Abstract
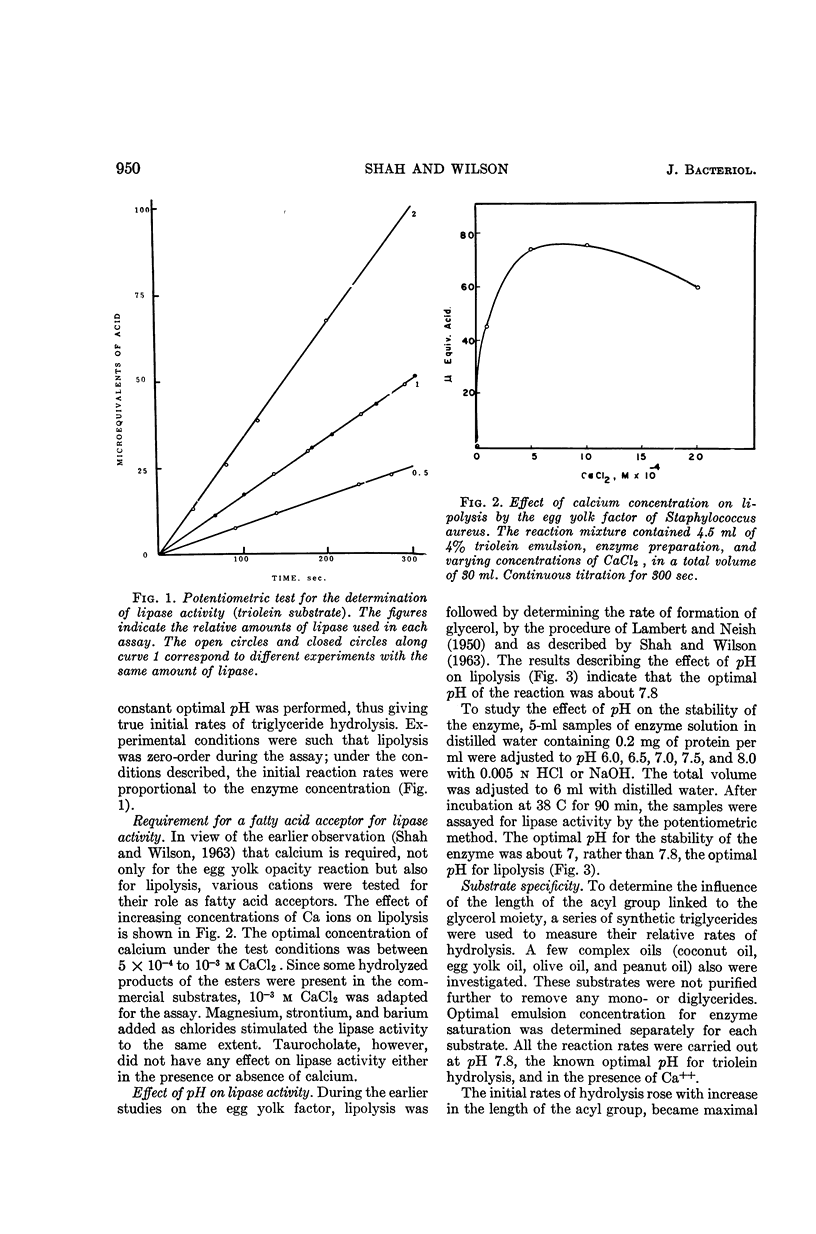
Shah, D. B. (University of Wisconsin, Madison), and J. B. Wilson. Egg yolk factor of Staphylococcus aureus. II. Characterization of the lipase activity. J. Bacteriol. 89:949–953. 1965.—The staphylococcal egg yolk factor was characterized as a lipase. The enzyme had an optimal pH of 7.8, but the optimal pH of stability was 7. Substrate specificity data showed that the relative rate of hydrolysis was lowest with triacetin as substrate, was maximal with tributyrin, and decreased as the chain length of the acyl moieties increased. The enzyme showed an absolute requirement for a fatty acid acceptor like calcium, when the acyl moiety of triglyceride was water-insoluble. Magnesium, strontium, and barium functioned equally well as fatty acid acceptors. The enzyme was able to hydrolyze coconut oil, peanut oil, olive oil, and egg yolk oil.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDER V. G., GILLESPIE W. A., HERDAN G. Production of opacity in egg-yolk broth by Staphylococci from various sources. J Pathol Bacteriol. 1953 Jul;66(1):205–210. doi: 10.1002/path.1700660123. [DOI] [PubMed] [Google Scholar]
- BURNS J., HOLTMAN D. F. Biochemical properties of virulent and avirulent staphylococci. Ann N Y Acad Sci. 1960 Nov 21;88:1115–1124. doi: 10.1111/j.1749-6632.1960.tb20101.x. [DOI] [PubMed] [Google Scholar]
- DESNUELLE P. Pancreatic lipase. Adv Enzymol Relat Subj Biochem. 1961;23:129–161. doi: 10.1002/9780470122686.ch4. [DOI] [PubMed] [Google Scholar]
- DESNUELLE P., SARDA L., AILHAUD G. [Inhibition of pancreatic lipase by diethyl-p-nitrophenyl phosphate in emulation]. Biochim Biophys Acta. 1960 Jan 29;37:570–571. doi: 10.1016/0006-3002(60)90532-1. [DOI] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Enzymatic activity of staphylocoagulase. I. Characterization of an esterase associated with purified preparations. J Bacteriol. 1959 Sep;78:407–412. doi: 10.1128/jb.78.3.407-412.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUMMOND M. C., TAGER M. Enzymatic activity of staphylocoagulase. II. Dissociation of plasma clotting from tributyrinase activity. J Bacteriol. 1959 Sep;78:413–421. doi: 10.1128/jb.78.3.413-421.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE W. A., ALDER V. G. Production of opacity in egg-yolk media by coagulase-positive staphylococci. J Pathol Bacteriol. 1952 Jan;64(1):187–200. doi: 10.1002/path.1700640119. [DOI] [PubMed] [Google Scholar]
- KELLER P. J., COHEN E., NEURATH H. Procarboxypeptidase. II. Chromatographic isolation, further characterization, and activation. J Biol Chem. 1958 Feb;230(2):905–915. [PubMed] [Google Scholar]
- MARCHIS-MOUREN G., SARDA L., DESNUELLE P. Purification of hog pancreatic lipase. Arch Biochem Biophys. 1959 Jul;83(1):309–319. doi: 10.1016/0003-9861(59)90036-0. [DOI] [PubMed] [Google Scholar]
- REID W. B., WILSON J. B. A study of the staphylococci associated with the bovine udder. Am J Vet Res. 1959 Sep;20:825–831. [PubMed] [Google Scholar]
- SARDA L., DESNUELLE P. Action de la lipase pancréatique sur les esters en émulsion. Biochim Biophys Acta. 1958 Dec;30(3):513–521. doi: 10.1016/0006-3002(58)90097-0. [DOI] [PubMed] [Google Scholar]
- SHAH D. B., WILSON J. B. EGG YOLK FACTOR OF STAPHYLOCOCCUS AUREUS. I. NATURE OF THE SUBSTRATE AND ENZYME INVOLVED IN THE EGG YOLK OPACITY REACTION. J Bacteriol. 1963 Mar;85:516–521. doi: 10.1128/jb.85.3.516-521.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


