Abstract
This study investigated age-related changes in the early processing of novel visual stimuli using ERPs. Well-matched old (n=30), middle-aged (n=30), and young (n=32) subjects were presented standard, target/rare, and perceptually novel visual stimuli under attend and ignore conditions. Our results suggest that the anterior P2 component indexes the motivational salience of a stimulus as determined by either task relevance or novelty. Its enhancement by focused attention does not decrease with age. Its responsiveness to novel stimuli is particularly striking in older adults. The age-related increase in the anterior P2 to novel visual stimuli does not appear to be due to impaired inhibitory control associated with aging. Rather, the enhanced anterior P2 to novel stimuli in older adults may be linked to age-related changes in the process of matching unusual visual stimuli to stored representations, which is indexed by the temporally overlapping anterior N2 component whose amplitude substantially decreases with age.
1. Introduction
There has been very limited examination of age-related changes in the early processing of novel visual stimuli. Because of their excellent temporal resolution, event-related potentials (ERPs) can be an effective tool for investigating early processing effects. Most ERP studies have focused on the novelty P3, a component that tends to peak between 350 and 500 ms after stimulus onset. Many studies have reported a decline in the amplitude of the novelty P3 among older subjects in response to novel auditory, tactile, or visual stimuli that consist of simple geometric figures that deviate from repeating standard stimuli (Fabiani & Friedman, 1995; Fjell & Walhovd, 2004; Friedman et al., 1998; Knight, 1987; Walhovd & Fjell, 2001; Yamaguchi & Knight, 1991). This often has been interpreted as reflecting age-related changes in prefrontal cortex that lead to a decreased ability to orient attention to novel events or to formulate and maintain templates for the different stimulus types used in the experiment (Fabiani et al., 1998; Fabiani & Friedman, 1995; Fjell & Walhovd, 2004). Interestingly, studies that have employed highly unusual, rather than simple visual stimuli as novels in a 3-stimulus novelty oddball task have tended to report no age-related changes in the overall size of the novelty P3 response (Beck et al., 1980; Snyder and Hillyard, 1979). This finding raises the possibility of a relatively preserved capacity of older individuals to direct attention to novel visual events if they are sufficiently unusual or interesting. Our research has found that cognitively high performing older adults actually generate a larger P3 to perceptually novel stimuli than both cognitively high performing young subjects and cognitively average performing old subjects, which we have argued may represent a successful compensatory neural mechanism, presumably in response to other age-related changes in neurophysiological function (Daffner et al., 2006a: Daffner et al, 2006b; Riis et al., 2008). The extent to which there were age-associated differences in earlier processing stages of novel visual stimuli and whether such differences varied as a function of cognitive status remained to be determined.
This study examined life-span changes in P2 effects in response to visual stimuli, which occur in the first few hundred milliseconds after stimulus onset. It is likely that there are at least two different visually-evoked P2 components that have different functional significance, one with a posterior-parietal scalp distribution (Ceponiene et al., 2008; Han et al., 2005; Khoe et al, 2006; Muller & Knight, 2002; Schendan & Kutas, 2007; Talsma & Kok, 2002) and the other with an anterior scalp distribution (Knight, 1997; Luck & Hillyard, 1994; Potts et al., 1996; Potts & Tucker, 2001). Most relevant to the current study is the anteriorly-distributed P2 component (Knight, 1997; Luck & Hillyard, 1994; Potts et al., 1996; Potts & Tucker, 2001). It has been conceptualized as either a marker of the activation of top-down control over the perceptual processing of task-relevant stimulus dimensions under attend conditions (Luck & Hillyard, 1994), or as a frontally mediated index of the motivational salience of a stimulus based on task relevance (Potts & Tucker, 2001). The motivational salience of a stimulus reflects a top-down, controlled process that designates certain features or their combinations as having potential significance to an individual on the basis of task demands, anticipated rewards, goals, or other factors (Corbetta & Shulman, 2002; Daffner et al., 2003; Itti & Koch, 2001; Navalpakkam & Itti, 2005). Motivational salience differs from stimulus salience, which reflects a bottom-up, stimulus-driven signal of potential attention-worthiness. Stimulus salience is believed to be a function of early stages of visual processing that determine the extent to which various features of a stimulus (color, edge orientation, motion, luminance) differ from their physical surroundings (Itti & Koch, 2001).
The anteriorly-distributed P2 has been shown to be elicited during a visual oddball task by infrequent stimuli designated as targets, but not when the same infrequent stimuli are passively viewed (Potts et al., 1996). The hypothesis linking the P2 with target processing has been strengthened by the observation that the latency of the target P2 correlates with target reaction time (Potts & Tucker, 2001). In addition, temporal probability has been demonstrated to modulate the amplitude of the anterior P2 to target stimuli, with infrequent target stimuli evoking a larger response than frequent target stimuli (Luck & Hillyard, 1994). The anterior P2 has been shown to be sensitive to stimulus dimensions, such as color, size, or orientation, that have been specified by task instructions as being significant (Luck & Hillyard, 1994). These findings suggest that the anterior P2 may index top-down processes involved in the evaluation of the motivational salience of a stimulus as defined by task-relevant features.
Of note, these influential studies on the anterior P2 have not included stimuli that were perceptually novel, nor subjects who were older adults. Studies using visual novelty oddball tasks that have included adult subjects with a wide range of ages have suggested that the anterior P2 is very responsive to perceptual novelty (e.g., highly unusual figures and shapes) (Beck et al., 1980; Knight, 1997; Riis et al., 2008; Snyder & Hillyard, 1979), especially among older adults (Beck et al., 1980; Riis et al., 2008). Such age-related changes have been observed under the traditional visual novelty oddball paradigm (Beck et al., 1980) as well as under a condition in which subjects controlled how long they looked at each visual stimulus (Riis et al., 2008). It was unclear whether there would be a similar pattern of response under an ignore condition. The reports by Beck et al. (1980) and Riis et al. (2008) suggest that the anterior P2 may be sensitive not only to specific stimulus dimensions or designated targets that are defined by task instructions, but also to stimuli that have ‘intrinsic’ motivational salience by virtue of their perceptual novelty and unusualness.
The current study systematically investigated age-related changes in the anterior P2 component in response to different stimulus types (standards, rares/targets, novels) and attentional demands. Subjects from three age groups (young, middle-aged, and old adults), classified as cognitively high or average performers, participated in two conditions, an Attend and an Ignore condition, during which the same types of visual stimuli were presented.
Since task relevance increases the potential significance of experimental events, we predicted that if the anterior P2 is a marker for motivationally salient stimuli, it would be larger under the Attend than the Ignore condition, a result that has been observed for the P2 to target/rare stimuli in young subjects (Potts et al., 1996). We hypothesized that there would be minimal, if any, age-related differences for this effect. This prediction was in keeping with the large body of evidence suggesting that unlike divided attention, focused attention, especially the enhanced processing of task-relevant information, is relatively well-preserved in older individuals (Curran et al., 2001; Czigler, 1996; Folk & Hoyer, 1992; Gazzaley et al., 2005; Hartley et al., 1992; Kok, 2000; Madden, 1990).
In contrast to findings on tasks of focused attention, older adults have been shown to have impaired ability to inhibit directing resources to potential distracters (Comalli et al., 1962; Plude & Hoyer, 1986; Rabbitt, 1965). Physiological evidence also supports the notion that older individuals have greater difficulty suppressing the processing of task-irrelevant stimuli (Alain & Woods, 1999; Andres et al., 2006; Chao & Knight, 1997; Gazzaley et al., 2005). Based on these observations, one might anticipate an age-associated increase in the P2 response to non-target, task-irrelevant events (Amenedo & Diaz, 1998), which should not be designated as motivationally salient. Such a hypothesis could account for the expected age-related increase in the anterior P2 to perceptually novel stimuli. One way in which this hypothesis was tested was by dividing older subjects into groups based on their performance on neuropsychological tests or experimental tasks, some of which placed considerable demands on the attentional control system. Finding that lower performing older subjects generate a larger P2 to novel stimuli than higher performing older subjects would support the hypothesis that age-related increases in the P2 response to novel stimuli may reflect a reduced capacity to inhibit the processing of task-irrelevant, non-target events.
We also considered the possibility that age-related changes in the anterior P2 might be influenced by a temporally overlapping ERP component that changes with age. To this end, we carried out focused analyses of age-related changes in the anterior N2 component, which tends to peak within 100-150 ms of the P2. The anterior N2 has been interpreted as indexing stimulus unfamiliarity or difficulty encoding that may reflect a mismatch between stimulus input and stored representations (Daffner et al., 2000a; Ferrari et al., in press; Folstein & Van Petten, 2008; Nittono et al., 2007).
2. Methods
2.1. Subject Criteria
After completing informed consent, participants underwent a detailed screening evaluation that included a structured interview to obtain a medical, neurological, and psychiatric history, a formal neurological examination, the completion of a neuropsychological test battery and questionnaires surveying mood and socioeconomic status. To be included in the study, participants had to be English-speaking, have ≥12 years of education, a Mini Mental State Exam (MMSE) score (Folstein et al., 1975) ≥26, and an estimated IQ on the American Modification of the National Adult Reading Test (AMNART) (Ryan & Paolo, 1992) ≥100 and be in one of three age groups: 18-30 years old (young subjects), 45-55 years old (middle-aged subjects), or ≥65 years old (old subjects). Subjects were excluded if they had a history of CNS diseases or major psychiatric disorders based on DSM-IV criteria (American Psychiatric Association, 1994), a history of clinically significant medical diseases, corrected visual acuity worse than 20-40 (as tested using a Snellen wall chart), a history of clinically significant audiological disease, a Geriatric Depression Scale score (Yesavage et al., 1981) of ≥10 for the old subjects or a Beck Depression Inventory (Beck & Steer, 1987) score of ≥10 for middle-aged and young subjects, or focal abnormalities on neurological examination consistent with a CNS lesion.
Participants completed a neuropsychological test battery that included assessments of estimated IQ (AMNART (Ryan & Paolo, 1992)), global cognitive status (MMSE (Folstein et al., 1975)), frontal-executive functioning (Digit Span, WAIS-III (Wechsler, 1997a)), Controlled Oral Fluency (COWAT) (Ivnik et al., 1996)), semantic access (Category Fluency (animals) (Spreen & Strauss, 1998)), verbal and visual memory (Logical Memory II, WMS-III (Wechsler, 1997b)), Visual Retention Test (Youngjohn et al., 1993), and language (Boston Naming Test (Tombaugh & Hubley, 1997)). Neuropsychological test scores were standardized using age-matched norms. Cognitively high performers were defined as scoring in the top 3rd (≥ 67th percentile) on ≥4 of the 6 cognitive tests based on age-matched norms. Cognitively average performers were defined as scoring in the middle 3rd (33rd to 66th percentile) on ≥3 of the 6 cognitive tests based on age-matched norms. To be included in the study, a subject had to meet criteria for being either a cognitively high or cognitively average performer. Composite percentile scores were computed for each subject by averaging percentile scores from each of the 6 tests (overall neuropsychological test composite percentile score), and the 2 tests that assess frontal-executive function (Digit Span and COWAT composite percentile score). We did not include subjects who scored in the bottom 3rd on neuropsychological tests to help exclude old subjects who may be suffering from mild cognitive impairment or in the very early stages of a dementing illness.
2.2. Subject Characteristics
Ninety-two participants were included in the study: 15 cognitively high and 15 cognitively average performing old subjects (age range: 65-82 years old), 15 cognitively high and 15 cognitively average performing middle-aged subjects (age range: 45-55 years old), and 16 cognitively high and 16 cognitively average performing young subjects (age range: 18-28 years old). Data from an additional 2 old subjects and 2 middle-aged subjects were excluded because of excessive EEG artifact (resulting in <25 artifact-free trials of any stimulus category). The three age groups had similar MMSE scores, overall neuropsychological test composite percentile scores, and Digit Span and COWAT composite percentile scores. Additionally, the groups were relatively well-matched on gender, years of education, and estimated IQ. The middle-aged subjects had more years of education than the young subjects, as many of the young subjects were still enrolled in school. The middle-aged subjects also had higher estimated IQs based on AMNART scores than the young subjects. (See Table 1 for subject characteristics, including demographic, neuropsychological test performance, and estimated IQ for each age group, and pertinent statistical analyses.) As expected, cognitively high performing subjects had a higher percentile score than cognitively average performing subjects on a composite of all 6 neuropsychological tests (73.2 (9.7) vs. 49.2 (10.2), F(1,86) = 139.81, p < 0.0001) and on the two tests emphasizing attention and executive functioning (75.6 (17.6) vs. 50.2 (18.4), F(1,86) = 43.87, p < 0.0001). The difference between high and average performers in composite scores was not modified by age.
Table 1.
Subject Characteristics
|
Young Mean (SD) |
Middle-Aged Mean (SD) |
Old Mean (SD) |
Age Effect p-value |
|
|---|---|---|---|---|
| Number of Subjects | 32 | 30 | 30 | |
| Gender (male:female) | 16:16 | 13:17 | 14:16 | ns |
| Age | 21.6 (0.6) | 49.9 (0.6) | 71.6 (0.6) | <0.001 |
| Years of Education | 15.1 (0.6) | 18.3 (0.6) | 16.4 (0.6) | <0.001a |
| MMSE | 29.0 (0.2) | 28.9 (0.2) | 28.9 (0.6) | ns |
| AMNART Estimated IQ | 119.2 (1.2) | 124.1 (1.3) | 119.4 (1.3) | <0.01a |
| Overall Neuropsych. Test Comp. Percentile | 59.3 (2.7) | 64.2 (2.8) | 61.8 (2.8) | ns |
| Digit Span & COWAT Composite Percentile | 64.8 (4.0) | 63.3 (4.1) | 62.6 (4.1) | ns |
Key: Middle-age vs. Young: p< 0.001. MMSE = Mini Mental State Exam; AMNART = American Modification of the National Adult Reading Test; Overall Neuropsych. Test Comp. Percentile = mean percentile score relative to age-matched norms for Digit Span WAIS-III, Controlled Oral Fluency (FAS), Logical Memory II WMS-III, Visual Retention Test, Boston Naming Test, and Category Fluency (animals); Digit Span & COWAT Composite Percentile = composite percentile score for Digit Span WAIS-III and Controlled Oral Fluency (FAS).
2.3. Experimental methods
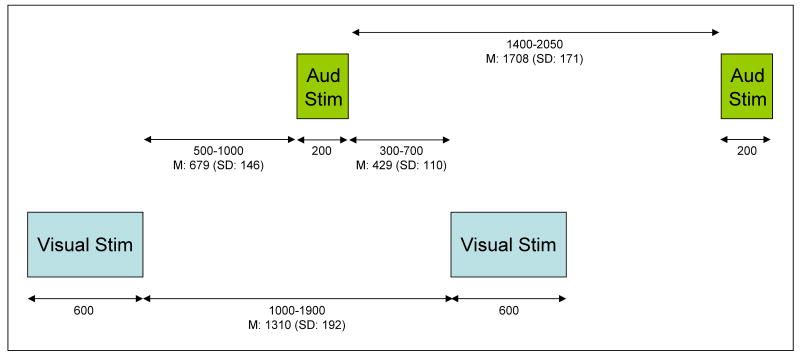
Each subject participated in two experimental conditions, the Attend condition and the Ignore condition, whose order was counterbalanced. Under both conditions, 250 line drawings were presented in 5 blocks of 50. Each drawing was shown one at a time at the center of a high-resolution computer monitor. All stimuli subtended a visual angle of approximately 2.75° along their longest dimension. There were three categories of visual stimuli: 1) a repetitive standard stimulus--70% frequency, 2) an infrequent target/rare stimulus--approximately 15% frequency, and 3) novel stimuli, randomly drawn from a set of unusual/unfamiliar line drawings (e.g., impossible or fragmented objects) shown only one time each--approximately 15% frequency, many of which came from the collection of drawings that have been used by Kroll and Potter (1984) and Kosslyn et al. (1994) (Figure 1). All visual stimuli appeared within a fixation box subtending a visual angle of approximately 3.5° × 3.5° that remained on the screen at all times. Three different sets of novel stimuli were used, counterbalanced across subjects and conditions. Under the Attend condition the standard stimulus was a square and the target a diamond, while under the Ignore condition, the standard stimulus was a rectangle with the long side vertically oriented and the rare stimulus was a rectangle with the long side horizontally oriented. Under both conditions, each visual stimulus was presented for 600 ms and was followed by a period in which only the fixation box remained on the screen. (Figure 2 presents the timing of stimulus presentations under each condition.) Under the Attend condition, subjects focused their attention on the visual stimuli and performed a visual novelty oddball task by responding to rare stimuli, designated as targets, with a foot pedal press. Left/right foot pedal press was counterbalanced across subjects.
Figure 1.
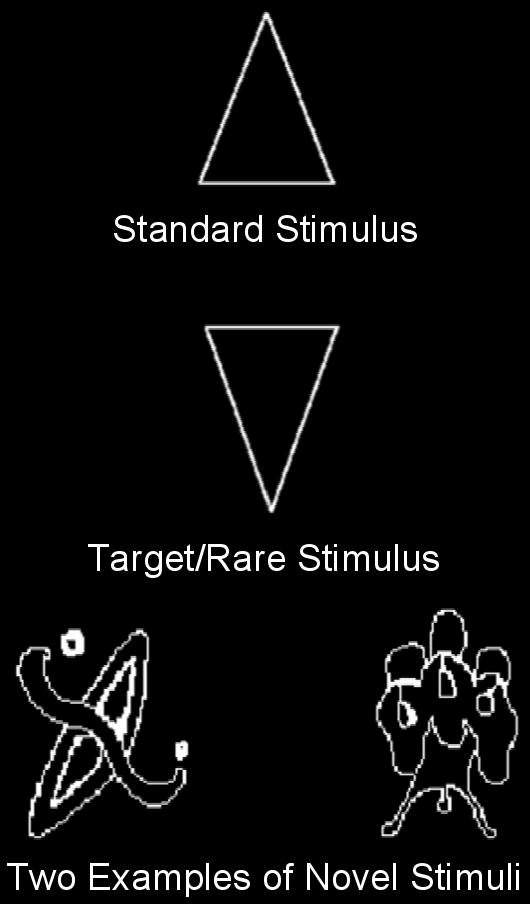
Examples of the repetitive standard stimulus (70% frequency), the target/rare stimulus (∼15% frequency), and of novel stimuli (∼15% frequency).
Figure 2.
Illustration of the timing of stimulus presentations under Attend and Ignore conditions. All durations are in milliseconds. M = mean. SD = standard deviation.
Under the visual Ignore condition, subjects were asked to perform a difficult experimental task in the auditory modality. A series of letters were binaurally presented (digitized voice) for 200 ms by computer via headphones during the period when only the fixation box appeared on the screen (see Figure 2). To reduce the influence of the ERPs elicited by auditory stimuli on the ERPs elicited by visual stimuli (Woldorff, 1993), the time between the onset of the auditory stimuli and the onset of the visual stimuli was varied between 500 and 900 ms (skewed toward shorter durations). Despite using this method, the influence of overlapping electrophysiologic activity remains a potential confound when comparing the ERPs to visual stimuli under Attend vs. Ignore conditions. Old and middle-aged participants performed an auditory 2-back letter task by responding with a foot pedal press to any letter that had been presented 2 letters before. In an effort to match overall n-back performance in the Ignore condition, the young subjects performed a 3-back task during blocks 1 and 2, and a 2-back task during blocks 3-5. N-back target letters were presented at a frequency of approximately 12% and occurred randomly with respect to the presentation of rare or novel visual stimuli. While performing the auditory n-back task, subjects were instructed that, in order to minimize artifacts from eye activity, they should keep their eyes focused on the center of the fixation box on the computer monitor in which the visual stimuli were presented. Thus, under the Ignore condition all subjects passively viewed the same types of visual stimuli as those responded to in the Attend condition. However, in the Ignore condition, these visual stimuli were irrelevant to the assigned task. Under both conditions, subjects were asked to respond quickly while trying to be as accurate as possible.
Although the types of visual stimuli presented were physically the same under both conditions, their roles differed as a function of the task assigned to subjects. Under the Attend condition, the infrequent target/rare stimuli required a response (foot pedal press) and are therefore referred to as target stimuli. Under the Ignore condition, the same kinds of stimuli used as targets under the Attend condition were task-irrelevant, as the paradigm required attending to the auditory modality. They are referred to as rare, and not target, stimuli because subjects were not instructed to generate a behavioral response to the visual events.
Subjects also participated in a third condition, a subject-controlled novelty oddball task (Riis et al., 2008), the results of which will not be discussed here.
2.4. ERP recording
An electrode cap (Electro-Cap International, Eaton, OH, USA) was used to hold 35 tin electrodes to the scalp, whose locations were based on the International 10-20 system. Electrodes were arranged in 5 columns (midline, 2 inner lateral, 2 outer lateral), each with 7 antero-posterior sites. All sites were referenced to the left mastoid, and the impedance between each recording site and reference was reduced to less than 5K ohms. An electrode was placed beneath the left eye (referenced to an electrode placed above the left eye) to monitor for blinks, to the right of the subjects' right eye (referenced to an electrode placed to the left of the left eye) to monitor for lateral eye movement, and over the right mastoid (referenced to the left one) to monitor for asymmetrical mastoid activity. (None was identified.) The EEG was amplified by an SA Instrumentation (San Diego, CA, USA) system, using a band filter with negative 3dB cutoffs of 0.01 and 40 Hz, and continuously digitized (200 Hz) by a computer yielding 1280 ms of data from each electrode site, beginning 100 ms before stimulus onset.
2.5. Data analysis
Behavioral data were collected under both conditions. In the Attend condition, target hit rate, false alarm rate, and median reaction time were calculated 200-1800 ms post-stimulus presentation. In the Ignore condition, n-back target hit rate, false alarm rate, and median reaction time were calculated 200-1800 ms post-stimulus presentation.
A continuous record of the raw EEG was stored on hard disk. Off-line, EEG epochs for each stimulus type (visual novel, target/rare, and standard events) were averaged separately. This report will focus on the response to visual stimuli under both conditions, and only data collected from the midline electrode sites will be reviewed. Trials with eye movements or amplifier blocking were excluded from data analysis. For all subjects, a blink correction program using principal component analysis was employed that computed the impact of the blink on the waveform in each channel (Dale, 1994).
Individual and grand mean ERPs were examined to determine the most appropriate P2 interval. The mean local positive peak latency between 100-250 ms after stimulus onset was calculated for each subject group in response to each visual stimulus type. The P2 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 80 ms interval centered at his or her group's mean midline local positive peak latency. To help assess the general effects of focused attention, the P2 elicited by repetitive standard stimuli was measured under the Attend and Ignore conditions. To isolate processing specific to the evaluation of novel and target/rare stimuli, novelty and target/rare difference waves (ERPs to novel stimuli – standard stimuli, and ERPs to target/rare stimuli – standard stimuli) were computed and the P2 component was measured1. The N2 component of a subject in response to each visual stimulus type was defined as the mean amplitude of the 80 ms interval centered at the mean midline local negative peak latency between 200-350 ms after stimulus onset that was calculated for his/her subject group. All components were measured with respect to the average of the 100 ms pre-stimulus baseline.
An analysis of variance (ANOVA), with age group (old, middle-aged, and young subjects) and cognitive status (high and average) as the between-subjects variables, and condition (Attend condition and Ignore condition), stimulus type (e.g., novelty difference wave and target/rare difference wave) and electrode site (FPz, Fz, FCz, Cz, CPz, Pz, Oz) as the within-subjects variables was performed. Analyses of scalp distribution focused on determining whether there were antero-posterior differences across subject groups. In examining scalp site interactions with other variables, both raw (Urbach & Kutas, 2002) and normalized (McCarthy & Wood, 1985) data were assessed. Statistics for the raw data are reported in the paper. Of note, all significant interactions between scalp site and other variables, such as age group, that are reported in the paper using raw data were confirmed using normalized data. Analyses that yielded significant interactions between subject group, condition, stimulus type, or electrode site resulted in planned contrasts between the levels of the variable. The Geisser-Greenhouse correction was applied for all repeated measures with greater than 1 degree of freedom.
3. Results
3.1. Behavior
3.1.1. Attend Condition
Old, middle-aged, and young subjects did not differ in performance under the Attend condition. No age-related differences were found between the groups for mean target hit rate (old: 0.96 (0.08), middle-aged: 0.96 (0.09), young: 0.99 (0.02) (mean (SD)), mean false alarm rate (old: 0.001 (0.003), middle-aged: 0.001 (0.003), young: 0.001 (0.004) (mean (SD)), or median reaction time (old: 708 ms (124), middle-aged: 752 ms (145), young: 719 ms (119) (mean (SD)). The cognitively high performing subjects tended to have a faster median reaction time than the cognitively average performing subjects (F(1,86) = 2.87, p = 0.09) (cognitively high: 705 ms (134), cognitively average: 750 ms (122) (mean (SD)). The magnitude of this effect was not modulated by age. There were no differences between the high and average performers in mean hit rate or mean false alarm rate, and there was no interaction between age and cognitive status for either measure.
3.1.2. Ignore Condition
Under the visual-Ignore condition, subjects focused on the auditory n-back task. There was a main effect for age (all age groups: F(2,89) = 5.07, p< 0.01; old vs. middle-aged: F(1,58) = 5.87, p< 0.02, old vs. young: F(1,60) = 11.18, p< 0.01; mid-aged vs. young: ns). The old subjects had a lower hit rate for n-back target letters than the middle-aged and young subjects, with no differences between the latter two age groups (old: 0.60 (0.20), middle-aged: 0.72 (0.19), young: 0.75 (0.15) (mean (SD)). No age-related differences were found in mean false alarm rate (old: 0.04 (0.05), middle-aged: 0.04 (0.03), young: 0.05 (0.03) (mean (SD)), or median reaction time (old: 898 ms (156), middle-aged: 935 ms (172), young: 917 ms (129) (mean (SD)). Cognitively high performing subjects were more accurate than the cognitively average performing subjects (F(1,86) = 4.25, p< 0.05) (mean hit rate: cognitively high: 0.72 (0.17), cognitively average: 0.66 (0.21) (mean (SD)). The magnitude of this effect was not modified by age. There were no differences between the high and average performers in mean false alarm rate or median reaction time, and no age by cognitive status interaction for either measure.
3.2. ERP Results
Cognitive status did not have a significant impact on the amplitude or distribution of the P2 elicited by any stimulus type under either condition, and there were no significant interactions between cognitive status and age, condition, or stimulus type. Therefore, we collapsed across cognitive groups for each age group in our data analysis.
Midline ERPs in response to novel, target/rare, and standard stimuli for the 3 age groups under Attend and Ignore conditions are illustrated in Figure 3. The effect of stimulus type (novelty P2 difference wave vs. target/rare P2 difference wave) was examined under the Attend and Ignore conditions separately. Figures 4 and 5 illustrate midline ERPs of the novelty and target difference waves respectively. Table 2 includes the mean (SEM) values of the amplitudes of the P2 difference waves across midline sites. The impact of direction of attention was also evaluated by comparing ERPs under the Attend vs. Ignore condition. Our analyses do not include a separate section on age-related changes, as they are incorporated into other Results sections. ERPs in response to auditory n-back events are not presented here because they are not the focus of this report and are not analyzed nor discussed further. However, they are available upon request to the senior author.
Figure 3.
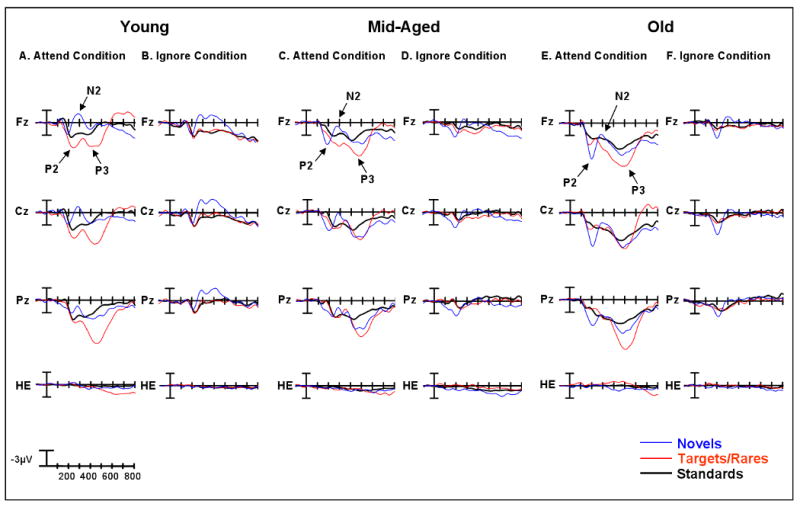
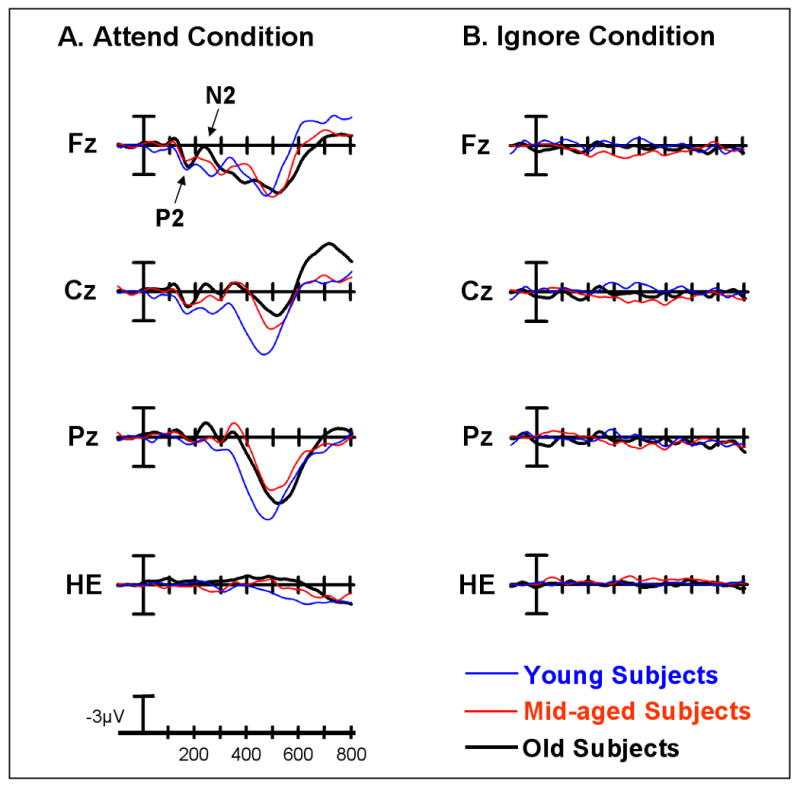
Midline grand average ERPs in response to standard, target/rare, and novel visual stimuli for old, middle-aged, and young subjects under the Attend Condition and the Ignore conditions. Arrows illustrate P2, N2, and P3 waves at Fz. HE represents the eye channel in Figures 3-5. Note that there is no evidence of P2-like electrical activity in the eye channel.
Figure 4.
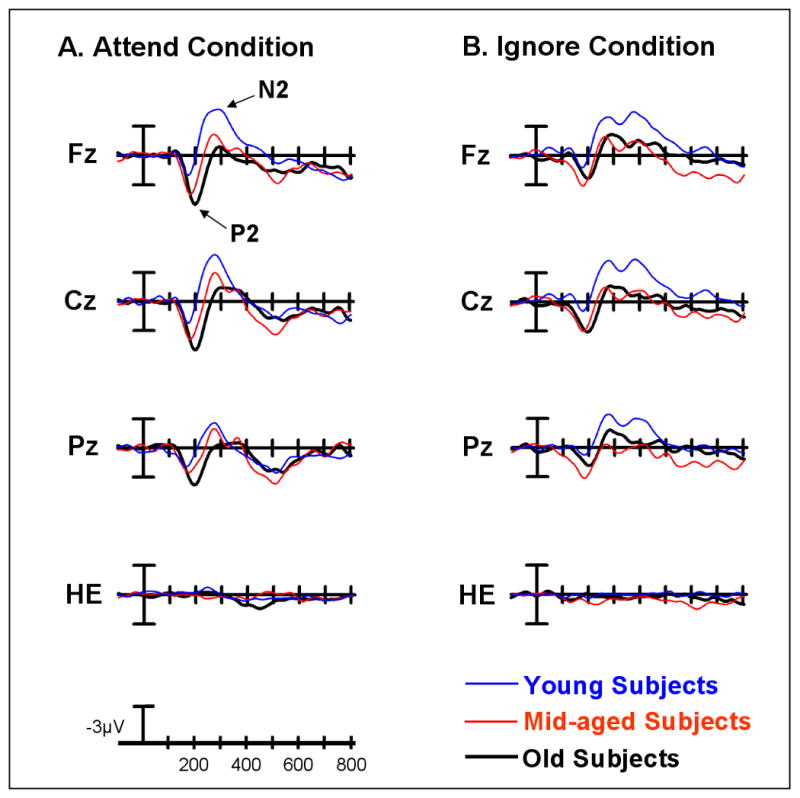
Midline grand average ERP plots of the novelty difference wave (novels-standards) for the young, middle-aged and old groups under the A) Attend Condition and the B) Ignore Condition. In (A) arrows mark the novelty P2 and N2 difference waves at Fz under the Attend condition.
Figure 5.
Midline grand average ERP plots of the target/rare difference wave (target/rare-standards) for the young, middle-aged and old subject groups under the A) Attend Condition and the B) Ignore Condition. In (A) arrows mark the target P2 and N2 difference waves at Fz under the Attend condition.
Table 2.
P2 and N2 Amplitudes at Midline Electrode Sites
| P2 Amplitude in μV (mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Novelty Diff Wave | Target Diff Wave | Standards | ||||
| Attend | Ignore | Attend | Ignore | Attend | Ignore | |
| Old | ||||||
| Fz | 3.44 (0.44) | 1.18 (0.48) | 1.24 (0.40) | -0.09 (0.48) | 3.20 (0.36) | 1.12 (0.39) |
| Cz | 3.60 (0.48) | 2.09 (0.44) | 0.78 (0.43) | -0.01 (0.41) | 2.80 (0.40) | 1.65 (0.35) |
| Pz | 2.79 (0.47) | 1.03 (0.54) | -0.07 (0.44) | -0.04 (0.40) | 2.25 (0.41) | 1.43 (0.34) |
| Mid | ||||||
| Fz | 2.7 (0.44) | 2.24 (0.48) | 1.43 (0.40) | 0.68 (0.48) | 2.44 (0.36) | 1.13 (0.39) |
| Cz | 2.79 (0.48) | 2.27 (0.44) | 1.26 (0.43) | 0.21 (0.41) | 2.10 (0.40) | 1.19 (0.35) |
| Pz | 1.85 (0.47) | 2.52 (0.54) | 0.42 (0.44) | -0.16 (0.40) | 2.19 (0.41) | 0.88 (0.34) |
| Young | ||||||
| Fz | 0.80 (0.42) | 0.50 (0.46) | 1.19 (0.39) | 0.20 (0.46) | 2.63 (0.35) | 2.20 (0.38) |
| Cz | 0.79 (0.46) | 0.29 (0.42) | 1.12 (0.41) | -0.06 (0.40) | 3.09 (0.38) | 1.99 (0.34) |
| Pz | 1.00 (0.46) | 0.43 (0.52) | 0.06 (0.42) | 0.37 (0.39) | 3.16 (0.39) | 1.49 (0.33) |
| N2 Amplitude in μV (mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Novelty Diff Wave | Target Diff Wave | Standards | ||||
| Attend | Ignore | Attend | Ignore | Attend | Ignore | |
| Old | ||||||
| Fz | -0.64 (0.57) | -2.32 (0.53) | 0.79 (0.47) | -0.72 (0.60) | 3.67 (0.39) | 0.77 (0.48) |
| Cz | -1.21 (0.62) | -1.67 (0.45) | -0.20 (0.50) | -0.78 (0.43) | 3.75 (0.45) | 0.91 (0.41) |
| Pz | -0.36 (0.59) | -1.79 (0.49) | -0.83 (0.48) | -0.71 (0.43) | 3.77 (0.43) | 1.31 (0.36) |
| Mid | ||||||
| Fz | -1.45 (0.57) | -1.24 (0.53) | 1.77 (0.47) | 0.67 (0.60) | 2.83 (0.39) | 1.22 (0.48) |
| Cz | -1.86 (0.62) | -0.86 (0.45) | 0.97 (0.50) | 0.37 (0.43) | 2.83 (0.45) | 0.82 (0.41) |
| Pz | -1.17 (0.59) | 0.17 (0.49) | 0.55 (0.48) | 0.32 (0.43) | 3.20 (0.43) | 0.62 (0.36) |
| Young | ||||||
| Fz | -3.39 (0.55) | -3.40 (0.51) | 1.95 (0.45) | -0.37 (0.58) | 2.76 (0.38) | 2.14 (0.46) |
| Cz | -3.22 (0.60) | -3.56 (0.44) | 1.60 (0.48) | -0.75 (0.42) | 3.35 (0.43) | 1.51 (0.40) |
| Pz | -1.60 (0.57) | -2.82 (0.48) | 0.23 (0.47) | -0.38 (0.41) | 3.96 (0.42) | 1.31 (0.35) |
3.2.1. Effects of Stimulus Type (Novel vs. Target/Rare)
Attend Condition
Under the Attend Condition, there was a main effect of stimulus type, with the novelty P2 difference wave being larger than the target P2 difference wave (F(1,86) = 23.42, p< 0.0001) (Figure 4A vs. 5A; Table 2). However, a stimulus type by age interaction (F(2,86) = 6.75, p< 0.002) revealed that only the old and middle-aged subjects generated a larger response to novels than to targets (effect of Stimulus Type: old subjects: F(1,28) = 26.88, p< 0.0001, mid-aged subjects: F(1,28) = 11.66, p< 0.005; young subjects: ns). No stimulus-related changes in the amplitude of the P2 difference wave were found for the young subjects. Additionally, there was a stimulus type by electrode site by age interaction (F(12,516) = 2.46, p< 0.02) which reflected age-related differences in the size and distribution of the novelty, but not the target, P2 difference wave. In response to novel stimuli, there was a main effect of age (all age groups: F(2,86) = 8.63, p< 0.0005; old vs. young: F(1,58) = 17.06, p< 0.0002; mid-aged vs. young: F(1,58) = 7.60, p< 0.01; old vs mid-aged: ns). Old and middle-aged subjects generated a larger P2 novelty difference wave than young subjects. The largest differences were found at frontocentral sites (Age x Electrode Site interaction, all age groups: F(12,516) = 3.52, p< 0.005; old vs. young: F(6,348) = 3.74, p< 0.02, mid-aged vs. young: F(6,348) = 6.21, p< 0.002; old vs. mid-age: ns). No differences were found in the amplitude or scalp distribution of the novelty P2 difference wave for the old and middle-aged groups. For the target P2 difference wave, there were no age-related differences in the amplitude or scalp distribution, which was largest at frontocentral sites for all age groups (effect of Electrode Site: F(6,516) = 58.62, p< 0.0001).
Ignore Condition
Under the Ignore condition, the difference waves in response to rare stimuli were relatively flat across all age groups (Figure 5B) (no main effect of Age, no main effect of Electrode Site, and no Age x Electrode Site interaction). This suggests that under the Ignore condition, regardless of age, the electrophysiological response to perceptually simple rare stimuli was very similar to the response to frequent, repetitive standard stimuli. Thus, the P2 difference wave in response to novel stimuli was larger than that in response to rare stimuli for all age groups (F(1,86) = 15.61, p< 0.0005) (Figure 4B vs. 5B). Similar to the findings under the Attend condition, the effect of stimulus type tended to be more robust in the old and middle-aged than the young subjects, who exhibited no effect of stimulus type (Stimulus Type x Age interaction, F(2,86) = 3.01, p = 0.09; effect of Stimulus Type, old: F(1,28) = 6.38, p< 0.02, mid-aged: F(1,28) = 13.90, p< 0.001; young: ns).
In response to novel stimuli, there was a main effect of age that was driven by the overall novelty P2 difference wave being smaller for young than middle-aged subjects (all age groups: F(2,86) = 4.05, p< 0.05; young vs. mid-aged: F(1,58) = 8.20, p< 0.01; young vs. old: ns; old vs. mid-aged subjects: ns). Of particular relevance to the anterior P2, the focus of this report, was the interaction between age and electrode site (all age groups: F(12,516) = 2.91, p< 0.01; old vs. young: F(6,348) = 4.80, p< 0.005, mid-aged vs. young: F(6,348) = 8.20, p< 0.01; old vs. mid-aged: ns). Both old and middle-aged subjects generated a larger novelty P2 difference wave than young subjects at frontocentral sites.
3.2.2. Effects of Direction of Attention (Attend vs. Ignore condition)
Novelty Difference Wave
The size of the novelty P2 difference wave was larger under the Attend condition than the Ignore condition (effect of Condition, F(1,86) = 6.51, p< 0.05) (Figure 4A vs. 4B). Although all groups exhibited the same direction of this effect, it was only significant for the old subjects (effect of Condition: old subjects, F(1,28) = 13.84, p< 0.001, no effect of Condition for the middle-aged or young subjects). No age-related differences in the scalp distribution of the novelty P2 difference wave were found between the Attend and the Ignore condition.
Target/Rare Difference Wave
The anterior P2 to targets under the Attend condition was larger than that to rares under the Ignore condition for all age groups (main effect of Condition: F(1,86) = 4.13, p< 0.05; no Condition x Age interaction) (Figure 5A vs. 5B).
P2 Response to Standard Stimuli
The P2 in response to standard stimuli was larger under the Attend condition than the Ignore condition (effect of Condition, F(1,86) = 40.95, p< 0.0001) (Figure 2; Table 2). Of particular importance, the magnitude of the difference between the P2 to standards under the Attend condition vs. the Ignore condition was similar for all age groups (no Condition x Age interaction). Young subjects generated a numerically larger overall P2 response to standard stimuli than the other groups; however this effect did not reach significance (effect of Age, F(2,86) = 2.19, p < 0.12), and was not modulated by condition (no Age x Condition interaction). There was a three-way interaction between age, condition, and electrode site (F(12,516) = 7.98, p< 0.0001), which will not be discussed further due to its lack of relevance to the prime issues addressed in this paper.
3.3. P2 amplitude vs. performance on neuropsychological tests and n-back task
As noted in the Introduction, one explanation for the age-related increase in P2 to perceptually novel stimuli is that it reflects impairment in the ability of older adults to inhibit the processing of task-irrelevant stimuli. This hypothesis was tested by examining the size of the P2 response generated by old subjects who were grouped according to their performance on the neuropsychological tests and the n-back task. We found that cognitively high performers did not differ from cognitively average performers in terms of the size of the anterior P2 in response to any stimulus type under either condition. We also carried out a median split of the old subjects based on their performance on tests of frontal-executive function. Again, we found no group difference in the anterior P2 amplitude to novel or target stimuli.
Another way in which we tested this hypothesis was to examine the relationship between performance on the auditory n-back task and the size of the P2 to task irrelevant novel visual stimuli under the visual-Ignore condition. A median split based on n-back accuracy rate (percent hits minus percent false alarms) was performed on the old subject group alone and the old and middle-aged group combined.2 In neither case was there a difference in the size of the P2 between high and low performers (F's < 1.0) and there was no interaction between group and electrode site.3
3.4. Relationship between the anterior P2 and the anterior N2
Figure 4 indicates that in contrast to old subjects, young subjects generated a large anteriorly-distributed N2 response to novel stimuli under both Attend and Ignore conditions, which was larger than the anterior N2 response to target/rare stimuli (Figure 5; Table 2). Although the N2 component was not the main subject of this report, we carried out focused analyses to examine whether there might be a link between age-related changes in the anterior P2 and anterior N2 components. Table 3 compares the key findings and statistical results of the difference wave analyses for the P2 and N2 components across conditions and stimulus types. Our goal was to examine the overall pattern of response of these two components. The most salient points are briefly described below.
Table 3.
Comparison of P2 and N2 Difference Waves
| Condition | P2 Description | N2 Description | P2 Statistics | N2 Statistics |
|---|---|---|---|---|
| Attend | ||||
| Novels vs. Targets | ||||
| Stimulus Type | N > T | N > T | <0.0001 | <0.0001 |
| Stim × Age | N > T only for Old and Mid. For Young, N = T | Magnitude of N > T largest for Young. For Old, N = T | <0.005 | <0.005 |
| Novels Only | ||||
| Age | Old = Mid > Young | Young > Mid = Old | <0.0005 | <0.05 |
| Age × ES | Old and Mid more anteriorly-distributed than Young | Young more anteriorly-distributed than Mid and Old | <0.005 | <0.005 |
| Targets Only | ||||
| Age | No difference | No difference | ns | ns |
| Age × ES | No difference | Young more posteriorly-distributed than Mid and Old | ns | <0.05 |
| Ignore | ||||
| Novels vs. Rares | ||||
| Stimulus Type | N > R | N > R | <0.0005 | <0.0001 |
| Stim × Age | Magnitude of N > R larger for Old and Mid than Young | Magnitude of N > R larger for Young than Mid and Old | <0.06 | <0.06 |
| Novels Only | ||||
| Age | Young < Mid = Old | Young > Mid = Old | <0.05 | <0.001 |
| Age × ES | Largest difference between Young vs. Mid and Old at F-C sites | Largest differences between Young vs. Mid and Old at F-C sites | <0.05 | <0.05 |
| Rares Only | ||||
| Age | ∼Flat difference waves | ∼Flat difference waves | ns | ns |
| Age × ES | ∼Flat difference waves | ∼Flat difference waves | ns | ns |
| Attend vs. Ignore | ||||
| Novels Only | ||||
| Condition | Attend > Ignore | No difference | <0.05 | ns |
| Condition × ES | Attend > Ignore, largest at F-C sites | Ignore > Attend at frontal sites | <0.0001 | <0.1 |
| Condition × Age | Attend > Ignore for Old only | Ignore > Attend for Old only | <.01 | <.1 |
| Condition × ES × Age | No difference | No difference | ns | ns |
| Targets/Rares Only | ||||
| Condition | Attend > Ignore | Ignore > Attend | <0.06 | <0.005 |
| Condition × ES | Attend > Ignore, largest at F-C sites | Ignore > Attend esp. anteriorly; Attend > Ignore at Oz | <0.0001 | <0.0001 |
| Condition × ES × Age | No difference | No difference | ns | ns |
| Condition × Age | No difference | No difference | ns | ns |
Key: Stim = Stimulus, N = Novels, T = Targets, R = Rares, Old = Old subjects, Mid = Middle-aged subjects, Young = Young subjects, ES = Electrode site, F-C = Frontocentral, ∼ = Relatively
3.4.1. Attend condition
The size of both the P2 and N2 was larger in response to novel than target stimuli (see Table 3 for statistical results). Whereas for the P2, the size of response of young subjects to novel and target stimuli did not differ, for the N2, the size of response of old subjects to novel and target stimuli did not differ. Considering novel stimuli alone, the P2 response of old and middle-aged subjects was larger than that of young subjects. In contrast, the N2 response of young subjects was larger than that of middle-aged and old subjects (see Figure 6). For both the P2 and N2, the largest differences between age groups were observed at anterior sites. Considering target stimuli alone, there were no age-related changes in either the P2 or N2 response. The scalp distribution of the P2 and N2 in response to target stimuli differed. The P2 to targets had a frontocentral distribution, while the N2 to targets had a posterior distribution. In contrast to the P2 response, where there was no interaction between age and electrode site, for the N2, young subjects generated a more posteriorly-distributed response.
Figure 6.
Bar graph illustrating the P2 and N2 responses (at FCz) to novel stimuli under the Attend condition.
3.4.2. Ignore Condition
The size of both the P2 and N2 was larger in response to novel than target stimuli. For the P2, the magnitude of the difference between response to novel and target stimuli tended to be larger for old and middle-aged subjects than young subjects, whereas for the N2, the magnitude of the difference tended to be larger for young subjects than for middle-aged and old subjects. Regarding novel stimuli alone, young subjects generated a smaller P2 response than middle-aged and old subjects, whereas young subjects generated a larger N2 response than middle-aged and old subjects. For both the P2 and N2, the largest difference between young subjects and the other two groups was observed at anterior electrode sites. Regarding rare stimuli alone, there was no effect of age, electrode site, nor age by electrode site interaction for either the P2 or N2.
3.4.3. Correlation Analysis
Across the entire group of subjects (n = 92), a strong inverse correlation was found between the size of the P2 response to novel stimuli and the size of the N2 response. This relationship was observed under both the Ignore and Attend conditions (as the size of the P2 increased, the size of the N2 decreased (became more positive)), which was most robust at frontocentral sites. For example, at FCz, under the Ignore condition, r = 0.64, p < 0.000001; under the Attend condition, r = 0.69, p < 0.000001. In general, as the age of subjects increased, the size of their P2 response to novel stimuli increased (age vs. P2 amplitude at FCz, Ignore condition: r = 0.23, p < 0.05; Attend condition: r = 0.39, p < 0.0005); whereas the size of their N2 decreased (age vs. N2 amplitude at FCz, Ignore condition: r = 0.30, p < 0.005; Attend condition: r = 0.34, p < 0.001).
4. Discussion
This study examined age-related changes in the anterior P2 response to different kinds of visual stimuli under attend and ignore conditions. Although the P2 was elicited ‘automatically’ under the Ignore condition, it was modulated by direction of attention in all subject groups. A larger P2 to standard stimuli was generated when attention was directed toward the visual oddball task than when visual stimuli were to be ignored. As was predicted, the magnitude of this effect did not differ across age groups. Consistent with other reports (Kok, 2000), this result provides additional evidence for the relatively preserved capacity of older adults to enhance the processing of task-relevant stimuli.
Under the Attend condition, all age groups generated a larger P2 response to novel and target stimuli than standard stimuli. It seems very unlikely that this difference is due to saccadic eye movements, or a frontal cortical potential signaling the initiation of a saccade (Brooks-Eidelberg & Adler, 1992), in response to the abrupt onset of a novel or target event. All stimuli were presented at fixation located at the center of the screen, and not eccentrically. No saccadic eye movements were necessary to process the events. Moreover, an examination of the eye channel revealed no evidence of P2-like activity (Figures 3-5). The enhanced P2 activity to novel and target events may be due to this component's sensitivity to the temporal probability of stimulus presentation, as novel and target stimuli were shown much less frequently than standard stimuli. However, it is unlikely that infrequency alone can account for the increased size of the P2 response, because under the Ignore condition the P2 elicited by infrequent rare stimuli was no different from the P2 elicited by frequent standard stimuli. Also, under the same condition, perceptually novel stimuli that were presented as infrequently as simple rare stimuli evoked a much larger P2 effect. This provides evidence that the P2 is sensitive to perceptual novelty regardless of a subject's focus of attention. However, perceptual novelty alone cannot completely explain enhanced P2 responses. Under the Attend condition, target stimuli had similar simple perceptual features as standard stimuli, but elicited a much larger P2 response than standard stimuli. This difference is unlikely to simply be the result of the motor response to targets, as Potts (2004) has shown that the anterior P2 to targets has the same scalp topography and estimated dipole location in overt (button press) and covert (silent counting) conditions.
One way to account for our findings is to suggest that motivational salience plays a critical role in determining the size of the anterior P2 response. Potts and colleagues (1996) emphasized that the motivational salience indexed by the anterior P2 is determined by task relevance. Our data suggest that the motivationally salient stimuli can be determined not only by task relevance, as defined by study conditions, but also by what has be labeled as the ‘intrinsic’ relevance assigned to novel events (Berlyne, 1960; Hunt, 1965). Novel events can be characterized as intrinsically motivating because of their potential importance to the adaptive capacity and survival of an organism (Daffner et al., 1994; Daffner et al., 2003; Hunt, 1965).
Given its anterior scalp distribution, positive deflection and sensitivity to stimulus salience and novelty, one might argue that the component we have labeled as the anterior “P2” is really a “P3a”. There are several reasons we do not believe that this is the case. Examining the ERPs in response to novel stimuli, one sees that the component labeled the anterior P2 clearly precedes the N2 component (see Figure 4). This temporal sequence would not be consistent with this positive deflection representing a P3a, which has so reliably followed (and not preceded) an anterior N2 response that some researchers have labeled the two components as a “N2-P3a” ERP complex (Baudena et al., 1995; Daffner et al., 1998; Folstein & Van Petten, 2008). Also, young subjects have been shown to generate a larger P3a response to novel than target stimuli (Friedman et al., 2001; Spencer et al., 1999), which was not observed for the component under discussion here. In addition, most reports have suggested an age-related decline (Fabiani & Friedman, 1995; Fjell & Walhovd, 2004) or no change in the size of the P3a (Beck et al., 1980; Snyder & Hillyard, 1979) to deviant visual stimuli, which would be inconsistent with the findings of the anterior P2. The nervous system has developed a variety of mechanisms to deal with novelty, which seem to be indexed by different ERP components. Whereas the P2 may be a marker for the motivational salience of novel stimuli, the P3a has been variably conceptualized as indexing processes involved in reorienting attention (Escera et al., 1998; Naatanen, 1990), shifting mental set (Barcelo et al., 2002; Barcelo et al., 2006), signaling an event's potential attention-worthiness (Daffner et al., 1998; Daffner et al., 2000b), or inhibiting inappropriate responses to non-target, novel events (Goldstein et al., 2002). The relatively early peak of the P2 component suggests the brain's capacity to make a rapid preliminary assessment of the potential motivational significance of visual stimuli. We suspect that the allocation of additional resources and the further processing of such stimuli are determined subsequently, and are likely indexed by the P3 component and slow waves that follow (Chong et al., 2008).
Under the Attend condition, neither the amplitude nor the distribution of the target P2 difference wave was modulated by age. This finding provides further support for the notion that older adults have a relatively well-preserved capacity to focus attention on task relevant stimuli (Kok, 2000). In contrast to the absence of age-related differences in target-specific P2 activity, old and middle-aged subjects exhibited greater novelty-specific P2 activity than the young subjects. The finding of an enhanced P2 activity to novel stimuli among older subjects is consistent with previous studies reporting age-related increases in the anteriorly-distributed P2 to novelty (Beck et al., 1980; Riis et al., 2008; Snyder & Hillyard, 1976). Additionally, only for the old subjects was novelty-specific P2 processing further modulated by direction of attention, with the novelty P2 difference wave under the Attend condition being larger than under the Ignore condition. Taken together, these results suggest that for middle-aged and, especially, old subjects the P2 is particularly sensitive to novel stimuli.
An appealing explanation for the age-associated increase in P2 amplitude is that it represents the inability of older subjects to inhibit the processing of task-irrelevant stimuli that should not be designated as motivationally salient (Amenedo & Diaz, 1998; Andres et al., 2006; Chao & Knight, 1997; Gazzaley et al., 2005; Rabbitt, 1965). If enhancement of the P2 novelty difference wave simply reflected a deficiency of inhibition, one might expect to see age-associated increases in the size of the P2 elicited by standard and rare4 stimuli under the Ignore condition and by standard stimuli under the Attend condition, but such differences were not found. However, it is plausible that older individuals retain sufficient capacity to filter out non-salient stimuli (e.g., repetitive standards), but are incapable of inhibiting the processing of more salient novel events, which is much more challenging. We directly tested the hypothesis linking the size of the novelty P2 response to the capacity to filter out task-irrelevant stimuli by dividing older subjects into groups based on their performance on neuropsychological tests of frontal-executive function or on the difficult n-back experimental task. According to this hypothesis, older individuals who perform worse on tasks that demand attentional control should generate a larger P2 to novel stimuli. However, our results did not support this hypothesis, as no relationship was found between the size of the P2 response to novel stimuli and various measures of attentional control.5
The anteriorly-distributed P2 and N2 components may reflect temporally overlapping processes that peak within 100-150 ms of each other. Given the divergent polarity of these components, age-associated changes in the amplitude of one of the components (e.g., increase in size) would be associated with effects in the opposite direction of the other component (e.g., decrease in size). In fact, one of the striking observations of this study is the strong inverse relationship between the amplitude of the anterior P2 and the amplitude of the anterior N2 in response to novel stimuli: the larger the P2, the smaller the N2 response, and vice versa. As subjects increased in age, there was a reliable decrease in the anterior N2 and increase in the anterior P2 in response to novel stimuli. Interestingly, this may reflect the continuation of age-related changes in electrophysiological responses to novelty that occur across the lifespan, with a reduction over time of anterior negativity and an augmentation of anterior positivity. For example, children respond to visual novelty by generating a large frontal negativity (labeled the Nc wave), but no P3a component (Courchesne, 1977; Courchesne, 1978). By adolescence, the P3a component is clearly present and the Nc wave has receded. What remains present is the anterior N2 component that is very responsive to novelty. By middle-age, the anterior N2 may be further attenuated by a frontally-distributed positivity that peaks earlier as the anterior P2.
Based on the current study and the findings in the literature, we suspect that in contrast to the anterior P2 that indexes the motivational salience of visual stimuli, the anterior N2 indexes the difficulty matching unusual visual stimuli to stored representations. Several laboratories have demonstrated that in young adult subjects, the anterior N2 is sensitive to stimulus unfamiliarity or encoding difficulty, and may reflect a mismatch or conflict between stimulus input and existing knowledge (Nittono et. al., 2007; Daffner et. al., 2000; Folstein and Van Petten, 2008; Ferrari et. al., in press). Our results demonstrate an age-related decline in the anterior N2 in response to novel visual stimuli. There are several potential explanations for this finding.
One possibility is that age-related augmentation in response to motivationally salient novel stimuli (as indexed by an enhanced P2) facilitates the process of encoding these novel events (as indexed by a diminished N2). However, it is unclear why aging would lead to such a processing advantage. Another possibility is that as individuals get older, they encounter an increasing number of visual stimuli and create a wider range of stored representations. Thus, for older individuals, even seemingly ‘unusual’ visual stimuli might be matched to one of their large warehouse of stored representations.6 If this were true, one would expect the observed age-related reduction in the size of the N2. Alternatively, normal aging may be associated with a reduced ability to process conflicting or ambiguous representations or encode unusual stimuli, which may manifest as a reduced N2 response (Denburg et al., 2007; Falkenstein et al., 2001; Mager et al., 2007; Zamarian et al., 2008). In this context, age-related increases in the anterior P2 could reflect a compensatory mechanism. However, if the anterior P2 and N2 reflect temporally overlapping components with opposite polarities, then regardless of the processes responsible, an age-related decline in the anterior N2 might be associated with an age-related increase in the anterior P2. Additional research is needed to specify the most critical neural and cognitive mechanisms that underlie these changes.
There are several limitations of the current study that warrant comment. Although the same types of visual stimuli were presented under the Attend and Ignore conditions, the Ignore condition included exposure to auditory stimuli, whereas the Attend condition did not. Additionally, task and task difficulty varied across the conditions. It is unclear whether the presentation of a second set of stimuli under the Ignore condition or differences in task difficulty across conditions contributed to the differential electrophysiological response to visual stimuli under the two conditions. The likelihood of the electrophysiological response to auditory events impacting upon the pertinent ERPs in response to the visual stimuli was reduced by varying the jitter between the presentation of auditory and visual stimuli, and by computing difference waves (that theoretically subtract out overlapping activity) (Woldorff, 1993). However, this possibility cannot be excluded.
Another potential confound in comparing the Attend and Ignore tasks is that the two conditions not only differed in terms of the focus of attention, but also in the requirement of a motor response to visual targets under the Attend condition. Methodologically, the use of a mental count rather than a motor response to targets might have been a way to avoid this problem. However, a mental count to targets during the Attend task would have meant that the two conditions would have differed not only in the direction of attention, but also the demands placed on working memory. It also is worth noting that under both conditions there was a requirement for motor preparation and output, as subjects also had to respond to n-back auditory targets under the Ignore condition. This had the advantage of providing more data on the performance of subjects.
The study's strengths include a relatively large sample size and subjects from a wide range of ages. Different age groups were reasonably well-matched for gender, estimated IQ, education, and cognitive status, as measured by neuropsychological test scores relative to their age-matched peers, reducing the likelihood that differences found between age groups were due to confounding variables (Daselaar & Cabeza, 2005; Salthouse & Ferrer-Caja, 2003). Moreover, there were no dramatic differences in task performance across age groups, reducing concerns that the age-related differences in ERPs observed were really a reflection of differences in level of performance across groups (Rugg & Morcom, 2005).
Based on our findings, we conclude that the anterior P2 component reflects the processing of the motivational salience of a stimulus as established by either task relevance or novelty. It is enhanced under attend conditions to a similar degree across age groups. Moreover, there are no age-related differences in anterior P2 activity in response to target events. These findings are consistent with the notion that older adults exhibit a fairly well-preserved capacity to focus attention on task-relevant information. There is a striking age-related enhancement of the anterior P2 component's sensitivity to novel stimuli. This effect does not appear to be due to reduced inhibitory control that has been associated with aging. Instead, the increased size of the anterior P2 to novel stimuli in older adults may be linked to age-related changes in the process of matching unusual visual stimuli to stored representations, which is indexed by the temporally overlapping anterior N2 component whose amplitude decreases considerably with age.
Acknowledgments
This research was funded in part by NIA grant R01 AGO17935 and by generous support from D. Wimberly and S. Muss. The authors thank Katherine K. Ryan for her assistance with data collection and Katie Gartner with her administrative help.
Footnotes
We also examined the relationship between the P2 elicited by novel vs. standard, and target vs. standard stimuli using analysis of variance, with stimulus type as a within-subjects variable. However, we elected to report the results of the difference wave analyses because it simplified the presentation of the major findings and allowed us to discuss novelty-specific and target-specific processing. The results of the analyses of the P2 response to novel vs. standard stimuli and target/rare vs. standard stimuli (not reported here) were very similar to those derived by computing novelty and target difference waves.
As expected, mean (± SD) performance was significantly different for high and low performers on the n-back task for the old subjects alone (.70 (.09) vs. .43 (.17), F(1,28) = 32.17, p < 0.00001) and old and middle-aged subjects combined (.77 (.10) vs. .48 (.17), F(1,58) = 65.75, p < 0.000001).
Consistent with this finding, correlation analyses revealed no relationship between accuracy rate on the n-back task and the size of P2 to novels across midline sites for old subjects alone or in combination with middle-aged subjects.
There is a long tradition of examining ERP responses to rare stimuli under ignore vs. attend conditions (e.g., (Ford & Pfefferbaum, 1991; Friedman et al., 1998; Snyder & Hillyard, 1976; Squires et al., 1975) that has largely focused on the P3 component. For example, studies have consistently found an increased P3b response to rare stimuli under attend conditions, in keeping with the sensitivity of this component to task relevance as well as infrequency. The current study showed that the size of the P2 to rare events also was very sensitive to direction of attention, consistent with the notion that it is an index of motivational salience. Most relevant to the current study is that there were no age-related differences in the degree of P2 enhancement in response to rare stimuli under the attend condition. We do not address the aforementioned literature further as it does not seem to be directly pertinent to the focus of our study.
The size of the anterior P2 was not sensitive to differences in cognitive status. This result differs from our previously reported finding about the P3 component in which, for example, cognitively high performing old subjects generated a larger P3 in response to novel stimuli than cognitively average old subjects (Daffner et al., 2006; Riis et al., 2008). This suggests that differences in the capacity to signal that visual events are motivationally salient, as indexed by the P2 component, do not appear to make a significant contribution to overall differences in cognitive competence, which seems to be determined by information processing that occurs at a later stage (Riis et al., 2008). Alternatively stated, differences in information processing between cognitively high and average performers do not appear to be expressed at the level of being able to identify motivationally salient stimuli, as indexed by the anterior P2.
A similar idea has been suggested as one possible explanation for the age-related decline in the size of the N400 (Kutas & Iragui, 1998).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Woods DL. Age-Related Changes in Processing Auditory Stimuli During Visual Attention: Evidence for Deficits in Inhibitory Control and Sensory Memory. Psychology and Aging. 1999;14(3):507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Amenedo E, Diaz F. Aging-related changes in processing of non-target and target stimuli during an auditory oddball task. Biological Psychology. 1998;48(3):235–267. doi: 10.1016/s0301-0511(98)00040-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Andres P, Parmentier FB, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44(12):2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Escera C, Corral MJ, Perianez JA. Task switching and novelty processing activate a common neural network for cognitive control. Journal of Cognitive Neuroscience. 2006;18(10):1734–1748. doi: 10.1162/jocn.2006.18.10.1734. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Perianez JA, Knight RT. Think differently: a brain orienting response to task novelty. NeuroReport. 2002;13(15):1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- Baudena P, Halgren E, Heit G, Clarke JM. Intracerebral potentials to rare target and distractor auditory and visual stimuli. III. Frontal cortex. Electroencephalography and Clinical Neurophysiology. 1995;94(4):251–264. doi: 10.1016/0013-4694(95)98476-o. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventory: Manual. The Psychological Corporation; San Antonio, Texas: 1987. [Google Scholar]
- Beck EC, Swanson C, Dustman RE. Long latency components of the visually evoked potential in man: effects of aging. Experimental Aging Research. 1980;6(6):523–545. doi: 10.1080/03610738008258385. [DOI] [PubMed] [Google Scholar]
- Berlyne D. Conflict, Arousal and Curiosity. McGraw-Hill; New York: 1960. [Google Scholar]
- Brooks-Eidelberg BA, Adler G. A frontal cortical potential associated with saccades in humans. Experimental Brain Research. 1992;89(2):441–446. doi: 10.1007/BF00228260. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cerebral Cortex. 1997;7(1):763–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb P, Daffner K. To Ignore or Explore: Top-Down Modulation of Novelty Processing. Journal of Cognitive Neuroscience. 2008;20(1):120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Comalli P, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. Journal of Genetic Psychology. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: comparison between children and adults. Science. 1977;197:589–591. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45(4):468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Curran T, Hills A, Patterson MB, Strauss ME. Effects of aging on visuospatial attention: an ERP study. Neuropsychologia. 2001;39(3):288–301. doi: 10.1016/s0028-3932(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Czigler I. Age, color processing and meaningfulness: an event-related potential study. International Journal of Psychophysiology. 1996;22(12):25–34. doi: 10.1016/0167-8760(96)00010-4. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Calvo V, Faust R, Scinto LFM, Holcomb PJ. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000a;37(6):737–747. [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Acar D, Calvo V, Faust R, Chabrerie A, Kennedy B, Holcomb P. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000b;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LFM, Cohen LG, Kennedy BP, West WC, Holcomb PJ. Regulation of attention to novel stimuli by frontal lobes: an event-related potential study. NeuroReport. 1998;9(5):787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ. Increased Responsiveness to Novelty is Associated with Successful Cognitive Aging. Journal of Cognitive Neuroscience. 2006;18(10):1759–1773. doi: 10.1162/jocn.2006.18.10.1759. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LFM, Weintraub S, Guinessey J, Mesulam MM. The impact of aging on curiosity as measured by exploratory eye movements. Archives of Neurology. 1994;51(4):368–376. doi: 10.1001/archneur.1994.00540160062009. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LFM, Weitzman AM, Faust R, Rentz DM, Budson AE, Holcomb PJ. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. Journal of Cognitive Neuroscience. 2003;15(2):294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Dale AM. Dissertation Abstracts International, 55-07B. 1994. Source localization and spatial discriminant analysis of event-related potentials: linear approaches (brain cortical surface) p. 2559. [Google Scholar]
- Daselaar SM, Cabeza R. Age-Related Changes in Hemispheric Organization. In: Cabeza R, Nyberg L, Park D, editors. Cognitive Neuroscience of Aging. Oxford University Press; 2005. pp. 325–353. [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, Wallace RB. The orbitofrontal cortex, real-world decision making, and normal aging. Annals of the New York Academy of Sciences. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Naatanen R. Neural mechanisms of involuntary attention to acoustic novelty and change. Journal of Cognitive Neuroscience. 1998;10(5):590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32(6):579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D, Cheng JC. Individual differences in P3 scalp distribution in older adults, and their relationship to frontal lobe function. Psychophysiology. 1998;35(6):698–708. [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Experimental Brain Research. 2001;138(2):258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Detecting novelty and significance. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21244. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Life-span changes in P3a. Psychophysiology. 2004;41(4):575–583. doi: 10.1111/j.1469-8986.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Folk CL, Hoyer WJ. Aging and shifts of visual spatial attention. Psychology and Aging. 1992;7(3):453–465. doi: 10.1037//0882-7974.7.3.453. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Ford JM, Pfefferbaum A. Event-related potentials and eyeblink responses in automatic and controlled processing: effects of age. Electroencephalography and Clinical Neurophysiology. 1991;78:361–377. doi: 10.1016/0013-4694(91)90098-o. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain's evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25(4):355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski VA, Cycowicz YM. Effects of aging on the novelty P3 during attend and ignore oddball tasks. Psychophysiology. 1998;35(5):508–520. doi: 10.1017/s0048577298970664. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Spencer KM, Donchin E. The influence of stimulus deviance and novelty on the P300 and novelty P3. Psychophysiology. 2002;39(6):781–790. [PubMed] [Google Scholar]
- Hartley AA, Kieley J, McKenzie CR. Allocation of visual attention in younger and older adults. Perceptual Psychophysiology. 1992;52(2):175–185. doi: 10.3758/bf03206771. [DOI] [PubMed] [Google Scholar]
- Hunt JM. Intrinsic motivation and its role in psychological development. In: Levine D, editor. Nebraska Symposium on Motivation Volume XIII. University of Nebraska Press; Lincoln, Nebraska: 1965. pp. 189–282. [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nature Reviews Neuroscience. 2001;2(3):194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE Token, WRAT-R Reading, AMNART, STROOP, TMT, and JLO. The Clinical Neuropsychologist. 1996;10(3):262–278. [Google Scholar]
- Knight RT. Aging decreases auditory event-related potentials to unexpected stimuli in humans. Neurobiology of Aging. 1987;8(2):109–113. doi: 10.1016/0197-4580(87)90019-4. [DOI] [PubMed] [Google Scholar]
- Knight RT. Distributed cortical network for visual attention. Journal of Cognitive Neuroscience. 1997;9(1):75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biological Psychology. 2000;54(13):107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints: a PET investigation. Brain. 1994;117:1055–1071. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: a comparison of lexical, object and reality decisions. Journal of Verbal Learning and Verbal Behavior. 1984;23(1):39–66. [Google Scholar]
- Kutas M, Iragui V. The N400 in a semantic categorization task across 6 decades. Electroencephalography and Clinical Neurophysiology. 1998;108(5):456–471. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Luck S, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Madden DJ. Adult Age Differences in Attentional Selectivity and Capacity. European Journal of Cognitive Psychology. 1990;2(3):229–252. [Google Scholar]
- Mager R, Bullinger AH, Brand S, Schmidlin M, Scharli H, Muller-Spahn F, Stormer R, Falkenstein M. Age-related changes in cognitive conflict processing: an event-related potential study. Neurobiology of Aging. 2007;28(12):1925–1935. doi: 10.1016/j.neurobiolaging.2006.08.001. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Naatanen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Sciences. 1990;13:201–288. [Google Scholar]
- Navalpakkam V, Itti L. Modeling the influence of task on attention. Vision Research. 2005;45(2):205–231. doi: 10.1016/j.visres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Nittono H, Shibuya Y, Hori T. Anterior N2 predicts subsequent viewing time and interest rating for novel drawings. Psychophysiology. 2007;44(5):687–696. doi: 10.1111/j.1469-8986.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Hoyer WJ. Age and the selectivity of visual information processing. Psychology and Aging. 1986;1(1):4–10. doi: 10.1037//0882-7974.1.1.4. [DOI] [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain and Cognition. 2004;56(1):5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Potts GF, Liotti M, Tucker DM, Posner MI. Frontal and Inferior Temporal Cortical Activity in Visual Target Detection: Evidence from High Spatially Sampled Event-Related Potentials. Brain Topography. 1996;9:3–14. [Google Scholar]
- Potts GF, Tucker DM. Frontal evaluation and posterior representation in target detection. Brain Research Cognitive Brain Research. 2001;11(1):147–156. doi: 10.1016/s0926-6410(00)00075-6. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. An age-decrement in the ability to ignore irrelevant information. Journal of Gerontoogy. 1965;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. NeuroImage. 2008;39(1):441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Morcom AM. The Relationship Between Brain Activity, Cognitive Performance, and Aging. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; 2005. pp. 132–154. [Google Scholar]
- Ryan J, Paolo A. A screening procedure for estimating premorbid intelligence in the elderly. The Clinical Neuropsychologist. 1992;6(1):53–62. [Google Scholar]
- Salthouse TA, Ferrer-Caja E. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging. 2003;18(1):91–110. doi: 10.1037/0882-7974.18.1.91. [DOI] [PubMed] [Google Scholar]
- Snyder E, Hillyard SA. Long-latency evoked potentials to irrelevant, deviant stimuli. Behavioral Biology. 1976;16:319–331. doi: 10.1016/s0091-6773(76)91447-4. [DOI] [PubMed] [Google Scholar]
- Snyder E, Hillyard SA. Changes in visual event-related potentials in older persons. In: Hoffmeister F, Muller C, editors. Bayer Symposium VIII: Brain Function in Old Age. Springer-Verlag; New York, New York: 1979. pp. 112–125. [Google Scholar]
- Spencer KM, Dien J, Donchin E. A componential analysis of the ERP elicited by novel events using a dense electrode array. Psychophysiology. 1999;36:409–414. doi: 10.1017/s0048577299981180. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd. Oxford University Press; New York, New York: 1998. p. 599. [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two variables of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Hubley AM. The 60-item Boston Naming test: norms for cognitively intact adults aged 25 to 88 years. Journal of Clinical and Experimental Neuropsychology. 1997;19(6):922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39(6):791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Walhovd K, Fjell A. Two-and three-stimuli auditory oddball ERP tasks and neuropsychological measures in aging. NeuroReport. 2001;12(14):3149–3153. doi: 10.1097/00001756-200110080-00033. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale WAIS-III Administration and Scoring Manual. 3rd. The Psychological Corporation; San Antonio, Texas: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale WMS-III Administration and Scoring Manual. 3rd. The Psychological Corporation; San Antonio, Texas: 1997b. [Google Scholar]
- Woldorff MG. Distortion of ERP averages due to overlap from temporally adjacent ERPs: analysis and correction. Psychophysiology. 1993;30(1):98–119. doi: 10.1111/j.1469-8986.1993.tb03209.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Knight RT. Age effects on the P300 to novel somatosensory stimuli. Electroencephalography and Clinical Neurophysiology. 1991;78:297–301. doi: 10.1016/0013-4694(91)90184-6. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Rose TL, Lapp D. Validity of the Geriatric Depression Scale in Subjects with Senile Dementia. Veterans Administration Medical Clinic; Palo Alto, California: 1981. [Google Scholar]
- Youngjohn JR, Larrabee GJ, Crook TH. New adult- and education-correction norms for the Benton Visual Retention Test. The Clinical Neuropsychologist. 1993;7:155–160. doi: 10.1080/13854049308401517. [DOI] [PubMed] [Google Scholar]
- Zamarian L, Sinz H, Bonatti E, Gamboz N, Delazer M. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology. 2008;22(5):645–657. doi: 10.1037/0894-4105.22.5.645. [DOI] [PubMed] [Google Scholar]