Abstract
Background
Pseudorabies virus (PRV), a neurotropic herpesvirus of pigs, serves as an excellent model system with which to investigate the herpesvirus life cycle both in cultured cells and in vivo. Real-time RT-PCR is a very sensitive, accurate and reproducible technique that can be used to detect very small amounts of RNA molecules, and it can therefore be applied for analysis of the expression of herpesvirus genes from the very early period of infection.
Results
In this study, we have developed and applied a quantitative reverse transcriptase-based real-time PCR technique in order to profile transcription from the whole genome of PRV after lytic infection in porcine kidney cells. We calculated the relative expression ratios in a novel way, which allowed us to compare different PRV genes with respect to their expression dynamics, and to divide the PRV genes into distinct kinetic classes. This is the first publication on the whole-genome analysis of the gene expression of an alpha-herpesvirus by qRT2-PCR. We additionally established the kinetic properties of uncharacterized PRV genes and revised or confirmed data on PRV genes earlier examined by traditional methods such as Northern blot analysis. Our investigations revealed that genes with the same expression properties form clusters on the PRV genome: nested overlapping genes belong in the same kinetic class, while most convergent genes belong in different kinetic classes. Further, we detected inverse relationships as concerns the expressions of EP0 and IE180 mRNAs and their antisense partners.
Conclusion
Most (if not all) PRV genes begin to be expressed from the onset of viral expression. No sharp boundary was found between the groups of early and late genes classified on the basis of their requirement for viral DNA synthesis. The expressions of the PRV genes were analyzed, categorized and compared by qRT2-PCR assay, with the average of the minimum cycle threshold used as a control for the calculation of a particular R value. In principle, this new calculation technique is applicable for the analysis of gene expression in all temporally changing genetic systems.
Background
The pseudorabies virus (PRV; also called Aujeszky's disease virus or suid herpesvirus type 1) is a member of the alpha-herpesviruses and an important pathogen of pigs, causing Aujeszky's disease [1]. The PRV is a valuable model organism in herpesvirus research [2] and also a powerful tool in neuroscience, employed to map neural circuits [[3-5], and [6]] and gene delivery [7]. The PRV life cycle is primarily controlled at the level of transcription. The genes of α-herpesviruses are divided into three major temporal classes (immediate-early, IE or α; early, E or β, and late, L or γ), which are regulated in a coordinated, cascade-like fashion [[8] and [9]]. First, the IE genes are expressed, independently of de novo protein synthesis from the virus. The products of these genes are transcription factors and other regulatory proteins. PRV IE180 protein [homologous to herpes simplex virus (HSV) ICP4 protein] is the major regulator of the transcription of the E genes, which in turn are mainly involved in replication of the viral DNA. Blockers of DNA synthesis inhibit the E gene expression to a much lesser extent than the L gene expression. On a finer scale, the E genes can be subdivided into β1 (E) and β2 (E/L) genes. Finally, the L genes are transcribed, encoding mainly structural proteins involved in virion assembly. The expression of the L genes is partially (γ1, leaky L genes) or completely (γ2, true L genes) dependent on the viral DNA replication. HSV encodes 5 IE genes: icp0, icp4, icp22, icp27 and icp47. By contrast, PRV has been reported to express ep0 (homologous to icp0 of HSV; [10] and ul54 (the homolog of icp27 of HSV; [11]) proteins in the E kinetics, and it lacks the icp47 gene. The ie180 gene of PRV has been shown to be a true IE gene in various experimental systems [[12] and [13]]; however, there is no consensus as to whether the US1 protein (Rsp40; homologous to HSV ICP22) is expressed in the IE [14] or the E kinetics [15]. Herpesviruses are capable of two types of infections: lytic (or productive) and latent [2]. In the lytic pathway, the entire transcription machinery of the herpesvirus is initiated, and the progress of infection eventually leads to the production of new virions and the lysis of infected cells. In contrast, in latency, only a limited segment (LAT region) of the herpesvirus genome is transcriptionally active, no new virus particles are produced and the cells survive the infection. A characteristic feature of the organization of the herpesvirus genome is the presence of nested genes producing 3'-coterminal transcripts. The read-through of overlapping genes is regulated by the ICP27 protein, which helps in the recognition of the internal polyA signals, resulting in differential transcript lengths [16]. The gene expression of α-herpesviruses, such as HSV types 1 and 2, Varicella-zoster virus, bovine herpesvirus type 1 and PRV has been investigated earlier by traditional methods including Northern blot analysis, ribonuclease protection assay, and end-point RT-PCR analysis. However, each of these techniques is associated with a number of disadvantages. For example, Northern blot analysis is labor-intensive, allows only semiquantitative determination of the mRNA level and is unsuitable for multiple mRNA analysis. Moreover, hybridization-based membrane arrays profile changes in a nonlinear fashion, tending to overemphasize large alterations, and they are insensitive to smaller variations. Ribonuclease protection assay-based methods require the use of polyacrylamide gel electrophoresis and typically utilize radioactively labeled probes. The limitations of endpoint PCR technique are the time-consuming procedure, the poor precision and the variable endpoints between samples. Over the past few years, microarray techniques have revolutionized practically all disciplines of molecular biology, including herpesvirus research. As compared with traditional methods, microarray analysis is superior in that it is applicable for the simultaneous analysis of a large number of genes, and even whole genomes. The disadvantage of DNA chip technology is associated with the uncertain quality control: it is impossible to assess the identity of DNA immobilized on any microarray. Further, there are many artifacts associated with image and data analysis. Real-time RT-PCR is an alternative to microarray techniques for the analysis of transcription from multiple genes. The main advantage of real-time PCR is that it is more sensitive to low-fold changes than other high-throughput assays. In addition, the real-time PCR technique provides a reproducible quantitation of DNA copies, and has a large dynamic range, and various controls can be included to ensure accuracy, such as a loading control to verify equal cDNA loading, a no-primer control to prove a measure of non-amplification-related background, a no-template control to screen for contamination of reagents or false amplification, and a no-RT control with confirm the absence of DNA contamination. In contrast to microarray techniques, in real-time PCR the parameters for each gene can be optimized individually. Moreover, the identity of PCR products can be confirmed through melting curve analysis, restriction endonuclease analysis, or DNA sequencing. In fact, real-time PCR is often used to verify gene expression data obtained by microarrays. Nevertheless, considerable pitfalls may be associated with this technique. The major limitations of real-time RT-PCR relative to microarray techniques are the higher cost and labor intensiveness for a large number of samples. Another disadvantage of real-time PCR as compared with blotting techniques is that only the accumulation, but not the size of the transcripts can be monitored. Microarray techniques have recently been applied to investigate herpesvirus gene expression [[17-19] and [20]]; to analyze the effects of the deletion of particular viral genes or of the specific experimental conditions on whole-genome viral gene expression [[21,22], and [23]]; and to analyze the impact of virus infection on the expression of cellular genes [[24-26] and [27]]. The expression of PRV genes has been studied by traditional methods, but many its genes have not yet been characterized at all. Flori and co-workers [27] investigated the dialog between PRV and epithelial cells, but obtained poor resolution for viral transcripts that did not provide conclusive data on the temporal expression of the PRV genes. To date, Real-time RT-PCR has not been frequently utilized in herpesvirus research for global gene expression analysis. With this technique Oster and Höllsberg [28] carried out a kinetic analysis of 35 genes of human herpesvirus 6B, a β-herpesvirus, and Dittmer and colleagues [29] performed a whole-genome profiling of the rhesus monkey rhadinovirus, a γ-herpesvirus. As far as we are aware, no genome-wide expression data obtained by qRT2-PCR have been published so far on α-herpesviruses. In the present study, we describe the development and utilization of a real-time RT-PCR assay for the global analysis of PRV gene expression. We applied a novel method that allows the kinetic characterization of individual PRV genes, and also the comparison of the expression dynamics of different viral genes. Expression profiles were constructed on the basis of relative expression ratios (Rs) calculated as described in the Materials section. This method is applicable for the analysis of gene expression in any genetic system that progressively changes in time.
Results
Experimental design
For each gene, a minimum of 3 independent replicates were carried out for statistical confidence, and the median of these values along with the standard error was calculated. In these experiments, porcine kidney (PK)-15 epithelial cells were infected with PRV with a low multiplicity of infection [MOI; 0.1 plaque-forming units (pfu)/cell]. The reason for this was that our preliminary experiments indicated that infecting cells with a low dose of the virus produced a much better resolution of the cycle threshold (Ct) values for PRV genes at different time points than infecting with a higher MOI (data not shown). However, the use of a low MOI led to a larger proportion of the cells remaining uninfected; accordingly, in order to avoid the initiation of a new infection cycle we chose a relatively short maximal incubation period. Initially, we attempted an 8-h investigation period, but observed a second infection wave of the virus (data not shown), and therefore we restricted our analysis to 6 h post-infection (pi). The transcription of PRV genes was monitored at 5 time points: 0, 1, 2, 4 and 6 h. Prior to PRV infection, cells were either untreated or treated with cycloheximide (CHX), a protein synthesis blocker, or phosphonoacetic acid (PAA), an inhibitor of DNA synthesis. We used strand-specific primers for the reverse transcription, one of the reasons for this being that we intended to exclude the distorting effects of potential overlapping antisense RNAs transcribed from the antiparallel DNA strands, which cannot be distinguished by oligo-dT- or random priming-based RT (this issue will be described elsewhere). Another reason was that it produced a much higher amount of specific cDNAs than oligo-dT priming (data not shown), which was especially important under our experimental conditions with a low MOI. We utilized the mathematical model for relative quantification described by Soong et al. [30]; however, we calculated the R values in a different way: we used the average of the maximal ECt-sample values for each gene as a control, which was normalized with the average of the corresponding 28S values (ECt-reference). Thus, in our calculation, a particular R value indicates the ratio of the mRNA level of a gene at a given time point to the maximal RNA level of this gene (maximal gene expression occurred at 6 h pi for all genes except us3 and llt2, which peaked at 4 h and at 2 h pi, respectively). The relative amounts of the transcripts of different genes cannot be compared because the primer efficiencies may vary both in RT and in PCR. Even so, use of the maximal expression as the control value allows a comparison of the expression dynamics of viral genes both at individual time points and across the entire infection period. The specificity of the PCR products was confirmed by quality control experiments, including Tm analysis and PAGE (formation of a single product with the appropriate molecular weight was accepted); if some doubts remained, the amplification products were subjected to DNA sequencing or restriction endonuclease analysis using amplicons containing recognition sites for a particular restriction endonuclease [see Additional file 1]. The accuracy of sampling and qRT2-PCR analysis were ensured by using loading controls, as well as no-RT, no-primer and no-template controls (Figure 1).
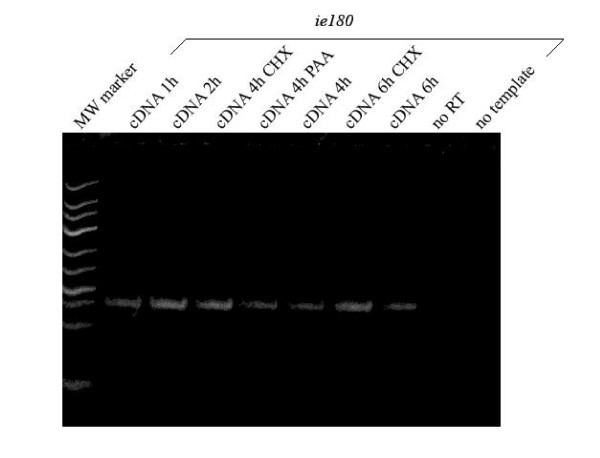
Figure 1.
Controls for qRT2-PCR. Polyacrylamide gel electrophoresis of the products from 30 cycles of real-time PCR on cDNA derived from PK-15 cells infected by the PRV for 1, 2, 4 or 6 h or from cells infected by the virus for 4 or 6 h and treated with CHX or PAA. A no-template control was used for each primer pair, consisting of water, to test for false-positive results, and no-RT control was used to ensure the absence of DNA contamination. The GeneRuler™ Low Range DNA Ladder (Fermentas) is shown at the left side of the photo. The cDNAs of ie180 gene (a) and ul30 gene (b) were used for the presentation.
Reproducibility of the internal control
A major concern regarding the choice of internal reference gene control in viral gene expression studies is the constancy of the reference gene products throughout the entire examination period. We tested whether the 28S ribosomal RNA of the pig fulfilled this requirement, and found that this gene was suitable as reference gene for the study of PRV infection, at least in our 6-h examination period. The 28S rRNA values were highly reproducible: the mean value ± SD of all measurements the experiments was 8.03 ± 0.61 cycles throughout (Figure 2).
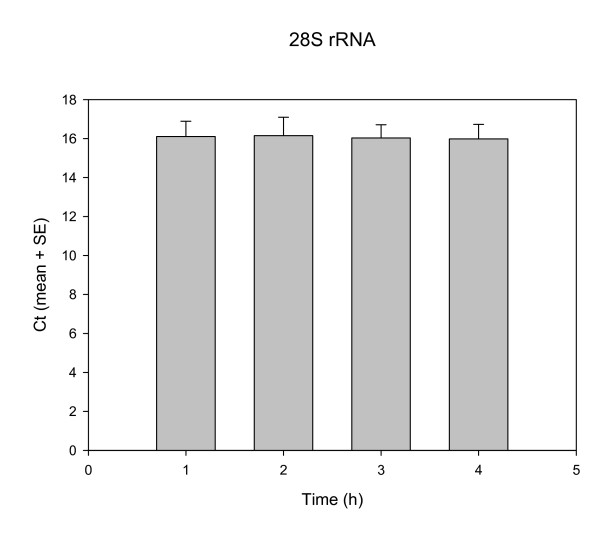
Figure 2.
Reproducibility of the reference control. The constancy of the 28S rRNA level (shown by the similar Ct values) throughout the 6-h infection period indicates that this gene serves as an appropriate reference control.
Classification of PRV genes in terms of dependence on de novo protein synthesis and DNA replication
Traditionally, lytic herpesvirus genes are classified into distinct kinetic groups on the basis of the effects of protein and DNA synthesis inhibition on the gene expression. In general, inhibitory drugs are applied to infected cells for a prolonged incubation period (24, 48 or 72 h; [[31,32] and [33]]. Instead, similarly to Stingley et al [18], we used shorter incubation periods (2, 4 and 6 h for CHX, and 4 and 6 h for PAA) and compared the inhibitory effects of these drugs by calculating the R value ratios for the treated and untreated samples.
CHX analysis
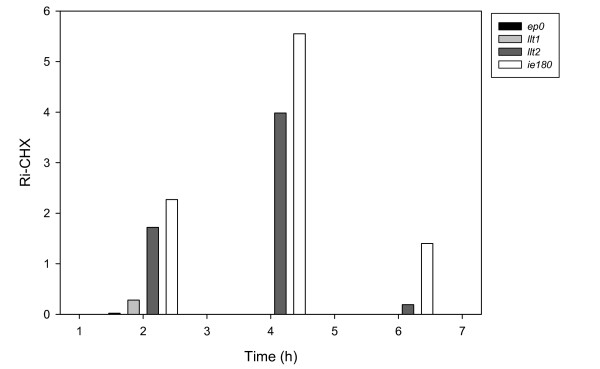
To test the requirement of de novo protein synthesis for PRV mRNA production, PK-15 cells were untreated or treated with 100 μg/ml CHX prior to the infection of cells with the virus. At the indicated time points, RNA was isolated and converted to cDNA, which was subsequently analyzed by qRT2-PCR. The degree of inhibition [1-Ri-CHX) × 100] was found to range between 97.3 and 100% for all but one protein encoding PRV gene (the ie180 gene) and two antisense transcripts [the long-latency transcript-1 (llt1), antiparallel to ep0, and the long-latency transcript-2 (llt2), antiparallel to ie180). IE180 mRNA displayed a significantly increased level of expression in the CHX-treated samples at the analyzed 3 time points: 2.27-fold at 2 h; 5.55-fold at 4 h; 1.4-fold at 6 h pi (Figure 3). We explain this phenomenon in that the IE180 proteins exert an inhibitory effect on their own synthesis (upon the binding of their own promoters; [34]), which is resolved by CHX blocking protein synthesis from the IE180 mRNAs. The other exception for the negative CHX effect is llt2: a 1.72-fold increase at 2 h and a 3.983-fold increase at 4 h. Interestingly, at 6 h pi llt2 is significantly repressed by CHX. The repression of llt1 expression at 2 h pi is relatively low (Ri-CHX-2h = 0.281), but this antisense transcript is significantly blocked by CHX at 4 and 6 h pi. Overall, our CHX analysis indicated that the only true IE protein-encoding gene of PRV is ie180. While the icp27 and icp0 genes in the HSV are IE genes, their PRV homologs ul54 [11] and ep0 [10] genes were earlier described as E genes, which was confirmed by our CHX analysis. The icp22 gene of HSV is expressed with IE kinetics; our analysis revealed that its homologous counterpart, the us1 gene of PRV, is significantly blocked by CHX, and hence it is not an IE gene. As a result of CHX treatment, LLT2 exhibits elevated levels at 2 and 4 h pi, which indicates that IE180 transcription factor exerts a negative effect on its expression. Thus, LLT2 appears to be an IE transcript. However, llt2 displays fairly low relative expression at 1 h pi [see Additional file 2], and therefore it cannot be regarded as an IE gene beyond doubt.
Figure 3.
Effects of the CHX on the expression of ie180, llt1, llt2 and ep0 genes. This Figure shows the dependence of the PRV gene expression on de novo protein synthesis on two genes (ie180 and ep0) and two antisense transcripts (llt1 and llt2) overlapping these genes. PK-15 cells infected with PRV were incubated in the presence or absence of CHX for 2, 4 or 6 h. The CHX-mediated inhibition of viral gene expression (Ri-CHX) was calculated as follows: Ri-CHX = RCHX/RUT, where RCHXis the R value of the CHX-treated samples; and RUTis the R value of the untreated samples.
PAA analysis
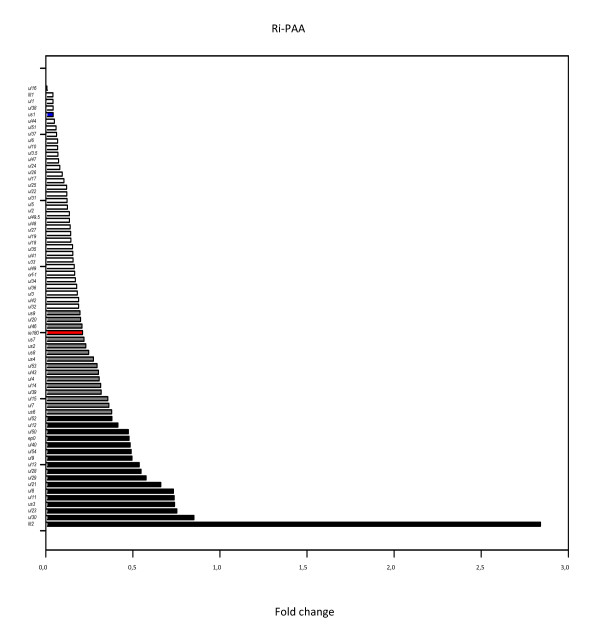
For examination of the dependence of the PRV genes on DNA replication, PK-15 cells were infected with the virus in the absence or presence of 400 μg/ml PAA, an inhibitor of DNA polymerase. It was expected that PAA would exert a more drastic effect on the L genes because the expressions of these genes are highly dependent on DNA replication. However, PAA must affect the E gene expression, too. The reason for this is that the gene expression is dependent not only on the promoter activity, but also on the copy numbers of the genes, which are higher after DNA replication than in the initial phase of infection when the PRV DNA is represented in a single copy in a cell (at least in our system). The inhibitory effect of PAA on gene expression was calculated by using the ratio of the R values for the PAA-treated/untreated samples at 6 h pi (Ri-PAA = R6h-PAA/R6h-UT): a low ratio indicates a strong inhibitory effect and vice versa. The PRV genes were ranked from the least inhibited (highest Ri-PAA) to the most inhibited (lowest Ri-PAA). We expected that such a ranking of the PRV genes on the basis of the inhibitory effect would provide natural clusters of E and L genes. Indeed, the results obtained conformed fairly well to the data published earlier on HSV and PRV genes, though there is a certain degree of disagreement between the data published by the different authors on the HSV genes, and only incomplete expression data are available for the PRV genes. Moreover, in many cases a PRV gene is characterized without comparative analysis, e.g. "it appears 3 h pi". For comparison of the HSV and PRV genes, we used five main sources for the HSV data: microarray data published by Wagner [35,36]; various expression data compiled by Roizman [37,38]; and the data collected by Mettenleiter [39]. For the PRV genes, we used the data compiled by Mettenleiter [39]. We classified the analyzed but uncategorized PRV genes via the following principles: if the mRNA was detected between 1 and 2 h pi,, between 3 and 4 h pi, or 5 h pi, the particular gene was classified as E, E/L or L, respectively. In the upper part of the ranking list (Table 1, Figure 4), [see Additional file 3], we mostly find typical E genes, with typical L genes in the bottom part of the list. However, we could not draw a clear-cut line between the E and L genes; instead, we put the 15 genes with intermediate values in the E/L group. The precise locations of borderlines between kinetic classes were drawn arbitrarily, because we found smooth transitions between the Ri-PAA values. We chose a typical E gene (ul52) of the HSV as the last member of the E group, and a typical L gene (ul32) of the HSV as the first member of the L group. The 3 protein-encoding genes with the highest Ri-PAA values were ul30 (0.851), ul23 (0.752) and us3 (0.739). These high Ri-PAA values indicate that PAA does not exert a significant cytotoxic effect on the gene expression apart from blocking DNA replication. The 2 LLT transcripts display a surprising response to PAA treatment: the level of LLT1 increases to 2.94-fold at 4 h, and drops to 0.007-fold at 6 h pi relative to the untreated sample; while the level of LLT2 increases markedly close to 40-fold at 4 h, and 3-fold at 6 h pi (this phenomenon will be discussed later). The genes with the lowest Ri-PAA values are all but one (us1: 0.042) L genes in the HSV: ul16 (0.000), ul1 (0.041) and ul38 (0.042). The strong inhibitory effect of PAA on the us1 expression is surprising because it is an IE gene in the HSV. The ie180 gene expression is also significantly inhibited by PAA (0.211); however, we found that ie180 and to a certain extent us1 were expressed in an "irregular" manner in other analyses, too (see later). ul11 and ul21, which are L genes in HSV, do not appear to belong in the E kinetic class. Both genes were characterized as L genes in the PRV by De Wind et al. [59] using Northern blot analysis. Furthermore, ul13 is an L gene in the HSV, but its transcripts were detected at 2 h pi in the PRV. The ul2 and ul5 genes are E genes in the HSV, but were characterized as E/l and L genes, respectively, in the PRV by Dean and Cheung [55,60], respectively, which is in agreement with our results. We classified the kinetically still uncharacterized PRV genes as follows; E genes: us3, ul29 and ul30, L genes: ul17, ul18, ul27, ul32, ul33, ul35, ul37 and ul41. Peaking of the us3 gene expression at 4 h can be explained by the important role of US3 protein kinase in blocking the apoptotic pathway of the compromised cell in the E phase of infection [61]. Our kinetic classifications accorded more strongly with those published on the HSV than with those on the PRV. The reason may be that the expression profiling of PRV genes was performed by others with low resolution techniques. Our data allow the following conclusions: (1) there is no sharp boundary between the E and L genes; (2) classification of the PRV genes on the basis of the Ri-PAA values yielded similar results those for the homologous HSV genes. A noteworthy exception is the us1 gene, which according to our PAA analysis is expressed in L kinetics; (3) the ie180 and llt genes cannot be classified on the basis of the Ri-PAA data alone, because they display unique expression kinetics; (4) LLT1 and LLT2 respond differently to CHX and PAA treatment, indicating that these antisense transcripts are, at least partially, under different regulation. We assume that the putative antisense promoter (ASP; [10]) controls the expression of LLT2.
Table 1.
Function and kinetic grouping of PRV genes
| Name | Transcription Kinetics | Function(s)b | References | ||||||
| Wagner37 | Wagner38 | Roizman39 | Roizman40 | Mettenleiter41 | Mettenleiter41 | Our dataa | |||
| orf-1 | - | - | - | - | - | ND | L | unknown | |
| ul54 (*ICP27) | IE | IE | IE | IE | IE | E | E | transcription regulation | [40] |
| ul53 (gK) | L | EL | L | L | L | (EL) 3 h pi | E/L | viral egress | [40] |
| ul52 | E | E | E | E | E | (E) 2 h pi | E | DNA replication | [40] |
| ul51 | L | EL | L | L | L | E | L | viral egress/oralgen unknown | [40] |
| ul50 (*dUTPase) | E | E | E | E | E | (EL) 3 h pi | E | dUTPase, viral replication | [27] |
| ul49.5 (gN) | L | L | L2 | L2 | L | L | L | virion entry | [39] |
| ul49 (*VP22) | E? | E | L | L | E | (EL) 4 h pi | L | virion formation, tegumentation | [27] |
| ul48 (*VP16, IE-TIF) | ? | EL | L | L | L | (L) 8 h pi | L | gene regulation, viral egress | [41] |
| ul47 (*VP13/14) | E | EL | L2 | L2 | L | L | L | secondary envelopment | [41] |
| ul46 (*VP11/12) | E | EL | L | L | L | E | E/L | unknown function, tegument protein | [41] |
| ul27 (gB) | E | EL | L1 | L1 | E | ND | L | cell-cell spread, virus entry | [27] |
| ul28 (*ICP18.5) | E | EL | L | L | E | E | E | DNA cleavage and packaging | [27] |
| ul29 (*ICP8) | E | E | E | E | ND | ND | E | DNA replication | [27] |
| ul30 | E | E | E | L | E | ND | E | DNA replication | [27] |
| ul31 | L | EL | L2 | L2 | L | (L) 6 h pi | L | nuclear egress | [42] |
| ul32 | L | EL | L2 | L2 | L | ND | L | DNA packaging | [2] |
| ul33 | L | EL | ? | L | L | ND | L | encapsidation of viral DNA | [43] |
| ul34 | L | L | ? | L | ND | (EL) 3 h pi | L | nuclear egress | [14] |
| ul35 (*VP26) | L | L | L2 | L2 | L | ND | L | capsid protein | [44] |
| ul36 (*VP1/2) | ? | EL | L2 | L2 | L | (EL) 3-9 h pi | L | tegumentation and egress | [45] |
| ul37 | L | E | L | L | L | (E) 2 h pi | L | secondary envelopement, egress | [45] |
| ul38 (*VP19c) | L | L | L2 | L2 | L | (E) 2 h pi | L | capsid protein | [46] |
| ul39 (RR1) | E | E | E | E | E | (E) E/L | E/L | nucleotide synthesis | [46] |
| ul40 (RR2) | E | E | E | E | E | E | E | nucleotide synthesis | [47] |
| ul41 (VHS) | L | EL | L | L | L | ND | L | RNase, gene regulation | [48] |
| ul42 | L | E | E | E | E | L | L | DNA replication | [47] |
| ul43 | L | EL | ? | L | E | E | E/L | unknown | [49] |
| ul44 (gC) | L | L | L2 | L2 | L | L | L | viral entry, virion attachment | [50] |
| ul26 | E | L | L | L | L | L | L | scaffold protease | [52] |
| ul25 | L | EL | L | L | L | L | L | capsid protein | [51] |
| ul24 (*VP24) | L | L | L | L | L | L | L | unknown | [52] |
| ul23 (TK) | E | E | E | E | E | E | E | nucleotide synthesis | [47] |
| ul22 (gH) | L | L | L2 | L2 | L | L | L | viral entry, cell-cell spread | [47] |
| ul21 | L | E | ? | L | L | L | E | capsid maturation | [47] |
| ul20 | L | EL | L | L | L | ND | E/L | capsid transport | [2] |
| ul19 (*VP5) | L | EL | L1 | L1 | L | (L) 16 h pi | L | capsid protein | [53] |
| ul18 (*VP23) | L | EL | L | L | L | ND | L | capsid protein | [27] |
| ul17 | L | EL | L | L | L | ND | L | DNA cleavage and encapsidation | [2] |
| ul16 | ? | EL | ? | L | L | ND | L | unknown, interacts with UL11 | [27] |
| ul15 | L | EL | L | L | L | ND | E/L | DNA cleavage and encapsidation | [27] |
| ul14 | L | EL | ? | L | L | L | E/L | DNA cleavage and packaging | [47] |
| ul13 (*VP18.8) | L | EL | L | L | L | (E) EL | E | protein phosphorylation | [47] |
| ul12 (*AN) | E | E | E | E | E | (E) EL | E | alkaline nuclease | [47] |
| ul11 | L | EL | L(?) | L | L | L | E | secondary envelopement | [47] |
| ul10 (gM) | L | E | L | L | L | E | L | egress, secondary envelopement | [54] |
| ul9 (*OBP) | E | E | L(?) | E | E | E | E | ori depentent DNA synthesis | [54] |
| ul8 (*OBPC) | E | E | E | E | E | (E/L) 3-5 h pi | E | DNA replication | [54] |
| ul7 | ?E | E | ? | ND | L | (E/L) dE | E/L | unknown | [54] |
| ul6 | L | E | ? | ND | L | (E/L) dE | L | capsid protein, portal protein | [54] |
| ul5 | E | E | E | E | E | (L) 6 h pi | L | DNA replication | [55] |
| ul4 | E | E | ? | L | L | (L) 6 h pi | E/L | unknown | [55] |
| ul3.5 | - | - | - | - | - | E/L | L | replication, cell-to-cell spread | [55] |
| ul3 | L | L | L2 | L2 | L | E/L | L | unknown | [55] |
| ul2 (*UNG) | E | E | E | L | E | E/L | L | DNA repair | [55] |
| ul1 (gL) | E | EL1 | L | L | L | E/L | L | viral entry, cell-to-cell spread | [55] |
| ep0 (*ICP0) | IE | IE | IE | IE | E | E | E | gene regulation | [10] |
| llt1 | L | ND | ND | ND | ND | ND | L | latency | [56] |
| llt2 | L | ND | ND | ND | ND | ND | E | latency | [56] |
| ie180 (*ICP4) | IE | IE | IE | IE | IE | IE | E/L | gene regulation | [13] |
| us1 (*RSp40/ICP22) | IE | IE | IE | IE | IE | IE | L | regulator of gene expression | [27] |
| us3(PK) | E | E | E | E/L | E | E | E | nuclear egress | [27] |
| us4(gG) | E | E | L | L | E/L | E | E/L | unknown | [57] |
| us6 (gD) | L | E | L1 | E/L | E/L | ND | E/L | entry | [27] |
| us7 (gI) | E | E | L | L | L | (L) 6 h pi | E/L | cell-to-cell spread | [27] |
| us8 (gE) | E | E | L2 | L2 | L | E | E/L | cell-to-cell spread | [58] |
| us9 (*11K) | E | E | ? | L | L | L | E/L | anterograde spreed of virus | [58] |
| us2 (*28K) | E | E | ? | L | L | (L) 5 h pi | E/L | unknown | [27] |
This Table demonstrates the function and kinetic classification of PRV genes in comparison with available data on HSV and PRV genes.
a PRV genes were classified on the bases of the ratio of the PAA-treated and untreated samples, at 6 h pi.
b Gene functions in italics rely on studies of the HSV-1 homologs.
Figure 4.
Clustering of genes on the basis of the effects of PAA on gene expression. Horizontal bars show the PRV genes ranked on the basis of Ri-PAA values in an increasing sequence. Early and late gene clusters were separated by a set of genes labeled as E/L using arbitrarily given threshold values.
Kinetics of PRV gene expression in untreated cells
Real-time RT-PCR is a high-resolution technique, which allows a detailed analysis of gene expression dynamics and a reliable temporal classification of viral mRNAs without drug treatment. The data from these experiments are presented in detail in the text [see Additional file 2 and 4]. The us3 PRV gene peaks at 4 h, llt2 at 2 h, while the other genes reach their maximum at 6 h within the 0-6 h examination period.
0-6 h incubation period
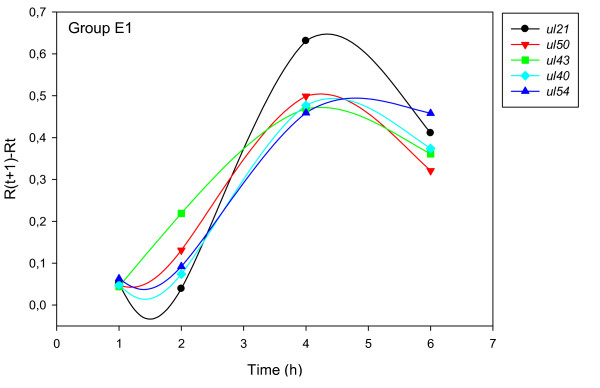
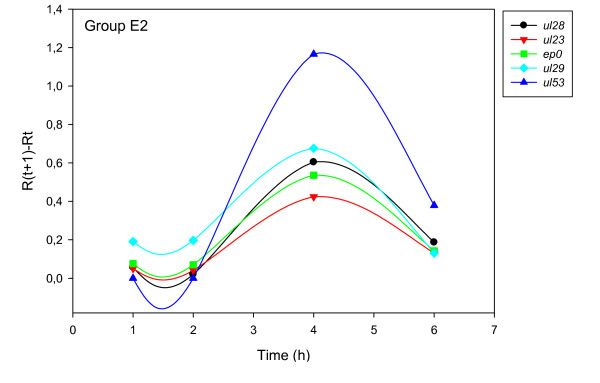
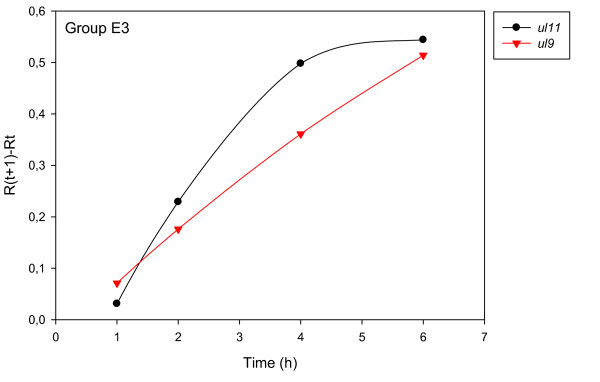
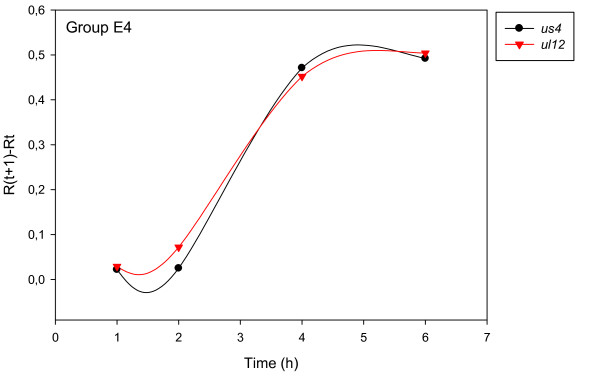
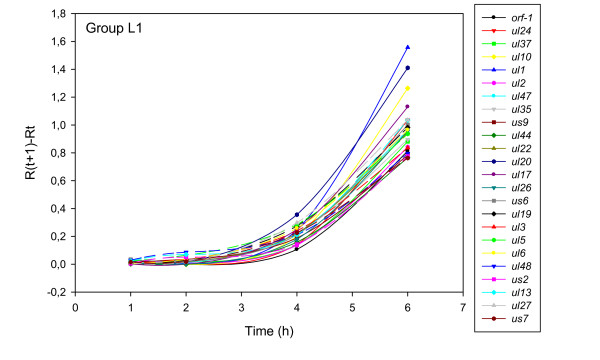
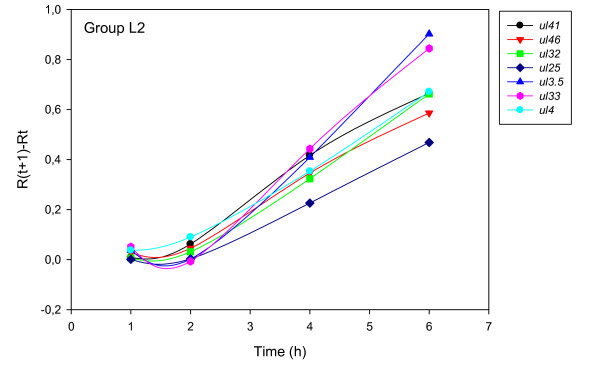
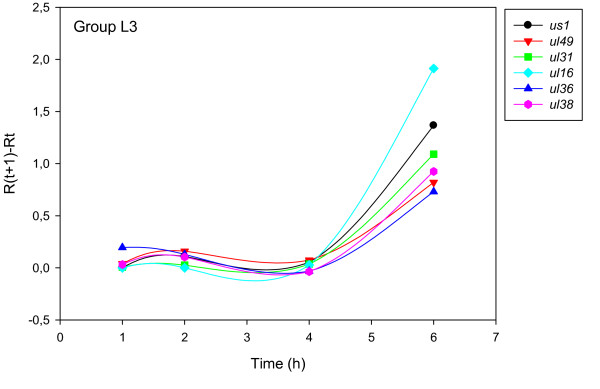
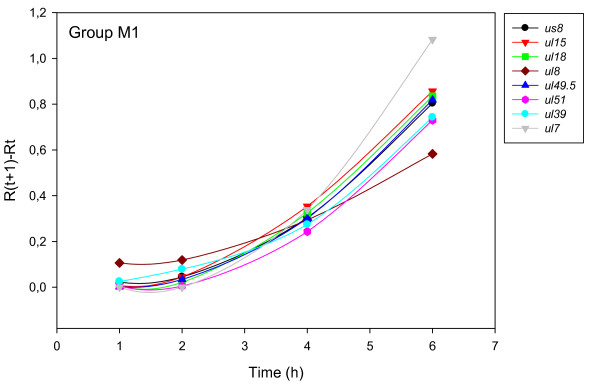
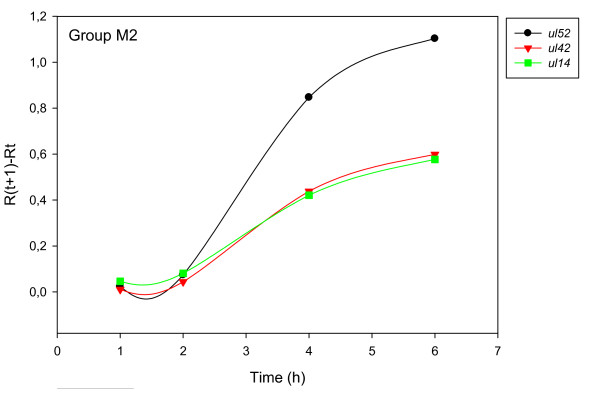
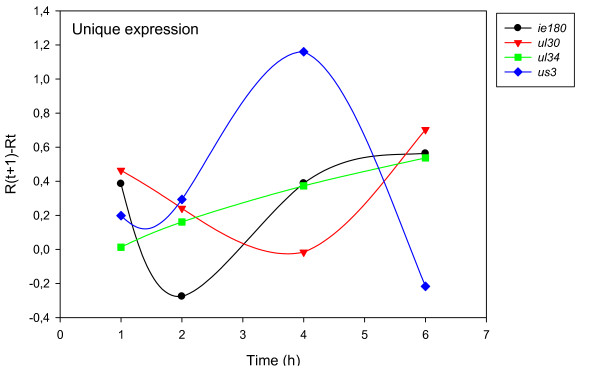
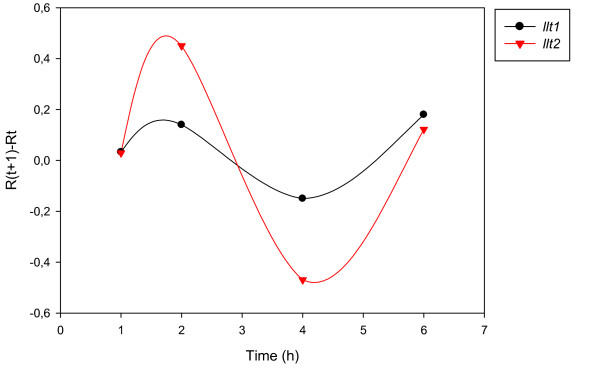
The accurate and reproducible data generated by real-time PCR allow the analysis of gene expression throughout the entire time course of infection. In order to classify genes on the basis of their expression kinetics, we performed Pearson's correlation analysis for each gene pair of the PRV, using the net increase (RΔ) in each time interval for the comparison [see Additional file 4b]. Genes were clustered into the same group if their pairwise correlation coefficients were high, ranging from r ≥ 0.9 to 0.99 depending on the group. Surprisingly, genes belonging in a particular group very rarely give similarly high values as any of the genes belonging in other groups. Albeit Pearson's coefficient expresses the kinetic properties of a gene in single numbers, the expression curves of genes belonging in the same group proved to be very similar (Figures 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15). We distinguished 10 gene sets. Group L1 of PRV genes (each pair shared r ≥ 0.989 values) contains 24 genes, of which 22 were identified as L and two as E/L genes by our PAA analysis. The only exception was ul13 which although an L gene in the HSV, behaved as an E gene in all of our analyses. The profile curves of the genes clustered on the basis of Pearson's correlation of pairwise RΔ values, gave very similar patterns of expression within the groups. In each period of time, the expression curves can also be characterized by the formula: RΔ(t+1) - RΔt (Table 2). For example, group L1 was characterized by hardly any change in the first 2 h, a moderate rise in mRNA level between 2 and 4 h, and a sudden increase in mRNA copy number from 4 to 6 h (0/+/++). Group L2 (r ≥ 0.99) contains one E/L and 6 L genes. The expression profiles of these genes are characterized by a continuous intensive increase from 2 to 6 h (0/+/+). Our PAA analysis revealed that all of the 6 members of group L3 (r ≥ 0.99) are L genes, except for us1, which is an IE gene in the HSV. In our system, therefore, most of the data (except the relatively high R2h for us1), suggest that it is an L gene. This group is characterized by the expression profile 0+/0-/+++. Group E1 (r ≥ 0.91; with 5 members) and group E2 (r ≥ 0.901, with 5 members) comprise E genes, except for ul53, which is an L gene in the HSV. However, this gene is characterized as an E gene in the PRV [39], which was confirmed by our PAA analysis. These genes are characterized by the expression profile: 0+/+/-. Group E3 (r = 0,964, 2 members: ul11 and ul9), expression profile: +/+/0+; Group E4 (r = 0.996, 2 members: ul12 and us4), profile: 0/+/0. Group M1 (r ≥ 0.933) is composed of 8 members, shown by our PAA analysis to exhibit either E or L kinetics. The expression profiles of these genes are very similar (0/+/++), which draws attention to the fact that PAA analysis alone is not sufficient for the grouping of genes with a similar expression profile. The 3 members of group M2 exhibit a very high correlation (r ≥ 0.998) and similar expression profiles (0/++/+). Group LLT (r > 0.946) contains 2 llt genes that have an unusual expression profile (++/--/+++). The following 4 genes cannot be clustered on the basis of Pearson's coefficients: ie180 (--/++/++), ul30 (--/--/++), ul34 (+/+/+) and us3 (+/++/---), which are likewise unusual Indeed, these genes have peculiar expression profiles (Figure 16). The expression profile can also be visualized by heatmap presentation, using the expression data generated with the RΔtvalues (Figure 17).
Figure 5.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 6.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 7.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 8.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 9.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 10.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 11.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 12.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 13.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 14.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Figure 15.
Grouping and plotting of PRV genes using their RΔ values. Pearson's correlation analysis was performed to group the PRV genes into clusters on the basis of similar expression dynamics (using pairwise RΔ values) over the four time intervals.
Table 2.
Grouping PRV genes on the basis of expression curves
| group | L1 | L2 | L3 | E1 | E2 | E3 | E4 | M1 | M2 | LLT |
| profile | 0/+/+++ | 0/+/+ | 0/0/+++ | 0/+/- | 0/++/-- | +/+/0+ | 0/+/0 | 0/+/++ | 0/++/+ | ++/--/+++ |
| gene | ie180 | ul30 | ul34 | us3 | ||||||
| profile | --/++/++ | --/--/++ | +/+/+ | +/++/--- |
Gene expression profiles of PRV gene clusters were characterized by plus and minus signs at various time intervals [Rt(2h-1h) - R(t1h-0h) ; Rt(4h-2h) - R(t2h-1h); Rt(6h-4h) - R(41h-2h)]. The numbers of plus or minus signs indicate the relative rate of increase or decrease of transcripts.
Figure 16.
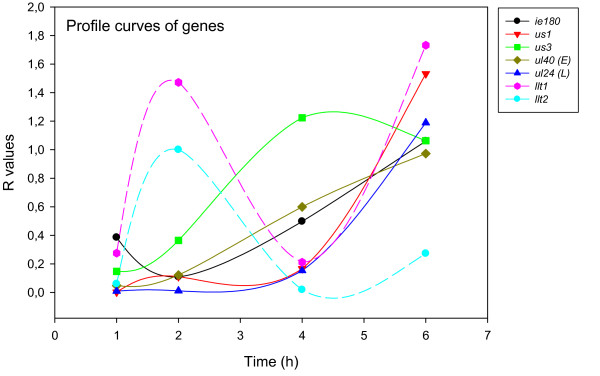
Expression kinetics of some selected genes via their R values. This Figure shows the expression curves involving the R values of the only IE (ie180) gene, a typical E gene (ul40) and a typical L (ul24) gene, two genes with irregular expression profiles: us1 (local minimum at 4 h pi) and us3 (maximal expression at 4 h pi), and llt1 and llt2.
Figure 17.
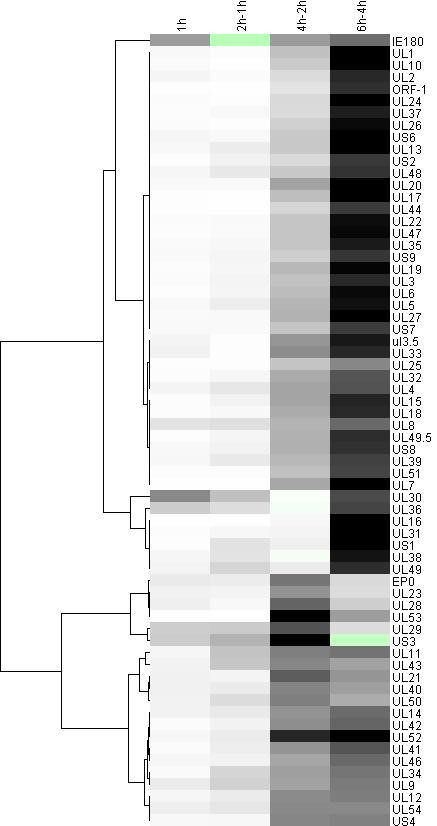
Clusters of PRV RΔ values identified by the hierarchical clustering method. The dendogram depicts the whole-genome profiling of protein-coding PRV genes, following de novo infection of PK-15 cells. Green indicates negative RΔ values, white low RΔ values, gray intermediate RΔ values, and black the highest level of increase in viral mRNA detected between two time points.
Co-regulated gene clusters
We utilized our data to analyze whether the expression properties of PRV genes localized in adjacent genomic loci display similarities.
PAA analysis
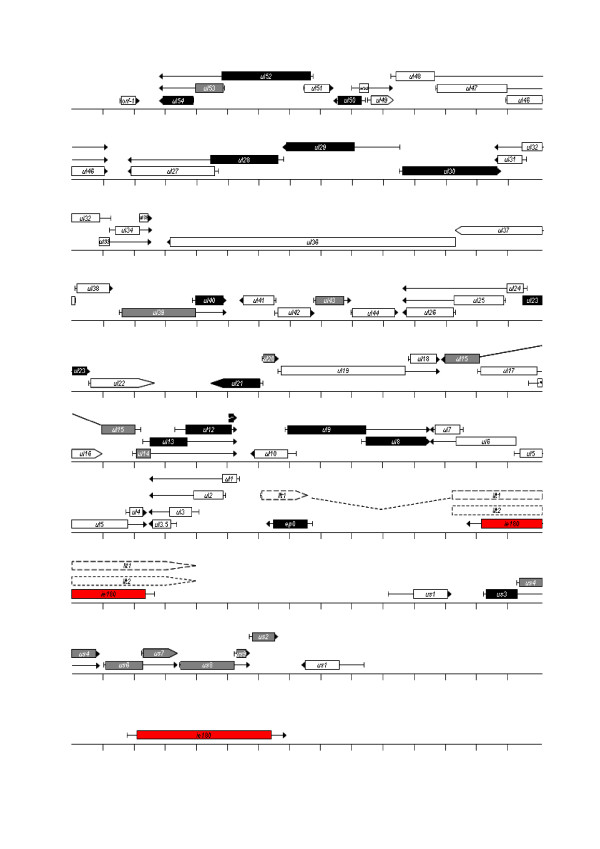
Figure 18 shows an intriguing relationship between genes categorized on the basis of the data of the PAA experiments. Nested 3' co-terminal genes were found to belong in the same kinetic classes, indicating the existence of shared regulatory mechanisms of gene expression. An exception is the late ul27 gene, which forms a cluster overlapping in parallel with the ul28 and ul29 genes expressed with E kinetics. Of the 11 divergent pairs, 6 genes were found to belong in the same and 5 in different kinetic classes. A further special feature of the PRV genome is that the convergent genes mostly belong in different kinetic classes (Figure 18). The potential role of such organization of the herpesvirus genome is discussed later. It may be noted that most US genes belong in the E/L kinetic class accounting for 37.5% of this group.
Figure 18.
PRV genome. PRV genes are represented as arrows, which indicate their relative lengths, orientations and kinetic classes determined by PAA analysis. Red indicates IE kinetics, black E kinetics, gray E/L kinetics, and white L kinetics.
Pearson's correlation
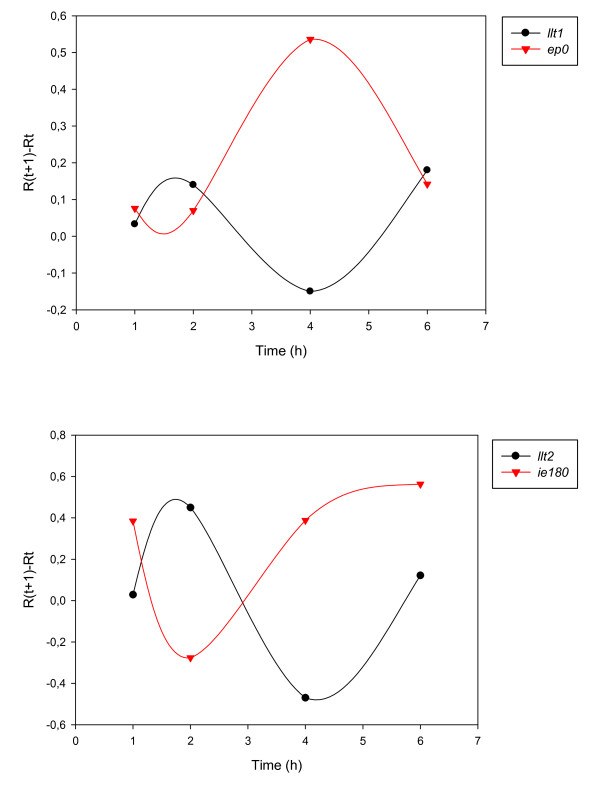
Analysis of the gene clusters generated by using Pearson's correlation coefficients revealed that genes within the same clusters are often located in neighboring genomic positions. Group L1 is composed of 24 members. The adjacent ul1, ul2, ul3, ul5 and ul6 genes are members of this group. The high pairwise correlation coefficients and the similar profile curves of the RΔ values of these genes cannot be explained by the nested co-localization alone, because ul5 and ul6 are divergent genes and do not overlap with ul1, ul2 and ul3. The ul44 gene is convergent with ul24 and ul26; and ul22 is divergent with ul20. Moreover, ul27 is convergent with ul47 and ul48. It should e borne in mind that ul26 and ul27, an adjacent gene pair in the HSV, are relocalized into a remote position in the PRV, due to a large inversion, but retain their expression properties. This is also true for ul44, ul47, and ul48 (there is no ul45 gene in the PRV). Several genes (us2, us6, us8 and us9) in the US region of the PRV genome belong in this group, too. The ul17 and ul19 genes, which are oriented parallelly, but non-overlapping, likewise belong in group L1. The ul34 and ul35 genes are in a divergent position with ul37. Group L2 is composed of 7 members. The ul32 and ul33 genes are divergently oriented, while ul4 and ul3.5 are convergent genes. Group L3 is a small group with 6 members. The ul36 and ul38 genes are arranged in a divergent orientation. Group E2: Two of the 4 members of this group, ul28 and ul29, are overlapping genes. Group E3: Two (ul11 and ul12) of the 4 members of this group are divergently oriented with the third member, ul9. Group M1: ul7 and ul8 are a convergent gene pair. The ul49.5 and ul51 are L genes oriented in parallel and separated by the divergent early ul50 gene. Furthermore, ul15 and ul17 are a convergent adjacent gene pair. Genes with similar expression profiles appear to be under a common regulation, which cannot be explained solely by the nested localization (these genes also have common promoters) of genes. Group LLT: the llt1 and llt2 expression patterns show a high degree of similarity. However, there is noteworthy inverse relationship in the expression profiles of LLT1 and LLT2 with those of their complementary partners, EP0 and IE180 mRNAs, respectively (Figure 19).
Figure 19.
Inverse expression profiles of sense/antisense transcripts. The expression curves of RΔ reveal show an inverse relationship between the mRNAs of genes and their antisense transcripts: ep0/llt1 and ie180/llt2.
Discussion
Gene expressions of α-herpesviruses have already been analyzed by various techniques including Northern-blot and microarray analysis. Different authors have often assigned the same genes to different kinetic groups, which may be explained by the poor resolution of the applied techniques. Only partial data are available on the expression of PRV genes. Flori et al. [27] carried out genome-wide gene expression analysis via a microarray technique, but this approach did not produce evaluable data on the kinetic properties of the PRV genes. We employed a novel qRT2-PCR technique using (1) strand-specific primers for the RT to obtain a higher yield and to eliminate the effects of the potential antisense transcripts; and (2) the average maximal ECt values as the controls for the calculation of relative expression ratios (Rs). Other approaches, such as the models of Pfaffl's [62] and Souazé et al., [63], and the ΔCt and ΔΔCt models [64] are simpler than the model we used; however, they all neglect the variation in the amplification efficiencies between cDNA samples, which was present in our system. Traditionally, herpesvirus genes are grouped into kinetic classes on the basis of gene expression inhibitory effects of protein and DNA synthesis-blocking reagents. The IE genes should not be significantly inhibited by any of the above substances, the E genes are inhibited only by DNA synthesis blockers, and the expression of the L genes is substantially inhibited by both protein and DNA synthesis blockade. We grouped the PRV genes into IE, E, E/L and L categories on the basis of the results of CHX and PAA treatment (Table 1), [see Additional file 3]. Our findings conformed well with those published for the HSV genes. The expression of the ie180 gene is enhanced by CHX treatment. The explanation of this phenomenon lies in the fact that the ie180 protein normally inhibits its own expression a short time after the onset of virus infection, a feature which is absent in CHX-treated cells due to novel protein synthesis being blocked by the drug. The other PRV genes were inhibited to a significant extent by CHX at every examined time point. Consequently, ie180 is the only IE gene of the PRV. The ep0 and ul54 genes (IE genes in the HSV) were shown to be E genes by others [[10] and [11]], and this was confirmed by our analyses. Interestingly, us1, which is an IE gene in the HSV, appeared to be an L gene in most of our analyses: its expression was significantly inhibited by PAA; it afforded a low amount of mRNAs at 1 h pi, and a high rate of increase of transcript level at 4-6 h pi, all of these being L characteristics. Furthermore, together with a set of L genes, us1 belongs in group L2 according to the clustering of the genes on the basis of pairwise Pearson correlation coefficients. The moderately high relative amount of us1 transcripts at 2 h pi is the only E-like characteristic of this gene. However, us1 displays a high Ct value, suggesting a low absolute amount of transcripts at 2 h pi. We may mention here that the Ct value of a transcript is dependent on the primer efficiency; these data therefore cannot be reliably used to the estimate the absolute transcript amount. If the us1 gene has an important function early in the lytic cycle of the virus, it might fulfill this without de novo synthesis by being released upon infection from the tegument layer of the virus where it might be incorporated. Our data do not demonstrate a clear-cut demarcation line between the groups of E and L genes, suggesting that the categorization of herpesvirus genes on the basis of the requirements for DNA replication might be arbitrary. The kinetic types of genes identified by our PAA analysis resemble homologous HSV genes more than the published PRV genes, which can be explained by the semiquantitative methods used for the kinetic analysis of the PRV genes. The present study revealed that temporal classification of herpesvirus genes is also possible through study of the gene expression of untreated cells in consequence of the high sensitivity of the real-time RT-PCR technique. As a further interesting point, we detected mRNAs by qRT2-PCR in all but 3 PRV genes as early as 1 h pi [see Additional file 4]. This observation indicates that PRV genes are either leaky or subject to regulatory mechanisms which have not yet been elucidated. We found that E genes are generally expressed in a higher proportion than L genes at 1, 2 and 4 h, as indicated by their higher R values at these time points [see Additional file 4a]. The E gene products proved to display a higher net increase (RΔ) than the L genes within the 0-1 h, 1-2 h and 2-4 h pi periods. However, by the 4-6 h pi interval, this trend had definitely reversed, i.e. the L genes exhibited high R(6h-4h) values (Additional file 4b). Unexpectedly, the ratio of increase (Ra) between 1 and 2 h was higher for a number of L genes than any of the E genes [see Additional file 4c]. Since the R2h values of these L genes were lower than those of the E genes, the high R(2h/1h) value is a result of the very low R1h values of late genes. Most of the L genes also have higher R(4h/2h) and R(6h/4h) values than those of E genes, which is explained by the fact that the E genes reach a relatively high expression level by 1 h pi. The classification of the PRV genes through the CHX and PAA analyses led to results very similar those generated by the analysis of untreated cells via the following calculation: R(6h-4h)/R(1h-0h) [see Additional file 5]. The differences between the results of the two approaches can be explained in that PAA analysis alone gives only a rough picture of the gene expression; a detailed kinetic analysis of the viral gene expression furnishes a more sophisticated picture. Some genes classified as E or L by PAA analysis display "irregular" kinetics under untreated conditions. The ie180 and perhaps us1 genes cannot be classified by PAA analysis alone. Analysis of the transcription kinetics of overlapping transcript sets is an important issue. It is not known whether downstream genes on polycistronic mRNAs are translated, and if so, to what extent. This makes interpretation of the mRNA expression data difficult because the mRNA levels cannot be correlated with the amounts of the corresponding proteins, which are the workhorse molecules in regulating cellular physiology. In principle, a downstream gene is translated if it is transcribed from its own promoter. Theoretically, downstream genes could also be translated from a polycistronic RNA if alternative splicing removed upstream gene(s) from the pre-mRNA, or if potential IRES-like sequences helped recruit ribosomes, thereby initiating a cap-independent translation from the downstream gene (we have found no data in the literature concerning these possibilities). Thus, analysis of the downstream genes without discriminating between transcription from their own promoters or by read-through from upstream genes might result in a false categorization. The PRV genes were also classified by analysis of the gene expression throughout the entire examination period by using Pearson's correlation analysis. Genes with similar expression profiles (high pairwise r values) were placed in the same group. Genes in the same kinetic class (identified by PAA analysis) are generally situated in the same group generated by the use of Person's correlation. Moreover, members of a particular group (with high r values) rarely display high correlation coefficients with genes belonging in different groups. The PRV genes conformed well to 10 patterns of expression. Only 4 viral genes (ie180, ul30, ul34and us3) did not fit into any of the 10 groups. We found an inverse expression profile of the sense/antisense partners in the ep0-llt1 and ie180-llt2 genes (Figure 19). Combination of the untreated data with the results of the CHX and PAA analyses revealed an interesting relationship for coordinated regulation of sense/antisense partners. The CHX analysis suggested that IE180 protein facilitates LAP (latency-associated transcript promoter) activity, and inhibits ASP (putative antisense promoter activity). The PAA treatment resulted in a significantly elevated antisense transcript level at 3 of the 4 time points in LLTs, which indicates the existence of another regulatory level besides IE180 protein action. We assume that transcription from one DNA strand negatively influences the expression of transcripts from the complementary DNA strand. The interaction can occur at the level of transcription (RNA polymerase moving in one direction along one of the DNA strands inhibits RNA polymerase moving in another direction) and/or translation by forming double-stranded RNAs by the sense and antisense transcripts. As an example, PAA has a negative effect on the transcription of ie180 (the level of IE180 mRNA is reduced to a quarter) at 4 h pi, which results in a lower rate of transcription from ie180 (thereby facilitating the expression of LLT2); and a lower amount of inhibitory IE180 proteins, which also facilitates LLT2 expression. Overall, the LLT2 level increases 39.4-fold relative to the untreated conditions. Furthermore, genes with the same kinetic properties exhibit a distinctive distribution pattern along the PRV genome. Nested genes appear to belong in the same kinetic group. Additionally, convergent genes and gene clusters in most (3 out of 11) cases belong to different kinetic classes. It should be remembered that the PAA-based approach and the time intervals applied for kinetic analysis produce only a coarse resolution of gene expression. It is possible that convergent genes allocated to the same kinetic classes might display different expression profiles on a finer scale. The above genome organization principles may point to the existence of as yet unknown regulatory mechanisms. It may be speculated whether a read-through of transcription across convergent genes is the basis of this regulation. Moreover, groups formed in terms of high Pearson's correlation coefficients contain many genes localized at adjacent loci on the PRV genome. Most genes with high correlation coefficients are not nested genes. This means that the similarity of their expression profiles cannot be explained by the control of a common promoter. Further, several genes in the same group are separated by genes that display different kinetic profiles. These results suggest the existence of a genetic mechanism that synchronizes gene expression on a higher-order (chromatin?) scale.
Conclusion
Although the relative amounts of the transcripts between genes cannot be compared because primer efficiencies may vary both in RT and in PCR, our method allows a comparison of genes on the basis of their expression dynamics. We detected the expression of almost all genes from the earliest time point (1 h), Further, we have found that a sharp boundary cannot be drawn a between the groups of E and L genes in terms of expression profiles. We have also found that the PRV has only a single IE gene (the ie180 gene) and that the us1 gene is expressed in L kinetics. Our kinetic studies revealed that PAA analysis alone is not sufficient for the classification of genes on the basis of their expression profiles. The analysis of gene expression throughout the 6 h examination period demonstrated that these genes can be subdivided into further clusters, and that genes showing significant differences in response to PAA treatment can have very similar expression characteristics in untreated conditions. In our analysis, nested genes displayed the same expression profiles, whereas convergent genes mostly exhibited different kinetic properties. It should be noted that a finer resolution of the gene expression might expose slight differences in the expressions of nested genes. Approximately half of the divergent genes belong in the same class, and the other half of them in different kinetic classes. The new calculation technique that we have developed is also applicable to evaluate the loss-of-function phenotypes of mutant herpesviruses viruses and, in principle, for the analysis of gene expression in every temporally changing genetic system.
Methods
Cells, viruses and infection conditions
Monolayer cultures of immortalized porcine kidney 15 (PK-15) cells were maintained at 37°C in an atmosphere of 95% air, 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% fetal bovine serum (Gibco) and 80 μg gentamicin per ml. Strain Kaplan of pseudorabies virus (PRV-Ka) was used for gene expression analysis. The virus stock was prepared by infecting PK-15 cells with 10 plaque forming units (pfu)/cell PRV-Ka followed by incubation of the cells until a complete cytopathic effect was observed. For the analysis of the PRV transcriptome, rapidly-growing semi-confluent PK-15 cells were infected with a low multiplicity of infection (MOI; 0.1 pfu/cell) of the virus, and incubated for 1 h, after which the virus suspension was removed and the cells were washed with phosphate-buffered saline (PBS). Subsequently, new culture medium was added to the cells, which were further cultivated for 0, 1, 2, 4 or 6 h. Cells were incubated in the presence or absence of 100 μg/ml cycloheximide (CHX), a translation inhibitor, or 400 μg/ml phosphonoacetic acid (PAA), an inhibitor of DNA synthesis (both purchased from Sigma-Aldrich) 1 h prior to virus infection. Mock-infected cells, treated in the same way as infected cells, were used as controls.
Preparation of viral DNA
Viral DNA was used to test the efficiency and specificity of the primers applied in real-time PCR. The viral DNA was purified as follows. Monolayers of PK-15 cells were infected with the PRV at an MOI of 10, and cultivated at 37°C until a complete cytopathic effect was observed. Subsequently, culture medium was collected without disrupting the cells and clarified by centrifugation at 4,000 rpm for 10 min using a Sorvall GS-3 rotor. Next, the virus in the supernatant fluids was sedimented on a 30% sucrose cushion by ultracentrifugation at 24,000 rpm for 1 h using a Sorvall AH-628 rotor. The sedimented virus was resuspended in sodium Tris-EDTA buffer. After the addition of proteinase-K (l00 μg/ml final concentration) and sodium dodecyl sulfate (SDS; 0.5% final concentration), the lysate was incubated at 37°C for 1 h, which was followed by phenol-chloroform extraction and dialysis.
Primers
Primers were designed through use of the Primer Express (Applied Biosystems) and FastPCR Professional (Primer Digital Ltd.) oligonucleotide design software according to the given guidelines. Primer pairs were designed from the 3'-end regions of ORFs for each gene (Table 3). The ul26 and ul26.5, and orf1 and orf1.2 genes contain overlapping ORFs; and we therefore, did not employ distinct primers for them. Primer specificity was verified by BLAST searches of the GenBank database (National Center for Biotechnology Information (NCBI) website http://www.ncbi.nlm.nih.gov/BLAST/. All primers were purchased from Bio Basic Inc. (Mississauga, Ontario, Canada).
Table 3.
Primer sequencesa
| Nameb | Forward primer sequence (5'-3')c | Reverse primer sequence (5'-3')d | Primer coordinatese | Size of amplicon (bp)f | |||||||||||||||
| orf1 | GCC | TCG | TGT | TTC | TGA | TCC | TGC | TC | AAC | ACG | ACA | GAC | TCC | CGG | AGC | A | 2204-2252 | 49 | |
| ul54 | TGC | AGC | TAC | ACC | CTC | GTC | C | TCA | AAA | CAG | GTG | GTT | GCA | GTA | AA | 2730-2795 | 66 | ||
| ul53 | CCA | AGG | CGC | TGT | ACC | TCT | G | TGT | GCC | GCT | CAT | AGT | GCA | G | 3912-3977 | 66 | |||
| ul52 | CGC | GCA | ACT | TTC | ACT | TCC | ACG | CA | TGC | GCT | CGA | AGA | AGC | TCT | CGT | A | 5325-5376 | 93 | |
| ul51 | GCT | CAT | GCA | CCT | GTA | CCT | CTC | G | ACG | TCG | GAC | ATC | ACC | ACG | TTG | C | 7989-8096 | 108 | |
| ul50 | CTT | CTT | CGA | GGT | CTT | TGC | GC | ATG | TCG | TAT | CCG | GCG | TCC | T | 8921-8971 | 51 | |||
| ul49.5 | TGA | CAT | TTT | ATA | TCT | GCC | TCC | TGG | TGC | AGC | ATC | CGG | GTG | C | 9447-9525 | 79 | |||
| ul49 | CAT | CAC | CGT | GTG | CGA | G | CTT | TTC | CCT | TCC | GCC | CG | 10247-10329 | 83 | |||||
| ul48 | AGG | TGC | GGA | TCA | AGA | TGG | AG | CAG | TAC | GTG | TGG | TCG | CGG | T | 11305-11359 | 55 | |||
| ul47 | GAG | CTC | ATG | GAC | GCG | CTC | CTG | GCG | CAT | GAC | GGC | GTC | 13684-13767 | 84 | |||||
| ul46 | TGC | TGT | GGT | CGA | CGT | TCG | TCT | GGG | CCT | TGT | GCT | TGA | A | 14435-14494 | 60 | ||||
| ul27 | ACT | ACG | AGG | ACT | ACA | ACT | ACG | TGC | G | GTC | ACC | CGC | GTG | CTG | ATC | 17512-17575 | 64 | ||
| ul28 | GCG | CTC | TAC | TTT | GCG | GTG | GAG | AA | GAA | CTC | GCT | GAC | GCA | CCA | ATC | G | 19781-19879 | 117 | |
| ul29 | CTG | ATC | CTG | CGC | TAC | TGC | G | ACT | GCA | TCG | TGA | TCC | CCG | 22609-22675 | 67 | ||||
| ul30 | TCA | TCA | CGA | AGA | AGA | AGT | ACA | TCG | G | CCT | TCA | TGA | GCA | TCT | TGC | CG | 27959-28015 | 57 | |
| ul31 | GTG | GCA | GAC | CAT | GTT | CGT | GT | GGT | CTC | CCG | TCT | CCC | TTC | TT | 28809-28859 | 51 | |||
| ul32 | TGC | TCA | GCT | ACT | CGG | AGA | ACA | AC | CAC | GGG | CTC | GAT | GCA | GTC | 29841-29893 | 53 | |||
| ul33 | CGC | GCG | AGC | TGG | AAG | T | TGC | GTG | TGG | GCC | AGA | TAA | 31031-31094 | 64 | |||||
| ul34 | AGC | ACA | ACA | ACG | TGA | TCC | TGG | GAG | AGG | CTC | ATG | CCG | GTG | 31696-31747 | 52 | ||||
| ul35 | ATC | ATG | TCC | TTC | GAC | CCG | AAC | GAG | CGT | CTG | CGC | GGT | GA | 32238-32288 | 51 | ||||
| ul36 | CGT | CGG | TGG | GTA | TTA | GAG | ACC | A | GAA | CAA | GAG | CCA | TGG | ATT | TTC | G | 34451-34501 | 51 | |
| ul37 | CTA | CGA | CAT | GGA | CTT | TGT | GCA | GGA | AGT | TGG | TGT | GCT | GCG | CCA | CGT | A | 44556-44611 | 56 | |
| ul38 | CAC | CCG | GAA | CTC | GTG | CAT | GTA | CAG | AAG | AAA | CAC | TCC | TCC | CAC | G | 46118-46273 | 156 | ||
| ul39 | GCT | GGC | CAA | GTT | CAA | GAC | G | CGC | ACA | TGT | CGA | TGA | GCA | G | 48701-48759 | 59 | |||
| ul40 | GGA | CTT | TCC | CAT | GGC | GC | GCT | CGA | AAA | AGT | TGG | TGT | GCT | T | 49802-49854 | 53 | |||
| ul41 | TGA | AGA | ACG | AGA | CGC | GGG | TGT | GTT | TCC | AAA | ACA | GGC | CC | 50475-50525 | 51 | ||||
| ul42 | GCT | CCC | CGA | GCG | TCG | CAT | GAT | GCA | GTA | GTC | GTT | GAA | CTC | 52118-52173 | 56 | ||||
| ul43 | CTG | GTG | CAG | GCG | TAC | GTG | A | GGA | TTT | AAT | GCT | AGT | GGC | GCA | 53904-53954 | 51 | |||
| ul44 | TCG | TGA | GCA | GCA | TGA | TCG | T | GTC | GCC | ATG | ATG | ACC | AGC | 55368-55437 | 70 | ||||
| ul26 | TTC | TTC | CTC | GGC | GTC | GTC | AAC | TG | GTA | GTT | GCT | CAG | CAG | GTA | CAG | CA | 56962-57087 | 126 | |
| ul25 | GGC | AGT | TTG | GCG | TCT | CCA | G | CCA | GGC | AGA | GAA | AGT | ACA | GGA | GG | 57336-57386 | 51 | ||
| ul24 | TGT | GCT | TCG | TCA | TCG | AGC | TC | TGG | GCG | TGT | TGA | GGT | TCC | 59249-59305 | 57 | ||||
| ul23 | ATG | ACG | GTC | GTC | TTT | GAC | CGC | CAC | CGC | TGA | TGT | CCC | CGA | CGA | TGA | A | 59818-59898 | 81 | |
| ul22 | GGC | GGC | CAT | CAC | CGT | GAG | AAT | AGC | CCT | CGG | AGG | AGA | 62574-62647 | 74 | |||||
| ul21 | TCA | GCT | GTT | TCG | GGC | GC | ATT | GAG | GAC | GAT | GGA | GAT | GTT | GG | 64500-64551 | 52 | |||
| ul20 | GAG | AAC | GAC | GCG | CTG | CTG | AG | CAG | GAG | GCT | CAC | CAC | GTG | 66226-66309 | 84 | ||||
| ul19 | TCT | TTG | CGG | AGA | AGG | CCA | G | GCT | CTC | GGT | GCG | CGC | 69841-69905 | 65 | |||||
| ul18 | TGG | TGC | TGA | ACA | TGA | TCT | TCC | GGA | TGA | GCG | ACA | GCA | GGA | T | 71443-71502 | 60 | |||
| ul17 | GGC | GTT | TTC | CTC | TTC | GAC | TAC | TAC | CAC | CCT | TAT | AAC | CTC | CCC | GC | 74663-74713 | 51 | ||
| ul16 | CTG | TGG | CGC | CAG | GCG | GAC | AA | GAT | CTT | GCG | GCG | GGG | GAG | CAT | G | 75670-75780 | 111 | ||
| ul15 | CAG | AAG | CAA | AAG | ACC | CCC | G | CGA | GTT | GAA | CTG | CTT | GAC | GAA | A | 72204-72254 | 51 | ||
| ul14 | CGG | ACA | AGA | AAA | ACC | CCG | AG | CCT | GTT | TGG | CCG | CCA | TAA | A | 77167-77217 | 51 | |||
| ul13 | AGC | CAC | CTG | GAC | GTC | AAG | G | CCA | TGA | GGC | TAA | AGT | CCC | CG | 78083-78173 | 91 | |||
| ul12 | GCA | GAC | GGA | GAT | GCG | CTT | CCG | AGA | ACA | GGT | ACT | TGG | CG | 79394-79449 | 56 | ||||
| ul11 | ATG | GGA | CAG | TGT | TGC | TGC | C | TCA | AAG | TCC | TCG | AAC | GCG | T | 80084-80178 | 95 | |||
| ul10 | CAT | TTT | GTG | TTT | CTC | GCC | CTC | TTT | ACC | CGT | GCC | CTT | GCA | GG | 81390-81443 | 54 | |||
| ul9 | CAA | GTT | CAA | GCA | CCT | GTT | CGA | TGA | GGC | TGT | CGT | TGA | CGC | 83337-83392 | 56 | ||||
| ul8 | CCG | CTG | ATC | CTG | CCC | TG | GAA | GAT | GGG | CTC | CAT | GTG | G | 86355-86405 | 51 | ||||
| ul7 | TCC | GCG | GGT | TCG | CCT | TC | AGC | GAG | AGC | ACG | CGG | TC | 87217-87271 | 55 | |||||
| ul6 | CAG | GAG | CTG | ATC | CGC | TGC | TGT | TGG | AGT | ACG | AGA | CGG | ACA | C | 87687-87765 | 79 | |||
| ul5 | TGG | ACA | TGG | CCA | CCT | ACG | T | ACC | GCG | CGA | TGG | TCA | T | 91530-91592 | 63 | ||||
| ul4 | GAG | CGA | GAC | CGA | CCA | TGA | C | TTA | TTT | CGT | AAA | ACG | AGT | AGG | CCA | 91849-91899 | 51 | ||
| ul3.5 | CAA | CGG | AGC | ATC | AAC | GCC | TC | ATT | GAT | CGC | GGT | AGC | AAC | AGG | A | 92478-92658 | 181 | ||
| ul3 | CTG | GGC | GCA | GCA | CGT | TCC | ACG | GAC | GCG | ACC | ATA | 93502-93552 | 51 | ||||||
| ul2 | TTC | AGG | ACC | TGC | CCG | CAC | TTT | GGA | ACT | CAG | TCC | ACG | CTC | CAG | TCG | A | 93914-93999 | 86 | |
| ul1 | CTG | GTC | AAC | CCC | TTT | GTC | G | CGC | CTC | ATT | TAA | GGG | CTC | TC | 94928-94987 | 60 | |||
| ep0 | GGG | TGT | GAA | CTA | TAT | CGA | CAC | GTC | TCA | GAG | TCA | GAG | TGT | GCC | TCG | 96956-97006 | 51 | ||
| ie180 | CAT | CGT | GCT | GGA | CAC | CAT | CGA | G | ACG | TAG | ACG | TGG | TAG | TCC | CCC | A | 103988-104056 | 69 | |
| us1 | AGC | TCA | ACG | AGC | GCG | ACG | TCT | A | CGG | AAG | CTA | AAC | TCG | GAC | GCG | A | 116466-116602 | 137 | |
| us3 | GGG | CTT | TCC | TGA | TTT | ACA | AGA | TGT | AAG | GGC | GGC | GGA | CG | 119245-119295 | 51 | ||||
| us4 | ACC | TCG | ATC | TAC | ATC | TGC | GTC | G | GGC | CCT | GGT | GAT | CGC | CAT | 120764-120850 | 87 | |||
| us6 | TGG | AAC | GAC | GAG | AGC | TTC | AGG | GTA | GAA | CGG | CGT | CAG | GAA | TCG | 121690-121749 | 60 | |||
| us7 | CCC | GGG | AAG | ATA | GCC | ATG | AAG | AAG | ATC | AGG | AGG | ACG | ACG | 123144-123199 | 56 | ||||
| us8 | CTT | CGA | CGT | CTG | GTT | CCG | C | GGT | CAC | GCC | ATA | GTT | GGG | C | 125136-125202 | 67 | |||
| us9 | CAG | GAC | GAC | TCG | GAC | TGC | TA | AGG | AAC | TCG | CTG | GGC | GT | 125395-125453 | 59 | ||||
| us2 | CGG | CTG | GAC | ACG | GAG | TG | AGT | TCA | GGT | ACT | GGA | TCC | CGT | T | 126171-126222 | 52 | |||
| 28S | GGG | CCG | AAA | CGA | TCT | CAA | CC | GCC | GGG | CTT | CTT | ACC | CAT | T | 54 | ||||
a This table shows the list of primer pairs used for analysis of PRV gene expression:
b name of the primers
cforward primer sequences
dreverse primer sequences
e Coordinates refer to the PRV genome sequence in strain Ka (NCBI Reference Sequence: NC_006151.1).
fsize of the amplified products (amplicons) Underlined sequences were not available from strain Ka, and therefore we sequenced these DNA segments.
Polymerase chain reaction
A conventional PCR technique was used to check on the potential DNA contamination and the quality and specificity of the primers. The PCR reaction was carried out by standard methods on a Veriti™ 96-Well Thermal Cycler (Applied Biosystems) with the following parameters: 1 cycle of 94°C for 4 min; 30 cycles of 94°C for 1 min, 60°C for 1 min and 72°C for 2 min; and 1 cycle of 72°C for 7 min. The PCR reaction was carried out by using the GC Rich PCR System (Roche). The complete DNA sequence of the PRV is a composite of 6 different strains [65], but the variation between them is very small (around 1%). Most of the sequence data (86.7%) have been derived from strain Kaplan (PRV-Ka), which we used in our experiments. To circumvent the problem of genetic background differences, we tested 2 or more primers for RT and PCR reactions for non-Ka sequences and selected those that performed best; alternatively, we sequenced the particular DNA region and designed new primers on the basis of the sequence data. For each gene, we designed and tested various primers, and selected those, which did not produce primer dimers or other nonspecific products. For those genes where we could not eliminate the problem of primer dimer formation, in every cycle we applied an extra extension step with an elevated temperature (below the Tm of the specific product and well above the Tm of the primer dimers) for the detection.
RNA preparation
PK-15 cells (5 × 106 cells per flask) were washed in PBS and harvested for RNA isolation at 0, 1, 2, 4 and 6 h pi. For quantitative RT2-PCR, total RNA was extracted from the cells with the NucleoSpin RNA II Kit (Macherey-Nagel GmbH and Co. KG) according to the manufacturer's instructions. Briefly, cells were collected by low-speed centrifugation, lysed in a buffer containing the chaotropic ions needed for the inactivation of RNases and providing the conditions for binding of nucleic acids to a silica membrane. Next, contaminating DNA was removed with RNase-free rDNase solution (included in the NucleoSpin RNA II Kit). Subsequently, the RNA solution was treated with Turbo DNase (Ambion Inc.) to remove potential residual DNA contamination. Finally, RNA was eluted in RNase-Free Water (supplied with the kit) in a total volume of 60 μl. RNA concentration was determined in triplicate by spectrophotometric analysis of the absorbance at 260 nm with a BioPhotometer Plus (Eppendorf). The RNA solution was stored at -80°C until use.
Quantitative real-time RT-PCR
A two-step quantitative real-time RT-PCR was carried out for the transcriptional analysis. For each gene, a minimum of 3 independent replicates (separate infections) were performed.
Reverse transcription (RT) Total RNA isolated from infected cell cultures subjected to drug treatment (CHX or PAA) was reverse-transcribed into cDNA for PCR analysis. RTs were performed in 5 μl of solution containing 0.07 μg of total RNA, 2 pmol of the gene-specific primer, 0.25 μl of dNTP mix (10 μM final concentration), 1 μl of 5× First-Strand Buffer, 0.25 μl (50 units/μl) of SuperScript III Reverse Transcriptase (Invitrogen) and 1 U of RNAsin (Applied Biosystems Inc.) and the mixture was incubated at 55°C for 60 min. The reaction was stopped by raising the temperature to 70°C for 15 min. No-RT control reactions (RT reactions without Superscript III enzyme) were run to test the potential viral DNA contamination by conventional PCR. RNA samples with no detectable DNA contamination were used for quantitative RT-PCR reactions. First-strand cDNAs were diluted 10-fold with DEPC-treated water (Ambion Inc.), then subjected to real-time PCR analysis.
Real-time quantitative PCR experiments were performed with a Rotor-Gene 6000 cycler (Corbett Life Science). All reactions were carried out in 20-μl reaction mixtures containing 7 μl of cDNAs, 10 μl of ABsolute QPCR SYBR Green Mix (Thermo Fisher Scientific), 1.5 μl of forward and 1.5 μl of reverse primers (10 μM each). The running conditions were as follows: (1) 15 min at 95°C, followed by 30 cycles of 94°C for 25 sec (denaturation), 60°C for 25 s (annealing), and 72°C for 6 s (extension). The absence of nonspecific products or primer dimers was indicated by observation of a single melting peak in melting curve analysis. An additional extension and detection step was applied for those primers that produced primer dimmers: for 2 s at a temperature just below the Tm of the specific product and substantially above the Tm of the primer dimers. With this technique we could eliminate nonspecific fluorescent signals produced by primer dimers. Following the PCR reaction, melting curve analysis was performed to control amplification specificity (specificity was defined as the production of a single peak at the predicted temperature and the absence of primer dimers) by measuring the fluorescence intensity across the temperature interval from 55°C to 95°C. The 28S ribosomal (r)RNA used as the loading control (reference gene) was amplified in each run. H2O was included as a no-template control, and cDNA derived from the reverse-transcribed RNAs of non-infected cells was used as a negative mock-infected control. We applied SYBR Green-based real-time PCR because of the lower costs and simpler protocol than for TaqMan probe-based methods for instance. It has recently been demonstrated that the SYBR-based method of detection is as sensitive and specific, and has a similar dynamic range to that of the TaqMan-based technique [66].
Calculation of relative expression ratio (R)
We calculated the R value by using the following equation:
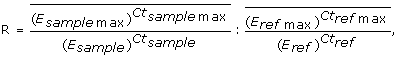 |
where R is the relative expression (quantification) ratio; E is the efficiency of amplification; Ct is the cycle threshold value; sample refers to any particular gene at a given time point; and ref is the 28S rRNA, which was used as a reference gene and was amplified in each run. Average Ct values with their standard error (SE) values and amplification efficiencies with SE are shown in Additional file 6. This equation is similar to that used by Soong et al [30]. However, instead of individual values, we used the average maximal value of ECt for each gene as the control. The relative copy numbers of mRNAs were calculated by normalizing cDNAs to 28S rRNA using the Comparative Quantitation module of the Rotor-Gene 6000 software (Version 1.7.28, Corbett Research), which automatically calculates the real-time PCR efficiency sample-by-sample. Thresholds were set automatically by the software.
Analysis and presentation of data
Data were analyzed by the Microsoft Excel program, using the average and the standard deviance functions. The inhibitory effect of CHX or PAA was calculated via the ratio of the drug-treated and untreated R values at 2, 4 and 6 h pi for CHX: Ri-CHX = RCHX/RUT, or 4 and 6 h pi for PAA: Ri-PAA = RPAA/RUT. Thus, a low value indicates a high inhibitory effect and vice versa. The net increase in a product was calculated by subtracting the R value at time point t+1 from that at t (RΔ = R(t+1)-Rt), where t = 0, 1, 2, 4 or 6 h and (t+1) = 1, 2, 4 or 6 h. The ratios of adjacent R values (rate of change; Ra) were calculated with the following equation: Ra = R(t+1)/Rt; t = 1, 2, 4 or 6 h; (t+1) = 2, 4 or 6 h. Pearson's correlation analysis was used to evaluate qRT2-PCR data, as an alternative method for the grouping of PRV genes into kinetic classes. Pearson's correlation coefficient (r) was calculated as follows.
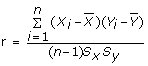 |
A correlation is a number between -1 and +1 that measures the degree of association between two variables [labeled here as X and Y, which are the RΔ values of two different genes in the same time interval (i)]. 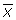 and
and 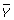 are the average values, n is the sample number, and SX and SY are the standard deviances (errors) for X and Y, respectively. A positive value for the correlation implies a positive association and a negative value implies a negative or inverse association. Genes were clustered by using a complete linkage hierarchical clustering method with a centered correlation similarity metric with Cluster 3.0 software (Stanford University). To view the clustering results generated by Cluster 3.0, we used Alok Saldanha's Java TreeView.
are the average values, n is the sample number, and SX and SY are the standard deviances (errors) for X and Y, respectively. A positive value for the correlation implies a positive association and a negative value implies a negative or inverse association. Genes were clustered by using a complete linkage hierarchical clustering method with a centered correlation similarity metric with Cluster 3.0 software (Stanford University). To view the clustering results generated by Cluster 3.0, we used Alok Saldanha's Java TreeView.
Gel electrophoresis
Larger DNA fragments generated by conventional PCR were run on 1% agarose/TBE gels containing ethidium bromide and visualized under UV illumination, using Marker 16 (Lambda DNA/Eco130I; Fermentas) to size DNA fragments. Smaller DNA fragments generated by qRT2-PCR were run in a 12% polyacrylamide gel to ensure that the amplified products had the correct size. DNA fragments were visualized with ethidium bromide staining under UV illumination. A GeneRuler™ Low Range DNA Ladder (Fermentas) was included in each run.
Restriction endonuclease analysis
If there were doubts concerning the identity of the amplified product, restriction endonuclease analysis was performed to confirm the specificity of the qRT2-PCR products.
Construction of recombinant plasmids
Plasmids containing PRV DNA fragments were constructed for DNA sequencing analysis, which was performed if primers designed on the basis of sequence data relating to non-Ka strains performed badly; or if the identity of amplicons generated by real-time PCR was uncertain. PRV DNAs were subcloned by using two methods: PCR amplification of the particular DNA segment; or subcloning of the desired DNA region by standard molecular cloning protocols. Amplified products were subcloned to the pGEM (Promega) vector in accordance with the manufacturer's instructions. PRV BamHI fragments to be sequenced were subcloned to the pRL525 vector [67].
DNA sequencing
Subcloned DNA fragments were subjected to DNA sequencing with the ABI Prism™ 3730xl DNA sequencer (AME Bioscience Ltd.). DNA sequences were analyzed by using the Chromas Lite 2.01 software (Technelysium Pty Ltd).
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
DT carried out the standard and real-time PCR, the agarose and polyacrylamide gel electrophoresis, and the DNA sequencing, and participated in the evaluation of the primary data. JT took part by performing the reverse transcription reactions, purified PRV RNA, and propagated PK-15 cells. PP participated in performing the reverse transcription reactions. ZB coordinated the study, propagated viruses and isolated viral DNAs. All authors have read and approved the final manuscript.
Supplementary Material
Validation of qRT2-PCR products by restriction endonuclease analysis. In a few cases, the specificity of PCR products was confirmed by restriction endonuclease assay. Polyacrylamide gel electrophoresis of qRT2-PCR products, using primers ul54 (lanes 1 and 2) and us4 (lanes 3 and 4). Amplicon specificity was confirmed by restriction endonuclease analysis, using SalI (lane 2) and HpaII (lane 4) enzymes. The GeneRuler™ Low Range DNA Ladder is shown on the left.
Grouping PRV genes according to their expression in untreated cells. Data of R values of untreated samples.
PRV genes ranked on the basis of the effect of PAA on gene expression. PRV genes were classified on the basis of the inhibitory effect of PAA (Ri-PAA) on gene expression. Early genes are separated by E/L genes from late genes. The ie180 gene was classified as IE gene because its expression was not inhibited by CHX treatment. a R values of PAA-treated samples at 4 h post-infection. b Relative expression ratios of PRV genes after PAA-treatment at 6 h post-infection. c Ri-PAA values at 4 h post-infection. d PRV genes ranked on their Ri-PAA (6 h pi) values. e Ri-PAA values of PRV genes after 6 hours post-infection.
PRV genes ranked on the basis of their expression profiles. a. R values Genes were ranked on the basis of their R values at different time points. a PRV genes ranked on the basis of R values at 1 h pi. b Relative expression ratios of PRV genes at 1 h pi. c Order of R values of PRV genes at 2 h pi. d R values after 2 hours post infection. e PRV genes ranked on the basis of R values at 4 h pi. f R values at 4 h pi. b. RΔ values Genes were ranked on the basis of their RΔ values at different time intervals. a Order of RΔ values of PRV genes in the interval 1 h-2 h. b RΔ values in the interval between 1 and 2 h. c PRV genes ranked on the basis of their RΔ values in the infection period from 2 to 4 hours. d RΔ values in the interval 2 h-4 h. e PRV genes ranked on their basis of their RΔ values in the interval 4 h-6 h. f RΔ values in the infection period: 4 h-6 h. c. Ra values Genes were ranked on the basis of their Ra values at different time intervals. a Order of Ra values of PRV genes in the interval 1 h-2 h. b Ra values in the interval between 1 and 2 hours. c PRV genes ranked on the basis of their Ra values in the infection period from 2 to 4 hours. d Ra values in the infection period from 2 to 4 hours. e PRV genes ranked on their basis of their Ra values in the interval 4 h-6 h. f Ra values in the interval 4 h-6 h.
PRV genes ranked on the basis of their R(6h-4h)/R1h ratios. R(6h-4h)/R1h values.
Average cycle threshold (a) and amplification values (b) with standard errors.a Average of cycle threshold (Ct) values based on almost three separated reactions. b Standard error of the mean.
Acknowledgments
Acknowledgements
This study was supported by Hungarian National Fund for Human Frontiers Science Program Young Investigator grant (No. RGY0073/2006) to Z.B.
Contributor Information
Dóra Tombácz, Email: dora@sb4.szote.u-szeged.hu.
Judit S Tóth, Email: jtoth@sb4.szote.u-szeged.hu.
Pál Petrovszki, Email: petrovszki@gmail.com.
Zsolt Boldogkői, Email: boldog@sb4.szote.u-szeged.hu.
References
- Aujeszky A. A contagious disease, not readily distinguishable from rabies, with unknown origin. Veterinarius. 1902;25:387–396. [Google Scholar]
- Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 2001;Chapter 1 doi: 10.1002/0471142301.ns0105s09. [DOI] [PubMed] [Google Scholar]
- Boldogkõi Z, Sík A, Dénes A, Reichart A, Toldi J, Gerendai I, Kovács KJ, Palkovits M. Novel tracing paradigms-genetically engineered herpesviruses as tools for mapping functional circuits within the CNS: present status and future prospects. Prog Neurobiol. 2004;72:417–445. doi: 10.1016/j.pneurobio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Boldogkõi Z, Bálint K, Awatramani GB, Balya D, Busskamp V, Viney TJ, Lagali PS, Duebel J, Pásti E, Tombácz D, Tóth JS, Takács IF, Scherf BG, Roska B. Genetically timed, Activity sensor and Rainbow transsynaptic viral tools. Nature Methods. 2009;6:127–130. doi: 10.1038/nmeth.1292. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Enquist LW, Pomeranz LE. The alpha-herpesviruses: molecular pathfinders in nervous system circuits. Trends Mol Med. 2008;14:134–140. doi: 10.1016/j.molmed.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogkõi Z, Szabó A, Vrbová G, Nógrádi A. Pseudorabies virus-based gene delivery to rat embryonic spinal cord grafts. Hum Gene Ther. 2002;13:719–729. doi: 10.1089/104303402317322285. [DOI] [PubMed] [Google Scholar]
- Demarchi JM, Schmidt CA, Kaplan AS. Patterns of Transcription of Human Cytomegalovirus in Permissively Infected Cells. J Virol. 1980;35:277–286. doi: 10.1128/jvi.35.2.277-286.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of Herpesvirus Macromolecular Synthesis I. Cascade Regulation of the Synthesis of Three Groups of Viral Proteins. J Virol. 14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AK. Cloning of the latency gene and the early protein 0 gene of pseudorabies virus. J Virol. 1991;65:5260–5271. doi: 10.1128/jvi.65.10.5260-5271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wu CY. Characterization and expression of the pseudorabies virus early gene UL54. J Virol Methods. 2004;119:129–136. doi: 10.1016/j.jviromet.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Feldman LT, Demarchi JM, Ben-Porat T, Kaplan AS. Control of abundance of immediate-early mRNA in herpesvirus (pseudorabies)-infected cells. Virology. 1982;116:250–262. doi: 10.1016/0042-6822(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Ihara S, Feldman L, Watanabe S, Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983;131:437–454. doi: 10.1016/0042-6822(83)90510-X. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Ehrlich C, Klupp BG, Mettenleiter TC. Characterization of the replication origin (Ori(S)) and adjoining parts of the inverted repeat sequences of the pseudorabies virus genome. J Gen Virol. 2000;81:1539–1543. doi: 10.1099/0022-1317-81-6-1539. [DOI] [PubMed] [Google Scholar]
- Zhang G, Leader DP. The structure of the pseudorabies virus genome at the end of the inverted repeat sequences proximal to the junction with the short unique region. J Gen Virol. 1990;71:2433–2441. doi: 10.1099/0022-1317-71-10-2433. [DOI] [PubMed] [Google Scholar]
- Hann LE, Cook WJ, Uprichard SL, Knipe DM, Coen DM. The role of herpes simplex virus ICP27 in the regulation of UL24 gene expression by differential polyadenylation. J Virol. 1998;72:7709–7714. doi: 10.1128/jvi.72.10.7709-7714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J, Angulo A, Amaratunga D, Guo H, Jiang Y, Wan JS, Bittner A, Frueh K, Jackson MR, Peterson PA, Erlander MG, Ghazal P. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J Virol. 1999;73:5757–5766. doi: 10.1128/jvi.73.7.5757-5766.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingley SW, Ramirez JJ, Aguilar SA, Simmen K, Sandri-Goldin RM, Ghazal P, Wagner EK. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J Virol. 2000;74:9916–9927. doi: 10.1128/JVI.74.21.9916-9927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Grinfeld E, Craigon M, Vierlinger K, Roy D, Forster T, Ghazal P. Transcriptomal analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J Gen Virol. 2005;86:2673–2684. doi: 10.1099/vir.0.80946-0. [DOI] [PubMed] [Google Scholar]
- Aguilar JS, Devi-Rao GV, Rice MK, Sunabe J, Ghazal P, Wagner EK. Quantitative comparison of the HSV-1 and HSV-2 transcriptomes using DNA microarray analysis. Virology. 2006;348:233–241. doi: 10.1016/j.virol.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Yang WC, Devi-Rao GV, Ghazal P, Wagner EK, Triezenberg SJ. General and specific alterations in programming of global viral gene expression during infection by VP16 activation-deficient mutants of herpes simplex virus type 1. J Virol. 2002;76:12758–74. doi: 10.1128/JVI.76.24.12758-12774.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A, Devi-Rao GV, Rice MK, Gary LW, Bloom DC, Sandri-Goldin RM, Ghazal P, Wagner EK. Immediate-early expression of the herpes simplex virus type 1 ICP27 transcript is not critical for efficient replication in vitro or in vivo. J Virol. 2004;78:10470–10478. doi: 10.1128/JVI.78.19.10470-10478.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaca G, Hargett D, McLean TI, Aguilar JS, Ghazal P, Wagner EK, Bachenheimer SL. Inhibition of the stress-activated kinase, p38, does not affect the virus transcriptional program of herpes simplex virus type 1. Virology. 2004;329:142–156. doi: 10.1016/j.virol.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Blanchard Y, Le MN, Le CM, Blanchard P, Leger J, Jestin A. Cellular gene expression survey of PseudoRabies Virus (PRV) infected Human Embryonic Kidney cells (HEK-293) Vet Res. 2006;37:705–723. doi: 10.1051/vetres:2006027. [DOI] [PubMed] [Google Scholar]
- Brukman A, Enquist LW. Suppression of the interferon-mediated innate immune response by pseudorabies virus. J Virol. 2006;80:6345–6356. doi: 10.1128/JVI.00554-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray N, Enquist LW. Transcriptional response of a common permissive cell type to infection by two diverse alphaherpesviruses. J Virol. 2004;78:3489–3501. doi: 10.1128/JVI.78.7.3489-3501.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flori L, Rogel_Gaillard C, Cochet M, Lemonnier G, Hugot K, Chardon P, Robin S, Lefèvre F. Transcriptomic analysis of the dialogue between Pseudorabies virus and porcine epithelial cells during infection. BMC Genomics. 2008;9:123. doi: 10.1186/1471-2164-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øster B, Bundgaard B, Höllsberg P. Viral gene expression patterns in human herpesvirus 6B-infected T cells. J Virol. 2002;76:7578–7586. doi: 10.1128/JVI.76.15.7578-7586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer DP, Gonzalez CM, Vahrson W, DeWire SM, Hines-Boykin R, Damania B. Whole-genome transcription profiling of rhesus monkey rhadinovirus. J Virol. 2005;79:8637–8650. doi: 10.1128/JVI.79.13.8637-8650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong R, Tabiti K. Detection of colorectal micrometastasis by quantitative RT-PCR of cytokeratin 20 mRNA. Proceedings of the American Association for Cancer Research. 2000;41:391. XP002149389. [Google Scholar]
- Stewart JP, Janjua NJ, Sunil-Chandra NP, Nash AA, Arrand JR. Characterization of Murine Gammaherpesvirus 68 Glycoprotein B (gB) Homolog: Similarity toEpstein-Barr Virus gB (gpllO) J Virol. 1994;68:6496–6504. doi: 10.1128/jvi.68.10.6496-6504.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, O'Neill J, Barbosa MS. Late temporal gene expression from the human cytomegalovirus pp28US (UL99) promoter when integrated into the host cell chromosome. J Gen Virol. 2001;82:1147–1155. doi: 10.1099/0022-1317-82-5-1147. [DOI] [PubMed] [Google Scholar]
- Sanfilippo CM, Blaho JA. ICP0 Gene Expression Is a Herpes Simplex Virus Type 1 Apoptotic Trigger. J Virol. 2006;80:6810–6821. doi: 10.1128/JVI.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Sebastián S, Tabarés E. Negative regulation of herpes simplex virus type 1 ICP4 promoter by IE180 protein of pseudorabies virus. J Gen Virol. 2004;85:2125–2130. doi: 10.1099/vir.0.80119-0. [DOI] [PubMed] [Google Scholar]
- Some Genetic Functions Encoded by Herpes Simplex Virus type 1 http://darwin.bio.uci.edu/~faculty/wagner/table.html
- The Genetic and Transcription Map of the HSV-1 Genome http://darwin.bio.uci.edu/~faculty/wagner/hsvimg04z.jpg
- Roizman B. The function of herpes simplex virus genes: A primer for genetic engineering of novel vectors. Proc Nat Acad Sci. 1996;93:11307–11312. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Campadelli-Fiume G. Alphaherpes viral genes and their functions. In: Arvin A, et al, editor. Human Herpesviruses - Biology, Therapy and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. pp. 70–92. [PubMed] [Google Scholar]
- Pseudorabies virus gene annotations http://www.fli.bund.de/prv_gene_annotations+M52087573ab0.html
- Baumeister J, Klupp BG, Mettenleiter TC. Pseudorabies virus and equine herpesvirus 1 share a nonessential gene which is absent in other herpesviruses and located adjacent to a highly conserved gene cluster. J Virol. 1995;69:5560–5567. doi: 10.1128/jvi.69.9.5560-5567.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras F, Dezelee S, Simonet B, Nguyen X, Vende P, Flamand A, Masse MJ. The left border of the genomic inversion of pseudorabies virus contains genes homologous to the UL46 and UL47 genes of herpes simplex virus type 1, but no UL45 gene. Virus Res. 1999;60:29–40. doi: 10.1016/S0168-1702(98)00146-4. [DOI] [PubMed] [Google Scholar]
- Fuchs W, Klupp BG, Granzow H, Osterrieder N, Mettenleiter TC. The Interacting UL31 and UL34 Gene Products of Pseudorabies Virus Are Involved in Egress from the Host-Cell Nucleus and Represent Components of Primary Enveloped but Not Mature Virions. J Virol. 2002;76:364–378. doi: 10.1128/JVI.76.1.364-378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Fan Y, Baines JD. Characterization of the UL33 gene product of herpes simplex virus 1. Virology. 2000;266:310–8. doi: 10.1006/viro.1999.0090. [DOI] [PubMed] [Google Scholar]
- McNabb DS, Courtney RJ. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992;66:2653–63. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Fuchs W, Granzow H, Nixdorf R, Mettenleiter TC. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J Virol. 2002;76:3065–3071. doi: 10.1128/JVI.76.6.3065-3071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Kaliman A, Boldogkõi Z, Aszódi A, Fodor I. Sequence and expression analyses of the UL37 and UL38 genes of Aujeszky's disease virus. Acta Vet Hung. 2000;48:125–136. doi: 10.1556/AVet.48.2000.1.14. [DOI] [PubMed] [Google Scholar]
- de Wind N, Berns A, Gielkens A, Kimman T. Ribonucleotide reductase-deficient mutants of pseudorabies virus are avirulent for pigs and induce partial protective immunity. J Gen Virol. 1993;74:351–359. doi: 10.1099/0022-1317-74-3-351. [DOI] [PubMed] [Google Scholar]
- Ambagala AP, Hinkley S, Srikumaran S. An early pseudorabies virus protein down-regulates porcine MHC class I expression by inhibition of transporter associated with antigen processing (TAP) J Immunol. 2000;164:93–99. doi: 10.4049/jimmunol.164.1.93. [DOI] [PubMed] [Google Scholar]
- Powers L, Wilkinson KS, Ryan P. Characterization of the prv43 gene of pseudorabies virus and demonstration that it is not required for virus growth in cell culture. Virology. 1994;199:81–88. doi: 10.1006/viro.1994.1099. [DOI] [PubMed] [Google Scholar]
- Robbins AK, Watson RJ, Whealy ME, Hays WW, Enquist LW. Characterization of a pseudorabies virus glycoprotein gene with homology to herpes simplex virus type 1 and type 2 glycoprotein. J Virol. 1986;58:339–347. doi: 10.1128/jvi.58.2.339-347.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin K, Dezelee S, Masse MJ, Bras F, Flamand A. The UL25 Protein of Pseudorabies Virus Associates with Capsids and Localizes to the Nucleus and to Microtubules. J Virol. 2000;74:474–482. doi: 10.1128/JVI.74.1.474-482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezélée S, Bras F, Vende P, Simonet B, Nguyen X, Flamand A, Masse MJ. The BamHI fragment 9 of pseudorabies virus contains genes homologous to the UL24, UL25, UL26, and UL 26.5 genes of herpes simplex virus type 1. Virus Res. 1996;42:27–39. doi: 10.1016/0168-1702(96)01293-2. [DOI] [PubMed] [Google Scholar]
- Yamada S, Imada T, Watanabe W, Honda Y, Nakajima-Iijima S, Shimizu Y, Sekikawa K. Nucleotide sequence and transcriptional mapping of the major capsid protein gene of pseudorabies virus. Virology. 1991;185:56–66. doi: 10.1016/0042-6822(91)90753-X. [DOI] [PubMed] [Google Scholar]
- Dijkstra JM, Fuchs W, Mettenleiter TC, Klupp BG. Identification and transcriptional analysis of pseudorabies virus UL6 to UL12 genes. Arch Virol. 1997;142:17–35. doi: 10.1007/s007050050056. [DOI] [PubMed] [Google Scholar]
- Dean HJ, Cheung AK. A 3'coterminal gene cluster in pseudorabies virus contains herpes simplex virus UL1, UL2, UL3 gene homologs and a unique UL3.5 open reading frame. J Virol. 1993;67:5955–5961. doi: 10.1128/jvi.67.10.5955-5961.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PR, Croen KD, Ostrove JM, Straus SE. Structural and Kinetic Analyses of Herpes Simplex Virus Type I Latencyassociated Transcripts in Human Trigeminal Ganglia and in Cell Culture. J Clin Invest. 1990;86:235–241. doi: 10.1172/JCI114689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl M, Gulden H van der, de Wind N, Gielkens A, Anton Berns A. Identification of Two Genes in the Unique Short Region of Pseudorabies Virus; Comparison with Herpes Simplex Virus and Varicella-zoster Virus. J Gen Virol. 1990;71:1747–1755. doi: 10.1099/0022-1317-71-8-1747. [DOI] [PubMed] [Google Scholar]
- Brideau AD, Banfield BW, Enquist LW. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J Virol. 1998;72:4560–70. doi: 10.1128/jvi.72.6.4560-4570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N, Peeters B, Zijderveld A, Gielkens ALJ, Berns AJM, Kimman TG. Mutagenesis and characterization of a 41-kilobase-pair region of the pseudorabies virus genome: transcription map, search for virulence genes, and comparison with homologs of herpes simplex virus type 1. Virology. 1994;200:784–790. doi: 10.1006/viro.1994.1242. [DOI] [PubMed] [Google Scholar]
- Dean HJ, Cheung AK. Identification of the pseudorabies virus UL4 and UL5 (helicase) genes. Virology. 1994;202:962–7. doi: 10.1006/viro.1994.1419. [DOI] [PubMed] [Google Scholar]
- Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. Pseudorabies virus US3 protein kinase mediates actin stress fiber brakedown. J Virol. 2003;77:9074–80. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souazé F, Ntodou-Thomé A, Tran CY, Rostène W, Forgez P. Quantitative RT-PCR: limits and accuracy. Biotechniques. 1996;21:280–285. doi: 10.2144/96212rr01. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔ C(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Klupp BG, Hengartner CJ, Mettenleiter TC, Enquist LW. Complete, annotated sequence of the pseudorabies virus genome. J Virol. 2004;78:424–440. doi: 10.1128/JVI.78.1.424-440.2004. Erratum in: J Virol 2004, 78(4):2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin J, Vahrson W, Hines-Boykin R, Dittmer DP. Real-time quantitative PCR analysis of viral transcription. Methods Mol Biol. 2005;292:449–480. doi: 10.1385/1-59259-848-x:449. [DOI] [PubMed] [Google Scholar]
- Elhai J, Wolk CP. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of qRT2-PCR products by restriction endonuclease analysis. In a few cases, the specificity of PCR products was confirmed by restriction endonuclease assay. Polyacrylamide gel electrophoresis of qRT2-PCR products, using primers ul54 (lanes 1 and 2) and us4 (lanes 3 and 4). Amplicon specificity was confirmed by restriction endonuclease analysis, using SalI (lane 2) and HpaII (lane 4) enzymes. The GeneRuler™ Low Range DNA Ladder is shown on the left.
Grouping PRV genes according to their expression in untreated cells. Data of R values of untreated samples.
PRV genes ranked on the basis of the effect of PAA on gene expression. PRV genes were classified on the basis of the inhibitory effect of PAA (Ri-PAA) on gene expression. Early genes are separated by E/L genes from late genes. The ie180 gene was classified as IE gene because its expression was not inhibited by CHX treatment. a R values of PAA-treated samples at 4 h post-infection. b Relative expression ratios of PRV genes after PAA-treatment at 6 h post-infection. c Ri-PAA values at 4 h post-infection. d PRV genes ranked on their Ri-PAA (6 h pi) values. e Ri-PAA values of PRV genes after 6 hours post-infection.
PRV genes ranked on the basis of their expression profiles. a. R values Genes were ranked on the basis of their R values at different time points. a PRV genes ranked on the basis of R values at 1 h pi. b Relative expression ratios of PRV genes at 1 h pi. c Order of R values of PRV genes at 2 h pi. d R values after 2 hours post infection. e PRV genes ranked on the basis of R values at 4 h pi. f R values at 4 h pi. b. RΔ values Genes were ranked on the basis of their RΔ values at different time intervals. a Order of RΔ values of PRV genes in the interval 1 h-2 h. b RΔ values in the interval between 1 and 2 h. c PRV genes ranked on the basis of their RΔ values in the infection period from 2 to 4 hours. d RΔ values in the interval 2 h-4 h. e PRV genes ranked on their basis of their RΔ values in the interval 4 h-6 h. f RΔ values in the infection period: 4 h-6 h. c. Ra values Genes were ranked on the basis of their Ra values at different time intervals. a Order of Ra values of PRV genes in the interval 1 h-2 h. b Ra values in the interval between 1 and 2 hours. c PRV genes ranked on the basis of their Ra values in the infection period from 2 to 4 hours. d Ra values in the infection period from 2 to 4 hours. e PRV genes ranked on their basis of their Ra values in the interval 4 h-6 h. f Ra values in the interval 4 h-6 h.
PRV genes ranked on the basis of their R(6h-4h)/R1h ratios. R(6h-4h)/R1h values.
Average cycle threshold (a) and amplification values (b) with standard errors.a Average of cycle threshold (Ct) values based on almost three separated reactions. b Standard error of the mean.