Abstract
Nonalcoholic fatty liver disease (NAFLD) may have distinct histological features in children and adults, but to date limited data are available on the spectrum and significance of histological lesions in pediatric patients. We conducted a multicenter study of children with well-characterized, biopsy-proven NAFLD to (1) assess the presence and significance of a constellation of histological lesions and (2) identify clinical and laboratory predictors of disease severity. One hundred thirty children with NAFLD seen from 1995 to 2007 in five centers in the United States and Canada were studied. Clinical and laboratory data were collected. Slides stained with hematoxylin-eosin and trichrome were evaluated by two liver pathologists. The NAFLD activity score (NAS) and the pattern of liver injury (type 1 or adult versus type 2 or pediatric nonalcoholic steatohepatitis [NASH]) were recorded. Fibrosis was staged using a published 7-point scale. The median age was 12 years (range, 4-18 years); 63% were boys, and 52% were Caucasian. Fibrosis was present in 87% of patients; of these, stage 3 (bridging fibrosis) was present in 20%. No patient had cirrhosis. The median NAS was 4. Overlapping features of both type 1 (adult pattern) and type 2 (pediatric pattern) NASH were found in 82% of patients. Compared with patients with no or mild fibrosis, those with significant fibrosis were more likely to have higher lobular and portal inflammation scores (P < 0.01), perisinusoidal fibrosis (P < 0.001), and NAS ≥5 (P < 0.005). Serum aspartate aminotransferase levels were the only clinical or laboratory data that independently predicted severity of fibrosis (P = 0.003).
Conclusion:
Our results highlight the limitations of published proposals to classify pediatric NAFLD, and identified histological lesions associated with progressive disease.
Nonalcoholic fatty liver disease is the most common form of chronic liver disease in both children and adults.1,2 It is estimated that nonalcoholic fatty liver disease (NAFLD) affects about 20% to 30% of adults and 10% of children in the United States.3,4 NAFLD represents a wide spectrum of conditions ranging from fatty liver to nonalcoholic steatohepatitis (NASH) to advanced fibrosis and cirrhosis.5 Steatosis without significant liver cell injury or fibrosis is the most common form of NAFLD in both adults and children.1,2 Studies in the adult population have variably suggested that steatosis is a benign nonprogressive condition, and NASH is recognized as a potentially serious condition with significant risk of morbidity and mortality.6-9
Liver biopsy remains the standard for establishing the diagnosis of NASH.10,11 The histological criteria for NASH in the adult population have evolved over the last two decades since the seminal publication of Ludwig et al.12 The principal histological features of adult NASH include the presence of macrovesicular steatosis (characterized by a single or a few large droplets of fat and displacement of the nucleus), ballooning degeneration of hepatocytes, and a mixed lobular inflammation with or without a degree of portal inflammation.10,11 Other features that are commonly present include zone 3 perisinusoidal–pericellular fibrosis; Mallory-Denk bodies (Mallory's hyaline); megamitochondria; acidophil bodies; and iron in hepatocytes, sinusoidal lining cells, or both.
A growing body of evidence suggests that children with NASH frequently show histopathological features that differ from those of adults characterized mainly by lack of zone 3 accentuation and the predominance of portal-based inflammation and fibrosis.13 Schwimmer et al.14 identified this recently as type 2 NASH, with the aforementioned features in adults as type 1 NASH. The prevalence of this pattern in a wide range of pediatric cases as well as other other histopathological lesions and their relevance and prognostic significance in children with NAFLD remains to be determined. Thus, we conducted this study of biopsies and clinical information from five North American centers to document the histological features of pediatric NAFLD, determine the frequency and prognostic value of these features, and perform clinico-pathological correlations.
Patients and Methods
Patient Characteristics
This was a multicenter, retrospective cohort study. Children with NAFLD who attended one of five medical centers and who had undergone a liver biopsy for clinical reasons between 1995 and 2007 were identified from institutional databases. One hundred thirty children with NAFLD were included from the following institutions: Children's Hospital at the Cleveland Clinic (n = 23), Eugenio Litta Children's Hospital at the Mayo Clinic (n=30), Cincinnati Children's Hospital Medical Center (n = 19), Children's Memorial Hospital in Chicago (n = 34), and The Hospital for Sick Children in Toronto (n = 24). The study was reviewed and approved by the Institutional Review Board at each of these institutions. Patients were included in the study if they met the following criteria: (1) age 3 to 18 years (inclusive) with biopsy-proven NAFLD; (2) alcohol consumption of <20 g/day for boys and <10 g/day for girls; and (3) appropriate exclusion of other liver diseases (such as viral hepatitis, α1-anti-trypsin disease, autoimmune hepatitis, and Wilson disease). We excluded 22 patients due to inadequate biopsy (see details in Histological Features) (n = 14) or biopsy unavailable for review (n=8); consequently, 108 patients were included in the final analysis.
Histological Features
Liver biopsy samples were evaluated by two hepato-pathologists (E. B. and L. Y.) blinded to clinical and laboratory data. The pathologists reviewed the biopsies independently and met twice to evaluate discordant results. All patients underwent a percutaneous needle liver biopsy. Each biopsy was scored using the formalin-fixed, paraffin-embedded sections stained with hematoxylin-eosin and Masson trichrome. The histological features assessed and scored were steatosis (amount), inflammation (portal and lobular), and hepatocyte ballooning (defined as enlarged hepatocytes that were distinct and had clear cytoplasm); other features assessed but not scored were types of steatosis (macrovesicular, microvesicular, mixed), hepatocyte cytoplasmic clearing, Mallory-Denk bodies (Mallory's hyaline), acidophil bodies, and megamitochondria. The stage of fibrosis was assigned. Relavent features were graded according to the NAFLD activity scoring (NAS) system proposed by Kleiner et al.15 Briefly, grade of steatosis was scored as 0=<5%, 1=5% to 33%, 2=>33% to 66%, 3=>66%; grade of lobular inflammation was scored as 0 = no foci, 1 = <2 foci/200× field, 2 = 2-4 foci/200× field, 3 = >4 foci/200× field; and grade of ballooning was scored as 0 = none, 1 = few ballooning cells, 2 = many cells/prominent ballooning. The grade of steatosis (0-3), lobular inflammation (0-3), and ballooning (0-2) were then combined to determine the NAS (0-8) as proposed. Fibrosis was scored as 0 = none, 1 = zone 3 perisinusoidal fibrosis (1a, 1b) or portal/periportal (1c), 2 = perisinusoidal and portal/periportal fibrosis, 3=bridging fibrosis, and 4 = cirrhosis. The liver biopsy samples were also given a diagnostic classification based on pattern. These included (1) type 1 NASH, which is a pattern similar to adult NASH defined by the zone 3 dominance of steatosis, ballooning, and zone 3 perisinusoidal fibrosis; (2) type 2 or pediatric NASH, which is defined by steatosis along with dominance of portal inflammation and/or fibrosis in the absence of ballooning and zone 3 perisinusoidal fibrosis; and (3) overlap when characteristics from both type 1 and 2 were present.14 In particular, when type 2 NASH had either ballooning and/or zone 3 perisinusoidal fibrosis, the case was considered to be overlapped. To control for biopsy size, the length of the biopsy was measured with a hand ruler, and the number of portal areas on one cross-section was counted. Although no firm adequacy parameters have been established for fatty liver disease, the histologic diagnosis of diffuse liver disease requires biopsies that are at least 1.5 cm in length and contain at least six complete portal tracts.16 Thus, to minimize the potential for sampling error,17 the quality of liver biopsies was considered inadequate for histologic diagnosis, and patients were excluded from the analysis if the biopsy was <1.5 cm, fragmented, or had <6 portal tracts.
Clinical and Laboratory Evaluation
The laboratory evaluation included liver biochemistry (serum aspartate aminotransferase [AST], alanine aminotransferase [ALT], total alkaline phosphatase activity, gamma glutamyl transpeptidase, total bilirubin, albumin levels, and prothrombin time), fasting blood glucose, fasting lipid profiles (triglyceride, total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein cholesterol), and serum autoantibodies, including anitnuclear antibody, smooth muscle antibody, antibody to the liver/kidney microsome type 1, and antimitochondrial antibody. All patients underwent abdominal imaging with ultrasonography, computed tomography, and/or magnetic resonance that confirmed the presence of changes consistent with fat within the liver.
Body mass index (BMI) was calculated for every patient. BMI percentile was determined according to age and sex based on data from the Centers for Disease Control and Prevention.18 Obesity was defined as a BMI >95th percentile for age and sex.19 Abnormalities in the fasting levels of triglycerides and HDL cholesterol were adjusted according to age, sex, and race or ethnic group (>95th percentile for triglycerides; <5th percentile for HDL cholesterol) as recommended. Diabetes mellitus was diagnosed based on standard criteria as recommended by the American Diabetes Association. Hypertension was defined as a systolic or diastolic value that exceeded the 95th percentile for age, sex, and height. Hypercholesterolemia was defined as a fasting total cholesterol level ≥200 mg/dL.
Statistical Analysis
Descriptive statistics were computed for all factors. These include medians, 25th and 75th percentiles for continuous factors, and frequencies for categorical factors. Wilcoxon rank sum tests for continuous factors and Pearson's chi-square or Fisher's exact tests for categorical variables were used to assess which factors were significantly associated with presence of significant fibrosis (stage ≥2). In addition, Spearman's correlation coefficients were used to evaluate the associations between fibrosis stage and other continuous characteristics of subjects. Receiver operating characteristic (ROC) analysis was performed to assess the value of AST as a predictor of severe fibrosis. The area under the ROC curve and corresponding 95% confidence interval (CI) were estimated. P < 0.05 was considered statistically significant. SAS version 9.1 software (SAS Institute, Cary, NC) and R version 2.4.1 software (R Institute for Statistical Computing) were used for all analyses.
Results
Patient Characteristics
The main clinical and laboratory characteristics of our patient population are summarized in Table 1. The median age of the patients at the time of diagnosis was 12 years (range, 4-18 years). Obesity (BMI >95%) was present in 82% of patients. Sixty-three percent of the patients were males. Fifty-two percent of the patients were Caucasian, 1% were African American, 18% were Asian, and 30% were Hispanic. The median BMI was 31 kg/m2 (Q25, Q75: 27, 35). Seven percent of the patients were diagnosed with diabetes mellitus, 6% with hypertension, and 38% with hyperlipidemia (in most cases, hypertriglyceridemia alone or in combination with low HDL cholesterol). The median AST was 70 U/L, and the median ALT was 116 U/L with a median AST/ALT ratio of 0.6. The median gamma glutamyl transpeptidase was slightly elevated at 53 U/L. Serum total bilirubin and serum albumin were essentially within the normal range in all patients. The median fasting blood glucose was normal at 91 mg/dL; however, the median fasting insulin level was elevated at 28.6 mg/dL. The most common presenting symptom for these patients was abdominal pain, which occurred in approximately 18% of cases.
Table 1.
Demographic and Clinical Characteristics of Patients
| BMI, kg/m2 | 30.7 (26.9, 35.2) |
| Obese (BMI >95%) | 88 (81.4) |
| Overweight (BMI >85%) | 94 (87) |
| Bilirubin, mg/dL | 0.5 (0.3, 0.7) |
| AST, U/L | 70.5 (55, 115) |
| ALT, U/L | 116 (73, 166) |
| Alkaline phosphatase, U/L | 240 (132, 348) |
| GGT, U/L | 53 (38, 85) |
| Albumin, g/dL | 4.5 (4.2, 5.1) |
| Prothrombin time, seconds | 11.8 (10.7, 12.8) |
| Fasting glucose, mg/dL | 91 (84, 99) |
| Fasting insulin, IU/mL | 28.6 (19.1, 49.2) |
| Cholesterol, mg/dL | 190 (157, 207) |
| Triglycerides, mg/dL | 152 (110, 246) |
| HDL, mg/dL | 38 (32, 44) |
| Platelet count, ×109/L | 285 (250, 351) |
| AST/ALT ratio | 0.6 (0.5, 0.8) |
| Male sex | 68 (63.0) |
| Ethnicity | |
| Asian | 15 (17.9) |
| Hispanic | 25 (29.8) |
| White | 44 (52.4) |
| Abdominal pain | 19 (18.5) |
| Diabetes | 7 (6.9) |
| Hyperlipidemia | 38 (38.4) |
| Hypertension | 6 (7.7) |
Values are expressed as the median (Q25, Q75) or n (%).
Abbreviation: GGT, gamma glutamyl transpeptidase.
Histological Features
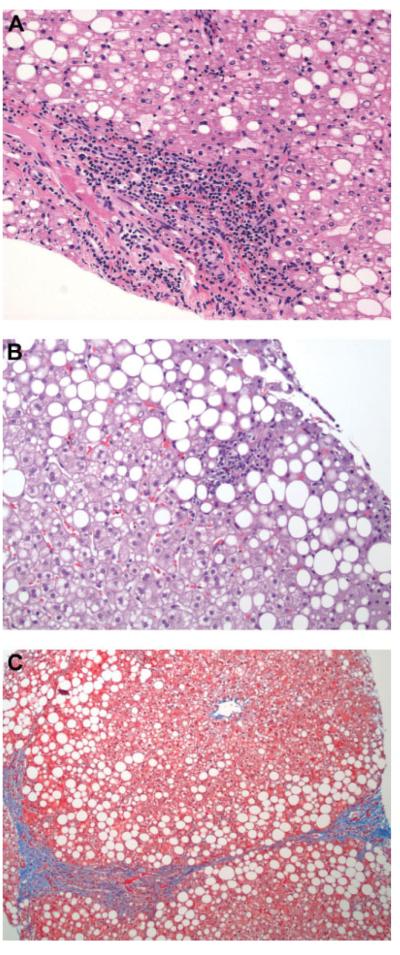
The histological features of the patients included in this study are summarized in Table 2. Steatosis was present in all cases, and it was of moderate to severe grade in more than half (58%) of the patients. Macrovesicular steatosis was present in all patients and combined with microvesicular in nearly 20% of patients. Zone 3 distribution of steatosis was seen in one-quarter of the patients, whereas zone 1 steatosis was found in less than 10% of patients. The most common distributions of steatosis were either azonal (scattered lobular) in 28 (27%) patients or panacinar in 41 (39%) patients. Lobular inflammation was present in most patients and was mild to moderate. Seventy-three percent of patients had ballooning of the hepatocytes that was in most cases of grade 1 (few ballooned cells). Portal-based injury (inflammation, fibrosis, or both) was seen in 91 (85%) patients but was associated with zone 3 injury in most cases. Based on these histological features, overlapping features of both type 1 or adult NASH and type 2 or pediatric NASH were found in the majority (n = 79 [82%]) of patients. Eight patients had type 1 NASH, nine patients had type 2 pediatric NASH, and 12 patients were classified as having fatty liver. The median NAS was 4 (inter-quartile range, 3-5). Some degree of fibrosis was present in 87% of patients (n = 94). Forty-three percent showed stage 1 fibrosis (n = 46), and 74% of these patients (n = 34) were stage 1c (portal, periportal). Twenty-four percent had stage 2 fibrosis (zone 3 perisinusoidal plus periportal) (n = 26), whereas 20% of the biopsy specimens revealed stage 3 (bridging) fibrosis (n = 22). No patient had cirrhosis. Figure 1 shows selected liver pathology images for these patients, including images of portal inflammation, lobular inflammation, and bridging fibrosis.
Table 2.
Histological Characteristics of Patients
| Steatosis amount | |
| 0 | – |
| 1 | 45 (41.6) |
| 2 | 37 (34.3) |
| 3 | 26 (24.1) |
| Steatosis type | |
| Macro | 87 (81.3) |
| Mixed | 20 (18.7) |
| Steatosis distribution (zone) | |
| One | 10 (9.6) |
| Three | 25 (24.0) |
| Azonal (scattered) | 28 (26.9) |
| Panacinar | 41 (39.4) |
| Ballooning | |
| 0 | 27 (25.0) |
| 1 | 58 (53.7) |
| 2 | 23 (21.3) |
| Lobular inflammation | |
| 0 | 9 (8.3) |
| 1 | 59 (54.6) |
| 2 | 34 (31.5) |
| 3 | 6 (5.6) |
| Portal inflammation | |
| 0 | 17 (15.7) |
| 1 | 77 (71.3) |
| 2 | 14 (13.0) |
| Fibrosis score | |
| 0 | 14 (13.0) |
| 1 | 46 (42.6) |
| 2 | 26 (24.1) |
| 3 | 22 (20.4) |
| Advanced fibrosis | 22 (20.4) |
| Z3PSF | 54 (50.5) |
| Mallory bodies | 13 (12.2) |
| Megamitochondria | 5 (4.6) |
| Acidophil bodies | 22 (22.2) |
Values are expressed as n (%).
Abbreviations: Z3PSF, zone 3 perisinusoidal fibrosis
Fig. 1.
Liver biopsy samples from pediatric patients with NAFLD. Liver biopsy specimens were formalin-fixed, paraffin-embedded, and stained with hematoxylin-eosin. The specimens shown are representative of the various histopathological features found in children with NAFLD. (A) Portal inflammation. (B) Lobular inflammation. (C) Bridging fibrosis. (Original magnification ×400.)
Histological Predictors of Progressive Disease in Pediatric NAFLD
As in other forms of chronic liver disease, the presence of fibrosis in NAFLD likely indicates more advanced and severe liver injury. Longitudinal studies in adult patients with NAFLD have clearly established the presence of liver fibrosis as the most important prognostic factor in these patients. Thus, to understand the potential significance of the variety of histological lesions found in our patient population, we sought associations between these histological features and the extent of fibrosis. Stage of fibrosis showed a positive but relatively weak correlation with NAS (r = 0.32, 95% CI 0.14-0.51, P < 0.001), as well as with ballooning (r = 0.25), lobular inflammation (r = 0.24), and portal (r = 0.20) inflammation (P < 0.05 in all cases). Correlation of portal inflammation and fibrosis has been recently shown in another large series of NAFLD biopsies.20 Degree of steatosis did not have a correlation to fibrosis. We next divided patients into two groups: (1) patients with significant, clinically relevant fibrosis on liver biopsy (stage ≥2 [n = 48]) and (2) patients with no or mild fibrosis (stage 0-1 [n = 60]) (Table 3). Compared with patients with no or mild fibrosis, those with significant fibrosis were more likely to have higher lobular inflammation and portal inflammation scores (P < 0.01), perisinusoidal fibrosis (P < 0.001), and NAS ≥5(P < 0.005). No differences were found between these two groups for the degree of steatosis or presence of ballooning of hepatocytes, as well as with other histological lesions (Table 3).
Table 3.
Associations of Histological Lesions with Presence of Significant Fibrosis (Stage ≥2)
| Factor | Fibrosis 2/3 (n = 48) |
Fibrosis 0/1 (n = 60) |
P Value* |
|---|---|---|---|
| Steatosis amount | 0.17 | ||
| 0 | 3 (6.3) | 2 (3.3) | |
| 1 | 13 (27.1) | 27 (45.0) | |
| 2 | 18 (37.5) | 19 (31.7) | |
| 3 | 14 (29.2) | 12 (20.0) | |
| Steatosis type | 0.63 | ||
| Macro | 40 (83.3) | 47 (79.7) | |
| Mixed | 8 (16.7) | 12 (20.3) | |
| Zone | 0.061 | ||
| One | 5 (10.6) | 5 (8.8) | |
| Three | 6 (12.8) | 19 (33.3) | |
| Azonal | 17 (36.2) | 11 (19.3) | |
| Panacinar | 19 (40.4) | 22 (38.6) | |
| Ballooning | 0.065 | ||
| 0 | 9 (18.8) | 18 (30) | |
| 1 | 25 (52.1) | 33 (55) | |
| 2 | 14 (29.2) | 9 (15) | |
| Lobular Inflammation | 0.027 | ||
| 0 | 1 (2.1) | 8 (13.3) | |
| 1 | 25 (52.1) | 34 (56.7) | |
| 2 | 18 (37.5) | 16 (26.7) | |
| 3 | 4 (8.3) | 2 (3.3) | |
| Portal Inflammation | 0.025 | ||
| 0 | 4 (8.3) | 13 (21.7) | |
| 1 | 35 (72.9) | 42 (70.0) | |
| 2 | 9 (18.8) | 5 (8.3) | |
| Z3PSF | 38 (80.9) | 16 (26.7) | <0.001 |
| Mallory bodies | 9 (19.2) | 4 (6.7) | 0.050 |
| Megamitochondria | 2 (4.2) | 3 (5.0) | 0.99 |
| Acidophil bodies | 12 (27.9) | 10 (17.9) | 0.23 |
| NAS | 0.005 | ||
| 0–2 | 1 (2.1) | 11 (18.3) | |
| 3,4 | 19 (39.6) | 29 (48.3) | |
| ≥5 | 28 (58.3) | 20 (33.3) |
Values are expressed as n (%).
Abbreviation: Z3PSF, zone 3 perisinusoidal fibrosis.
P values correspond to Wilcoxon rank sum tests for continuous factors and ordinal scores, Pearson's chi-square test for megamits and NAS, and Fisher's exact test otherwise. Boldface indicates statistically significant values.
Noninvasive Clinical and Laboratory Predictors of Progressive Disease in Pediatric NAFLD
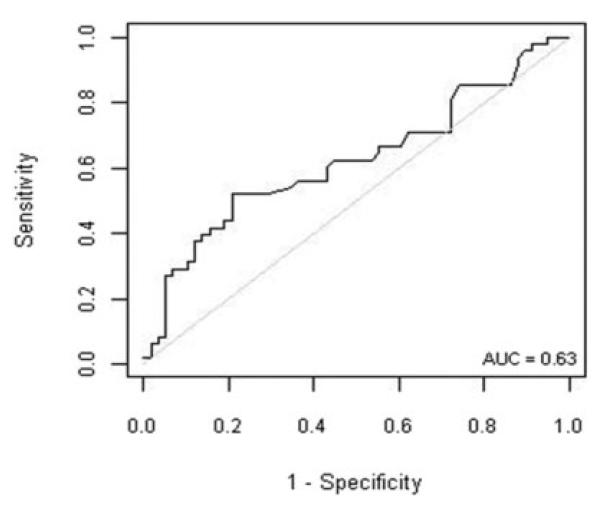
A wide variety of clinical and laboratory features were analyzed as possible predictors of histological disease severity (Table 4). None of the clinical features significantly correlated with stage of fibrosis, including presence of any comorbidities or BMI. From the laboratory markers, serum AST levels showed a positive correlation with stage of fibrosis (r = 0.29, 95% CI 0.10-0.47, P = 0.003). Moreover, serum AST level was the only laboratory marker that distinguished patients with significant fibrosis from those patients with no or mild fibrosis, and it was significantly higher in the former (P < 0.03). We next performed ROC curve analysis to establish the use of serum AST. Figure 2 displays the ROC curve for AST and prediction of clinical significant fibrosis (stage ≥ 2). For AST, the area under the ROC curve was estimated to be 0.63 (95% CI 0.51-0.74). Thus, AST does not appear to be a good predictor of the presence of severe fibrosis in children with NAFLD.
Table 4.
Associations of Clinical Features with Presence of Significant Fibrosis (Stage ≥2)
| Factor | Fibrosis 2/3 (n = 48) | Fibrosis 0/1 (n = 60) | P Value* |
|---|---|---|---|
| BMI, kg/m2 | 30.6 (27.6, 34.5) | 30.8 (25.9, 36.6) | 0.7 |
| Bilirubin, mg/dL | 0.5 (0.4, 0.7) | 0.4 (0.3, 0.6) | 0.14 |
| AST, U/L | 92.0 (55.0, 160.0) | 66.5 (52.0, 88.0) | 0.03 |
| ALT, U/L | 120.0 (73.0, 214.0) | 114.5 (67.0, 155.0) | 0.28 |
| AST/ALT ratio | 0.7 (0.6, 0.8) | 0.6 (0.5, 0.8) | 0.24 |
| Alkaline phosphatase, U/L | 216.0 (129.5, 303.5) | 250.0 (150.0, 361.0) | 0.32 |
| GGT, U/L | 65.0 (37.0, 91.0) | 50.0 (38.0, 76.5) | 0.27 |
| Albumin, g/dL | 4.6 (4.2, 39.5) | 4.5 (4.2, 5.1) | 0.77 |
| Prothrombin time, seconds | 11.8 (10.8, 12.8) | 12.0 (10.4, 12.7) | 0.69 |
| Glucose, mg/dL | 95.0 (83.0, 102.0) | 89.0 (84.0, 95.0) | 0.15 |
| Insulin, μU/mL | 27.0 (20.6, 46.5) | 30.6 (15.1, 50.2) | 0.91 |
| Cholesterol, mg/dL | 185.0 (156.0, 205.0) | 194.5 (160.5, 211.5) | 0.45 |
| Triglycerides, mg/dL | 157.5 (112.5, 248.0) | 144.0 (108.0, 231.0) | 0.83 |
| HDL, mg/dL | 38.0 (34.0, 42.0) | 38.0 (30.0, 45.0) | 0.83 |
| Platelet count, ×109/L | 276.5 (241.0, 337.5) | 303.0 (256.0, 359.0) | 0.085 |
| Male sex | 31 (64.6) | 37 (61.7) | 0.76 |
| Ethnicity | 0.1 | ||
| Asian | 10 (26.3) | 5 (10.9) | |
| Hispanic | 8 (21.1) | 17 (37.0) | |
| White | 20 (52.6) | 24 (52.2) | |
| Abdominal pain | 12 (25.5) | 7 (12.5) | 0.089 |
| Diabetes | 4 (8.5) | 3 (5.5) | 0.7 |
| Hyperlipidemia | 18 (38.3) | 20 (38.5) | 0.99 |
| Hypertension | 3 (8.6) | 3 (7.0) | 0.99 |
Values are expressed as the median (Q25, Q75) or n (%).
Abbreviation: GGT, gamma glutamyl transpeptidase.
P values correspond to Wilcoxon rank sum tests for continuous factors and ordinal scores; Pearson's chi-square for diabetes, hypertension, and zone; and Fisher's exact test otherwise.
Fig. 2.
ROC depicting serum AST as a predictor of significant fibrosis in patients with NAFLD. AST does not appear to accurately predict the presence of fibrosis in these patients. Approximate area under the ROC curve = 0.63.
Discussion
With the growing prevalence of childhood obesity, pediatric NAFLD has become the most frequent chronic liver disease in children and adolescents in industrialized countries.2 Currently, a liver biopsy remains the standard for the diagnosis of NAFLD or exclusion in appropriately chosen subjects.10,21 A liver biopsy provides key information regarding the amount of steatosis, degree of liver damage, and changes in the overall liver architecture as assessed by inflammatory activity and fibrosis scores, respectively. Recent longitudinal natural history studies from both United States and Europe have demonstrated that a liver biopsy provides useful information regarding prognosis in adult patients with NAFLD.6-9 These studies showed that adult patients with histological fatty liver tend to have a benign nonprogressive clinical course, whereas patients with NASH at baseline may develop complications related to chronic liver disease during follow-up as well as a significant increase in overall and liver-related mortality.6-9 Thus, a diagnosis of NASH in these patients may have significant clinical implications and may result in a more aggressive therapeutic approach toward metabolic risk factors as well as recruitment into clinical trials. Histological changes are also currently used as primary endpoints for monitoring response to therapy during therapeutic intervention trials in adult and pediatric NAFLD. In a recent attempt to standardize the histological diagnostic criteria to be used in comparing pretreatment and posttreatment biopsies, the NASH Clinical Research Network, which was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases, developed the NAS.15 The NAS was based on the classification proposed earlier by Brunt et al.22 and consists of the unweighted sum of scores for each of the following lesions: steatosis, lobular inflammation, and hepatocellular ballooning.15
It has been apparent from early studies in children that pediatric NAFLD may represent a unique challenge due to its distinct histological pattern of disease, which is characterized mainly by the presence of a predominant portal-based injury.14 This histological pattern has been referred to as type 2 or pediatric NASH to differentiate it from the typical zone 3 adult pattern of disease, which is known as type 1 NASH. Type 2 NASH has been reported to be the most common pattern in children and to be more frequently associated with Hispanic ethnicity, younger age, and male sex.14 Similar to recent reports,23-25 our study demonstrates that although portal-based injury (inflammation, fibrosis, or both) is seen in a large proportion of children with NAFLD, it is associated with the zone 3 pattern of injury in most cases. Based on these histological characteristics, overlapping features of both type 1 adult NASH and type 2 pediatric NASH were found in the vast majority of our patients. Finally, 44 of the 48 patients categorized as borderline by the NAS in our series showed the presence of portal inflammation. These results suggest the need for a more reproducible scoring system—perhaps a modified pediatric NAS incorporating portal inflammation into the scoring.
Although NAFLD is very common in the pediatric population, the data on prognosis of NAFLD in children remain scant. Case series have demonstrated that advanced fibrosis and cirrhosis may occur in children and young adults.4,26,27 Moreover, Feldstein et al.28 recently reported the first longitudinal study showing that, as in adults, NAFLD in children may progress to end-stage liver disease and require liver transplantation. However, because fewer than half of the patients in that study had a baseline liver biopsy, the prognostic value of individual histological features could not be examined. It is possible that there may be key histological features found on liver biopsy in children with NAFLD that may be predictive of more severe disease and progression for fibrosis. If identified, these patients could be targeted for more intensive intervention soon after diagnosis and before severe hepatic damage occurs. To understand the potential significance of a variety of histological lesions found in our patient population, we examined the potential associations of these features with the presence and severity of fibrosis. Stage of fibrosis showed a positive but relatively weak correlation with NAS, as well as with ballooning, lobular inflammation, and portal inflammation, though to a lesser extent. Degree of steatosis did not reveal a correlation with stage of fibrosis. We next divided patients into a group with significant clinically relevant fibrosis on liver biopsy (stage ≥2), and a group with no or mild fibrosis (stage 0-1). Compared with the latter group, the group with significant fibrosis was more likely to have higher lobular and portal inflammation scores, perisinusoidal fibrosis, and NAS ≥5. No differences were found between these two groups in terms of degree of steatosis or presence of ballooning of hepatocytes, as well as with other histological lesions. Thus, the lobular inflammation score appeared to be the driving force in this group. Thus, our results provide important clues into the potential clinical and prognostic relevance of histological lesions related to disease activity in pediatric NAFLD. Future, prospective, and longitudinal studies are needed to validate these results and further characterize the prognostic value of liver histology in children with NAFLD.
Because of the invasive nature, expense, and potential risks of liver biopsy, there has been great interest in the development of reliable noninvasive alternatives.21 Similar to a recent large study,24 in our series serum AST levels were the best predictor of increasing severity of fibrosis. However, neither AST levels alone nor the combination of routinely available laboratory and clinical data used to create prediction models showed sufficient specificity or sensitivity to predict the presence of severe fibrosis in these patients. Other approaches such as measuring specific circulating markers of fibrogenesis or fibrosis, or changes in tissue elasticity appear to be more promising techniques for staging disease in pediatric NAFLD.29,30
The current study has several strengths. First, we included one of the largest cohorts of children with well-characterized, biopsy-proven NAFLD reported to date. Second, we studied children with NAFLD seen in different areas of the United States and Canada; thus, the population included a large variety of ethnic backgrounds and a wide range of histological severity. The biopsy specimens were extensively reviewed by two hepato-pathologists at separate and joint readings. However, our study has some limitations, including the fact that patients were seen at large referral tertiary care medical centers, and although the results may be extrapolated to other similar medical centers, the results may not apply to children with NAFLD from the community. In most cases, a liver biopsy was performed due to persistently elevated serum aminotransferases; therefore, whether or not the prevalence and significance of histological lesions of pediatric NAFLD is any different among patients in whom liver enzymes may not be increased to this extent requires investigation. Finally, due to the retrospective nature of our study, the roles of some previously described and potentially useful noninvasive routine clinical or laboratory markers such as waist circumference,31 tanner stage, or autoantibodies24 for noninvasive diagnosis in pediatric NAFLD could not be assessed due to unavailable or insufficient data.
In conclusion, this study demonstrates that although portal-based injury is frequently found in children, it is almost universally associated with some elements of the zone 3 pattern identified in adults. Furthermore, our data identified the potential prognostic value of histological lesions of disease activity and suggested portal inflammation may be a useful variable to incorporate into assessment of pediatric NAFLD cases.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- CI
confidence interval
- HDL
high-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- ROC
receiver operating characteristic
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 3.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. HEPATOLOGY. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM. Pathology of fatty liver disease. Mod Pathol. 2007;20(Suppl 1):S40–S48. doi: 10.1038/modpathol.3800680. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sorensen TI, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. HEPATOLOGY. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 10.Wieckowska A, Feldstein AE. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–395. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 11.Yeh MM, Brunt EM. Pathology of nonalcoholic fatty liver disease. Am J Clin Pathol. 2007;128:837–847. doi: 10.1309/RTPM1PY6YGBL2G2R. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 13.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43:413–427. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 14.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. HEPATOLOGY. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. HEPATOLOGY. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 17.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 20.Brunt EM, Kleiner DE, Wilson LA, Unalp A, Behling CE, Lavine JE, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. HEPATOLOGY. 2009;49:809–820. doi: 10.1002/hep.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. HEPATOLOGY. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226–232. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 24.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–1971. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. HEPATOLOGY. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 26.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Kinugasa A, Tsunamoto K, Furukawa N, Sawada T, Kusunoki T, Shimada N. Fatty liver and its fibrous changes found in simple obesity of children. J Pediatr Gastroenterol Nutr. 1984;3:408–414. doi: 10.1097/00005176-198406000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders F, Angulo P. Nonalcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. doi: 10.1136/gut.2008.171280. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobili V, Parkes J, Bottazzo G, Marcellini M, Cross R, Newman D, Vizzutti F, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2008;136:160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Nobili V, Vizzutti F, Arena U, Abraldes JG, Marra F, Pietrobattista A, et al. Accuracy and reproducibility of transient elastography for the diagnosis of fibrosis in pediatric nonalcoholic steatohepatitis. HEPATOLOGY. 2008;48:442–448. doi: 10.1002/hep.22376. [DOI] [PubMed] [Google Scholar]
- 31.Manco M, Bedogni G, Marcellini M, Devito R, Ciampalini P, Sartorelli MR, et al. Waist circumference correlates with liver fibrosis in children with non-alcoholic steatohepatitis. Gut. 2008;57:1283–1287. doi: 10.1136/gut.2007.142919. [DOI] [PubMed] [Google Scholar]