Abstract
Neurokinins (NK) released from terminals of dorsal root ganglion (DRG) neurons may control firing of these neurons by an autofeedback mechanism. In this study we used patch clamp recording techniques to determine if NKs alter excitability of rat L4-S3 DRG neurons by modulating K+ currents. In capsaicin (CAPS)-responsive phasic neurons substance P (SP) lowered action potential (AP) threshold and increased the number of APs elicited by depolarizing current pulses. SP and a selective NK2 agonist, [βAla8]-neurokinin A (4–10) also inhibited low threshold inactivating K+ currents isolated by blocking non-inactivating currents with a combination of high TEA, (−) verapamil and nifedipine. Currents recorded under these conditions were heteropodatoxin-sensitive (Kv4 blocker) and α-dendrotoxin insensitive (Kv1.1 and Kv1.2 blocker). SP and NKA elicited a >10 mV positive shift of the voltage dependence of activation of the low threshold currents. This effect was absent in CAPS-unresponsive neurons. The effect of SP or NKA on K+ currents in CAPS-responsive phasic neurons was fully reversed by an NK2 receptor antagonist (MEN10376) but only partially reversed by a PKC inhibitor (bisindolylmaleimide). An NK1 selective agonist ([Sar9, Met11]-substance P) or direct activation of PKC with phorbol 12,13-dibutyrate, did not change firing in CAPS-responsive neurons, but did inhibit various types of K+ currents that activated over a wide range of voltages. These data suggest that the excitability of CAPS-responsive phasic afferent neurons is increased by activation of NK2 receptors and that this is due in part to inhibition and a positive voltage shift in the activation of heteropodatoxin-sensitive Kv4 channels.
Keywords: dorsal root ganglia, nociception, hyperexcitability, K+ channels, neurokinins, autofeedback
INTRODUCTION
Dorsal root ganglion (DRG) neurons are a heterogeneous population of cells (Rush, et al., 2007, Waxman, et al., 2002, Wood, et al., 2002) that respond to temperature changes and/or a variety of chemical and mechanical stimuli (Averbeck, et al., 2000, Gschossmann, et al., 2000). A subpopulation of small and medium diameter (Liu, et al., 2006, Rau, et al., 2006) capsaicin (CAPS)-sensitive DRG neurons that are thought to have a nociceptive function (Besson, 1999) synthesize and release substance P (SP) and neurokinin A (NKA, (Hökfelt, et al., 2001, Hökfelt, et al., 1982)). They also express all three subtypes of neurokinin receptors (Brechenmacher, et al., 1998) and exhibit excitatory responses to exogenous SP (Abdulla, et al., 2001, Maggi, 1997, Nakamura-Craig and Gill, 1991, Sculptoreanu and de Groat, 2007, Yamane, et al., 2007). These observations have raised the possibility that neurokinins may act in an autofeedback manner to regulate afferent terminal excitability (Morrison, et al., 1999, Sculptoreanu and de Groat, 2003, Sculptoreanu, et al., 2004).
Previous studies revealed that SP and NKA elicit various excitatory effects in CAPS-responsive (CR) DRG neurons, including: (1) increase in Ca2+ currents (Sculptoreanu and de Groat, 2003); (2) enhancement of CAPS-evoked TRPV1 currents (Sculptoreanu, et al., 2008); (3) decrease in the threshold for action potential (AP) generation (Sculptoreanu and de Groat, 2007) and (4) an increase in the number of APs triggered by long duration depolarizing current pulses (600 ms, (Sculptoreanu and de Groat, 2007)). The effects of SP on firing are mimicked by K+ channel blocking agents 4-amino-pyridine (4-AP) and heteropodatoxin II (HPTx, (Sculptoreanu and de Groat, 2007)), and are suppressed by KW-7158 (Sculptoreanu, et al., 2004), an agent that enhances the opening of K+ channels. These findings led to the proposal that part of the excitatory effect of neurokinins on DRG neurons is attributable to a suppression of K+ channels (Sculptoreanu and de Groat, 2007).
In phasic CR neurons, a broad spectrum PKC inhibitor, bisindolylmaleimide I HCl (BIM, 0.5µM), only partially reversed the effect of SP on firing (Sculptoreanu and de Groat, 2007). In addition, PDBu, a PKC activator, did not significantly change phasic firing in CR DRG neurons and had an inhibitory action on firing in tonic CR neurons. These data suggested that the change in firing induced by SP and NKA was caused by activation of an intracellular signaling mechanism distinct from activation of phospholipase C and protein kinase C (Sculptoreanu and de Groat, 2007).
In the present experiments whole cell patch clamp techniques in dissociated lumbosacral DRG neurons from adult rats were used to examine the mechanisms that might underlie the neurokinin-induced effects on firing and, in particular, the types of K+ channels modulated by neurokinin receptor activation.
METHODS
Experimental animals
Experiments were performed in DRG neurons from adult male Sprague Dawley rats (100–250g; Harlan, Indianapolis, Indiana USA). Care and handling of the animals was in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee.
Preparation of dissociated neurons
Neurons were isolated from L4-S3 DRG of adult rats using methods previously described (Sculptoreanu and de Groat, 2003). Briefly, freshly dissected ganglia were minced and washed in cold, oxygenated DMEM (Sigma). This was followed by a brief, 10 min dissociation at 37°C in DMEM containing 0.5 mg/ml trypsin (Sigma). At the end of this step, DMEM containing 1 mg/ml collagenase B (Boehringer-Mannheim) and 0.5 mg/ml trypsin inhibitor type 1S (Sigma) was added and the dissociation was continued for another 25–60 min. During dissociation the neurons were gently triturated with siliconized Pasteur pipettes every 20 min. After the ganglia were dissociated into individual neurons the cell suspension was layered on 25 ml of 50% adult bovine serum (Sigma) and DMEM in centrifuge tubes and centrifuged again at 800 rpm. This step removed most of the debris and broken cells. The pellet containing DRG neurons was resuspended in DMEM containing 10% heat inactivated horse serum and 5% fetal bovine serum (Sigma), and plated on collagen coated 35 mm petri dishes (Collaborative Research, Biocoat). Neurons were plated at low density (2000–3000 per dish). Primary cultures were kept in a 95% air, 5% CO2 incubator at 37°C.
Patch clamp techniques
Gigaohm seal whole cell recordings of CAPS currents, K+ currents and evoked APs were recorded in DRG neurons 3–5 days in culture using patch clamp techniques. Cultures older than 5 days in which slowly decaying capacitive responses became significant were not used. Immediately before recording, the serum containing media was replaced with phosphate buffered saline for AP, CAPS current and total voltage dependent current measurements or with 60 mM TEA and Cl−-free external solution to which verapamil and nifedipine (5 µM each) were added, for K+ current measurements. The 5 µM concentration of nifedipine has no measurable effect on inactivating K+ currents (Zhabyeyev, et al., 2000). The phosphate buffer solution consisted of (in mM): 138 NaCl, 2.6 KCl, 0.9 CaCl2, 0.5 MgCl2, 1.5 KH2PO4, 8.1 Na2HPO4. The pipette (intracellular) solution used to measure APs and total voltage dependent currents contained (in mM): 120 KCl, 10 K2HPO4, 10 NaCl, 2 MgCl2, 1 EGTA, 10 HEPES, pH adjusted to 7.4 with HCl. The Cl−-free buffer used to isolate low threshold inactivating K+ currents consisted of (in mM): 136 CH3SO3H, 60 TEA(OH), 60 TRIS, 4 Ca(OH)2, 2 Mg(OH)2, 10 HEPES, 4 KOH buffered to pH 7.4. In these experiments the pipette (intracellular) solution contained (in mM): 140 KOH, 134 CH3SO3H, 2 MgCl2,2 EGTA, 10 HEPES, pH adjusted to 7.4 with CH3SO3 H. Mg-ATP (3 mM), cAMP (0.3 mM) and tris-GTP (0.5 mM) were added to the intracellular solutions just prior to the experiments.
The long and short axes of the neurons were measured in some experiments with an eyepiece micrometer. In these neurons the membrane capacitance (pF) varied linearly as a function of average cell diameter (μm). Whole cell currents were voltage clamped using an Axopatch 200A amplifier (Axon Instruments, Foster City, California). Pulse generation, current recording and data analysis used pClamp software (Axon Instruments). Currents were sampled at 50–500 µs intervals, and filtered at 2 kHz. Capacitive currents and up to 80% of the series resistance were compensated. A p/4 protocol was used to subtract uncompensated capacitive currents and leak currents.
As described previously (Gold, et al., 1996, Sculptoreanu, et al., 2004) K+ currents in DRG neurons can be divided into at least six categories distinguishable in terms of voltage dependence of activation, inactivation and pharmacology. Activation curves for K+ currents were normalized by Goldman-Hodgkin-Katz relation GHKK/GHKKmax and plotted versus test voltages, where the GHKK was determined by the method of Clay (Clay, 2000), IK = qV/kT[exp(q(V − EK))kT) − 1]/[exp(qV/kT) − 1], where q is the unit electric charge, k is the Boltzmann constant, and T is absolute temperature (at room temperature kT/q is 25 mV, and EK is 72 mV). To simplify the analysis of peak outward currents (which consisted of a mixture of non-inactivating and inactivating currents), we assumed that these currents can be roughly divided into low threshold (LT) and high threshold (HT) components (Gold, et al., 1996, Sculptoreanu, et al., 2004) that when normalized can be fitted (SigmaPlot, Jandel Scientific) with a sum of two Boltzmann curves (I = (ALT/(1 + exp(−(V − VLT(0.5))/kLT) + (AHT/(1 + exp(−(V − VHT(0.5))/kHT) + constant; where ALT and AHT are the maximum amplitudes of the LT and HT activation curves; V, the test pulse voltage; V0.5, half-activation voltage and k, slope factor for each component. Since outward currents in our experiments were blocked by a combination of TEA and 4-AP, we assume that these currents were primarily K+ currents.
Inactivation curves of two types of K+ currents, one that inactivated at more hyperpolarized (A1) and one that inactivated at more depolarized voltages (A2), were obtained by subtracting non-inactivating currents from total currents and were fitted with a sum of two Boltzmann curves (I = (A1/(1 + exp((V − V1(0.5))/k2) + (A2/(1 + exp((V − V2(0.5))/k2) + constant; terms as defined for activation). In experiments that recorded IK in high TEA (60 mM) solution containing verapamil and nifedipine (5µM), which suppressed a large fraction of non-inactivating currents, the voltage dependence of activation and inactivation were fitted by two Boltzmann equations, with the AHT (activation) and A2 inactivating components being only a small percentage (<15%) of the total current. Data for inactivation (constant V-EK) were plotted as IK/IK(max) versus the prepulse voltages used to generate inactivation curves.
Total outward currents (activating and non-inactivating) and firing were analyzed in the same cells by switching from voltage clamp to current clamp recording. CAPS (0.5 µM) responsiveness (Sculptoreanu, et al., 2008) was determined after recording control currents and firing. APs were generated by rectangular current pulse injections 5 ms long and 50–500 pA in intensity, followed by a 100 ms interpulse at the holding potential and a second pulse, 600 ms long. In general, the sequence consisted of at least two control recordings of activation and inactivation curves and evoked APs followed by pharmacological studies in which drugs were tested on either total outward currents, firing, or on inactivating and non-inactivating currents. Activation and inactivation curves and evoked APs were repeated in the presence of drugs. The effects of drugs reached steady state within 3 min of application and were stable throughout the duration of the experiments (15–50 min). Recording was done at room temperature in static chambers fitted with a rapid mixing setup for addition of drugs as described previously (Sculptoreanu, et al., 2008).
Drugs
Neurokinin agonists (substance P, [βAla8]-neurokinin A (4–10) (Calbiochem), NK3 agonist [MePhe7]-neurokinin B (Calbiochem), NK1 selective agonist [Sar9, Met11]-substance P (Calbiochem), NK2 antagonist (MEN 10,376, Sigma), NK3 antagonist (SB 235,375, gift from SmithKline Beecham) and the Kv4 channel blocker HPTx (Alomone Labs) were prepared in external solutions. Nifedipine and (−) verapamil, 4-AP and TEA were obtained from Sigma. Capsaicin (Calbiochem), phorbol 12, 13-dibutyrate (Research Biochemicals), and the PKC inhibitor bisindolylmaleimide I HCl (Calbiochem) were dissolved in DMSO (100 mM) and used at less than 0.01% of their stock concentration. At these dilutions, DMSO alone had no effect on currents or firing. Stock solutions (10–100 mM) were stored at −20°C and diluted in the external recording solution just before experiments. Extracellularly applied drugs were pipetted from stock solutions at 50 times the final concentration and rapidly mixed in the recording chamber to give, after dilution the stated concentrations. With this technique, effects of drugs such as TTX (Yamane, et al., 2007) or capsaicin (Sculptoreanu, et al., 2008, Srinivasan, et al., 2008) reached steady state within seconds of drug application. Steady state effects of each drug concentration were measured for at least 2–3 min before changing the drug concentration or adding a new drug.
Data analysis
Results are reported as mean ± SEM. Statistical testing was carried out using a stepwise procedure depending upon the number of groups being compared. When only two means were involved in a comparison, a two-tailed t-test with unequal variances was used. A comparison was considered statistically significant if P<0.05. When more than two means were involved, a one-way analysis of variance was first carried out in order to obtain a global test of the null hypothesis. If the global P-value for the test of the null hypothesis was <0.05, we carried out post-hoc comparisons between the different groups using the Holm-Sidak Test (Glantz, 2005).
RESULTS
Properties of phasic and tonic neurons
Data presented in this paper were obtained in part from neurons that were used in earlier studies to measure effects of neurokinins on firing (Sculptoreanu and de Groat, 2007) and CAPS-evoked currents (Sculptoreanu, et al., 2008). The cultured lumbo-sacral DRG neurons ranged in size from 18–50 µm diameter (average of long and short axes) and had capacitances of 20–90 pF. The neurons could be subgrouped based on firing patterns (phasic and tonic) and CAPS responsiveness (Sculptoreanu and de Groat, 2007). In response to depolarizing current injections 600 ms in duration at 0.45 nA stimulus intensity, which produced the maximum firing, tonic neurons (n = 24) fired 5–12 APs (APs, 10.4 ± 1.2 APs/600ms). Phasic neurons (n = 106, Fig. 1A and B) were smaller (44 ± 2 pF) than tonic neurons (70 ± 8 pF) and characteristically fired at most 4 APs (mean, 1.5 ± 0.3/600 ms, at 0.45 nA) in response to depolarizing current injections 600 ms in duration, but were otherwise comparable to tonic neurons with respect to resting potentials and membrane resistances. Capsaicin applied in a concentration (0.5 µM, Fig. 1 C) that is near the EC50 of the concentration response curve, induced inward currents (0.05–5.00 nA) in 62 of 106 phasic neurons and 19 of 24 tonic neurons.
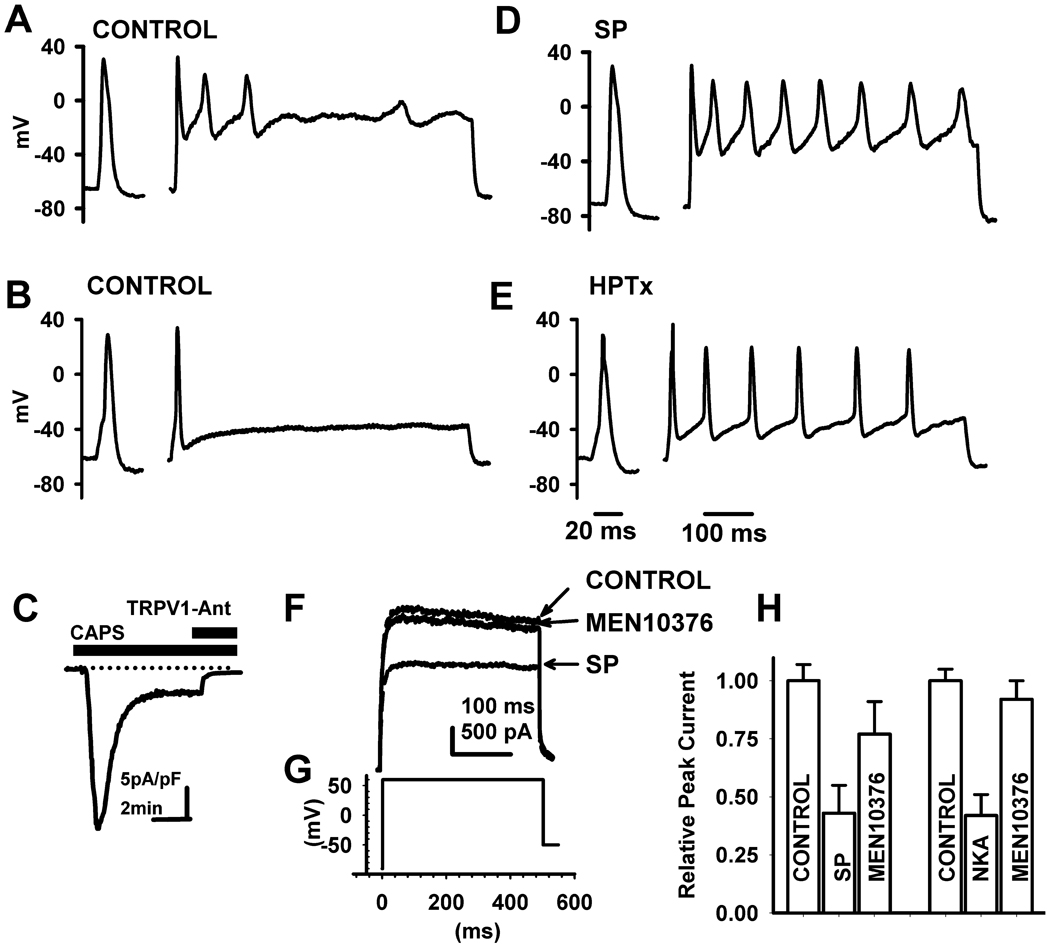
Fig. 1.
Effect of neurokinins on firing and K+ currents in capsaicin responsive (CR) DRG neurons. Action potentials generated in two different in CAPS-responsive (CR) phasic DRG neurons by rectangular current pulse injections 5 ms in duration and 200 pA (A, D) or 50 pA (B, E) in intensity, followed by a 100 ms interpulse at the holding potential and a second pulse of the same intensity as the first in the sequence, 600 ms in duration. Control recordings before treatment (A, B) and the enhancement of firing by substance P (SP, 0.5 µM, B) or by heteropodatoxin II (HPTx, 0.05 µM, E). SP (D) and HPTx (E) significantly increased the number of action potentials (APs) triggered by the second pulse in the sequence without significantly altering the duration of single AP triggered by the first brief pulse. C, Inward current evoked by CAPS (0.5 µM) is blocked by a TRPV1 antagonist (TRPV1-Ant, 5 µM). F, Total K+ currents activated by a test pulse to +60 mV from a holding potential of −80 mV were partially blocked by SP (0.5 µM) and the NK2 (0.5 µM) selective antagonist MEN10376 reversed the inhibitory effect of SP in the same cell shown in C. H, Summary of the effects of substance P or a selective NK2 agonist, [βAla8]-neurokinin A (4–10) (NKA, 0.5 µM) on total K+ currents in CR phasic DRG neurons and reversal of the effects by the NK2 (0.5 µM) selective antagonist MEN10376. Scale bar in A, B, D and E is 20 ms for the first action potential in the sequence and 100 ms for the second long stimulus pulse. Scale bars in F are 100 ms and 500 pA and in H are 5 pA/pF and 2 min.
Effect of neurokinins on firing in capsaicin responsive (CR) and unresponsive (CU) DRG neurons
In CR phasic neurons, application of neurokinins (substance P, SP, n = 30, or [βAla8]-neurokinin A (4–10), NKA, n = 19, not shown) lowered the threshold for inducing an AP and increased firing (Fig 1 D), an effect that is similar to that of HPTx (0.05 µM, Fig. 1 E). The effect of SP on the AP threshold and AP number generated by long duration 600 ms current pulses was restricted to CR neurons whether they were phasic or tonic. SP (0.5 µM) increased the firing 318% and 94%, in 13 phasic CR neurons and 6 tonic neurons respectively, and lowered the thresholds for firing by 12 mV in phasic CR neurons and 6 mV in tonic CR neurons (Table 1). On the other hand, in phasic or tonic CU neurons SP did not change AP number or the threshold for firing (Table 1).
Table 1.
Effect of substance P (0.5 µM) on firing and action potential threshold in phasic and tonic CAPS-responsive and CAPS-unresponsive DRG neurons.
| CAPS-responsive | CAPS-responsive | |||||||
|---|---|---|---|---|---|---|---|---|
| AP number (/600 ms) | AP threshold (mV) | AP number (/600 ms) | AP threshold (mV) | |||||
| control | +SP | control | +SP | control | +SP | control | +SP | |
| (N=30) | (N=6) | |||||||
| Phasic | 2.4 ± 0.6 | 7.8 ± 0.7** | −22.6 ± 0.5 | −34.5 ± 0.7 ** | 1.5 ± 0.2 | 2.2 ± 0.4 NS | −25.6 ± 2.0 | −24.8 ± 2.8 NS |
| (N=19) | (N=7) | |||||||
| Tonic | 8.4 ± 1.4 | 12.6 ± 1.3 * | −34.0 ± 1.3 | −39.6 ± 0.9** | 5.7 ± 1.1 | 6.0 ± 0.8 NS | −29.6 ± 1.1 | −29.6 ± 1.2 NS |
Averages; SEM; t-test relative to values in control experiments, 2 tailed, unequal variance, level of significance: NS-not significant
P<0.05, or
P<0.001.
Pharmacological characterization of low threshold and high threshold total K+ currents and inactivating and non-inactivating K+ currents in DRG neurons
By using normal intracellular and extracellular solutions we were able to study cell firing under current clamp conditions and currents under voltage clamp conditions (Fig. 1 F–H). We presume that depolarization-evoked outward currents represent voltage gated K+ currents because they were inhibited by a combination of TEA and 4-AP (Fig. 2 A and B). Thus, these currents will be termed K+ currents throughout the paper. In the first series of experiments the protocol used to isolate different types of voltage dependent K+ currents and to examine the effects of pharmacological agents consisted of a test pulse to +60 mV from a holding potential of −80 mV to generate total inactivating and non-inactivating currents, followed by a brief 50 ms interpulse to −50 mV, a prepulse 850 ms in duration to −20 mV, to eliminate the inactivating currents followed by a second pulse identical to the first pulse, but 500 ms in duration (Fig. 2 C). Another protocol used a combination of hyperpolarizing and depolarizing prepulses to elicit non-inactivating currents, which were subtracted from the total K+ currents to identify the inactivating currents (Fig. 2 D and E).
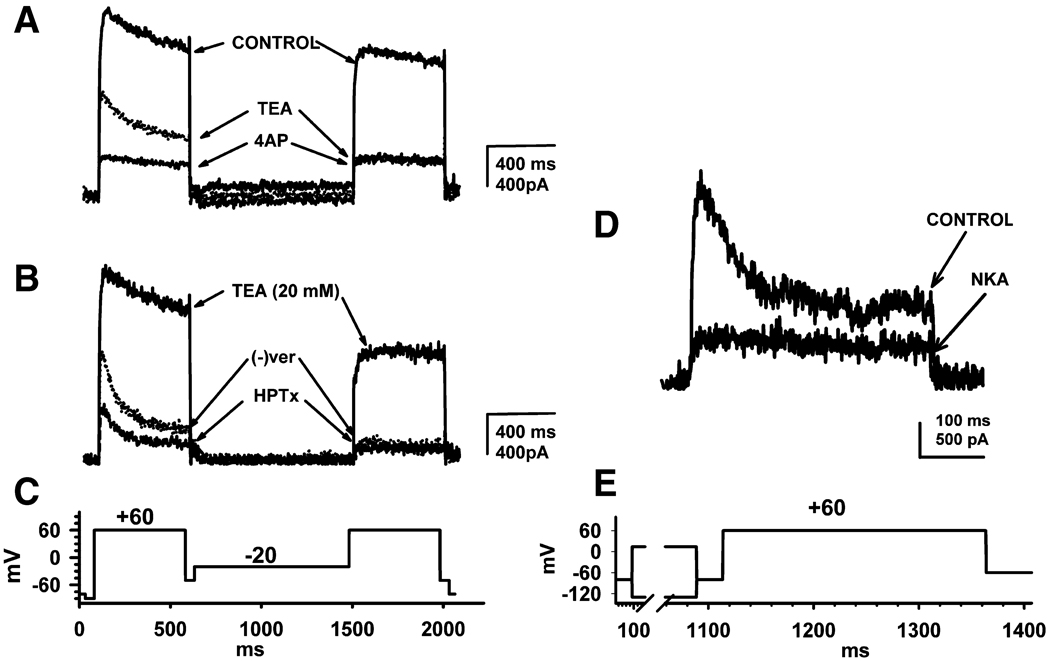
Fig. 2.
Pharmacological and voltage separation of inactivating and non-inactivating potassium-currents in CR phasic DRG neurons. A, B, Total outward-currents (on the left) and inactivating-currents (on the right) elicited by the multi pulse stimulation protocol shown in panel C. A, TEA (20 mM) partially reduces total outward currents before prepulse leaving a residual fast inactivating current and reduces by >70% the non-inactivating current after prepulse. Subsequent application of 4-AP (50 µM) blocks >50% of the TEA-resistant total currents before prepulse but does not alter the residual TEA-resistant non-inactivating currents after prepulse. B. In the presence of TEA (20 mM), which reduced the total outward current before prepulse and markedly reduced the non-inactivating current after prepulse, (−) verapamil ((−) ver, 5 µM) reduces by >70% the TEA-resistant non-inactivating current after pre-pulse and unmasks a more rapidly inactivating current before prepulse. In the presence of TEA and (−) verapamil, application of HPTx (0.05 µM), reduces by >50% the currents activated before prepulse but has no effect on the non-inactivating currents after prepulse. C,. D, Effect of a selective NK2 agonist [βAla8]-neurokinin A (4–10) (NKA) on inactivating K+ currents in a CR phasic DRG neuron measured using the stimulation protocol shown in panel E. NKA largely inhibited (5µM, D) the inactivating current obtained by subtraction of non-inactivating currents from the total currents. The difference between the two currents is represented by the fast inactivating current labeled "CONTROL" in D and by the residual non-inactivating current after block by NKA. Records in A, B and D were obtained from three different CR phasic neurons. Scale bars to the right of current traces A and B are 400 ms and 400 pA; scale bar below the current trace in D is 100 ms and 500 pA.
In CR phasic neurons the K+ currents consisted of a mixture of LT and HT currents and of a mixture of both inactivating and non-inactivating currents. TEA (20 mM, n = 14) suppressed the total K+ currents recorded before prepulse to a lesser extent (32 ± 4% reduction; Fig. 2 A) than the non-inactivating currents recorded after prepulse, which were reduced by 74 ± 6%. Application of a low concentration of 4-AP (50 µM, n = 7), in the presence of TEA, suppressed >50% of the remaining current before prepulse but had no effect on the non-inactivating current after prepulse (Fig. 2 A). In the presence of TEA (20 mM), application of (−) verapamil (5 µM, n = 12) (Catacuzzeno, et al., 1999, Madeja, et al., 2000) blocked 78 ± 6% of the residual TEA resistant non-inactivating currents (after prepulse), with little or no effect on inactivating currents elicited before prepulse (Fig. 2 B). Application of low concentrations of HPTx (0.05 µM, n = 6) in the presence of TEA and (−) verapamil, blocked up to 50% of the currents activated before prepulse (Fig. 2 B) but had no effect on the non-inactivating currents after prepulse, an effect which resembled that of low concentrations of 4-AP (Fig. 2 A). In 5 neurons, low concentrations of HPTx (0.05 µM) applied in the absence of TEA reduced by 17 ± 4% the currents before prepulse but had no effect on the non-inactivating currents after prepulse. After blocking the LT inactivating currents with 4-AP (1 mM, n = 4, not shown), HPTx (0.05 µM) did not alter total outward currents suggesting that the two agents affected the same currents at the concentrations tested in our experiments.
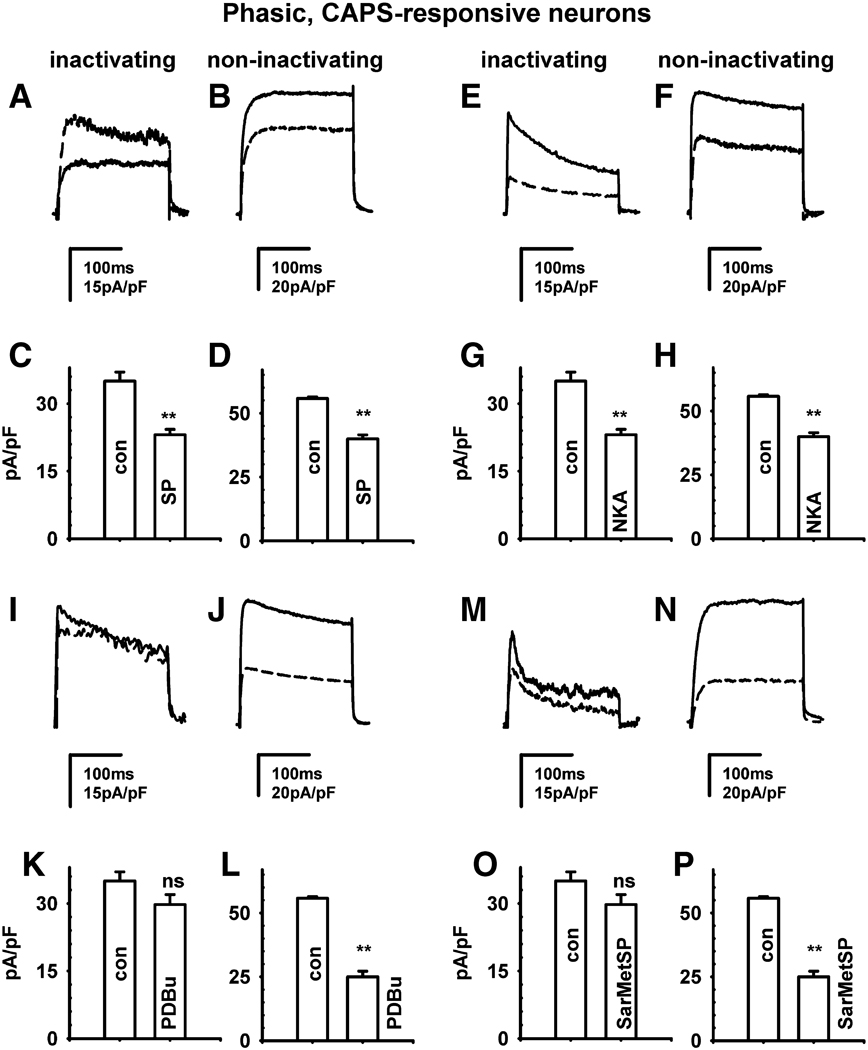
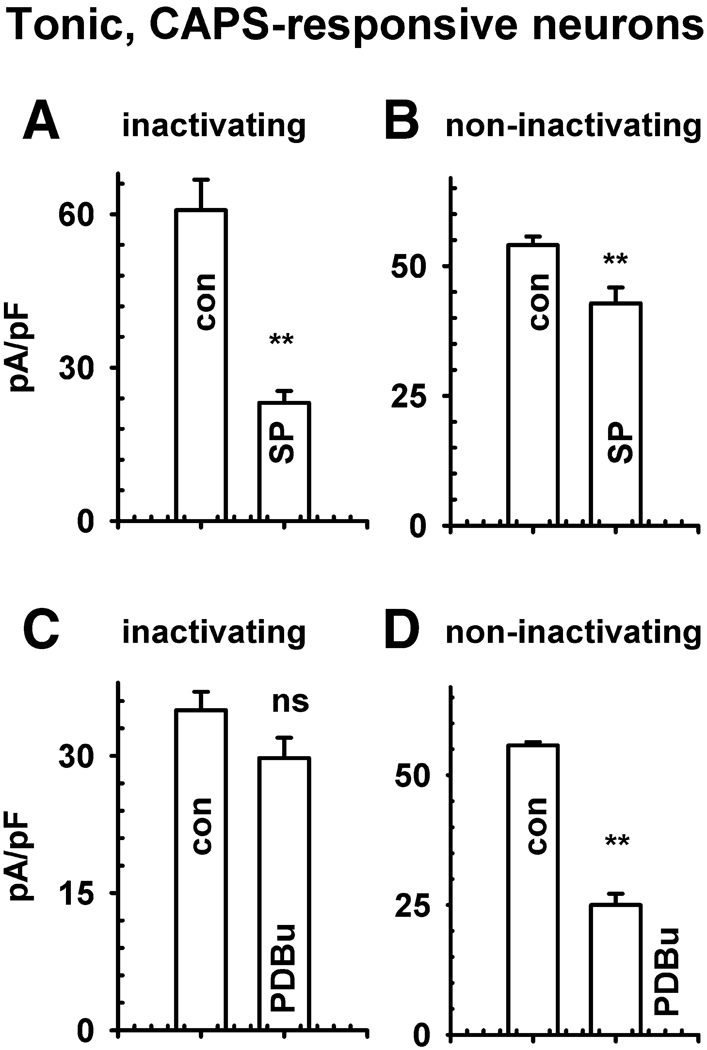
In CR phasic neurons, SP (0.5 µM, Fig. 3 A–D) reduced total outward currents by 20–62% (Fig. 1 F, H, P<0.001) as well as the inactivating K+ currents by 54 ± 2% (n = 13 neurons, Fig. 3 A and C, P<0.01) and the non-inactivating currents by 28 ± 1% (Fig. 3 B and D, P<0.01). NKA (0.5 µM, Fig. 3 E–H) produced a similar suppression of the inactivating (n = 7 neurons, Fig. 3 E and G, P<0.01) and non-inactivating currents (Fig. 3 F and H, Table 1, P<0.01). A selective NK2 antagonist (MEN10376, 0.5 µM, Fig. 1 H) completely reversed the inhibitory effect of SP (n = 7 neurons, P>0.1) and NKA (n = 5 neurons, P>0.1) on total outward currents. The NKA and SP inhibition of total K+ currents was unaffected by an NK3 antagonist (SB 235,375, 0.5 µM, n = 3–4, P>0.1). The NK3 agonist [MePhe7]-neurokinin B (0.5 µM, n = 4, not shown, P>0.1), NK1 agonist [Sar9, Met11]-substance P (Sar-MetSP, 0.5 µM, P>0.1), and phorbol 12, 13-dibutyrate (PDBu, 0.5 µM, P>0.1) did not significantly change the inactivating current (P>0.05) but markedly reduced (60–70%) the non-inactivating current (Fig. 3 I–P, P<0.01). In tonic CR neurons SP also elicited a much larger suppression of inactivating (60% decrease, Fig. 4 A, P<0.01) than non-inactivating currents (18% decrease; Fig. 4 B, P<0.01); whereas PDBu suppressed non-inactivating (Fig. 4 D, P<0.01) currents without significantly changing the inactivating currents (Fig. 4 C, P>0.1).
Fig. 3.
Effect of neurokinins and a phorbol ester on K+ currents in CR phasic neurons. Effects of substance P (SP, A, B, C, D); [βAla8]-neurokinin A (4–10) (NKA, E, F, G, H), a protein kinase C activator, phorbol 12,13-dibutyrate (PDBu, 0.5 µM, I, J, K, L) and a selective NK1 agonist, [Sar9, Met11]-substance P (Sar-MetSP, M, N, O, P) on phasic CR DRG neurons. Inactivating currents were obtained by subtraction of non-inactivating currents from the total currents using a stimulus protocol as described in Fig. 2 E. Non-inactivating currents are the currents generated by a test pulse to +60 mV after a prepulse to +4 mV. The upper tracing in each panel represents the control recording and the lower tracing is the current recorded after the various agents. Effects on inactivating K+ currents of SP (A), NKA (E), PDBu (I) and Sar-MetSP (M). C, G, K, O, Averages of peak amplitude in control and after drugs obtained from similar experiments as in A, E, I, M in response to SP (n = 13), NKA (n = 7), PDBu (n = 10) and Sar-MetSP (n = 6). B, F, J, N, Effects of SP (B), NKA (F), PDBu (J) and Sar-MetSP (N) on non-inactivating K+ currents. D, H, K, P, Averages of peak amplitude in control and after drugs obtained from similar experiments as in B, F, J, N in response to SP (n = 13), NKA (n = 7), PDBu (n = 10) and Sar-MetSP (n = 6). Statistical difference using a two tailed t-test, unequal variance difference: *P<0.05, **P<0.01; ns, not significant. Scale bars in A, E, I, M is 15 pA/pF and 100 ms and 20 pA/pF and 100 ms in B, F, J, N.
Fig. 4.
Effect of substance P and a phorbol ester on K+ currents in CR tonic neurons. Effects of substance P (SP, A–B) and phorbol 12, 13-dibutyrate (PDBu, C–D) in CR tonic DRG neurons. A, C. Averages of peak amplitudes of inactivating K+ currents in control (con) conditions before drugs and after SP (0.5 µM, A, n = 6) and PDBu (0.5 µM, E, n = 4). B, D, Averages of peak amplitudes of non-inactivating currents in control (con) and a SP (B) or PDBu (D) in the same experiments as in A and C. Stimulus protocols were the same as described in Fig. 3. Statistical difference using a two tailed t-test, unequal variance difference: * P<0.05, ** P<0.01; ns, not significant.
Effects of drugs on K+ current activation and inactivation in capsaicin responsive DRG neurons
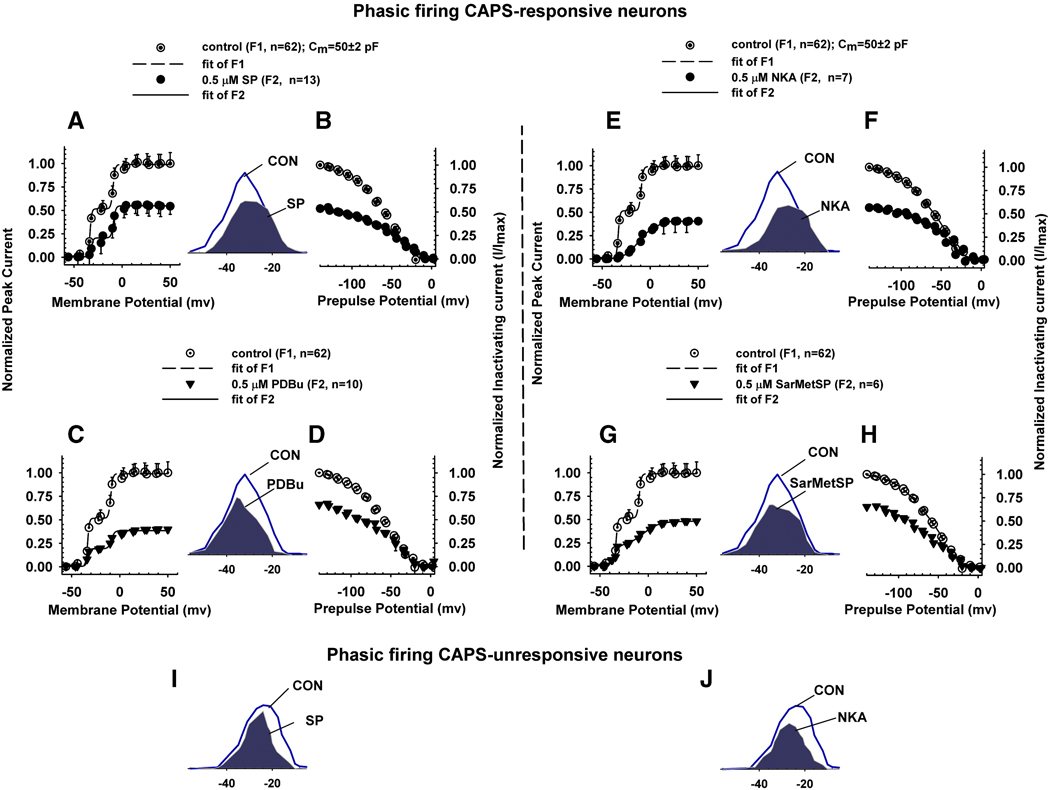
To evaluate the effects of neurokinins on the kinetics and voltage dependence of activation and inactivation of K+ currents, a combined two pulse activation inactivation protocol was used (Sculptoreanu, et al., 2004), consisting of a series of rectangular prepulses from a holding potential of −90 mV. A test pulse, 1000 ms in duration, ranging from −140 to +90 mV was used to generate K+ current activation curves. The inactivation curve was measured after a brief, 23 ms interpulse at −80 mV and a second test pulse to +60 mV, 250 ms in duration. For activation the average peak amplitudes of currents were normalized using the GHK equation as described in Methods. The outward currents before and after drugs were fitted with a sum of two Boltzmann equations (Fig. 5, and Table 2–Table 4) that represent the LT and HT K+ currents. The fractional block after drugs was determined from the ratio of maximum outward currents after the drugs to that before drug application in every cell. The parameters for activation curves determined by nonlinear regression analysis of total K+ currents in control experiments (n = 62, Fig. 5 A, C, E, G, dotted circles) and after addition of drugs (Fig. 5 A, C, E, G, filled symbols) were obtained from the fitted curves. Similarly, the parameters for the inactivation curves of currents that inactivated at more hyperpolarized (A1) and at more depolarized voltages (A2) were obtained from the best fits used to generate the curves in figure 5 before (Fig. 5 B, D, F, H, dotted circles) and after addition of drugs (Fig. 5 B, D, F, H, filled symbols).
Fig. 5.
Effect of drugs on current-voltage relationship of activation and inactivation of K+ currents in CR phasic neurons. Activation curves (A, C, E, G) for total outward currents and inactivation curves (B, D, F, H) of inactivating outward currents obtained by subtracting the non-inactivating currents from total currents in CR phasic neurons. Activation curves of LT and HT currents (see Methods) were generated by the first test pulse of a range of intensities 1000 ms in duration (Fig. 2 E) of a two pulse activation-inactivation protocol. Inactivation curve was measured as peak outward current during a second test pulse to +60 mV, 200 ms in duration following the first 1000 ms test pulse to a range of voltages and preceded by a brief interpulse interval (23 ms). Data for activation curves are average peak current density (pA/pF) normalized to α(V/25)[exp((V−EK)/25)]/[exp(V/25)−1], where α is a normalization factor (same for all points) chosen so that the average of the results at +38, +40, … +50 mV was unity in control or a fraction of unity after block with various drugs (see Methods). A–H. Effect of SP (A, B), PDBu (C, D), NKA (E, F) and Sar-MetSP (G, H) on the activation (A, C, E, G) and inactivation (B, D, F, H). Data was fitted by a sum of two Boltzmann equations, as explained in the Methods section. SP (A, filled circles, n = 13) inhibited total K+ currents and changed the voltage dependence of activation. SP (B, filled circles) selectively inhibited low threshold inactivating currents and changed the voltage dependence of inactivation. PDBu (C, filled triangles, n = 10) had no significant effect on low threshold inactivating currents and inhibited high threshold K+ currents. PDBu (D, filled triangles) inhibited inactivating currents and changed their voltage dependence. NKA (E, filled circles, n = 7) inhibited the low threshold component of the total K+ currents and changed their voltage dependence. NKA (F, filled circles) inhibited the inactivating currents. Sar-MetSP (G, filled triangles, n = 6) inhibited the high threshold component of the total K+ currents to a larger extent than the low threshold currents and changed the voltage dependence of activation. Sar-MetSP (H, filled triangles) inhibited the high threshold component of the inactivating currents. Insets between A, B; E, F; C, D; and G, H are scaled up overlaps between activation and inactivation curves (window currents) before (line) and after addition of drugs (shaded). See Table 2 and Table 3 for quantitative measurements of activation and inactivation. I, J, In CU phasic neurons SP and NKA did not shift the voltage dependence of the early phase of activation. For simplicity only window currents in control experiments and after addition of drugs are shown.
Table 2.
Parameters of Boltzmann fits of K+ current activation and inactivation before and after addition of substance P (SP) or phorbol 12, 13-dibutyrate (PDBu) in CAPS-responsive and unresponsive phasic neurons.
| ACTIVATION | INACTIVATION | ACTIVATION | INACTIVATION | ACTIVATION | INACTIVATION | |
|---|---|---|---|---|---|---|
| Phasic, CR | control (n=62) |
control (n=62) |
SP (n=13) |
SP (n=13) |
PDBu (n=10) |
PDBu (n=10) |
| LT, A1 | 0.53 ± 0.03 | 0.74 ± 0.05 | 0.20 ± 0.01** | 0.33 ± 0.06** | 0.18 ± 0.05** | 0.36 ± 0.12** |
| k1 | 1.1 ± 2.1 | 19 ± 6 | 1.0 ± 0.4NS | 31 ± 9NS | 1.0 ± 0.3NS | 21 ± 3NS |
| V10.5 | −34.8 ± 0.5 | −71 ± 3 | −21.8 ± 0.3* | −82 ± 2 NS | −33.6 ± 4.0NS | −75 ± 15* |
| HT, A2 | 0.47 ± 0 .03 | 0.26 ± 0.04 | 0.34 ± 0.04** | 0.24 ± 0.09NS | 0.19 ± 0.07** | 0.35 ± 0.17NS |
| k2 | 1.9 ± 6.0 | 8 ± 5 | 1.2 ± 0.2NS | 14 ± 3* | 1.6 ± 0.4NS | 9 ± 3 NS |
| V20.5 | −9.3 ± 0.3 | −39 ± 4 | −8.9 ± 0.2NS | −39 ± 4 NS | −8.6 ± 0.4NS | −38 ± 3 NS |
| Phasic, CU | control (n=44) |
control (n=44) |
SP (n=14) |
SP (n=14) |
PDBu (n=7) |
PDBu (n=7) |
| LT, A1 | 0.62 ± 0.07 | 0.59 ± 0.09 | 0.23 ± 0.02** | 0.12 ± 0.06 ** | 0.32 ± 0.01* | 0.20 ± 0.06* |
| k1 | 4.4 ± 0.8 | 13 ± 6 | 3 ± 2NS | 9 ± 7NS | 4.3 ± 0.2NS | 8 ± 4NS |
| V10.5 | −17 ± 1 | −71 ± 7 | −20 ± 1NS | −92 ± 8NS | −13.6 ± 0.3NS | −99 ± 5NS |
| HT, A2 | 0.38 ± 0.07 | 0.41 ± 0.04 | 0.20 ± 0.02* | 0.39 ± 0.05NS | 0.11 ± 0.01** | 0.40 ± 0.06NS |
| k2 | 4 ± 1 | 7 ± 5 | 2.1 ± 0.7NS | 11 ± 2NS | 1.9 ± 0.7NS | 11 ± 3NS |
| V20.5 | 7 ± 2 | −37 ± 6 | 4 ± 1NS | -47 ± 3NS | 11 ± 1NS | −44 ± 3NS |
Parameters for Boltzmann fits of low threshold activated and hyperpolarized voltage inactivating currents (ALT and A1, k1, V1) and high threshold activated and depolarized voltage inactivating K+ current (AHT and A2, k2, V2) for the activation and inactivation curves were determined as explained in Methods. The smooth curves shown in Fig. 3 were fitted with these values. Averages; SEM; t-test relative to values in control experiments, 2 tailed, unequal variance, level of significance: NS-not significant
P<0.05, or
P<0.001.
Table 4.
Parameters of Boltzmann fits of K+ current activation and inactivation before and after addition of substance P (SP) or phorbol 12, 13-dibutyrate (PDBu) in CAPS-responsive and unresponsive tonic neurons.
| ACTIVATION | INACTIVATION | ACTIVATION | INACTIVATION | ACTIVATION | INACTIVATION | |
|---|---|---|---|---|---|---|
| Tonic, CR | control (n=16) |
control (n=16) |
SP (n=6) |
SP (n=6) |
PDBu (n=6) |
PDBu (n=6) |
| LT, A1 | 0.5 ± 0.1 | 0.72 ± 0.06 | 0.19 ± 0.02** | 0.3 ± 0.1** | 0.47 ± 0.07NS | 0.17 ± 0.05** |
| k1 | 3.4 ± 0.8 | 16 ± 2 | 1.6 ± 0.5NS | 20 ± 4NS | 5 ± 1NS | 14 ± 6NS |
| V10.5 | −16 ± 2 | −70 ± 3 | −9 ± 1* | −60 ± 8NS | −14 ± 2NS | −81 ± 6* |
| HT, A2 | 0.5 ±0 .1 | 0.29 ± 0.04 | 0.35 ± 0.04* | 0.11 ± 0.05* | 0.14 ± 0.06** | 0.25 ± 0.02NS |
| k2 | 3.9 ± 0.7 | 1.1 ± 0.5 | 4 ± 3NS | 3.4 ± 0.7NS | 5 ± 1NS | 6 ± 1* |
| V10.5 | 1.5 ± 0.6 | −33.4 ± 0.4 | 1.3 ± 0.5NS | −31 ± 2NS | 7 ± 1* | −42 ± 1NS |
| Tonic, CU | control (n=8) |
control (n=8) |
SP (n=4) |
SP (n=4) |
PDBu (n=4) |
PDBu (n=4) |
| LT, A1 | 0.31 ± 0.01 | 0.83 ± 0.07 | 0.20 ± 0.03** | 0.48 ± 0.04** | 0.22 ± 0.04** | 0.38 ± 0.05** |
| k1 | 0.6 ± 0.2 | 13 ± 1 | 0.5 ± 0.1NS | 16 ± 2NS | 3 ± 1* | 12 ± 3NS |
| V10.5 | −20.1 ± 0.1 | −58 ± 2 | −21.0 ± 0.3NS | −63 ± 3NS | −24 ± 2NS | −70 ± 4* |
| HT, A2 | 0.69 ±0 .04 | 0.17 ± 0.07 | 0.41 ± 0.02** | 0.20 ± 0.04NS | 0.33 ± 0.03* | 0.19 ± 0.05 NS |
| k2 | 6.3 ± 0.7 | 5.3 ± 0.8 | 4 ± 1NS | 0.7 ± 0.4* | 3 ± 1NS | 8 ± 2NS |
| V10.5 | 0.7 ± 0.9 | −12 ± 2 | −0.6 ± 0.9NS | −24 ± 1** | 1 ± 1NS | −9 ± 3* |
Parameters for Boltzmann fits of low threshold activated and hyperpolarized voltage inactivating currents (ALT and A1, k1, V1) and high threshold activated and depolarized voltage inactivating K+ current (AHT and A2, k2, V2) for the activation and inactivation curves were determined as explained in Methods. Averages; SEM; t-test relative to values in control experiments, 2 tailed, unequal variance, level of significance: NS-not significant
P<0.05, or
P<0.001.
In CR phasic neurons SP inhibited the maximum amplitudes of the LT K+ currents (62%, Fig. 5 A, filled circles, n=13; Table 2, LT, P<0.001) and HT K+ currents (28%, Table 2, HT, P<0.001) and shifted the voltage dependence of LT K+ currents by 13 mV in the depolarizing direction without changing the voltage dependence of activation of HT K+ currents (Fig. 5 A, filled circles, HT, Table 2). SP also shifted the voltage dependence of inactivation of A1 K+ currents negatively by 11 mV but did not change significantly the voltage dependence of inactivation of A2 K+ currents (A2, Table 2). In CR phasic neurons NKA (0.5 µM) markedly inhibited the LT K+ currents (77% inhibition, Fig. 5 E, filled circles, n = 7, Table 3, P<0.001) but had only a modest and statistically insignificant inhibitory effect on the HT K+ currents (15 % inhibition, P>0.05). NKA (Fig. 5 F, filled circles) inhibited the inactivating A1 K+ currents (by 55%, Table 3, P<0.001) but had no effect on the inactivating A2 K+ currents (Table 3, P>0.1) and shifted the voltage dependence of activation positively for both LT K+ currents (by +19.5 mV, P<0.001) and HT K+ currents (by +20.3 mV, Table 3, P<0.001). Higher concentrations of NKA (5 µM) nearly fully inhibited the inactivating currents obtained after subtraction of non-inactivating currents (Fig 2 D, P<0.001). SP and NKA shifted the voltage dependence of inactivation of A1 K+ currents by −11 and −14 mV respectively, but had small or no effects on the inactivation of A2 K+ currents (Table 2, Table 3, P>0.1). In CR phasic neurons an NK1-selective agonist, Sar-MetSP (n = 5, Fig. 3 K, L, O, P, Table 3, P<0.05) inhibited the A1 K+ currents to a lesser extent than either SP or NKA (Table 3, 24% reduction, P<0.05, ANOVA) but did not alter significantly the voltage dependence of activation (Table 3). In CR phasic neurons PDBu did not change the voltage dependence of either LT K+ currents or HT K+ currents (Table 2). The net effect of current inhibition and voltage shift after NKA and SP was a >20% reduction of the window currents in the critical range of voltages near the threshold of firing and this voltage dependent reduction (i.e. near firing threshold) was small or absent after PDBu or Sar-MetSP (Fig. 5, insets). In tonic CR neurons, SP reduced the maximum amplitudes of LT K+ currents by 62% (P<0.01) and reduced HT K+ currents by 30% (Table 4, P<0.05).
Table 3.
Parameters of Boltzmann fits of K+ current activation and inactivation before and after addition of [βAla8]-neurokinin A (4–10) (NKA) or [Sar9, Met11]- substance P (SarMetSP) in CAPS-responsive and unresponsive phasic neurons.
| ACTIVATION | INACTIVATION | ACTIVATION | INACTIVATION | |
|---|---|---|---|---|
| Phasic, CR | NKA (n=7) |
NKA (n=7) |
SarMetSP (n=6) |
SarMetSP (n=6) |
| LT, A1 | 0.12 ± 0.01** | 0.33 ± 0.08** | 0.21 ± 0.04* | 0.56 ± 0.18NS |
| k1 | 9 ± 2* | 19 ± 6NS | 2 ± 1NS | 22 ± 6NS |
| V10.5 | −15 ± 3** | −85 ± 15* | −35 ± 1NS | −73 ± 9NS |
| HT, A2 | 0.40 ± 0.05NS | 0.26 ± 0.09NS | 0.27 ± 0.05* | 0.14 ± 0.09** |
| k2 | 16.5 ± 0.3** | 12 ± 2* | 9 ± 3NS | 6 ± 2NS |
| V20.5 | 11 ± 1** | −43 ± 7NS | −6 ± 3NS | −30 ± 3NS |
| Phasic, CU | NKA (n=6) |
NKA (n=6) |
SarMetSP (n=5) |
SarMetSP (n=5) |
| LT, A1 | 0.29 ± 0.05** | 0.41 ± 0.04* | 0.04 ± 0.04** | 0.33 ± 0.07* |
| k1 | 5 ± 1NS | 15 ± 2NS | 4 ± 3NS | 17 ± 4NS |
| V10.5 | −15 ± 2NS | −61 ± 3NS | −0.6 ± 0.6 ** | −70 ± 8NS |
| HT, A2 | 0.12 ± 0.05* | 0.12 ± 0.03** | 0.35 ± 0.07NS | 0.12 ± 0.06** |
| k2 | 4 ± 2NS | 3.6 ± 0.3NS | 9 ± 2** | 3 ± 2* |
| V20.5 | 9 ± 4NS | −32.8 ± 0.4NS | −10 ± 1** | −38 ± 3 NS |
Parameters for Boltzmann fits of low threshold activated and hyperpolarized voltage inactivating currents (ALT and A1, k1, V1) and high threshold activated and depolarized voltage inactivating K+ current (AHT and A2, k2, V2) for the activation and inactivation curves were determined as explained in Methods. Control experiments in absence of drugs are shown in Table 2. The smooth curves shown in Fig. 3 were fitted with these values. Averages; SEM; t-test relative to values in control experiments, 2 tailed, unequal variance, level of significance: NS-not significant
P<0.05, or
P<0.001 compared to controls shown in table 2.
Effects of drugs on K+ current activation and inactivation in capsaicin unresponsive DRG neurons
In CAPS-unresponsive (CU) phasic neurons, LT K+ currents were inhibited by 63% (P<0.001) after application of SP and 53% (P<0.001) after NKA without a significant change in the voltage dependence of activation of LT K+ currents (P>0.1; Table 2, Table 3). In CU phasic neurons SP and NKA reduced the HT K+ currents by 47% (P<0.05) and 68% respectively (P<0.05; A2, Table 2, Table 3). In CU tonic neurons both LT and HT K+ currents (n = 8, Table 4) were activated at voltages negative to those in CR tonic neurons (n = 16, Table 4, P<0.001 ANOVA). In CU phasic neurons the NK1 agonist, Sar-MetSP, greatly reduced the amplitude of LT K+ currents (P<0.001; 94% reduction, LT, Table 3) whereas the HT K+ currents were not significantly reduced (P>0.1; 8% reduction, HT, Table 3). In these neurons the voltage dependence of activation of both LT K+ currents (+16.4 mV shift, P<0.001) and HT K+ currents (−17 mV shift P<0.001) was significantly shifted by Sar-MetSP (Table 3). In CU phasic neurons SP (Fig. 5 I; P>0.1) and NKA (Fig 5 J; P>0.1) did not shift the voltage dependence of the early phase of activation.
Effects of neurokinins, heteropodatoxin and α-dendrotoxin, on subtypes of K+ currents isolated by recording in the presence of 60 mM TEA, (−) verapamil and nifedipine
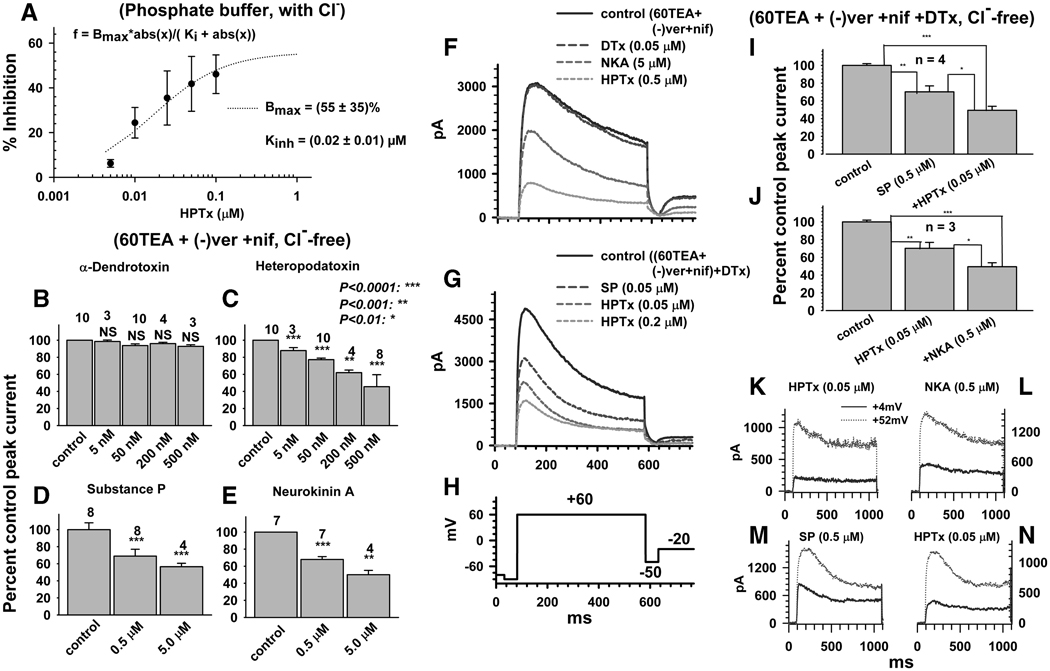
HPTx inhibited K+ currents in a concentration dependent manner by a maximum of 55% (at 1 µM; P<0.001) in physiological phosphate buffer (Fig. 6 A) and ≥60% (P<0.001) at 0.5 µM when K+ currents were recorded in high TEA (60 mM) in combination with (−) verapamil and nifedipine (5µM each, Fig 6 C). On the other hand, in the presence of 60 mM TEA, (−) verapamil and nifedipine (5 µM each), α-dendrotoxin (DTx, 5–500 nM), a blocker of Kv1.1 and 1.2 K+ channels (Yang, et al., 2004) did not inhibit K+ currents (Fig. 6 B; P>0.1). In these experiments, in unidentified neurons, SP, NKA (P<0.001) and HPTx (P<0.001) significantly inhibited the currents (20–30% block) and their inhibitory effects were additive (Fig. 6 I, J). The currents blocked by HPTx and SP or HPTx and NKA had similar kinetics whether tested at a voltage near the threshold (+4 mV) or near the maximum (+52 mV, Fig. 6 K–N).
Fig. 6.
Effect of K+ blockers and neurokinins on pharmacologically isolated low threshold inactivating K+ currents. A, Concentration dependence of HPTx block of total K+ currents recorded in phosphate buffer saline and normal pipette solution. In all subsequent panels Cl− was substituted with methylsulfonate and 60 mM of extracellular Na+ was replaced with 60 mM TEA. B–E, Concentration dependence of the effects of DTx (B), HPTx (C), SP (D) and NKA (E) on K+ current recorded after addition of 5 µM (−) verapamil (ver) and nifedipine (nif). F, Effect of sequential addition of DTx, NKA and HPTx on TEA-verapamil-nifedipine insensitive inactivating K+ currents. G, Effect of sequential addition of SP (0.5 µM) and HPTx (0.05 and 0.2 µM) on DTx-TEA-verapamil-nifedipine insensitive inactivating K+ currents. H, Pulse protocol for the experiments in F and G. I, J, Cumulative block of DTx-, TEA-, verapamil-, nifedipine-insensitive inactivating K+ currents by sequential addition of SP and HPTx (I) or HPTx and NKA (J). K, L, Time course of currents inhibited by HPTx (K), NKA in the presence of HPTx (L), in the same neuron as in K. M, N, Time course of currents inhibited by SP (M) and HPTx in the presence of SP (N), in the same neuron as M. Currents obtained by subtraction of currents before and after addition of drugs and represent currents activated at either +4mV (smallest of the two traces) or +52 mV (largest of the two traces).
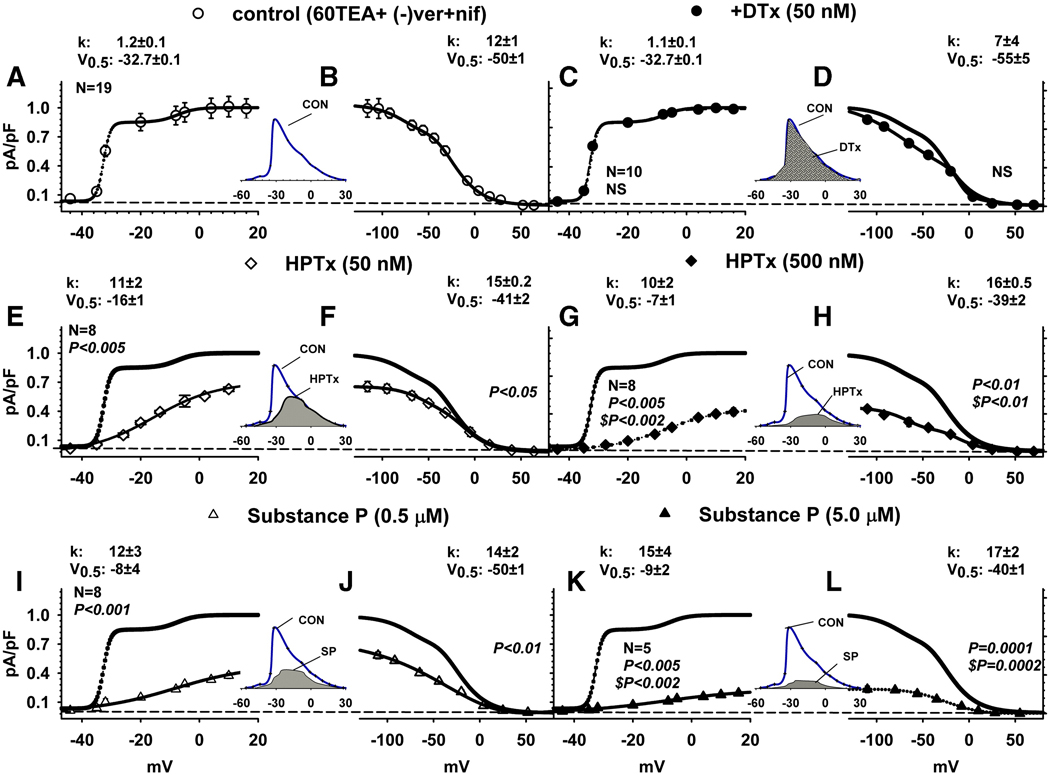
Activation and inactivation curves of inactivating currents isolated pharmacologically by treatment with high 60 mM TEA, 5 µM (−) verapamil and 5 µM nifedipine were fitted by a sum of two Boltzmann equations (Fig. 7, smooth and dotted lines). Under these conditions LT K+ currents represented >85% of the total current and were resistant to DTx (50 nM). After treatment with DTx, sequential application of increasing concentrations of HPTx (0.05 and 0.5 µM), SP (0.5–5) or NKA (0.5–5 µM), in unidentified DRG neurons, inhibited the K+ currents at all voltages (Fig. 7). HPTx shifted V0.5 for activation of the LT K+ currents from −32.7 ± 0.1 mV after DTx to −16 ± 1 mV after 0.05 µM HPTx (P<0.001) and −7 ± 1 mV after 0.5 µM HPTx, respectively (Fig. 7 C, E and G: P<0.001). HPTx also shifted the inactivation (V0.5) of A1 K+ currents by +9 (P<0.01) and +11 (P<0.01) mV for 0.05 and 0.5 µM HPTx, respectively (Fig. 7 D, F, H). Application of SP (0.5 and 5.0 µM) shifted positively both the voltage dependence of LT K+ currents (V0.5, by +25; P<0.001 and +24 mV; P<0.001, Fig. 7 C, I, K) and the A1 K+ currents (by +5; P<0.01 and +10 mV; P<0.001, Fig. 7 D, J, and L). The net effect of current inhibition and voltage shift by these agents was a concentration dependent reduction of the window currents in the critical range of voltages near the threshold of firing. The effects of SP on window currents in the presence of 60 mM TEA, (−) verapamil and nifedipine (5 µM each, Fig. 7, I, J, inset) were larger than the effects on window currents recorded in physiological solutions (Fig. 5 A, B, inset; P<0.01 ANOVA).
Fig. 7.
Effect of drugs on current-voltage relationship of activation and inactivation of pharmacologically isolated K+ currents. Activation curves (A, C, E, G, I, K) and inactivation curves (B, D, F, H, J, L) of inactivating K+ currents recorded in the presence of 60 mM TEA, 5 µM verapamil and nifedipine. Activation curves of LT and HT inactivating currents and inactivation curves of peak currents were obtained as in Fig. 5 and fitted by a sum of two Boltzmann equations, as explained in the Methods section. Data for activation curves of average peak current densities (pA/pF) were normalized as in Fig. 5. A–L. Control curves in the presence of 60 mM TEA, (−) verapamil and nifedipine (5 µM each, A, B), and after addition of DTx (0.5 µM, C, D), in the presence of DTX after addition of HPTx (0.05 µM , E, F or 0.5 µM, G, H) and in the presence of DTX after addition of SP (0.5 µM, G, H; or 5.0 µM, K, L) on the activation (A, C, E, G, I, K) and inactivation (B, D, F, H, J, L). DTx did not inhibit K+ currents (C, circles, n = 10) or change the voltage dependence of inactivation (D). HPTx (E–H, n = 8) inhibited the currents at all voltages in a concentration dependent manner. SP (I–L, n = 5–8) inhibited K+ currents and shifted the voltage dependence of activation at both concentrations and the voltage dependence of inactivation at higher concentrations. Insets between A, B; E, F; C, D; G, H; I, J; and K, L are scaled up overlaps between activation and inactivation curves (window currents) before (line) and after addition of drugs (shaded). Parameters of Boltzmann fits (see Methods) were considered statistically significant if P<0.05; a one-way analysis of variance was first carried out followed by a post-hoc comparisons between the different groups using the Holm-Sidak test.
DISCUSSION
The present experiments, which examined the mechanisms underlying the facilitatory effects of neurokinins on AP generation in lumbosacral DRG neurons, revealed that activation of NK2 receptors suppressed HPTx-sensitive, LT inactivating K+ currents and produced a >10 mV positive shift in the voltage dependence of activation of these currents. The effects occurred in CR DRG neurons in which neurokinins facilitated firing, but did not occur in CU neurons. These findings raise the possibility that neurokinins released at CR afferent terminals might be involved in an autofeedback mechanism that suppresses HPTx-sensitive Kv4-type channels (Kv4.2 and Kv4.3, (Winkelman, et al., 2005, Zarayskiy, et al., 2005)) and increases afferent nerve excitability.
Neurokinin mediated lowering of firing threshold correlates with selective blockade of LT K+ channels
In the first series of experiments, the effects of neurokinins were studied under conditions that allowed for measurements of firing properties and CAPS responsiveness in the same DRG neurons used to record K+ currents. In CR and CU neurons neurokinins suppressed various types of K+ currents (inactivating and non-inactivating as well as LT and HT). These currents regulate various properties of DRG neurons including: (1) membrane potential, (2) AP threshold and duration, (3) AP after-hyperpolarization, (4) pattern and frequency of firing. Due to this complexity of K+ channel function and the sensitivity of different channels to the modulatory actions of neurokinins it is difficult to make a direct link between neurokinin modulation of specific ion channels and changes in firing.
However, indirect evidence suggests that the increase in firing in CR neurons is correlated with an inhibition of LT inactivating K+ currents. First, SP and the NK2 selective agonist, [βAla8]-neurokinin A (4–10), reduced these currents in CR neurons in which they also reduced the voltage thresholds for evoking an AP and increased the number of APs induced by a long duration current pulse. All three effects were blocked by a selective NK2 antagonist (MEN10376) but unaffected by an NK3 antagonist (SB 235,375). The effects of SP and NKA were not mimicked by an NK1 (Sar-MetSP) or an NK3 (NKB) agonist indicating that effects were produced by selective activation of NK2 receptors. On the other hand, activation of all three types of neurokinin receptors suppressed non-inactivating HT K+ channels indicating that modulation of these channels is not sufficient to alter firing.
The aforementioned conclusion is supported by a comparison of the effects of neurokinins in CR and CU neurons. In CR neurons, SP (Fig. 5 A, B) or NKA (Fig. 5 E, F) elicited a positive voltage shift in the activation curve of LT K+ currents. This occurred in a critical voltage range for initiation of APs and was also evident as a positive voltage shift in the window current. The NK1 agonist, Sar-Met-SP, which did not facilitate firing in CR neurons, did not produce a positive voltage shift in the voltage dependence of activation of LT K+ currents or produce a positive voltage shift in the window current (Fig. 5). CU neurons that did not exhibit neurokinin enhancement of firing also did not exhibit a positive voltage shift in the voltage dependence of activation of LT K+ currents in response to SP or NKA, but did exhibit a neurokinin suppression of HT non-inactivating K+ currents indicating that the neurons do express neurokinin receptors. Thus, a suppression and positive voltage shift of the LT K+ window current seem to be important factors in the facilitatory effect of neurokinins on CR neurons.
Further support for the link between LT K+ current suppression and facilitation of firing is derived from an analysis of the effects of 4-AP and HPTx on DRG neurons. Rat DRG neurons express a diverse population of K+ channel types, which generate as many as six subtypes of currents distinguishable both in voltage dependence and pharmacology (Gold, et al., 1996). However, the Kv genes responsible for these K+ currents initially reported by Gold et al. (Gold, et al., 1996) were in subsequent work shown to be Kv1.1-Kv1.6, Kv2.1, Kv1.1, Kv1.6 (Ishikawa, et al., 1999, Yang, et al., 2004); and Kv4.2 and Kv4.3 channel subtypes (Winkelman, et al., 2005). The Kv1.1 and Kv1.2 currents are slow- or non-inactivating but give rise to A-type like K+ currents when combined with Kvβ subunits (Heinemann, et al., 1996) and therefore could be involved in neurokinin mediated facilitation of firing in CR neurons. The remainder are non-inactivating, delayed rectifier type (Gold, et al., 1996, Ishikawa, et al., 1999). The inactivating currents are all sensitive to 4-AP. One type (Kv1.4, (Pongs, 1992, Rasband, et al., 2001)) is also blocked by TEA (Gold, et al., 1996); other types are blocked by DTx (Kv1.1 and Kv1.2 (Yang, et al., 2004)) or HPTx (Kv4.2 and Kv4.3). In our experiments the 4-AP-sensitive inactivating currents recorded in CR DRG neurons in the presence of TEA were inhibited by HPTx (Fig. 2B) as well as by SP or NKA. Similarly, the inactivating currents recorded in the presence of TEA and (−) verapamil (Fig. 2A) or a combination of TEA, (−) verapamil, nifedipine and DTx, which should block non-inactivating K+ currents as well as Kv1.1, 1.2 and 1.4 inactivating currents, yielded an inactivating current that was inhibited by HPTx, SP or NKA (fig. 6). The similarity in the effects of neurokinins and heteropodatoxin on firing and on LT K+ currents isolated by various methods supports the view that inhibition of HPTx sensitive, TEA and DTx insensitive inactivating K+ currents contribute to the enhancement of firing by neurokinins and 4-AP.
Pharmacological separation of LT K+ currents that were inhibited by neurokinins
To conduct a more detailed analysis of activation and inactivation properties of neurokinin sensitive K+ currents, a combination of voltage protocols, ion substitution and pharmacological inhibition of K+ currents was used to separate the LT inactivating K+ currents from other pharmacologically distinct voltage activated K+ currents. Replacement of Cl− with methylsulfonate and treatment with TEA, (−) verapamil, nifedipine and DTx yielded inactivating outward currents that had properties (Fig. 7) very similar to those of the LT K+ currents recorded in physiological solutions (Fig. 5, table 2) including activation and inactivation V0.5’s, slope factors, HPTx sensitivity and HPTx and neurokinin-induced depolarizing shift in activation curves and window currents. The similarities in responses obtained in physiological solutions and under conditions used to isolate the currents validate the isolation methods and indicate that they did not markedly change the properties of the neurokinin-sensitive inactivating currents.
High concentrations of HPTx and SP markedly suppressed the window currents of the pharmacologically isolated LT inactivating K+ currents and almost completely blocked the window currents in the range of membrane potentials near the threshold for generation of APs (Fig. 7 G, H, K, L). These data indicate that HPTx and SP act on the same K+ channels. This view is supported by the finding that the blocking effect of submaximal concentrations of HPTx and SP or HPTx and NKA was also additive (Fig. 6 F, G). However, the maximum amplitude of the isolated inactivating LT K+ currents elicited by strong depolarizing pulses were not completely blocked by even high concentrations of SP, NKA and HPTx raising the possibility that another type of inactivating K+ current is present in CR phasic neurons. It is likely that HPTx, which is a less potent blocker of Kv4 potassium channels at depolarized potentials (Sanguinetti, et al., 1997), was applied in submaximal concentrations in our experiments. Unfortunately we were not able to construct a full concentration response curve for HPTx due to the high cost of this agent. It would have facilitated the interpretation of the data to have used maximal concentrations of the toxin to determine if the isolated LT inactivating currents could be completely blocked and therefore reflected an activation of a homogeneous population of channels. On the other hand, HPTx and the neurokinins were tested in concentrations that produced maximal enhancement of firing in CR DRG neurons, indicating that only a partial block of LT inactivating K+ currents is necessary to induce a prominent change in firing.
Intracellular signaling mechanisms underlying the effects of neurokinins on K+ channels
The intracellular signaling mechanisms underlying the effects of neurokinins on K+ channels are uncertain. Activation of neurokinin receptors (NK1, NK2 and NK3) is known to stimulate phospholipase C (Maggi, 1996, Maggi, 1997) and in turn induce the breakdown of phospholipids into diacyl glycerol which activates PKC (Sculptoreanu, et al., 2008, Torrens, et al., 1997), which in turn can phosphorylate and inhibit a number of K+ channels (Boland and Jackson, 1999, Hagiwara, et al., 2003, Richardson and Vasko, 2002).
We have also shown previously in DRG neurons that stimulation of NK2 receptors activates PKC to modulate TRPV1 (Sculptoreanu, et al., 2008) and voltage dependent Ca2+ channels (Sculptoreanu and de Groat, 2003). NK1 receptors also activate PKCε to enhance TRPV1 responses in L4–L6 DRG neurons of rats (Zhang, et al., 2007). A PKC inhibitor, bisindolylmaleimide, only partially reversed the effect of SP on firing (Sculptoreanu and de Groat, 2007) in CR phasic neurons. Furthermore activation of PKC with PDBu, did not mimic the effect of SP or NKA to shift the activation curve of the LT K+ current or diminish the window currents at the critical voltage range for AP generation although it did suppress non-inactivating currents. The lack of PDBu effect on the voltage dependence of LT K+ currents is similar to that reported for the effect of phorbol 12-myristate 13-acetate on Kv4.2 currents in dorsal horn neurons (Hu, et al., 2003). Taken together, these data suggest that while PKC-induced phosphorylation and inhibition of K+ channels may contribute to the increase in firing it is not the major mechanism underlying neurokinin induced hyperexcitability in DRG neurons.
Possible pathophysiological function of neurokinin receptors and Kv4 channels in afferent neurons
The expression of functional neurokinin receptors in the soma of DRG neurons (Brechenmacher, et al., 1998) raises the possibility that these receptors might also be expressed in the central and peripheral nerve terminals of these neurons. The most convincing evidence for functional receptors in peripheral terminals has been obtained in the urinary bladder where exogenously administered substance P increases the firing of mechano-sensitive afferent nerves (Morrison, et al., 1999). Administration of an NK2 agonist, NKA, decreased the pressure threshold and increased the discharge rate of bladder Aδ-fiber and C-fiber afferents. An NK2 selective antagonist abolished the sensitizing effect of NKA (Kibble and Morrison, 1996a, Kibble and Morrison, 1996b). SP is released from CAPS-sensitive C-fiber bladder afferent nerves and elicits inflammatory responses and induces bladder overactivity by activating multiple neurokinin receptors (Andersson, 2006, Lima-Junior, et al., 2007). NK1 and NK2 receptor antagonists reduce bladder hyperactivity induced by chemical irritants and other pathological conditions (Andersson, 1997, Andersson, 2006), however it is not known if this effect of antagonists is due to an action on neurokinin receptors on bladder afferent nerves that are linked with neuronal Kv4 channels or an action at other sites, such as bladder smooth muscle or second order neurons in the spinal cord or changes in the function of other channels (e. g., TRPV1 or Ca2+ channels) expressed on neuronal or non-neuronal cells (Andersson, 2006, Green, et al., 2006, Lima-Junior, et al., 2007).
However, modulation of A-type K+ channels in bladder afferent neurons has been linked with the generation of bladder hyperactivity in chronic cyclophosphamide-induced cystitis (Yoshimura and de Groat, 1999). As noted in the present experiments for unidentified CR DRG neurons, CR bladder afferent neurons normally exhibit phasic firing in response to long duration depolarizing current pulses and prominent LT inactivating K+ currents (Yoshimura, et al., 1996). In addition, administration of 4-AP, which suppresses these currents, induces tonic firing. In chronic, cyclophosphamide-induced cystitis, LT inactivating K+ currents are markedly reduced in CR bladder afferent neurons and phasic firing is converted to tonic firing (Yoshimura and de Groat, 1999). These changes are similar to those induced acutely by SP or NKA. Cyclophosphamide-induced cystitis also increases the firing of bladder afferent nerves elicited by bladder distension (Yu and de Groat, 2008). The changes in bladder afferent neuron excitability appear to be mediated by nerve growth factor (NGF, (Yoshimura, et al., 2006)) because NGF levels in the bladder are increased in cystitis (Zvara and Vizzard, 2007) and exogenous administration of NGF into the bladder wall or the spinal cord induces down-regulation of LT inactivating K+ currents and induces tonic firing in CR bladder afferent neurons (Yoshimura, et al., 2006). A reduction of A-type K+ currents has also been implicated in the induction of hyperexcitability of DRG neurons in other inflammatory conditions such as gastric ulcer (Dang, et al., 2004), pancreatitis (Xu, et al., 2006), ileitis (Stewart, et al., 2003), feline interstitial cystitis (Sculptoreanu, et al., 2005) as well as axotomy (Abdulla, et al., 2001, Everill and Kocsis, 1999, Yang, et al., 2003), although the subtypes of K+ channels that are suppressed are uncertain (Xu, et al., 2006). Clearly, further experiments are needed to determine if endogenously released neurokinins can increase the excitability of afferent nerves by suppressing Kv4 types K+ currents and thereby contribute to sensitization of afferent nerves in pathological conditions. In summary, the major finding in this study is that neurokinins activate NK2 receptors to selectively enhance firing in CR DRG neurons. The enhancement of firing was accompanied by a positive shift in the activation curve and a reduction in the maximum amplitude of HPTx-sensitive LT K+ currents. The former effect seems to be the important factor contributing to the changes in firing because neurokinins failed to enhance firing in CU neurons where they also suppressed the amplitude of LT K+ currents but did not alter the voltage dependence of activation of the currents.
ACKNOWLEDGMENTS
This work was supported by the following US National Institutes of Health (NIH) grant: NIDDK 45430 to W.C.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Stebbing MJ, Smith PA. Effects of substance P on excitability and ionic currents of normal and axotomized rat dorsal root ganglion neurons. Eur. J. Neurosci. 2001;13:545–552. doi: 10.1046/j.0953-816x.2000.01429.x. [DOI] [PubMed] [Google Scholar]
- Andersson KE. The overactive bladder: pharmacologic basis of drug treatment. Urol. 1997;50:74–84. doi: 10.1016/s0090-4295(97)00595-5. [DOI] [PubMed] [Google Scholar]
- Andersson KE. Tachykinins: role in detrusor overactivity? Eur. Urol. 2006;49:423–425. doi: 10.1016/j.eururo.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Averbeck B, Izydorczyk I, Kress M. Inflammatory mediators release calcitonin gene-related peptide from dorsal root ganglion neurons of the rat. Neurosci. 2000;98:135–140. doi: 10.1016/s0306-4522(00)00095-6. [DOI] [PubMed] [Google Scholar]
- Besson JM. The neurobiology of pain. Lancet. 1999;353:1610–1615. doi: 10.1016/s0140-6736(99)01313-6. [DOI] [PubMed] [Google Scholar]
- Boland LM, Jackson KA. Protein kinase C inhibits Kv1.1 potassium channel function. Am. J. Physiol. 1999;277:C100–C110. doi: 10.1152/ajpcell.1999.277.1.C100. [DOI] [PubMed] [Google Scholar]
- Brechenmacher C, Larmet Y, Feltz P, Rodeau JL. Cultured rat sensory neurones express functional tachykinin receptor subtypes 1, 2 and 3. Neurosci. Lett. 1998;241:159–162. doi: 10.1016/s0304-3940(98)00045-7. [DOI] [PubMed] [Google Scholar]
- Catacuzzeno L, Trequattrini C, Petris A, Franciolini F. Mechanism of verapamil block of a neuronal delayed rectifier K+ channel: active form of the blocker and location of its binding domain. Br. J. Pharmacol. 1999;126:1699–1706. doi: 10.1038/sj.bjp.0702477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay JR. Determining K+ channel activation curves from K+ channel currents. Eur. Biophys. J. 2000;29:555–557. doi: 10.1007/s002490000091. [DOI] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G573–G579. doi: 10.1152/ajpgi.00258.2003. [DOI] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J. Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- Glantz SA. Primer of biostatistics. New York: McGraw-Hill Medical Pub. Division; 2005. [Google Scholar]
- Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J. Neurophysiol. 1996;75:2629–2646. doi: 10.1152/jn.1996.75.6.2629. [DOI] [PubMed] [Google Scholar]
- Green SA, Alon A, Ianus J, McNaughton KS, Tozzi CA, Reiss TF. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J. Urol. 2006;176:2535–2540. doi: 10.1016/j.juro.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Gschossmann JM, Chaban VV, McRoberts JA, Raybould HE, Young SH, Ennes HS, Lembo T, Mayer EA. Mechanical activation of dorsal root ganglion cells in vitro: comparison with capsaicin and modulation by kappa-opioids. Brain Res. 2000;856:101–110. doi: 10.1016/s0006-8993(99)02353-7. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Nunoki K, Ishii K, Abe T, Yanagisawa T. Differential inhibition of transient outward currents of Kv1.4 and Kv4.3 by endothelin. Biochem. Biophys. Res. Commun. 2003;310:634–640. doi: 10.1016/j.bbrc.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Heinemann SH, Rettig J, Graack HR, Pongs O. Functional characterization of Kv channel beta-subunits from rat brain. J. Physiol. 1996;493:625–633. doi: 10.1113/jphysiol.1996.sp021409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Pernow B, Wahren J. Substance P: a pioneer amongst neuropeptides. J. Intern. Med. 2001;249:27–40. doi: 10.1046/j.0954-6820.2000.00773.x. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Vincent S, Dalsgaard CJ, Skirboll L, Johansson O, Schultzberg M, Lundberg JM, Rosell S, Pernow B, Jancso G. Distribution of substance P in brain and periphery and its possible role as a co-transmitter. Ciba Found. Symp. 1982:84–106. doi: 10.1002/9780470720738.ch6. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Glauner KS, Gereau RWt. ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. I. Modulation of A-type K+ currents. J. Neurophysiol. 2003;90:1671–1679. doi: 10.1152/jn.00340.2003. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Tanaka M, Black JA, Waxman SG. Changes in expression of voltage-gated potassium channels in dorsal root ganglion neurons following axotomy. Muscle Nerve. 1999;22:502–507. doi: 10.1002/(sici)1097-4598(199904)22:4<502::aid-mus12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kibble A, Morrison JFB. In vivo pelvic afferent responses to Neurokinin A agonist in the anaesthetized rat. J. Physiol. 1996a;491P:148P–149P. [Google Scholar]
- Kibble A, Morrison JFB. Changes in mechanosensitivity and sensitization of urinary bladder afferents in the anesthetized rat following administration of the selective NK2 antagonist SR48968. J. Physiol. 1996b;497P:18P–19P. [Google Scholar]
- Lima-Junior RC, Sousa DI, Brito GA, Cunha GM, Chaves MH, Rao VS, Santos FA. Modulation of acute visceral nociception and bladder inflammation by plant triterpene, alpha, beta-amyrin in a mouse model of cystitis: role of tachykinin NK1-receptors, and K+ATP channels. Inflamm. Res. 2007;56:487–494. doi: 10.1007/s00011-007-7023-4. [DOI] [PubMed] [Google Scholar]
- Liu M, Huang W, Wu D, Priestley JV. TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur. J. Neurosci. 2006;24:1–6. doi: 10.1111/j.1460-9568.2006.04889.x. [DOI] [PubMed] [Google Scholar]
- Madeja M, Muller V, Musshoff U, Speckmann EJ. Sensitivity of native and cloned hippocampal delayed-rectifier potassium channels to verapamil. Neuropharmacol. 2000;39:202–210. doi: 10.1016/s0028-3908(99)00110-0. [DOI] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins in the autonomic nervous system. Pharmacol. Res. 1996;33:161–170. doi: 10.1006/phrs.1996.0023. [DOI] [PubMed] [Google Scholar]
- Maggi CA. Tachykinins as peripheral modulators of primary afferent nerves and visceral sensitivity. Pharmacol. Res. 1997;36:153–169. doi: 10.1006/phrs.1997.0219. [DOI] [PubMed] [Google Scholar]
- Morrison J, Wen J, Kibble A. Activation of pelvic afferent nerves from the rat bladder during filling. Scand. J. Urol. Nephrol. Suppl. 1999;201:73–75. [PubMed] [Google Scholar]
- Nakamura-Craig M, Gill BK. Effect of neurokinin A, substance P and calcitonin gene related peptide in peripheral hyperalgesia in the rat paw. Neurosci. Lett. 1991;124:49–51. doi: 10.1016/0304-3940(91)90819-f. [DOI] [PubMed] [Google Scholar]
- Pongs O. Molecular biology of voltage-dependent potassium channels. Physiol. Rev. 1992;72:S69–S88. doi: 10.1152/physrev.1992.72.suppl_4.S69. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, Cooper BY, Johnson RD. Expression of TWIK-related acid sensitive K+ channels in capsaicin sensitive and insensitive cells of rat dorsal root ganglia. Neurosci. 2006;141:955–963. doi: 10.1016/j.neuroscience.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Johnson JH, Hammerland LG, Kelbaugh PR, Volkmann RA, Saccomano NA, Mueller AL. Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels. Mol. Pharmacol. 1997;51:491–498. [PubMed] [Google Scholar]
- Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur. J. Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Protein kinase C is involved in neurokinin receptor modulation of N- and L-type Ca2+ channels in DRG neurons of the adult rat. J. Neurophysiol. 2003;90:21–31. doi: 10.1152/jn.00108.2003. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp. Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp. Neurol. 2005;193:437–443. doi: 10.1016/j.expneurol.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Yoshimura N, de Groat WC. KW-7158 [(2S)-(+)-3,3,3-trifluoro-2-hydroxy - 2 - methyl - N- (5, 5,1 0 - trioxo- 4, 10 -dihydro thieno [3, 2-c] [1] benzothiepin - 9 -yl) propanamide] enhances A-type K+ currents in neurons of the dorsal root ganglion of the adult rat. J. Pharmacol. Exp. Ther. 2004;310:159–168. doi: 10.1124/jpet.104.065409. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Wolfe D, Goss J, Watkins S, de Groat WC, Sculptoreanu A, Glorioso JC. Protein kinase Cε contributes to basal and sensitizing responses of TRPV1 to capsaicin in rat dorsal root ganglion neurons. Eur. J. Neurosci. 2008;28:1241–1254. doi: 10.1111/j.1460-9568.2008.06438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J. Physiol. 2003;552:797–807. doi: 10.1113/jphysiol.2003.046409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens Y, Saffroy M, Glowinski J, Beaujouan JC. Substance P(6–11) and natural tachykinins interact with septide-sensitive tachykinin receptors coupled to a phospholipase C in the rat urinary bladder. Neuropeptides. 1997;31:243–251. doi: 10.1016/s0143-4179(97)90055-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Cummins TR, Black JA, Dib-Hajj S. Diverse functions and dynamic expression of neuronal sodium channels. Novartis Found. Symp. 2002;241:34–51. [PubMed] [Google Scholar]
- Winkelman DL, Beck CL, Ypey DL, O'Leary ME. Inhibition of the A-type K+ channels of dorsal root ganglion neurons by the long-duration anesthetic butamben. J. Pharmacol. Exp. Ther. 2005;314:1177–1186. doi: 10.1124/jpet.105.087759. [DOI] [PubMed] [Google Scholar]
- Wood JN, Akopian AN, Baker M, Ding Y, Geoghegan F, Nassar M, Malik-Hall M, Okuse K, Poon L, Ravenall S, Sukumaran M, Souslova V. Sodium channels in primary sensory neurons: relationship to pain states. Novartis Found. Symp. 2002;241:159–168. [PubMed] [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pasricha PJ. Enhanced excitability and suppression of A-type K+ current of pancreas-specific afferent neurons in a rat model of chronic pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G424–G431. doi: 10.1152/ajpgi.00560.2005. [DOI] [PubMed] [Google Scholar]
- Yamane H, de Groat WC, Sculptoreanu A. Effects of ralfinamide, a Na+ channel blocker, on firing properties of nociceptive dorsal root ganglion neurons of adult rats. Exp. Neurol. 2007;208:63–72. doi: 10.1016/j.expneurol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EK, Takimoto K, Hayashi Y, de Groat WC, Yoshimura N. Altered expression of potassium channel subunit mRNA and alpha-dendrotoxin sensitivity of potassium currents in rat dorsal root ganglion neurons after axotomy. Neurosci. 2004;123:867–874. doi: 10.1016/j.neuroscience.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Yang YL, Yao KH, Li ZW. Similarities of SP-, NKA- and NKB-induced currents in rat dorsal root ganglion neurons. Brain. Res. 2003;991:18–25. doi: 10.1016/s0006-8993(03)03451-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J. Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J. Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, White G, Weight FF, de Groat WC. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J. Physiol. 1996;494:1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Groat WC. Sensitization of pelvic afferent nerves in the in vitro rat urinary bladder-pelvic nerve preparation by purinergic agonists and cyclophosphamide pretreatment. Am. J. Physiol. Renal. Physiol. 2008;294:F1146–F1156. doi: 10.1152/ajprenal.00592.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarayskiy VV, Balasubramanian G, Bondarenko VE, Morales MJ. Heteropoda toxin 2 is a gating modifier toxin specific for voltage-gated K+ channels of the Kv4 family. Toxicon. 2005;45:431–442. doi: 10.1016/j.toxicon.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhabyeyev P, Missan S, Jones SE, McDonald TF. Low-affinity block of cardiac K+ currents by nifedipine. Eur. J. Pharmacol. 2000;401:137–143. doi: 10.1016/s0014-2999(00)00413-1. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, Zhao ZQ. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCε: a novel pathway for heat hyperalgesia. J. Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]