Abstract
Behavioral disinhibition has been characterized as a generalized vulnerability to externalizing disorders. Despite increasing evidence for its validity and heritability, the structural stability of behavioral disinhibition across adolescence and the strength and etiology of its relation to executive functions have not been studied. In this multivariate twin study, the authors assessed behavioral disinhibition using measures tapping substance use, conduct disorder, attention-deficit/hyperactivity disorder (ADHD), and novelty seeking at ages 12 and 17. Executive functions were assessed with laboratory-based cognitive tasks at age 17. Results indicated that, at age 12, behavioral disinhibition was dominated by ADHD and conduct problems and was highly heritable. At age 17, the contributions of the 4 components were more balanced, and the proportion of variance attributable to genetic factors was somewhat smaller, with additional variance due to shared environmental influences. At both ages, behavioral disinhibition was more closely related to response inhibition than other executive functions (working memory updating and task-set shifting), and this relationship was primarily genetic in origin. These results highlight the dynamic nature of behavioral disinhibition across adolescence and suggest that response inhibition may be an important mechanism underlying vulnerability to disinhibitory psychopathology.
Keywords: executive control, disruptive behavior, impulsivity, etiology, endophenotype
Since Gorenstein and Newman (1980) coined the term disinhibitory psychopathology and hypothesized an underlying diathesis and a neurobiological framework for this broad deficit in behavioral control, nearly 3 decades of research has documented the comorbidity among a wide range of behavioral problems that seem to fall under this rubric. The idea that behavioral disinhibition constitutes a generalized vulnerability to externalizing disorders has gained wide currency and consistent empirical support (Iacono, Malone, & McGue, 2008), and the genetic nature of individual differences in behavioral disinhibition has also been demonstrated (Krueger et al., 2002; Young, Stallings, Corley, Krauter, & Hewitt, 2000). Despite this progress, the nature of the developmental changes in behavioral disinhibition and its cognitive underpinnings remain subjects of intensive research efforts. How stable are the structure and etiology of behavioral disinhibition across development? Although the phrase behavioral disinhibition includes the term inhibition, is it really related to individual differences in inhibitory control abilities? If so, to what extent is that commonality attributable to genetic (as opposed to environmental) factors?
In this article, we report on a multivariate twin study designed to address these questions by focusing on the relationship between behavioral disinhibition and executive functions, a set of control processes that monitor and regulate lower level cognitive processes and thereby shape complex thought and behavior. We assessed behavioral disinhibition at two points during adolescence (ages 12 and 17 years old), when a wide range of disinhibitory behaviors are manifested with increasing prevalence. Our guiding hypothesis is that deficits in one component of executive control, the ability to inhibit or override prepotent responses (hereafter, response inhibition), may be central to the underlying pathology represented in behavioral disinhibition. Furthermore, since our recent work has suggested that individual differences in response inhibition are largely genetic in origin (Friedman et al., 2008), we predict that the relation between this cognitive ability and behavioral disinhibition will reflect genetic covariation.
The Nature and Etiology of Behavioral Disinhibition
Findings from epidemiological surveys in the United States and around the world have consistently shown a high prevalence of co-occurring childhood disorders, including oppositional defiant disorder (ODD), conduct disorder, and attention-deficit/hyperactivity disorder (ADHD; Boyle & Offord, 1991; Fergusson, Horwood, & Lynskey, 1994; Ford, Goodman, & Meltzer, 2003; Kandel et al., 1997). This pattern is generally mirrored by findings from clinical research (Biederman, Newcorn, & Sprich, 1991; Crowley & Riggs, 1995; Farrington & Van Kammen, 1990; Lahey, Loeber, Burke, Rathouz, & McBurnett, 2002).
Although not classified as disorders of childhood per se, substance use disorders are also common in youth (Young et al., 2002). Childhood conduct disorder is a strong predictor of substance use, abuse, and dependence, which typically develop subsequently. A review of 11 epidemiologic studies examining the comorbidity between adolescent substance use disorders and other psychiatric disorders reported a median prevalence rate of 46% for conduct disorder and/or ODD in youth reporting substance use or a substance use disorder (Armstrong & Costello, 2002). There is also an extensive literature linking personality characteristics such as novelty seeking, impulsivity, and (lack of) constraint with substance use disorders (Cloninger, Sigvardsson, & Bohman, 1988; Howard, Kivlahan, & Walker, 1997; McGue, Iacono, Legrand, Malone, & Elkins, 2001; Sher & Trull, 1994) and antisocial behavior (Caspi, Moffitt, Newman, & Silva, 1996; Finn, Mazas, Justus, & Steinmetz, 2002; Tremblay, Pihl, Vitaro, & Dobkin, 1994). Though these studies clearly implicate a common element of pathology of these childhood disorders and associated personality characteristics, their utility is limited by the absence of explicit hypotheses about the nature of this comorbidity. The current study is founded on the idea that what links these behavioral patterns together is an underlying deficit in the ability to inhibit impulses to act in ways that are attractive because of possible reward but are socially inappropriate and may result in negative consequences. This hypothesized deficit is behavioral disinhibition.
Multiple research groups have demonstrated that individual differences in behavioral disinhibition are largely genetic in origin (Krueger et al., 2002; Young et al., 2000). In a previous study from our group (Young et al., 2000), we explicitly tested a hierarchical model (also referred to as a common pathway model) of behavioral disinhibition, which hypothesizes that there are genetic and environmental influences that operate on an underlying common liability (i.e., a common factor) shared among adolescent externalizing problems. We applied this model to an adolescent twin sample, which included a small subsample of the longitudinal twins in the current study when they were 12 years of age. Using measures comparable to the current study, we reported a highly heritable general liability factor (a2 = .82). Krueger et al. (2002) tested a similar model of what they termed the externalizing spectrum (representing the covariance among symptoms of antisocial personality, conduct disorder, alcohol and drug dependence, and unconstrained personality style), reporting a similar estimate of heritability of .81 in their sample of 17-year-old twins. Taken together, these results provide compelling evidence that etiology of the common underlying pathology is primarily genetic, while leaving open questions about the nature of the pathology itself.
In the current study, we had the opportunity to examine whether there are developmentally specific aspects of behavioral disinhibition. We assessed the individual components of behavioral disinhibition in our twin sample at two critical points in adolescent development: near the onset of adolescence (age 12), a period of change when behavioral problems often rapidly emerge, and near the end of adolescence (age 17), when many youths are generally through the period of greatest risk for the development of these problems. Using these longitudinal data, we asked the following question that has not been addressed in previous studies: What are the similarities and differences in (a) the structure (i.e., the pattern of loadings on individual components of disinhibitory psychopathology and personality) and (b) the etiology (i.e., the genetic and environmental architecture) of behavioral disinhibition at two important points in adolescent development?
Relations Between Behavioral Disinhibition and Response Inhibition
Demonstrating that what connects the components of behavioral disinhibition is genetic in nature is only the first step toward understanding underlying mechanisms that lead to this confluence of problems. In this study, we also examined whether deficits in executive functions represent one key set of such explanatory mechanisms. We focused particularly on response inhibition because it has been previously suggested as the component of executive function that is most conceptually linked to disinhibitory psychopathology (e.g., Barkley, 1997).
The relationship between behavioral disinhibition and executive functions has never been explicitly tested at the level of latent variables. However, a number of studies have reported correlations between particular disinhibitory disorders and a variety of neuropsychological measures of executive function, most notably in children with ADHD. In a recent meta-analytic review of results from 83 studies evaluating the evidence for deficits in executive functions as primary etiological factors in the development of ADHD, Willcutt, Doyle, Nigg, Faraone, and Pennington (2005) concluded that although the effect sizes are modest and inconsistent, executive function deficits are one important component in ADHD.
Executive functions have also been hypothesized as a core component of risk for the development of substance use disorders (Giancola, Mezzich, & Tarter, 1998). A recent study of adolescent substance use disorders found that response inhibition ability predicted the onset of alcohol use–related problems and illicit drug use in adolescents independently of their ADHD and conduct disorder status (Nigg et al., 2006).
Although these studies provide some evidence for a link between particular externalizing disorders and executive functions, they did not examine how the more general concept of behavioral disinhibition relates to executive functions, especially deficits in the ability to inhibit prepotent (i.e., dominant or automatic) responses. Thus, the second question we addressed in this study was as follows: Can we empirically demonstrate that the covariance among adolescent externalizing disorders and novelty seeking is explained, in part, by deficits in an underlying cognitive process, namely, response inhibition? In other words, is behavioral disinhibition really a problem with inhibition?
The response inhibition construct under study here was previously validated through factor analytic work by Miyake, Friedman, et al. (2000), who demonstrated that three common executive functions (response inhibition, working memory updating, and task-set switching) were correlated but separable at the level of latent variables (see also Friedman et al., 2006). A replication and extension of this work demonstrated that these three executive functions share a highly heritable common factor that can be distinguished from that of IQ or speed (Friedman et al., 2008). In light of the finding that behavioral disinhibition itself is also highly heritable, the third question we asked in this study is as follows: Can the relationship between behavioral disinhibition and response inhibition be explained by shared genetic factors and, if so, to what extent?
Ours is the first study to empirically test this hypothesis. To do so, we applied a hierarchical genetic and environmental model in our adolescent twin sample to the two latent constructs of behavioral disinhibition and response inhibition. This model simultaneously estimates the contributions of genetic influences, shared (family) environmental influences, and nonshared (individual) environmental influences, as well as the correlations between the etiological factors of the two constructs. Our design was strengthened by the fact that we assessed psychiatric and personality characteristics longitudinally. Specifically, we compared the relationship between behavioral disinhibition and response inhibition in early and late adolescence.
Method
Participants
Participants were originally recruited from the Colorado Longitudinal Twin Study (LTS), which consists of same-sex twins whose emotional, cognitive, and behavioral development has been studied since birth. The LTS twin families were originally identified through the Colorado Department of Health’s Division of Vital Statistics between 1986 and 1990. Twins were ascertained preferentially for normal gestational periods and birth weights, as well as residing within a 2-hr driving distance of Boulder, CO. The LTS sample is largely Caucasian; approximately 8% report ethnic minority status. Retention of the LTS sample has been high; approximately 420 of the original 483 families (87%) are still active in the longitudinal studies (for more information about the LTS sample, see Rhea, Gross, Haberstick, & Corley, 2006).
We report on a subset of twins in the LTS sample who have participated in two waves of study in the Colorado Center for Antisocial Drug Dependence, a longitudinal, multicomponent study ongoing at the Institute for Behavioral Genetics and the Addiction Research and Treatment Services at the University of Colorado. Current analyses are based on data from 584 adolescents from 293 same-sex twin pairs: 159 monozygotic (MZ) pairs (177 male and 139 female; 2 co-twins did not participate) and 134 dizygotic (DZ) pairs (138 males and 130 female). Twins were eligible for participation in Wave 1 of the Center studies after completing the sixth grade; the mean age at time of assessment was 12.4 years (SD = 0.36, range 11.3–14.0 years). Wave 2 assessments were completed at the 5-year follow-up, when the twins were approximately 17 years old (M = 17.4, SD = 0.62, range 16.5–20 years). At the Wave 2 testing session, twins were also assessed with a battery of executive function tasks under a separate protocol. All research protocols and consent forms were reviewed and approved by the University of Colorado’s Institutional Review Board, and parental and participant informed consent (as appropriate) was obtained at each wave of assessment (child assent was also obtained at age 12). Participants were paid $30 for the Wave 1 Center study, $50 for the Wave 2 Center study, and $50 for the executive function study.
Previous reports on the LTS sample have demonstrated their representativeness in terms of general intelligence, as indicated by a normal IQ distribution (M = 106.6, SD = 13.6; Bishop et al., 2003), as well as their rates of childhood psychopathology (Ehringer, Rhee, Young, Corley, & Hewitt, 2006). For the current study sample, we provide lifetime prevalence rates for clinically significant levels of conduct disorder and ADHD, as well as rates of substance experimentation, in Table 1. As expected, rates of externalizing disorders were low (<2%) in early adolescence (age 12); however, these rates more than tripled by late adolescence (age 17). Substance experimentation (defined as any use by age 12) was relatively common in early adolescence, with rates ranging from 5.4% for tobacco to 24.9% for alcohol. As expected, these rates substantially increased by age 17, when approximately one in every five adolescents reported using tobacco and/or at least one illicit substance and more than half of the sample reported repeated alcohol use.
Table 1.
Prevalence of Self-Reported DSM–IV Symptoms of Externalizing Disorders and Rates of Substance Use in Longitudinal Twin Sample
| Behavioral measure | Age 12 years | Age 17 years |
|---|---|---|
| Externalizing disorders | ||
| Conduct disordera | 1.9% | 8.8% |
| ADHD–attentionb | 1.6% | 6.0% |
| ADHD–hyper/impulsiveb | 1.4% | 4.4% |
| Substance usec | ||
| Tobacco use | 5.4% | 20.3% |
| Alcohol use | 24.9% | 58.2% |
| Illicit drug use | 6.9% | 21.5% |
Note. DSM–IV = Diagnostic and Statistical Manual of Mental Disorders (4th ed.).
Three or more DSM–IV symptoms endorsed.
Six or more DSM–IV symptoms endorsed.
Age 12: use = any experimentation with substance; age 17: use = repeated use of substance.
Zygosity Determination
Zygosity for same-sex pairs was determined by two methods. During face-to-face assessment sessions throughout childhood, interviewers rated each twin pair on a nine-item assessment of physical characteristics (Nichols & Bilbro, 1966). The mean number of judgments for the 293 pairs was 18.9 for this longitudinally followed sample. Ratings were completed independently by two individuals at each occasion. These ratings were used to make a judgment of zygosity prior to the advent of routine DNA collection. A second zygosity determination was based on a more recent genotyping of 95% of the sample at a minimum of 11 highly informative short tandem repeat polymorphisms using standard polymerase chain reaction methods and ABI 377 genotyping technology. DNA was extracted from epithelial cells collected by noninvasive cheek swabbing (Meulenbelt, Droog, Trommelen, Boomsma, & Slagboom, 1995). The average heterozygosity of the markers exceeded .75 and gives a posterior probability of MZ misdiagnosis of less than .0001. Marker discordance for members of a twin pair indicated DZ origin, while marker concordance across all genotyped markers indicated their MZ origin. For all pairs in the current analyses, tester ratings and genotype data were in agreement.
Behavioral Assessments
Substance use
At age 12, early use with substances including tobacco, alcohol, marijuana, and other illicit drug categories was assessed with questions from the Monitoring the Future questionnaire (Johnston, O’Malley, & Bachman, 1999). Each question regarding frequency of use was recoded to a binary variable, coded 0 for never used and 1 for used at least one time. At age 17, substance use was assessed with the Composite International Diagnostic Instrument—Substance Abuse Module (CIDI–SAM; Cottler, Robins, & Helzer, 1989). The CIDI–SAM is a structured, face-to-face interview designed for administration by highly trained lay interviewers. The dependent variable for substance use at age 17 was the number of substances used repeatedly; the CIDI–SAM defines repeated use as smoking at least 20 cigarettes or the use of alcohol or illicit drugs on more than five occasions. The substances queried included tobacco, alcohol, marijuana, and eight other illicit drug categories.
Conduct disorder and attention-deficit/hyperactivity disorder composites
Twins were administered the Diagnostic Interview Schedule for Children—IV (DISC–IV; Shaffer, Fisher, & Lucas, 1997), a structured, face-to-face psychiatric interview that assesses Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM–IV]; American Psychiatric Association, 1994) Axis I disorders. A different field tester interviewed each member of the twin pair whenever possible. Rare exceptions to this procedure were necessary due to the costs of out-of-state travel, in cases where families had relocated outside Colorado during the time between Wave 1 and Wave 2. We used data from the DISC–IV to generate lifetime symptom counts for conduct disorder and ADHD (combining symptoms from the attention and hyperactive/impulsive subsections). Using the instructions provided by the instrument’s authors, computer algorithms were developed to determine the presence or absence of each symptom for each disorder. The outcome measure used in the current analysis was a count of the DSM–IV criteria met, based on the DISC–IV responses. Because questionnaire data had been collected on the LTS sample since early childhood, longitudinal parent- and teacher-reported behavior problems were also utilized. Composite scores were developed by combining DISC–IV symptom counts with scores from the attention subscale (for ADHD) and externalizing (for conduct disorder) subscale of the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1983), completed by parents, and the Teacher Report Form (TRF; Achenbach, 1991), completed by school teachers. Scores from the CBCL attention and externalizing subscales assessed at 4, 7, 9, 10, 11, and 12 years of age were averaged to provide a more stable estimate of the lifetime phenotype. Similarly, longitudinally collected scores up to age 16 (i.e., including the earlier assessments as well as those collected at ages 13, 14, 15, and 16) were used for the Wave 2 score. An identical method was applied to the teacher ratings for ages 7 though 16 (no teacher ratings were available for age 4). ADHD scores from the three raters were highly intercorrelated (p < .01). A composite score for ADHD symptoms was derived by summing the DISC–IV ADHD (lifetime) symptom count, the CBCL attention subscale score, and the TRF attention subscale, which were each first z-score transformed to provide uniform scaling. Likewise, we developed a composite score for conduct disorder symptoms by summing the DISC–IV conduct disorder (lifetime) symptom count, the CBCL externalizing subscale score, and the TRF externalizing subscale (as with the ADHD composite, conduct disorder scores from the three raters were also significantly intercorrelated, p < .01), which were z-score transformed prior to summing.
Novelty seeking
To assess novelty seeking at age 12, each twin completed the Junior Temperament and Character Inventory (J-TCI; Luby, Svrakic, McCallum, Przybeck, & Cloninger, 1999), which consists of 18 items. Novelty seeking can be characterized as high levels of exploratory behavior, enjoyment of novel experiences, and seeking of immediate rewards. Two example items for the novelty-seeking scale of the J-TCI are “I always like to spend time thinking about what I am going to do before I do it” (reversed) and “Even if I know I could get hurt, I can easily do things that are scary and dangerous.” Previous studies have reported Cronbach’s alpha coefficients ranging from .64 to .80 and estimates of test–retest reliability from .79 to .86 (Laucht, Becker, Blomeyer, & Schmidt, 2007; Lyoo et al., 2004; Schmeck, Goth, Poustka, & Cloninger, 2001). When the twins were reassessed at age 17, they completed Cloninger’s Tridimensional Personality Questionnaire—Short Form (TPQ; Heath, Cloninger, & Martin, 1994), which also provides a measure of novelty seeking based on 18 items. Two example items for the novelty-seeking scale of the TPQ are “I often try new things just for fun or thrills, even if most people think it is a waste of time,” and “I hate to make decisions based only on my first impressions” (reversed; Cloninger, 1987). As with the J-TCI, psychometric studies have shown the TPQ novelty-seeking scale to have good internal consistency (ranging from .62 to .82) and test–retest reliability (ranging from .65 to .80; Kuo, Chih, Soong, Yang, & Chen, 2004; Otter, Huber, & Bonner, 1995; Sher, Wood, Crews, & Vandiver, 1995).
Laboratory-Based Executive Function Measures
At the Wave 2 session, participants completed nine computerized tasks selected to tap three executive functions—response inhibition, working memory updating, and task-set shifting. Because our primary hypothesis concerned response inhibition, we present detailed methods for the three response inhibition tasks. However, to verify that response inhibition does in fact show discriminant validity in its relation to behavioral disinhibition, we also conducted one set of analyses that included the other two executive functions. Hence, we also briefly describe the essential requirements for the six additional executive function tasks (for additional details of the tasks, see Friedman et al., 2008). Task administration was computerized (Macintosh iBook computers) in PsyScope 1.2.5 (Cohen, MacWhinney, Flatt, & Provost, 1993). A button box with millisecond accuracy was employed for the tasks using reaction time (RT) measures, and a voice key was attached to the button box to record RTs for verbal responses.
Response inhibition
During each trial of the antisaccade task (adapted from Roberts, Hager, & Heron, 1994), participants were required to override their automatic tendency to move their eyes toward a cue when it briefly flashed (for 150 ms) on the left or right side of the computer screen. Instead, they had to quickly move their eyes in the opposite direction to see a small target (a box containing a 5/16-in. arrow pointing left, right, or up) that appeared immediately after the cue for 175 ms before being masked. The dependent measure was proportion of correct arrow identifications out of 90 trials.
In the stop-signal task (Logan, 1994), participants had to periodically stop a prepotent categorization response in response to an auditory signal. In the first block of 48 trials, used to build the prepotent response, participants categorized 24 words (e.g., duck, gun) as referring to animals or nonanimals as quickly and accurately as possible. In four subsequent blocks (96 trials each), participants continued categorizing the words but had to withhold the response when they heard a signal (a 100-ms-long tone) during 25% of the trials. The signals occurred at one of three equally probable delays that were customized for each participant based on his or her average RT during the first no-signal block. The dependent measure was the stop-signal RT, the estimated time at which the stopping process finishes.
The Stroop task (Stroop, 1935) required inhibiting a prepotent word reading response in favor of a color categorization response. In each of 180 trials (split into four mixed-trial blocks), participants named, as quickly and accurately as possible, the color (red, green, blue, orange, yellow, or purple) of the stimulus. The stimulus could be any one of three equally probable types: a colored string of asterisks, a color word printed in an incongruent different color (e.g., blue printed in red), or a colored neutral word (e.g., ship). The dependent measure was the difference between the average incongruent trial RT and the average asterisks trial RT.
Other executive function tasks
Participants completed six additional executive function tasks. The keep-track, letter-memory, and spatial-2-back tasks were designed to tap the ability to flexibly update the contents of working memory with new relevant information and delete no-longer-relevant information when necessary. In the keep-track task, participants saw lists of 15 words presented serially and at the end reported the most recently presented words belonging to two to four target categories. In the letter-memory task, participants continuously said aloud the three most recent letters in a serially presented list of unpredictable length, reporting the last three letters at the end of the list. In the spatial-2-back task, participants saw a series of 25 locations flash and had to indicate for each one whether it was the same as the one that had flashed two trials before. The dependent measure for all three tasks was proportion correct.
The number–letter, color–shape, and category-switch tasks assessed how efficiently participants could shift between two subtasks (respectively, classifying numbers vs. letters, colors vs. shapes, and size vs. animacy of words). Before each trial, a cue indicated which subtask to perform, with the cue sometimes being the same in two consecutive trials (repeat trials) and other times being different (switch trials). The dependent measures were switch costs, calculated as the RT difference between the switch and repeat trials.
Development of the Component Scores
Behavioral disinhibition measures
Males had higher mean scores than females for each behavioral disinhibition measure. Thus, each component score was sex corrected using standard linear regression methods, yielding standardized residual scores. Following the procedures we used in our earlier work on behavioral disinhibition (Young et al., 2000), we also residualized for age, which showed a small but significant positive correlation with most of the behavioral measures. An evaluation of skewness and kurtosis showed unacceptable nonnormality in the individual measures. Thus, a rank normalization procedure was used to transform each variable to approximate normality (Blom, 1958).
Executive function measures
All accuracy data were arcsine transformed to improve normality. For RT tasks, we applied a within-subject trimming procedure recommended by Wilcox and Keselman (2003) before computing mean RTs. Then, to reduce the influence of extreme scores and improve normality, for each variable, we replaced observations farther than three standard deviations from the group mean with values three standard deviations from the mean. There were no significant age or sex effects for executive function measures, except for a small but significant sex difference in antisaccade scores. The measures were transformed to z scores so that the variance of each measure would be comparable to the behavioral disinhibition measures.
Statistical Analyses
Phenotypic and genetic analyses
Structural equation models were fit to data using Mx (Neale, 1999), a statistical software package designed for structural equation modeling of family-level data. Mx provides an option for use of raw data files as input to accommodate variable missing data patterns and to produce unbiased estimates of the variances and covariances. Model fit was evaluated using a chi-square difference test (i.e., the log-likelihood of a constrained model is subtracted from that of a saturated model with parameters for all the covariance elements and means). Because the chi-square is sensitive to sample size, we used χ2/df < 2 and root-mean-square error of approximation (RMSEA) < .06 as indicators of good fit (Hu & Bentler, 1998). RMSEA takes into account the degrees of freedom of the models, which compensates for the effect of complexity in our multivariate genetic models. The significance of individual model parameters was evaluated using 95% confidence intervals.
The genetic analyses we conducted are based on the basic assumptions of twin studies. Because MZ twins share all their genes, whereas DZ twins on average share half of their alleles identically by descent, MZ correlations higher than DZ correlations for a behavioral measure suggest a genetic influence. Estimates of the proportions of variance in performance due to additive genetic (A), shared environmental (C), and nonshared environmental (E) influences are obtained through structural equation ACE models (Neale & Cardon, 1992) of MZ and DZ twin covariance matrices that incorporate standard assumptions. Specifically, the correlations between the additive genetic effects (As) are fixed at 1.0 and 0.5 for MZ and DZ twins, respectively. The correlation between the shared environmental effects (Cs) is fixed to one for both MZ and DZ twins, implying equal shared environmental effects for the different twin types. Other implicit assumptions of the model are that genetic and environmental effects are uncorrelated and act additively and that random mating is operating in the parent generation.
Results
The current study was motivated by three questions regarding the etiology of behavioral disinhibition and its relation to response inhibition at two critical points in adolescent development. Accordingly, we organize the reporting of the results around these questions.
Question 1: What Are the Similarities and Differences in the Structure and Etiology of Behavioral Disinhibition at Two Points in Adolescent Development?
Components of behavioral disinhibition
As summarized in Table 2, twin correlations in the MZ pairs were consistently higher than those in the DZ pairs for both Wave 1 (age 12) and Wave 2 (age 17), suggesting that individual differences in each of these four components of behavioral disinhibition are due, in part, to genetic factors. Results of univariate ACE model fitting are also shown in Table 2. A comparison of the modeling results at ages 12 (Wave 1) and 17 (Wave 2) shows that the etiology of individual components of behavioral disinhibition undergoes some changes during adolescent development.
Table 2.
Twin Correlations and ACE Model Parameters at Waves 1 and 2
| Wave 1 (age ~12 years) |
Wave 2 (age ~17 years) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Twin correlations |
Model parameter estimates (95% confidence intervals) |
Twin correlations |
Model parameter estimates (95% confidence intervals) |
|||||||
| Behavioral measure | MZ | DZ | a2 | c2 | e2 | MZ | DZ | a2 | c2 | e2 |
| Substance use | .61 | .31 | .58 (.24–.68) | .02 (.00–.31) | .40 (.32–.50) | .75 | .65 | .20 (.01–.42) | .55 (.35–.71) | .25 (.20–.31) |
| CD composite | .81 | .46 | .70 (.46–.85) | .11 (.00–.34) | .19 (.15–.24) | .75 | .50 | .49 (.25–.76) | .26 (.00–.46) | .25 (.20–.32) |
| ADHD composite | .79 | .59 | .41 (.17–.75) | .38 (.05–.59) | .21 (.16–.27) | .83 | .57 | .51 (.29–.83) | .32 (.00–.52) | .17 (.14–.27) |
| Novelty seeking | .50 | .25 | .50 (.20–.60) | .00 (.00–.24) | .50 (.40–.63) | .28 | .14 | .28 (.06–.41) | .00 (.00–.15) | .72 (.59–.87) |
Note. Maximum-likelihood correlations estimated from the model using Mx, which uses all available data. ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; DZ = dizygotic; MZ = monozygotic.
Substance use was more heritable early in adolescence (age 12), with an estimated heritability of 58%, in contrast to 20% in older adolescence (age 17). While a large portion of the variance in substance use at age 17 was due to shared environmental factors (), the same was not true for the earlier time point, where there was no evidence for shared environmental influences (). Model fitting results for the conduct disorder composite at age 12 were similar to those for substance use (, ). However, at age 17, the heritability of conduct disorder was substantially larger () than the heritability of substance use. The composite score for problems associated with ADHD was moderately heritable at both ages ( at age 12, at age 17), with shared environmental factors accounting for a significant portion of the variance at age 12 only (). At both ages, novelty seeking showed significant genetic influences ( at age 12, at age 17), without any influence of shared environment.
The behavioral disinhibition factor
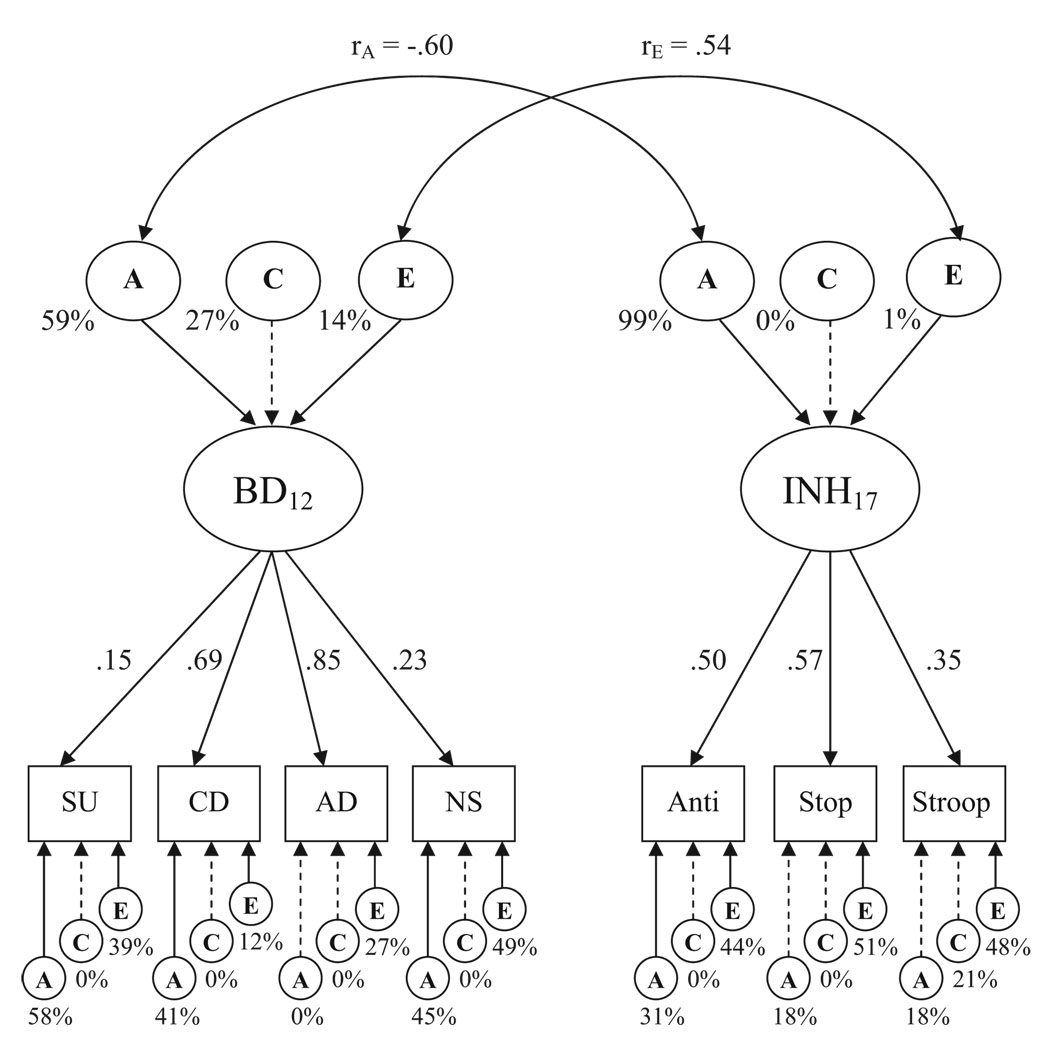
Results of the Wave 1 multivariate model are shown in Figure 1 (note that, to avoid redundancy, we do not present here a model that includes only the behavioral disinhibition factor, but it results in quite similar parameter estimates). This model was compared with a fully saturated model, which freely estimates twin means, variances, and covariances, to judge the fit of the hierarchical model. The chi-square difference test showed that the model provides a good fit to the data: χ2(200, N = 293) = 259.81, p = .003, RMSEA = .045. The relationship between our four component measures and the latent behavioral disinhibition factor is illustrated by the pattern of factor loadings in Figure 1. At age 12, the behavioral disinhibition latent factor was dominated by individual differences in the conduct disorder and ADHD composites (λs = .69 and .85, respectively). Substance use and novelty seeking made relatively smaller contributions (though both statistically significant at p < .05) at this young age. On the upper left side of Figure 1, the decomposition of the variance in behavioral disinhibition into its genetic and environmental components is illustrated. Results suggest that the behavioral disinhibition factor was highly heritable, with 59% of the variance explained by genetic influences. Shared environmental factors accounted for only a modest portion of the variance (27%, ns), and the remaining 14% was explained by significant nonshared environmental influences. With the exception of ADHD, the residual, measure-specific variances were also primarily genetically driven.
Figure 1.
Hierarchical model fitting results from Wave 1 (age 12). Observed variables, represented by boxes, are as follows: SU = substance use; CD = conduct disorder; AD = attention-deficit/hyperactivity disorder composite; NS = novelty seeking; Anti = antisaccade task; Stop = stop-signal task; Stroop = Stroop task. The latent variables, represented by ovals, are as follows: BD = behavioral disinhibition; INH = inhibiting of prepotent responses; A = additive genetic influences; C = environmental influences shared by twins; E = nonshared environmental influence. The double-headed arrows between the latent variables represent factor correlations. Factor loadings on each latent constructs are standardized. Proportions of variance are expressed as percentages. Solid lines indicate significant loadings/proportions of variance (p < .05); dashed lines indicate nonsignificant loadings/proportions of variance (p > .05).
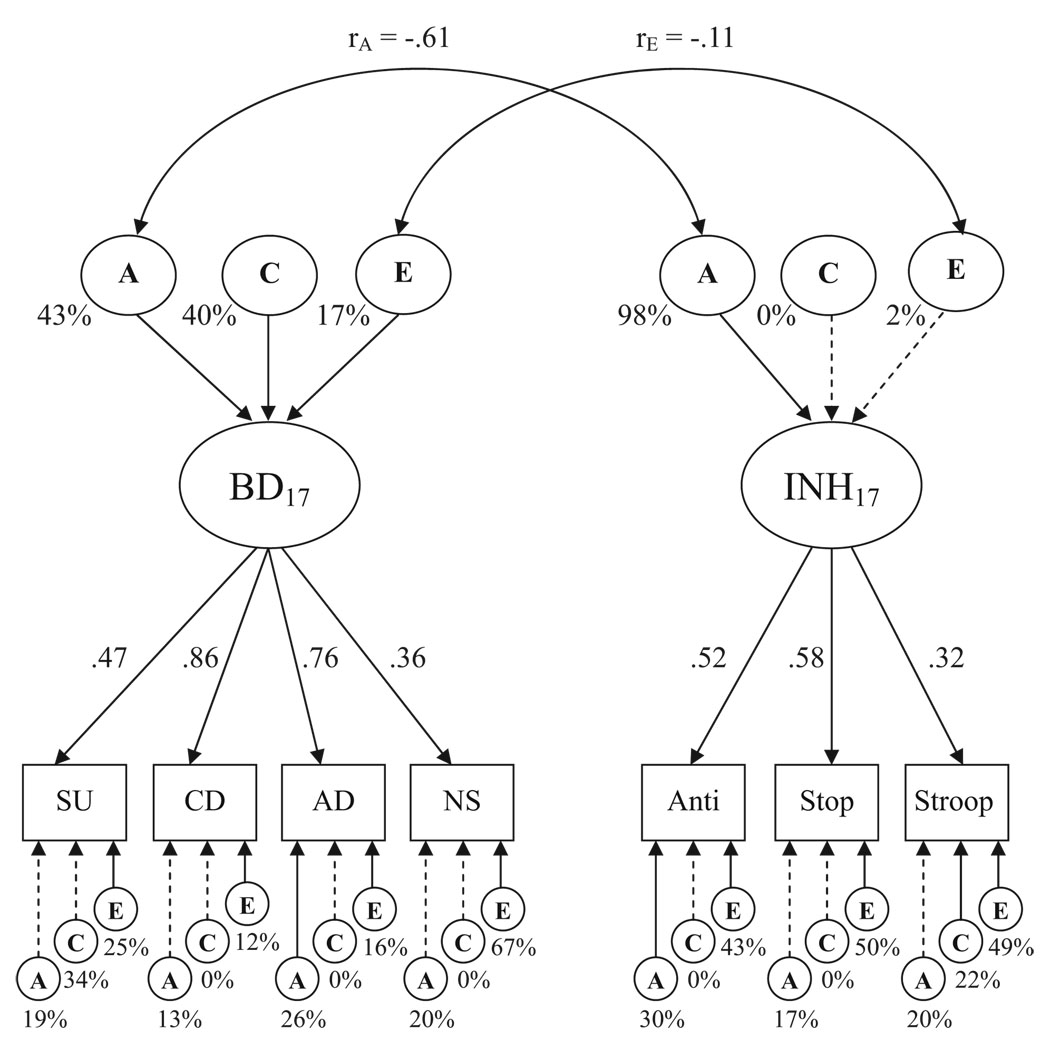
Figure 2 shows results from our parallel analyses of the Wave 2 data, collected when the same twins were 17 years old. Again, the model provided a good fit to the data: χ2(200, N = 293) = 299.60, p < .001, RMSEA = .058. The factor loadings showed a pattern that differed somewhat from Wave 1. Although conduct disorder and ADHD continued to show the highest loadings (λs = .86 and .76, respectively), substance use and novelty seeking were increasingly related to the latent behavioral disinhibition factor (both significant at p < .01). The heritability of behavioral disinhibition was more moderate (43%) in late adolescence, with greater influence by twins’ shared environment, which accounted for 40% (p < .05) of the variance in behavioral disinhibition.
Figure 2.
Hierarchical model fitting results from Wave 2 (age 17). Observed variables, represented by boxes, are as follows: SU = substance use; CD = conduct disorder; AD = attention-deficit/hyperactivity disorder composite; NS = novelty seeking; Anti = antisaccade task; Stop = stop-signal task; Stroop = Stroop task. The latent variables, represented by ovals, are as follows: BD = behavioral disinhibition; INH = inhibiting of prepotent responses; A = additive genetic influences; C = environmental influences shared by twins; E = nonshared environmental influence. The double-headed arrows between the latent variables represent factor correlations. Factor loadings on each latent constructs are standardized. Proportions of variance are expressed as percentages. Solid lines indicate significant loadings/proportions of variance (p < .05); dashed lines indicate nonsignificant loadings/proportions of variance (p > .05).
Question 2: Is Our Behaviorally Based Construct of Disinhibition Meaningfully Related to Laboratory-Based Measures of Response Inhibition?
We used phenotypic analyses to specify the extent of the relationship between behavioral disinhibition at each age and executive functions at age 17. To examine the discriminant validity for response inhibition, we also examined whether behavioral disinhibition was more strongly related to response inhibition than two other executive functions (working memory updating and task-set shifting) that have been shown to be behaviorally and neuropsychologically related but separable from response inhibition (Collette et al., 2005; Friedman et al., 2006; Miyake, Friedman, et al., 2000; Sylvester et al., 2003; Willcutt et al., 2001). Using confirmatory factor models (separately for the Wave 1 and Wave 2 behavioral disinhibition data), we estimated the phenotypic correlations among the four latent factors. Models were fit to pedigree data in Mx to accommodate the nonindependence of the data due to the twin correlations.
Results show that our model fits were good, χ2(540, N = 293) = 808.54, p < .001, RMSEA = .058, for age 12, and λ2(540, N = 293) = 822.13, p < .001, RMSEA = .059, for age 17. At age 12, behavioral disinhibition significantly correlated −.47 with response inhibition, −.27 with working memory updating, and −.20 with task-set shifting (all ps < .001). The correlation with response inhibition was significantly larger than the correlation with the other two executive functions, both Δχ2s(1) > 6.46, ps < .02. At age 17, behavioral disinhibition also significantly correlated with response inhibition (−.39), working memory updating (−.18), and task-set shifting (−.17). Again, the correlation with response inhibition was significantly larger than the correlation with working memory updating and task-set shifting, both Δχ2(1)s > 6.67, ps < .01. Taken together, these results indicate that response inhibition shows discriminant validity as a component of executive control and is more closely related to behavioral disinhibition than the other two executive functions examined. When only response inhibition and behavioral disinhibition were included in the models, the correlations changed slightly to −.44 at age 12 and −.41 at age 17.
Question 3: Can the Relationship Between Behavioral Disinhibition and Response Inhibition Be Explained by Shared Genetic Factors?
Having established that behavioral disinhibition is selectively related to response inhibition, we turned to examining the etiology of this relationship. To do so, we estimated genetic models that included both the behavioral disinhibition and response inhibition latent variables. As shown in Figure 1, the three component measures of response inhibition each make a significant contribution to the latent construct. Individual differences in response inhibition are due almost entirely to genetic influences (a2 = ~.99; note that because the parameters for the two-factor hierarchical model were estimated simultaneously by maximum likelihood, there were minor differences in the inhibiting part of the model at age 12 and age 17). Results provided no evidence of shared environmental influences on response inhibition.
The main focus of our analysis of this third question was the correlations between the latent genetic and environmental influences on our latent factors. Such correlations should be negative because the direction of the effect in response inhibition (i.e., higher scores represent better task performance) is opposite that in behavioral disinhibition (i.e., higher scores represent greater problem behavior). At age 12, the genetic influences in behavioral disinhibition correlated −.60 with the genetic influences on response inhibition at age 17. Although unique environmental factors accounted for only a small portion of the variance in behavioral disinhibition and response inhibition (14% and 1%, respectively), these environmental factors were somewhat overlapping (rE = .54), though not significantly so. It is important to point out that the correlation (−.44) derived from this model between the behavioral and cognitive factors was almost entirely due to the genetic correlation and only negligibly due to the environmental correlation .
The genetic correlation at age 17 was essentially the same as that at age 12 (rA = −.61; see Figure 2) even though the relative contribution of genetic factors to individual differences in behavioral disinhibition was somewhat smaller than at age 12 (.43 vs. .59). Moreover, as with age 12 behavioral disinhibition, the phenotypic correlation of −.41 between behavioral disinhibition and response inhibition at age 17 was almost completely due to the genetic correlation , while the environmental correlation made a minimal contribution . Hence, despite modest differences in the genetic and environmental etiology of the individual latent constructs at ages 12 and 17, the genetic association between behavioral disinhibition and response inhibition was quite comparable at the two points in development.
Secondary Analyses of ADHD Subscales
Results of our multivariate genetic analyses raise a follow-up question regarding the role of the ADHD measure in the latent behavioral disinhibition construct, as well as its relationship with response inhibition: Is it truly the comorbidity between the different components of behavioral disinhibition that accounts for its association with response inhibition, or is it mainly the effect of attention problems, which might be predicted based on previous studies of the relationship between ADHD symptoms and executive functions? To address this question, we tested two additional models, which separately included composite scores of the two DSM-guided subscales of ADHD. Attention problem and hyperactivity/impulsivity composites were constructed by applying the same method used in the full ADHD composite (i.e., summed z scores of self-, parent, and teacher reports).
Results from the age 12 data suggest that the hyperactivity/impulsivity subscale was more strongly related to the conduct disorder composite (rH/I-CD = .64) than the attention problems subscale (rATT-CD = .46). Factor loadings for the substance use and novelty-seeking measures were quite similar across these two subscale models. The latent behavioral disinhibition factor was somewhat more heritable in the attention problems model (a2 = .66) compared with the hyperactivity/impulsivity model (a2 = .57), but this difference was not statistically significant. Likewise, the genetic correlation between behavioral disinhibition and response inhibition was higher for the attention problems model (rA = −.65) as compared with the hyperactivity/impulsivity model (rA = −.41). However, based on the 95% confidence intervals for these estimates, this difference was also nonsignificant. Results from the age 17 data showed a similar pattern to those from age 12 in terms of the relationships among the observed variables. Although the genetic correlation between behavioral disinhibition and response inhibition was higher for the attention problems model (rA = −.71) than for the hyperactivity/impulsivity model (rA = −.46), the heritability estimates of the latent behavioral disinhibition factor were nearly identical in the model including the attention problems subscale and the model including the hyperactivity/impulsivity subscale (a2s = .43 and .45, respectively) at age 17. These results suggest that both the attention problems and hyperactivity/impulsivity components of ADHD are involved in behavioral disinhibition at both age 12 and age 17 and are genetically related to response inhibition.
Discussion
The goal of the current study was to answer three primary questions: (a) What are the similarities and differences in the structure and etiology of behavioral disinhibition at the beginning and end of adolescence? (b) Is behavioral disinhibition related to the ability to inhibit prepotent responses? (c) If so, can the relationship between behavioral disinhibition and response inhibition be explained by shared genetic liability? Our results provide clear answers to each of these questions.
Structure and Etiology of Behavioral Disinhibition Components Across Adolescence
Individual components of behavioral disinhibition
With respect to our first study question, we found that the structure and etiology of the individual components of behavioral disinhibition change somewhat from the beginning to the end of adolescence. Because the disorders under study are developmental in nature, it is perhaps not surprising to see differences in etiologic factors at different points in adolescence.
First, the heritability of substance use was substantially higher in early adolescence, demonstrating that while genes are still influencing substance use in late adolescence, there is a substantial increase in the shared environmental component of the variance in substance use from early to late adolescence. These results are consistent with previous studies suggesting that while early exposure to substances is moderately heritable (Legrand, McGue, & Iacono, 1999), later adolescent experimentation is mostly influenced by environmental factors (Hopfer, Crowley, & Hewitt, 2003). However, persistent substance use leading to abuse and dependence problems has been clearly shown to have a strong genetic etiology (Koopmans, Slutske, Heath, Neale, & Boomsma, 1999; Rhee et al., 2003; Rose, Dick, Viken, Pulkkinen, & Kaprio, 2001; Young, Rhee, Stallings, Corley, & Hewitt, 2006).
A similar yet much smaller shift in the pattern of twin resemblance from age 12 to 17 was observed for conduct disorder, which also lowered the estimate of heritability at Wave 2. Our data suggest that early substance use and conduct problems are more heritable than in late adolescence when some substance experimentation and delinquency may be more normative and, importantly, influenced by family, neighborhood, and peers contributing to shared environmental variance. Although published heritability estimates for adolescent conduct problems have been variable, likely due to differences in method of assessment (e.g., instrument, informant) and age at which the behaviors were studied, a recent review and meta-analysis of 51 available twin and adoption studies (Rhee & Waldman, 2002) suggests that approximately one third of the variation in antisocial behavior can be accounted for by genetic factors. Moreover, a recent study of preadolescent twins showed that combining behavioral data from multiple informants (i.e., child, parent, teacher) captures an index of conduct problems that are highly heritable (a2 = .96; Baker, Jacobson, Raine, Lozano, & Bezdijian, 2007). The fact that we were also able to use a multi-method, multi-informant measure of conduct problems may explain our moderate to high estimates of heritability.
The genetic and environmental etiology for ADHD was comparable at the two ages. ADHD is a highly stable trait across this adolescent time frame since, unlike conduct problems and substance use, symptoms of ADHD generally manifest earlier in childhood and persist (Kuntsi, Rijsdijk, Ronald, Asherson, & Plomin, 2005). The magnitude of genetic influences on ADHD symptoms in our sample is somewhat smaller than in previously published studies (reviewed in Thapar, 2002), due to higher DZ correlations in our sample than some reported previously (e.g., Rietveld, Hudziak, Bartels, van Beijsterveldt, & Boomsma, 2004). We attribute these higher DZ correlations to the fact that our ADHD composite comprises longitudinal scores from multiple raters (teacher, parent, and self), making it a highly reliable measure that offers a cross-setting index of problem behavior that is less vulnerable to rater contrast effects than parental ratings (Simonoff et al., 1998), which are commonly used in etiologic studies of ADHD.
Twin resemblance for novelty seeking decreased substantially from age 12 to age 17, resulting in a lower estimate of heritability (a2 = .28) in late adolescence, as compared with early adolescence (a2 = .50). Notably, this difference in heritability at the two ages was not due to diminished reliability in the novelty-seeking measure at age 17. In fact, internal consistency was quite comparable for the TCI (age 12; α = .68) and the TPQ (age 17; α = .72). We know of no previously published studies of the heritability of adolescent novelty seeking outside our own laboratory. However, constraint, defined as the tendency to inhibit behavioral impulses and to prefer boring but safe activities to exciting but dangerous activities (thus negatively correlated with novelty seeking; Waller, Lilienfeld, Tellegen, & Lyyken, 1991), has been shown to be moderately heritable in older adolescents and significantly related to a latent externalizing construct (Krueger et al., 2002).
Latent behavioral disinhibition factor
Several interesting differences emerged when comparing the hierarchical model of behavioral disinhibition at age 12 with the model at age 17. First, we note that at age 12, the latent behavioral disinhibition factor was more heavily weighted by ADHD and conduct problems and less related to early substance use, even though the factor loadings for all of the component measures were significant. This finding may be expected, given that the onset of both ADHD and conduct disorder symptoms typically predates the onset of substance use (Elkins, McGue, & Iacono, 2007; Loeber, Stouthamer-Loeber, & White, 1999; Young et al., 1995). At age 17, the loadings were generally higher (with the exception of ADHD), ranging from .36 for novelty seeking to .86 for conduct disorder, and were more comparable across the measures. Substance use, in particular, was more involved in the latent construct at age 17 (λ = .47) than at age 12 (λ = .15). We note that lifetime symptom counts and substance use measures were used at both time points, dictating that individual scores either remained unchanged or increased over time. Although this may have influenced the factor structure to some degree, the lifetime framework was chosen for two important reasons. First, measures of current or recent (e.g., past year) behavior do not accommodate the developmental fluctuations inherent in these behavioral syndromes (i.e., the fact that certain aspects of externalizing disorders are more salient and prevalent at certain ages). Lifetime scores, in contrast, provide a cumulative record of all symptoms manifest to that point in time and, hence, a more comprehensive and reliable measure of the underlying vulnerability. Second, for individuals who have previously received or are currently undergoing treatment for externalizing problems (e.g., psychoactive medications for ADHD symptoms), a lifetime score captures symptom information that predates treatment or other interventions such as juvenile detention.
In the current study, our twin sample was assessed at two ages (12 and 17 years) that basically flank adolescence. In contrast, our previous study on the etiology of behavioral disinhibition (Young et al., 2000), was conducted with twins who spanned the adolescent age range (ages 12–18 years), with an average age of 14.2 years. Considering the developmental nature of the behavioral problems under study, we might expect results from our current Wave 1 analyses to align more closely with our previous findings. Indeed the etiological structure of behavioral disinhibition at Wave 1 is quite similar to that in our previous report. It is important to note that in our previous article, we reported a parsimonious model for behavioral disinhibition in which nonsignificant parameters were dropped from the model. If the same procedure were carried out on the current Wave 1 model (i.e., dropping the nonsignificant influences of shared environment, C), the heritability of behavioral disinhibition would be consistently quite high. Interestingly, our Wave 2 findings are more comparable to our previous study in terms of the pattern of factor loadings, in that the contributions of the individual components of behavioral disinhibition are more balanced.
The genetic and environmental architecture at Wave 1 was also comparable to findings from the Minnesota Twin-Family Study (MTFS), which demonstrated a highly heritable “externalizing spectrum” construct (Hicks, Krueger, Iacono, McGue, & Patrick, 2004; Krueger et al., 2002). However, our Wave 2 results showed more modest genetic influences as well as modest shared environmental influences. Given the similar age at time of assessment (~17 years) in the LTS and MTFS samples, we attribute these differences in heritability to the fact that the MTFS twins were assessed with measures of more severe pathology, including symptoms of Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987) defined antisocial personality disorder, as well as symptoms of alcohol and drug dependence, rather than experimentation. Deliberately targeting these more deviant behaviors likely defines a more genetically influenced syndrome (e.g., Rhee & Waldman, 2002) but one that would not be meaningful in twins as young as 12 years.
As previously discussed, this developmental shift may suggest an increased salience in environmental factors that are shared by twins such as peers, neighborhood effects (e.g., drug availability), and degree of parental supervision. We recognize, however, that the hypothesized developmental changes from age 12 to age 17 cannot be disentangled from the possible effects of using slightly different measures at the two time points. Although we used age-appropriate measures of substance use and novelty seeking, they were not completely isomorphic (however, ADHD and conduct disorder composites came from the same instruments at both waves). Though justified, this methodological inconsistency should be considered when interpreting differences in the results across the two ages.
Phenotypic and Genetic Relation of Behavioral Disinhibition to Response Inhibition
With respect to the second study question, we found that behavioral disinhibition in both early (age 12) and late (age 17) adolescence was significantly related to response inhibition at age 17 (rs = −.44 at age 12 and −.41 at age 17). Moreover, behavioral disinhibition at each age was significantly more closely associated with response inhibition than with the two other executive functions (working memory updating and task-set shifting) measured at age 17. These results support the hypothesis that behavioral disinhibition does indeed share something in common with cognitive response inhibition.
The strength of the relations between behavioral disinhibition and response inhibition is particularly remarkable, given the quite different methodologies for measuring the two constructs (i.e., clinical measures and questionnaires for behavioral disinhibition vs. laboratory cognitive tasks for response inhibition). We note that our factor correlations are somewhat higher than those found between single cognitive or neuropsychological tasks and individual components of behavioral disinhibition such as ADHD (e.g., Willcutt et al., 2005). One likely explanation for these larger correlations is the power afforded by the latent variable approach. In particular, it appears that response inhibition may be a difficult construct to capture with individual measures (Friedman & Miyake, 2004) because of the large degree of task impurity (i.e., a substantial proportion of variance unrelated to inhibition) present in established response inhibition measures. However, the results of this study demonstrate that when measured as a latent variable, the response inhibition construct is tapping an important ability that has the potential to contribute to theoretical advances in our understanding of behavior problems.
With respect to our third study question, results of our biometrical twin analyses showed that at both ages, the association between behavioral disinhibition and response inhibition was almost entirely genetic in nature, providing the first empirical support for the hypothesis that the relationship between behavioral and cognitive inhibition is driven by a shared biological vulnerability.
One notable limitation of the current study is that fact that while behavioral disinhibition was assessed at both waves, executive functions were only assessed at Wave 2. Hence, it was not possible to examine how age 12 behavioral disinhibition related to concurrent response inhibition, only how it related to later response inhibition at age 17, leaving open the question of whether the concurrent relation would be similar to the longitudinal relation. Nevertheless, our longitudinal design was unique in that we were able to determine that behavioral disinhibition early in adolescence predicted response inhibition much later in adolescence. In this regard, we found that the strength of both the phenotypic and genetic relations between the latent factors was equally strong at both waves, despite the fact that there was a 5-year separation between the age 12 behavioral disinhibition assessment and age 17 response inhibition assessment. Similarly, in a previous study utilizing data from this twin sample, we found that attention problems throughout childhood and adolescence also showed remarkable stability in their relations to response inhibition. In fact, attention problems as early as age 7 showed essentially the same strength of relation to response inhibition at age 17 as attention problems at age 14 (Friedman et al., 2007). Results from both studies suggest substantial stability in the relationship between response inhibition and behavioral disinhibition during adolescence, despite the fact that that this developmental period involves considerable neuromaturation in the frontal and prefrontal cortex, areas critical for both executive and behavioral control (Banich, 2004; Chen et al., 2007; Collette et al., 2005; Paus, 2005; Schepis, Adinoff, & Rao, 2008; Steinberg, 2005).
An Alternative Model of Behavioral Disinhibition
In the current study, behavioral disinhibition has been characterized as a common latent factor representing a generalized vulnerability to various externalizing disorders. In contrast, alternative models have been presented in the literature that conceptualize behavioral (dis)inhibition as a heterogeneous dual process associated with two distinct, interacting neural circuits (Nigg, 2000, 2003, 2006). Specifically, Nigg (2003) argued that conduct disorder primarily involves a primary failure of reactive or motivational control process (i.e., detecting and responding to immediate contextual cues related to incentives, punishment, or social anxiety), with secondary breakdowns in effortful control of attention (Rothbart & Bates, 2006) or executive control processes. In contrast, according to Nigg, ADHD develops, in part, from a primary failure of neural systems of executive control, with secondary deficits in reactive or motivational controls. This model is put forth as a tool for examining differential developmental pathways (both biological and psychosocial) to disruptive behavior problems and other distinctions that have clinical relevance. Thus, while Nigg’s model helps to dissociate etiological mechanisms influencing ADHD versus conduct disorder, our focus here is on etiological factors explaining their association (i.e., comorbidity).
The primary models examined in the current study treat ADHD as a single continuum of symptoms. However, previous ADHD research by Nigg (Nigg, Stavro, et al., 2005) and others (e.g., Chhabildas, Pennington, & Willcutt, 2001) led us to question whether separable components of ADHD, namely, attention problems and hyperactivity/impulsivity problems, may be differentially related to other externalizing disorders as well as to response inhibition. In post hoc analyses, we examined these two ADHD domains at ages 12 and 17 and found that conduct problems were correlated less highly with attention problems than with hyperactivity/impulsivity problems. However, the correlations with substance use and novelty seeking were nearly identical for the two ADHD components. Attention problems were also more highly correlated with response inhibition than hyperactivity/impulsivity problems, but both components showed a highly significant genetic correlation with response inhibition. Thus, our findings partially mirror previous research but suggest that conduct problems and associated reactive or motivational problems (e.g., high risk taking, low fear of punishment) are also strongly associated with deficits in response inhibition and that the relationship is largely genetic.
Though our common pathway model takes a different perspective than Nigg’s (2001, 2003) dual-process model, the two approaches are complementary in the sense that, together, they examine both the unity (common underlying risks) and diversity (differential underlying risks) of externalizing problems and associated cognitive deficits. We would argue that both perspectives provide unique and valuable tools that can be used to better understand the nature and etiology of psychopathology.
Implications and Conclusions
Recently, Krueger, Markon, Patrick, and Iacono (2005) proposed that classifications in the upcoming fifth edition of the DSM should reflect the etiologic and clinical commonalities among the components of behavioral disinhibition, under the rubric of externalizing disorders. They pointed out that although the clustering of disorders for diagnostic purposes (based, for example, on comorbidity) is not a new concept, incorporating empirical evidence of biological connections among externalizing disorders and personality traits associated with aggression and impulsivity is an important development with useful implications for both diagnostic taxonomies and treatment planning. The current findings, which build on a growing literature demonstrating a common biological etiology among externalizing disorders, give further credence to such proposed changes.
This study also represents an important step toward integrating advances in cognitive models of executive control with clinical psychology. The concept of executive control, particularly response inhibition, is central to many theories of psychopathology, including ADHD (Barkley, 1997; Nigg, 2000, 2001) and substance use disorders (Garavan & Stout, 2005; Giancola et al., 1998). Our finding that the link between behavioral disinhibition and response inhibition is almost entirely genetic extends previous behavioral findings to a new level. Understanding the underlying biological processes that link behavioral disinhibition, measured with assessments of real-life behavior, and response inhibition, measured with cognitive laboratory tasks, promises to provide new insights into the nature of externalizing spectrum disorders. In this respect, response inhibition may be a valuable endophenotype for future neuropsychiatric and molecular genetic studies of disinhibited behavior problems. One cautionary note is that the usefulness of response inhibition as an endophenotype will depend in large part on how it is measured. In particular, using individual tasks is unlikely to be fruitful. As the factor loadings for the tasks demonstrate, although the latent response inhibition factor is almost entirely heritable, the individual measures are quite impure, with only a small portion of their variances tapping the latent factor. Thus, future studies would benefit from incorporating multiple measures of inhibition and using either latent variable analysis or aggregation to arrive at a purer measure of response inhibition abilities (Miyake, Emerson, & Friedman, 2000).
Response inhibition is also emerging as an important executive function related to psychiatric conditions outside the externalizing spectrum, such as schizophrenia (Donohoe, Corvin, & Robertson, 2006; Ross, Heinlein, Zerbe, & Radant, 2005; Thoma, Zoppelt, Wiebel, & Daum, 2007), unipolar depression (Kaiser et al., 2003), obsessive-compulsive disorder (Herrmann, Jacob, Unterecker, & Fallgatter, 2003; Kim, Kim, Yoo, & Kwon, 2007), and borderline personality disorder (Nigg, Silk, Stavro, & Miller, 2005). Thus, continued investigation of the links between response inhibition and an array of psychiatric illnesses may provide new insights into their underlying biological vulnerabilities.
Acknowledgments
Data collection was supported by National Institutes of Health (NIH) Grants MH63207, HD010333, and DA011015. Susan E. Young was supported by NIH Grant MH01865, Naomi P. Friedman by NIH Grant MH075814, and Brett C. Haberstick by National Institute on Alcohol Abuse and Alcoholism Grant AA07464. We thank Sally Ann Rhea and Scott Sabella for project coordination and data collection.
Contributor Information
Susan E. Young, Institute for Behavioral Genetics, University of Colorado at Boulder
Naomi P. Friedman, Institute for Behavioral Genetics, University of Colorado at Boulder
Akira Miyake, Department of Psychology, University of Colorado at Boulder.
Erik G. Willcutt, Institute for Behavioral Genetics and Department of Psychology, University of Colorado
Robin P. Corley, Institute for Behavioral Genetics, University of Colorado at Boulder
Brett C. Haberstick, Institute for Behavioral Genetics, University of Colorado at Boulder
John K. Hewitt, Institute for Behavioral Genetics and Department of Psychology, University of Colorado
References
- Achenbach TM. Manual for the Teacher Report Form and 1991 Profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Behavior Profile. Burlington: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed rev. Washington, DC: Author; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse or dependence and psychiatric comorbidity. Journal of Consulting and Clinical Psychology. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and environmental bases of childhood antisocial behavior: A multi-informant twin study. Journal of Abnormal Psychology. 2007;116:219–235. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Cognitive neuroscience and neuropsychology. Boston: Houghton Mifflin; 2004. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety and other disorders. American Journal of Psychiatry. 1991;148:564–577. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- Bishop EG, Cherny SS, Corley RP, Plomin R, DeFries JC, Hewitt JK. Developmental genetic analysis of general cognitive ability from 1 to 12 years in a sample of adoptees, biological siblings, and twins. Intelligence. 2003;31:31–49. [Google Scholar]
- Blom G. Statistical estimates and transformed beta variables. New York: Wiley; 1958. [Google Scholar]
- Boyle MH, Offord DR. Psychiatric disorder and substance use in adolescence. Canadian Journal of Psychiatry. 1991;36:699–705. [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chen AC, Porjesz B, Rangaswamy M, Kamarajan C, Tang Y, Jones KA, et al. Reduced frontal lobe activity in subjects with high impulsivity and alcoholism. Alcoholism: Clinical and Experimental Research. 2007;31:156–165. doi: 10.1111/j.1530-0277.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Chhabildas N, Pennington BF, Willcutt EG. A comparison of the neuropsychological profiles of the DSM-IV subtypes of ADHD. Journal of Abnormal Child Psychology. 2001;29:529–540. doi: 10.1023/a:1012281226028. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. The Tridimensional Personality Questionnaire: Version 4. St. Louis, MO: Washington University School of Medicine, Department of Psychiatry; 1987. [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Childhood personality predicts alcohol abuse in young adults. Alcoholism: Clinical and Experimental Research. 1988;12:494–505. doi: 10.1111/j.1530-0277.1988.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Collette F, Van der Linden M, Laureys S, Delfiore G, Degueldre C, Luxen A, Salmon E. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–423. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. British Journal of Addiction. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Crowley TC, Riggs PD. Adolescent substance use disorder with conduct disorder and comorbid conditions. In: Rahdert E, Czechowicz D, editors. Adolescent drug abuse: Clinical assessment and therapeutic interventions. Washington, DC: U.S. Department of Health and Human Services; 1995. pp. 49–111. [Google Scholar]
- Donohoe G, Corvin A, Robertson IH. Evidence that specific executive functions predict symptom variance among schizophrenia patients with a predominantly negative symptom profile. Cognitive Neuropsychiatry. 2006;11:12–32. doi: 10.1080/13546800444000155. [DOI] [PubMed] [Google Scholar]
- Ehringer MA, Rhee SH, Young SE, Corley RP, Hewitt JK. Genetic and environmental contributions to common psychopathologies of childhood and adolescence: A study of twins and their siblings. Journal of Abnormal Child Psychology. 2006;34:1–17. doi: 10.1007/s10802-005-9000-0. [DOI] [PubMed] [Google Scholar]
- Elkins IJ, McGue M, Iacono WG. Prospective effects of attention- deficit/hyperactivity disorder, conduct disorder, and sex on adolescent substance use and abuse. Archives of General Psychiatry. 2007;64:1145–1152. doi: 10.1001/archpsyc.64.10.1145. [DOI] [PubMed] [Google Scholar]
- Farrington DP, Van Kammen WB. Long term criminal outcomes of hyperactivity-impulsivity-attention deficits and conduct problems in childhood. In: Robins N, Rutter MR, editors. Straight and deviant pathways to adulthood. New York: Cambridge University Press; 1990. pp. 62–81. [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT. The comorbidities of adolescent problem behaviors: A latent class model. Journal of Abnormal Child Psychology. 1994;22:339–354. doi: 10.1007/BF02168078. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: Go/no go learning deficits, working memory capacity and personality. Alcoholism: Clinical and Experimental Research. 2002;26:186–206. [PubMed] [Google Scholar]
- Ford T, Goodman R, Meltzer H. The relative importance of child, family, school, and neighborhood correlates of childhood psychiatric disorder. Social Psychiatry & Psychiatric Epidemiology. 2003;39:487–496. doi: 10.1007/s00127-004-0782-0. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Haberstick BC, Willcutt EG, Miyake A, Young SE, Corley RP, Hewitt JK. Greater attention problems during childhood predict poorer executive functioning in late adolescence. Psychological Science. 2007;18:893–900. doi: 10.1111/j.1467-9280.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control processes: A latent variable analysis. Journal of Experimental Psychology: General. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychological Science. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive function are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Mezzich AC, Tarter RE. Disruptive, delinquent and aggressive behavior in female adolescents with a psychoactive substance use disorder: Relation to executive cognitive functioning. Journal of Studies on Alcohol. 1998;59:560–567. doi: 10.15288/jsa.1998.59.560. [DOI] [PubMed] [Google Scholar]
- Gorenstein EE, Newman JP. Disinhibitory psychopathology: A new perspective and a model for research. Psychological Review. 1980;87:301–315. [PubMed] [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: A comparison of the personality systems of Cloninger and Eysenck. Journal of Personality and Social Psychology. 1994;66:762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Jacob C, Unterecker S, Fallgatter AJ. Reduced response inhibition in obsessive-compulsive disorder measured with topographic evoked potential mapping. Psychiatry Research. 2003;120:265–271. doi: 10.1016/s0165-1781(03)00188-4. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: A twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Crowley TJ, Hewitt JK. Review of twin and adoption studies of adolescent substance use. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- Howard MO, Kivlahan D, Walker RD. Cloninger’s tridimensional theory of personality and psychopathology: Applications to substance use disorders. Journal of Studies on Alcohol. 1997;58:48–66. doi: 10.15288/jsa.1997.58.48. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychological Methods. 1998;3:424–453. [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Mally PM, Bachman JG. National survey results on drug use from the Monitoring the Future Study, 1975–1998: Vol. I. Secondary school students. Rockville, MD: National Institute on Drug Abuse; 1999. [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: Event-related potentials in a go/no go task. Psychiatry Research. 2003;122:169–184. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Johnson JG, Bird HR, Canino G, Goodman SH, Lahey BB, et al. Psychiatric disorders associated with substance use among children and adolescents: Findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) Study. Journal of Abnormal Child Psychology. 1997;25:121–132. doi: 10.1023/a:1025779412167. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim YY, Yoo SY, Kwon JS. Electrophysiological correlates of behavioral response inhibition in patients with obsessive-compulsive disorder. Depression and Anxiety. 2007;24:22–31. doi: 10.1002/da.20195. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiological connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biological Psychiatry. 2005;57:647–654. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Chih YC, Soong WT, Yang HJ, Chen WJ. Assessing personality features and their relations with behavioral problems in adolescents: Tridimensional Personality Questionnaire and Junior Eysenck Personality Questionnaire. Comprehensive Psychiatry. 2004;45:20–28. doi: 10.1016/j.comppsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Burke J, Rathouz PJ, McBurnett K. Waxing and waning in concert: Dynamic comorbidity of conduct disorder with other disruptive and emotional problems over 7 years among clinic-referred boys. Journal of Abnormal Psychology. 2002;111:445–567. doi: 10.1037//0021-843x.111.4.556. [DOI] [PubMed] [Google Scholar]
- Laucht M, Becker K, Blomeyer D, Schmidt MH. Novelty seeking involved in mediating the association between the dopamine D4 receptor gene exon III polymorphism and heavy drinking in male adolescents: Results from a high-risk community sample. Biological Psychiatry. 2007;61:87–92. doi: 10.1016/j.biopsych.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Legrand LN, McGue M, Iacono WG. Searching for interactive effects in the etiology of early-onset substance use. Behavior Genetics. 1999;29:433–444. doi: 10.1023/a:1021627021553. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthhamer-Loeber M, White HR. Developmental aspects of delinquency and internalizing problems and their association with persistent juvenile substance use between ages 7 and 18. Journal of Clinical Child Psychology. 1999;28:322–332. doi: 10.1207/S15374424jccp280304. [DOI] [PubMed] [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: A user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Luby JL, Svrakic DM, McCallum K, Przybeck TR, Cloninger CR. The Junior Temperament and Character Inventory: Preliminary validation of a child self-report measure. Psychological Reports. 1999;84:1127–1138. doi: 10.2466/pr0.1999.84.3c.1127. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Han CH, Lee SJ, Yune SK, Ha JH, Chung SJ, et al. The reliability and validity of the Junior Temperament and Character Inventory. Comprehensive Psychiatry. 2004;45:121–128. doi: 10.1016/j.comppsych.2003.12.002. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink: I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcoholism: Clinical and Experimental Research. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. American Journal of Human Genetics. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Emerson MJ, Friedman NP. Assessment of executive functions in clinical settings: Problems and recommendations. Seminars in Speech and Language. 2000;21:169–183. doi: 10.1055/s-2000-7563. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Neale MC. Mx: Statistical modeling. 5th ed. Richmond: Virginia Commonwealth University, Department of Psychiatry; 1999. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 1992. pp. 153–156. [Google Scholar]
- Nichols RC, Bilbro WC. The diagnosis of twin zygosity. Acta Genetica et Statistica Medica. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg J. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Response inhibition and disruptive behaviors: Toward a multiprocess conception of etiological heterogeneity for ADHD combined type and conduct disorder early-onset type. Annals of the New York Academy of Sciences. 2003;1008:170–182. doi: 10.1196/annals.1301.018. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Silk KR, Stavro G, Miller T. Disinhibition and borderline personality disorder. Development and Psychopathology. 2005;17:1129–1149. doi: 10.1017/s0954579405050534. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: Evidence for selective effects on ADHD symptom domains. Journal of Abnormal Psychology. 2005;114:706–717. doi: 10.1037/0021-843X.114.3.706. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Otter C, Huber J, Bonner A. Coninger’s Tridimensional Personality Questionnaire: Reliability in an English sample. Personality and Individual Differences. 1995;18:471–480. [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP. Colorado Twin Registry. Twin Research and Human Genetics. 2006;9:941–949. doi: 10.1375/183242706779462895. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Archives of General Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Rietveld MJ, Hudziak JJ, Bartels M, van Beijsterveldt CE, Boomsma DI. Heritability of attention problems in children: Longitudinal results from a study of twins, age 3 to 12. Journal of Child Psychology and Psychiatry. 2004;45:577–588. doi: 10.1111/j.1469-7610.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Hager LD, Heron C. Prefrontal cognitive processes: Working memory and inhibition in the antisaccade task. Journal of Experimental Psychology: General. 1994;123:374–393. [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcohol: Clinical and Experimental Research. 2001;25:1594–1604. [PubMed] [Google Scholar]
- Ross RG, Heinlein S, Zerbe GO, Radant A. Saccadic eye movement task identifies cognitive deficits in children with schizophrenia, but not in unaffected child relatives. Journal of Child Psychology and Psychiatry. 2005;46:1354–1362. doi: 10.1111/j.1469-7610.2005.01437.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Eisenber N, Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. Hoboken, NJ: Wiley; 2006. pp. 99–166. [Google Scholar]
- Schmeck K, Goth K, Poustka F, Cloninger CR. Reliability and validity of the Junior Temperament Character Inventory. International Journal of Methods in Psychiatric Research. 2001;10:172–182. [Google Scholar]
- Shaffer D, Fisher P, Lucas C. The Diagnostic Interview Schedule for Children: IV. New York: Columbia University, Ruane Center for Early Diagnosis, Division of Child Psychiatry; 1997. [Google Scholar]
- Shepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. American Journal of Addictions. 2008;17:6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: Alcoholism and antisocial personality. Journal of Abnormal Psychology. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Wood MD, Crews TM, Vandiver PA. The Tridimensional Personality Questionnaire: Reliability and validity studies and derivation of a short form. Psychological Assessment. 1995;7:195–208. [Google Scholar]
- Simonoff E, Pickles A, Hervas A, Silberg JL, Rutter M, Eaves LJ. Genetic influences on childhood hyperactivity: Contrast effects imply parental rating bias, not sibling interaction. Psychological Medicine. 1998;28:825–837. doi: 10.1017/s0033291798006886. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: FMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Thapar A. Attention deficit hyperactivity disorder: New genetic findings, new directions. In: Plomin R, DeFries JC, Craig IW, McGuffin P, editors. Behavioral genetics in the postgenomic era. Washington, DC: American Psychological Association; 2002. pp. 445–462. [Google Scholar]
- Thoma P, Zoppelt D, Wiebel B, Daum I. Context processing and negative symptoms in schizophrenia. Journal of Clinical and Experimental Neuropsychology. 2007;29:428–435. doi: 10.1080/13803390600744814. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Pihl RO, Vitaro F, Dobkin PL. Predicting early onset of male antisocial behavior from preschool behavior. Archives of General Psychiatry. 1994;51:732–739. doi: 10.1001/archpsyc.1994.03950090064009. [DOI] [PubMed] [Google Scholar]
- Waller NG, Lilienfeld SO, Tellegen A, Lykken DT. The Tridimensional Personality Questionnaire: Structural validity and comparison with the Multidimensional Personality Questionnaire. Multivariate Behavioral Research. 1991;26:1–23. doi: 10.1207/s15327906mbr2601_1. [DOI] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: Measures of central tendency. Psychological Methods. 2003;8:254–274. doi: 10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, Olson RK. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2001;110:157–172. doi: 10.1037//0021-843x.110.1.157. [DOI] [PubMed] [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug and Alcohol Dependence. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]
- Young SE, Mikulich SK, Goodwin MB, Hardy J, Martin CL, Zoccolillo MS, Crowley TJ. Treated delinquent boys’ substance use: Onset, pattern, relationship to conduct and mood disorders. Drug and Alcohol Dependence. 1995;37:149–162. doi: 10.1016/0376-8716(94)01069-w. [DOI] [PubMed] [Google Scholar]
- Young SE, Rhee SH, Stallings MC, Corley RP, Hewitt JK. Genetic and environmental vulnerabilities underlying adolescent substance use and problem use: General or specific? Behavior Genetics. 2006;36:603–615. doi: 10.1007/s10519-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics: Neuropsychiatric Genetics. 2000;96(B):684–695. [PubMed] [Google Scholar]