.
Notes: A) The straps developed by western blotting. Line 1. K562 blank; line 2. K562/A02 blank; line 3. K562/A02 + 2 μM DNR; line 4. K562/A02 +2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v); line 5. K562/A02 +2 μM DNR in conjunction with BrTet (0.5 μM); and line 6. K562/A02 + 2 μM DNR loaded with MNPs-Fe3O4 (0.1 v/v), synergia with BrTet (0.5 μM). B) The relative gradation calculated by the formula above.
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin; MNPs-Fe3O4, magnetic nanoparticles of Fe3O4; P-gp, P-glycoprotein.
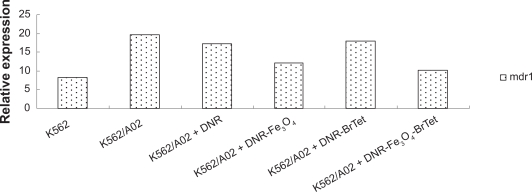
Mdr1 mRNA level
DNR in the absence or presence of MNPs-Fe3O4 or BrTet alone or in combination downregulated the mdr1 mRNA levels. The mdr1 relative expression of K562/A02 was 19.68, which was 3.23 times lower compared with K562 cells. The value of mdr1 expression was significantly decreased to 12.2 and 10.12 in the cells pretreated with DNR and loaded with MNPs-Fe3O4 and the combination of MNPs-Fe3O4 and BrTet, respectively, while BrTet connected with DNR showed a less significant amount of mdr1 compared with BrTet only. The values were 17.89 (Figure 4).
Figure 4.
Bar graph of mdr1 mRNA level in cells determined by real-time polymerase chain reaction after treatment with different reagents for 48 hours. The values were calculated as ΔCt.
Abbreviations: BrTet, 5-bromotetrandrine; DNR, daunorubicin.
Discussion
MDR is considered to be a major problem for clinical therapy related to hematological malignancies. Several calcium channel inhibitors, such as verapamil, nifedipine, azidopine, or tetrandrine, can inhibit the excretion of anticancer drugs, thus overcome MDR,15–16 but the toxicity of these drugs constrains both their application and their therapeutic effect. BrTet, a modified production of Tet, significantly reversed resistance to ADM in MCF-7/Dox cell line in vivo, and restrained tumor growth in athymic mice bearing cancer.17 In our former study, reversal effect of BrTet was more effective than that of Tet in K562/A02 cell line in vivo and in vitro.11 In this experiment, we also studied the cytotoxicity of BrTet in PBMCs, and the results showed that it was a safe, hypotoxic reversal agent and did not increase the drug accumulation in PMBCs in vitro. Since toxicity constrains the application of reversal reagents, we degraded half concentrations of BrTet and synergia with MNPs-Fe3O4. The results clearly indicated that the reversal activity of BrTet combined with MNPs-Fe3O4 was significantly greater than that of BrTet or MNPs-Fe3O4 alone at the same dose in the K562/A02 cell line, This result provides a synergia administration route in MDR reversal.
Using nanoparticle carriers polymerizing chemotherapeutics in MDR reversal is an exploratory study. Delivery systems of therapeutic nanoparticles could prevent drug degradation, change pharmacokinetics, and/or alleviate intracellular drug accumulation and disposition.18 In pharmacological studies, nanoparticle carriers could increase concentrations of anticancer drugs intracellularly, and retain certain drug accumulation after drug withdrawal.19 Several papers have also reported increasing concentrations of anticancer drugs though different nanoparticles when combined with these drugs.20,21 In our previous study, we used MNPs-Fe3O4 loaded with ADM in MDR reversal, and this strategy may have a MDR reversal effect through increasing cellular accumulation of DNR and downregulating the expression of mdr1.14 The overexpression of P-gp is the major cause of MDR development. Suppression of the expression of P-gp at either a transcriptional or a translational level is a critical approach to overcome MDR. In this trial, we also performed experiments on the mechanism of MDR reversal. As a result, MNPs-Fe3O4 combined with BrTet could significantly increase cellular accumulation, and downregulate the expression of P-gp, but this combination seemed to have a less obvious effect on inhibiting the mdr1 gene than that of MNPs-Fe3O4 loaded with DNR. As shown in other studies, BrTet may reverse MDR by retaining the route of translation from mdr1 mRNA to P-gp,22 which was in concordance with our results. How does the combination have the most obvious reversal effect? The mechanism may have something to do with targeted delivery.11 Targeted deliveries include passive and active targeting, and the former enhances permeability and retention. We consider that MNPs-Fe3O4 increase the DNR accumulation and retention through passive targeting, which has synergy with BrTet inhibiting P-gp in order to decrease active excretion. Therefore, this synergy could significantly reverse MDR compared with using these two reagents alone. This theory requires a penetrating mechanism study and an in vitro study.
Acknowledgments
This work was supported by 973 National Key Fundamental Research Project of China (No. 2006CB933205), 863 Project of People’s Republic of China (No. 2007AA022007), National Nature Science Foundation of People’s Republic of China (No. 30740062, 30872970) and Special-Purpose Science Research Foundation for High School (No. 20070286042). Jian Cheng and Weiwei Wu contributed equally to this work and report no conflicts of interest.
References
- 1.Obligacion R, Murray M, Ramzan I. Drug-metabolizing enzymes and transporters: expression in the human prostate and roles in prostate drug disposition. J Androl. 2006;27(2):138–150. doi: 10.2164/jandrol.05113. [DOI] [PubMed] [Google Scholar]
- 2.Ross DD. Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17(4):641–651. doi: 10.1016/j.beha.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 3.List AA. Multidrug resistance: clinical relevance in acute leukemia. Oncology (Williston Park) 1993;7(10):23–32. [PubMed] [Google Scholar]
- 4.Yuen AR, Sikic BI. Multidrug resistance in lymphomas. J Clin Oncol. 1994;12(11):2453–2459. doi: 10.1200/JCO.1994.12.11.2453. [DOI] [PubMed] [Google Scholar]
- 5.Valera ET, Scrideli CA, Queiroz RG, et al. Multiple drug resistance protein (MDR-1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122(4):166–171. doi: 10.1590/S1516-31802004000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurand A, Laroche-Clary A, Larrue A, et al. Quantification of the expression of multidrug resistance-related genes in human tumour cell lines grown with free doxorubicin or doxorubicin encapsulated in polyisohexylcyanoacrylate nanospheres. Anticancer Res. 2004;24(6):3781–3788. [PubMed] [Google Scholar]
- 7.Kukowska-Latallo JF, Candido KA, Cao Z, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 8.Gao H, Wang J, Shen X, Deng Y, Zhang W. Preparation of magnetic polybutylcyanoacrylate nanospheres encapsulated with aclacinomycin A and its effect on gastric tumor. World J Gastroenterol. 2004;10(14):2010–2013. doi: 10.3748/wjg.v10.i14.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng FY, Su CH, Yang YS, et al. Characterization of aqueous dispersions of Fe3O4 nanoparticles and their biomedical applications. Biomaterials. 2005;26(7):729–738. doi: 10.1016/j.biomaterials.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Fu L, Liang Y, Deng L, et al. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol. 2004;53(4):349–356. doi: 10.1007/s00280-003-0742-5. [DOI] [PubMed] [Google Scholar]
- 11.Wang JQ, Chen BA, Chen J, et al. Comparison of reversal effects of 5-bromotetrandrine and tetrandrine on P-glycoprotein-dependent resistance to adriamycin in human leukemia cell line K562/A02. Chin J Cancer. 2008;27(5):49–53. [PubMed] [Google Scholar]
- 12.Huang YZ, Shen JL, Yang PD, et al. Comparison of different cryopreservation systems for peripheral blood stem cells. Appl Physiol. 2008;24(1):125–128. [PubMed] [Google Scholar]
- 13.Chen BA, Sun Q, Wang X, et al. Reversal in multidrug resistance by magnetic nanoparticle of Fe3O4 loaded with adriamycin and tetrandrine in K562/A02 leukemic cells. Int J Nanomedicine. 2008;3(2):277–287. doi: 10.2147/ijn.s2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du YQ, Zhang DS, Ni HY, et al. Research on biocompatibility of magnetic Fe3O4 nanoparticles using on tumor thermotherapy. Nanjing Shi Da Xue Bao. 2006;42(3):324–330. [Google Scholar]
- 15.Tsuruo T, Iida H, Kitatani Y, Yokota K, Tsukagoshi S, Sakurai Y. Effect of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and Adriamycin in drug-resistant tumor cells. Cancer Res. 1984;44(10):4303–4307. [PubMed] [Google Scholar]
- 16.Shinoda H, Inaba M, Tsuruo T. In vivo circumvention of vincristine resistance in P388 leukemia with a novel compound, AHC-52. Cancer Res. 1989;49(7):1722–1726. [PubMed] [Google Scholar]
- 17.Jin J, Wang F, Wei H, et al. Reversal of multidrug resistance of cancer through inhibition of P-glycoprotein by 5-bromotetrandrine. Cancer Chemother Pharmacol. 2005;55(2):179–188. doi: 10.1007/s00280-004-0868-0. [DOI] [PubMed] [Google Scholar]
- 18.Couvreur P, Vauthier C. Nanotechnology: Intelligent design to treat complex diseases. Pharm Res. 2006;23(7):1417–1450. doi: 10.1007/s11095-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 19.Ghazal M, Eun SL, You HB. Enhanced intracellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm Res. 2007;24(9):1618–1627. doi: 10.1007/s11095-007-9277-5. [DOI] [PubMed] [Google Scholar]
- 20.Wong HL, Rauth AM, Bendayan R, et al. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23(7):1574–1585. doi: 10.1007/s11095-006-0282-x. [DOI] [PubMed] [Google Scholar]
- 21.Alexiou C, Jurgons R, Schmid R, et al. [Magnetic Drug Targeting – a new approach in locoregional tumor therapy with chemotherapeutic agents. Experimental animal studies] HNO. 2005;53(7):618–622. doi: 10.1007/s00106-004-1146-5. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Yang L, Chen Z, Shin DM. Application of nanotechnology in cancer therapy and imaging. CA Cancer J Clin. 2008;58:97–110. doi: 10.3322/CA.2007.0003. [DOI] [PubMed] [Google Scholar]