Summary
In the nervous system, neural stem cells (NSC) are necessary for the generation of new neurons and for cognitive function. Here we show that FoxO3, a member of a transcription factor family known to extend lifespan in invertebrates, regulates the NSC pool. We find that adult FoxO3−/− mice have fewer NSC in vivo than wild type counterparts. NSC isolated from adult FoxO3−/− mice have decreased self-renewal and an impaired ability to generate different neural lineages. Identification of the FoxO3-dependent gene expression profile in NSC suggests that FoxO3 regulates the NSC pool by inducing a program of genes that preserves quiescence, prevents premature differentiation, and controls oxygen metabolism. The ability of FoxO3 to prevent the premature depletion of NSC might have important implications for counteracting brain aging in long-lived species.
Introduction
Neural stem cells (NSC) can self-renew and generate all three neural lineages of the nervous system: neurons, astrocytes, and oligodendrocytes. During embryonic and post-natal development, NSC give rise to rapidly amplifying neural progenitors, which are responsible for the proper formation of the nervous system. The adult mammalian brain contains two residual populations of relatively quiescent NSC in the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus and in the subventricular zone (SVZ) of the cortex (Alvarez-Buylla and Temple, 1998; Zhao et al., 2008). In the adult brain, the generation of new neurons (neurogenesis) from NSC is thought to play an important role in learning and memory, spatial pattern separation, and odor discrimination (Clelland et al., 2009; Gheusi et al., 2000; Imayoshi et al., 2008). Both the number of NSC and neurogenesis decline with age and this age-dependent decline is correlated with a gradual loss of cognitive and sensory functions (Bondolfi et al., 2004; Kempermann et al., 1998; Tropepe et al., 1997). Conversely, the pool of NSC, neurogenesis, and cognitive performance in adults are preserved in a strain of long-lived mutant mice (Kinney et al., 2001; Sun et al., 2005). Thus, an intact pool of functional NSC may be crucial for preserving cognitive functions throughout life.
The polycomb family member Bmi-1 has been recently found to play an important role in NSC self-renewal by negatively regulating the cell cycle inhibitor p21CIP1 in embryonic NSC (Fasano et al., 2007), and p16INK4a and p19ARF in adult NSC (Molofsky et al., 2005; Molofsky et al., 2006). TLX, a nuclear receptor, also regulates NSC self-renewal during development and adulthood in a cell-autonomous manner (Zhang et al., 2008). Other mechanisms to regulate the self-renewal and multipotency of NSC throughout life remain largely unknown, but one intriguing possibility is that genes that regulate lifespan in invertebrates may have evolved to control stem cell pools in mammals.
FoxO transcription factors are necessary for the extreme longevity of mutants of the insulin pathway in invertebrates (Kenyon, 2005). In humans, single nucleotide polymorphisms in one of the four FoxO genes, FoxO3, has recently been associated with extreme longevity (Flachsbart et al., 2009; Willcox et al., 2008), raising the possibility that FoxO3 also regulates lifespan in mammals. FoxO factors can elicit a variety of cellular responses, including cell cycle arrest, differentiation, resistance to oxidative stress, and apoptosis (Salih and Brunet, 2008). FoxO factors have recently been found to regulate the self-renewal of adult hematopoietic stem cells (HSC), primarily by providing resistance to oxidative stress (Miyamoto et al., 2007; Tothova et al., 2007). Whether and how FoxO transcription factors regulate NSC is unknown.
FoxO transcription factors are inactivated in response to insulin or growth factors by phosphorylation by the protein kinase Akt, which results in their nuclear export (Salih and Brunet, 2008). Activation of the PI3K-Akt pathway, for example by ablation of the gene encoding the PTEN phosphatase, promotes the self-renewal of neural progenitor cells (Groszer et al., 2006; Li et al., 2002; Sinor and Lillien, 2004). However, the role of the PI3K-Akt pathway in the NSC pool in vivo has not been examined and the PI3K-Akt pathway has many other downstream targets in addition to FoxO factors.
Here we show that the transcription factor FoxO3, a member of a gene family that extends lifespan in invertebrates, is necessary for the regulation of the NSC pool in mice. We also identify the program of genes regulated by FoxO3 in NSC. Our findings suggest that FoxO3 regulates the NSC pool by inducing a program that promotes quiescence, prevents premature differentiation, and controls oxygen metabolism. FoxO3's ability to regulate NSC homeostasis may protect normal cognitive function in organisms that live to an advanced age.
Results
FoxO3 is expressed in adult NSC/neural progenitors in vivo and in vitro
To determine if FoxO3 protein is expressed in NSC niches in the adult mouse brain, we used an antibody that recognized FoxO3 but did not significantly detect FoxO1, FoxO4, or FoxO6 in cells (Figures S1A-S1C). We stained brain sections of adult FoxO3+/+ and FoxO3−/− mice with this antibody and found that FoxO3 is expressed in both the SGZ and the SVZ (Figure S2A). Western blotting experiments confirmed that FoxO3 is highly expressed in NSC niches in vivo (Figure S2B).
NSC niches contain NSC, committed progenitors, and differentiated progeny. To determine if FoxO3 is expressed in NSC in vivo, we stained brain sections with antibodies to FoxO3 and to Sox2, a marker of NSC/neural progenitors, or to NeuN, a marker of neurons (Figure 1A). These experiments revealed that FoxO3 is expressed in Sox2-positive cells in the SGZ and the SVZ (Figure 1A). FoxO3 is expressed in a subset of NeuN-negative cells (Figure 1A, bottom panels, white arrows) and in bromodeoxyuridine (BrdU)-positive label-retaining cells, which are thought to be NSC (Figure S2C, see Figure 2). These results indicate that FoxO3 is expressed in adult NSC, as well as in other cells in the NSC niches. The fact that FoxO3 staining overlaps with the nuclear staining of Sox2 and BrdU further suggests that FoxO3 is nuclear in adult NSC and therefore likely to be active in these cells (Figures 1A and S2C).
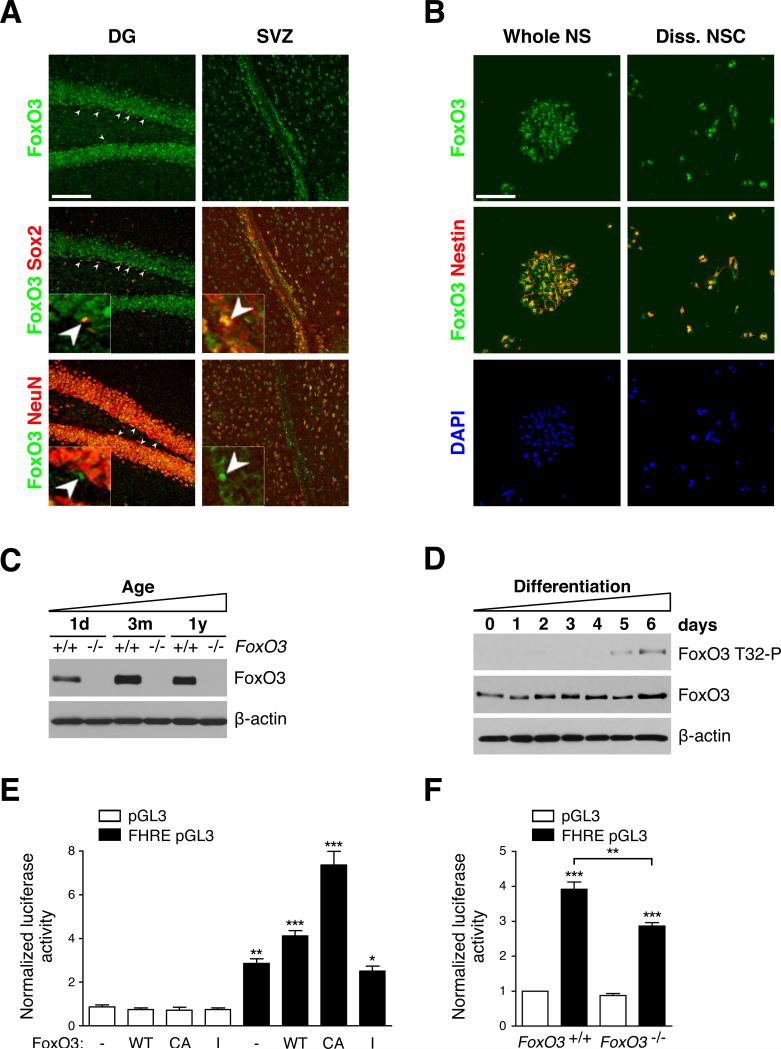
Figure 1. FoxO3 is expressed and active in NSC.
(A) FoxO3 is expressed in adult NSC in vivo. Immunohistochemistry on brain sections of 3 month-old mice with antibodies to FoxO3 (‘Ct’), Sox2, and NeuN in the dentate gyrus (DG) of the hippocampus and in the subventricular zone (SVZ) of. Sox2-positive/FoxO3-positive and NeuN-negative/FoxO3-positive nuclei are shown by white arrowheads. Higher magnification images are included. Scale bar: 100 μm.
(B) FoxO3 is expressed in purified NSC in culture. Immunocytochemistry on NSC isolated from 3 month-old mice with antibodies to FoxO3 (‘Ct’) and to Nestin. DAPI was used to stain nuclei. NSC were either grown as neurospheres (whole NS) (left panels) or freshly dissociated NSC (Diss. NSC) (right panels). Scale bar: 100 μm.
(C) FoxO3 expression in NSC at different ages. Western blots of protein lysates from secondary neurospheres in self-renewing conditions from FoxO3+/+ and FoxO3−/− littermates at 3 different ages, 1 day-old (1d), 3 month-old (3m), and 1 year-old (1y), probed with antibodies to FoxO3 (‘NFL’) and to β-actin. The data presented are representative of 3 independent experiments.
(D) FoxO3 phosphorylation is increased in differentiated progeny. Western blots of protein lysates of dissociated wild-type adult NSC in self-renewing conditions (day 0) or in differentiation conditions for increasing lengths of time (days 1-6) (Figure S3A). Western blots were probed with antibodies to FoxO3 (‘NFL’), to Phospho-T32 (FoxO3 T32-P) and to β-actin. Blots representative of 3 independent experiments.
(E) A FoxO-dependent reporter gene is responsive to FoxO3 in NSC. Luciferase assays in wild-type adult NSC using a promoter containing three FoxO binding sites driving the luciferase reporter gene (FHRE pGL3) or a control promoter (pGL3) and FoxO3 expression vectors (-: empty vector; WT: wild-type; CA: constitutively active; I: inactive). Values represent mean ± SEM from 3 independent experiments. One-way ANOVA, Bonferroni post-tests, *: p<0.05, **: p<0.01, ***: p<0.001.
(F) Endogenous FoxO3 is active in self-renewing NSC. Luciferase assays in FoxO3+/+ and FoxO3−/− adult NSC using FHRE pGL3 or control pGL3 as in E. Values were normalized to the first column. Values represent mean ± SEM from 3 independent experiments. One-way ANOVA, Bonferroni post-tests, **: p<0.01, ***: p<0.001.
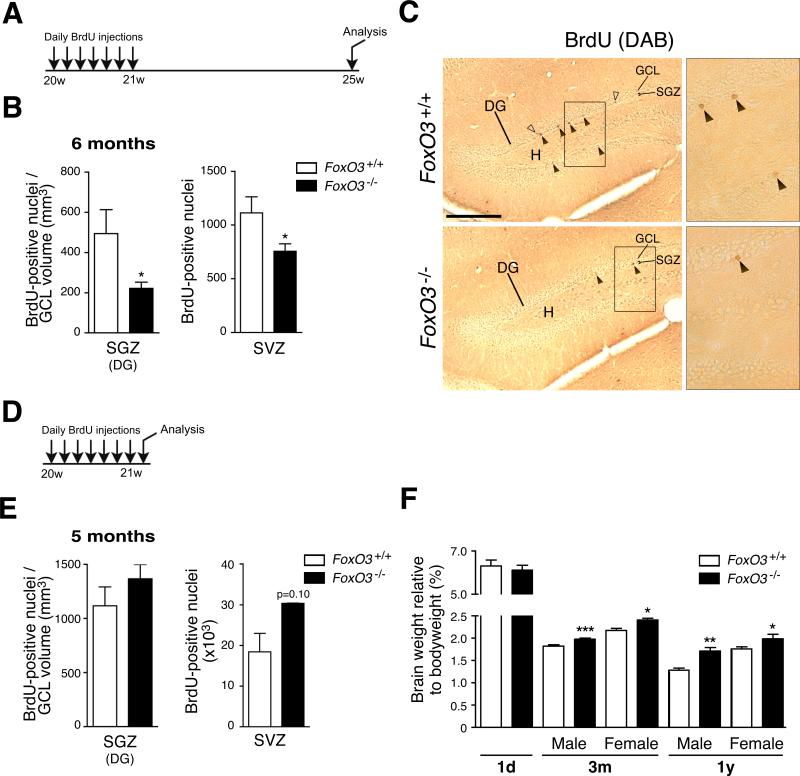
Figure 2. The ablation of FoxO3 results in a decrease in NSC number in vivo.
(A) Experimental design for the quantification of NSC in vivo. 5 month-old FoxO3+/+ and FoxO3−/− littermates were injected daily with BrdU for 7 days and sacrificed one month after the last BrdU injection.
(B) Quantification of label-retaining NSC in vivo. Number of BrdU-positive cells one month after 7 days of daily BrdU injection in SGZ of the DG (left panel) and the SVZ (right panel, Figure S4A). The number of BrdU-positive cells in the SGZ was normalized to the volume of the granular cell layer (GCL) (Figure S4C). Values represent mean ± SEM (left panel) and mean ± SD (right panel) from 5 animals for FoxO3+/+ and 4 animals for FoxO3−/− mice. Mann-Whitney test, *: p<0.05.
(C) Examples of label-retaining NSC in the DG of FoxO3+/+ and FoxO3−/− mice. Coronal sections of adult FoxO3+/+ and FoxO3−/− mouse brains showing BrdU-positive nuclei one month after 7 days of daily BrdU injection in the SGZ. Filled arrowhead: label-retaining NSC. Empty arrowhead: BrdU-positive cells that have migrated into the GCL. DG: dentate gyrus, H: hilus. Scale bar: 200 μm. Right panels represent higher magnifications of the rectangles in the left panels.
(D) Experimental design for the quantification of proliferating NSC and neural progenitors in vivo. 5 month-old FoxO3+/+ and FoxO3−/− littermates were injected daily with BrdU for 7 days and sacrificed one day after the last BrdU injection.
(E) Quantification of proliferating NSC and neural progenitors in vivo. Number of BrdU-positive cells one day after 7 days of daily BrdU injections in the SGZ (left panel) and the SVZ (right panel). The number of BrdU-positive cells in the SGZ was normalized to the GCL volume (Figure S4D). Values represent mean ± SEM (left panel) and mean ± SD (right panel) from 3 animals for each genotype. Mann-Whitney test, p=0.40 (left panel) and p=0.10 (right panel).
(F) Brain weight is increased in adult FoxO3−/− mice compared to FoxO3+/+ littermates. Brain weights were measured for FoxO3−/− and FoxO3+/+ animals in mice at different ages (1 day-old (1d), 3 month-old (3m), and 1 year-old (1y)). Values represent mean ± SEM from 4-6 mice (1d), 20-23 males and 6-9 females (3m), and 4-7 males and females (1y). Mann-Whitney test, *: p<0.05; **: p<0.01; ***: p<0.001.
To confirm that FoxO3 is expressed in NSC, we isolated NSC from both the DG and the SVZ regions of the post-natal or adult mouse brain. Mouse NSC form clonal spheres called neurospheres, which are composed of NSC and more committed neural progenitors (Reynolds and Weiss, 1992). Immunostaining experiments on whole neurospheres or on dissociated NSC revealed that FoxO3 is expressed in NSC/progenitors derived from both post-natal and adult animals and is co-expressed with Nestin, a NSC/progenitor marker (Figures 1B and S3A).
To quantify the levels of FoxO3 in NSC as a function of age, we used Western blotting on protein extracts of neurospheres from NSC isolated from mice at three different age milestones: birth (1 day-old), adulthood (3 month-old) and middle age (1 year-old). These experiments confirmed that FoxO3 is expressed in NSC from mice at all ages (Figure 1C). FoxO3 protein expression is lower in NSC from neonates than in NSC from adults, but there was no significant change in FoxO3 expression in NSC from young versus middle-aged adults (Figure 1C). Thus, FoxO3 is expressed in NSC/progenitors from both developing and adult mice, with higher expression in adult NSC.
FoxO3 activity is higher in self-renewing NSC than in differentiated progeny
To assess FoxO3 activity in NSC, we compared the phosphorylation status of FoxO3 in self-renewing versus differentiating adult NSC using Western blotting with phospho-specific antibodies to Threonine 32 (T32), one of the three sites of FoxO3 phosphorylated by Akt (Brunet et al., 1999). A larger percentage of FoxO3 was phosphorylated at T32 in differentiated progeny than in self-renewing NSC (Figures 1D and S3B). As T32 phosphorylation is correlated with FoxO3 inactivation (Brunet et al., 1999), these results suggest that FoxO3 is more active in self-renewing NSC than in their differentiated progeny.
To quantify FoxO3 activity in NSC, we performed luciferase assays using a luciferase reporter gene under the control of three Forkhead binding sites (Brunet et al., 1999). We first verified that this FoxO reporter gene was responsive to FoxO3 in NSC by transfecting wild-type adult NSC with active or inactive forms of FoxO3 (Figure 1E). We next tested the activity of endogenous FoxO3 in NSC and found that this FoxO reporter gene was active in adult FoxO3+/+ NSC and that its activity was significantly decreased in FoxO3−/− NSC (Figure 1F). The remainder of the activity of the FoxO reporter gene in FoxO3−/− NSC is likely due to partial compensation by other FoxO family members. Taken together, these findings indicate that FoxO3 is active in self-renewing NSC.
The ablation of FoxO3 results in a decrease in NSC number in vivo
To determine if FoxO3 regulates the NSC pool in vivo, we performed BrdU injection experiments in adult FoxO3−/− and FoxO3+/+ mice (Figure 2). FoxO3−/− mice are viable and normal in outward appearance, though they are prone to cancer and die at around 1 to 1.5 years of age (data not shown, Paik et al., 2007). BrdU was injected daily into adult FoxO3−/− and FoxO3+/+ littermates for seven days and the mice were sacrificed either one month (Figure 2A) or one day (Figure 2D) after the last BrdU injection. We counted BrdU-positive cells in the NSC niches one month after the last BrdU injection (Figures 2B, 2C, and S4A). These label-retaining cells are thought to be the relatively quiescent NSC that remain in NSC niches whereas neural progenitors and differentiated cells migrate away from NSC niches (Bondolfi et al., 2004). FoxO3−/− mice displayed a significant reduction in the number of label-retaining NSC compared to FoxO3+/+ mice in both the SGZ (Figure 2B, left panel) and the SVZ (Figures 2B, right panel, and S4A). This result suggests that in the absence of FoxO3, adult mice have fewer NSC. In contrast, when mice were sacrificed one day after the BrdU injections (Figure 2D), FoxO3−/− mice tended to have more BrdU-positive cells than FoxO3+/+ mice in the SGZ (p=0.40) and the SVZ (p=0.10) (Figure 2E). As BrdU-positive cells in the short-term BrdU paradigm are mostly neural progenitors, these results indicate that in FoxO3−/− mice, the number of progenitors is not decreased and may even be slightly increased. Consistent with this finding, brains from adult FoxO3−/− mice were significantly heavier than brains from wild-type counterparts. In contrast, brains from FoxO3−/− mice had similar weight as wild-type counterparts at birth (Figure 2F). Together, these results suggest that FoxO3 loss may lead to the short-term amplification of progenitors, resulting in the exhaustion of the NSC pool over time.
The absence of FoxO3 leads to the depletion of NSC in adult mice
To independently test if FoxO3 prevents the progressive depletion of NSC in vivo, we isolated NSC from adult FoxO3−/− and FoxO3+/+ mice and tested the ability of these cells to form primary neurospheres. The frequency of primary neurospheres formed at low cell density from freshly isolated NSC can be used as an estimate of the pool size of NSC in the brain and indirectly as a measure of self-renewal (Kippin et al., 2005). We found that NSC from adult FoxO3−/− mice formed primary neurospheres at a significantly lower frequency than NSC from FoxO3+/+ littermates (Figure 3A). Although neurospheres can fuse, which can limit the interpretation of these experiments (Reynolds and Rietze, 2005), these results confirm that FoxO3 regulates the NSC pool. These findings are also consistent with the possibility that FoxO3 is necessary for NSC self-renewal.
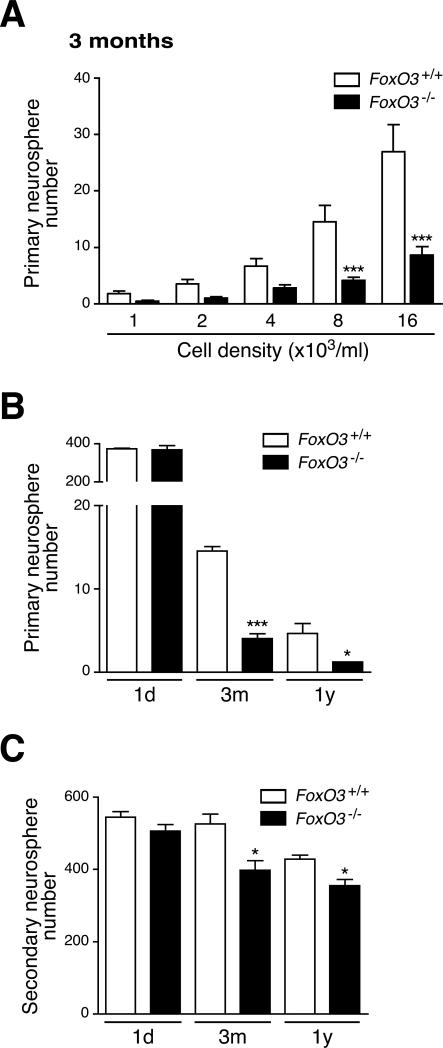
Figure 3. FoxO3 regulates the NSC pool and NSC self-renewal in adult mice, but not in neonates.
(A) FoxO3−/− NSC from adult mice display a defect in primary neurosphere formation. NSC isolated from 3 month-old FoxO3+/+ or FoxO3−/− littermates were seeded at the indicated cell densities. The number of neurospheres formed after one week was counted. Values represent mean ± SEM from 7 independent experiments performed with 5 littermates for each genotype. Two-way ANOVA (p<0.0001 for the genotype variable), Bonferroni post-tests, ***: p<0.001.
(B) FoxO3−/− NSC from adult mice, but not from neonates, display a defect in primary neurosphere formation. Frequency of primary neurospheres formed from NSC at low-density (8,000 cells/ml) from mice at different ages (1 day-old, 1d; 3 month-old (3m); 1 year-old (1y)). Values represent mean ± SEM from 2 independent experiments with 2 littermates (1d), 7 independent experiments with 5 littermates (3m), and 3 independent experiments with 3-5 littermates (1y) for each genotype. Two-way ANOVA with Bonferroni post-tests, *: p<0.05; ***: p<0.001.
(C) FoxO3−/− NSC from adult mice, but not from neonates, display a defect in secondary neurosphere formation. Dissociated cells from primary neurospheres from FoxO3+/+ or FoxO3−/− littermates were seeded at 4,000 cells/ml. The number of secondary neurospheres formed after one week was counted. Values represent mean ± SEM from 2 independent experiments with 2 littermates (1d), 4 independent experiments with 5 littermates (3m), and 4 independent experiments with 3 to 5 littermates for each genotype (1y) for each genotype. Two-way ANOVA with Bonferroni post-tests, *: p<0.05.
To compare the ability of FoxO3 to regulate the NSC pool at different ages, we isolated NSC from FoxO3−/− and FoxO3+/+ mice at different ages. FoxO3−/− NSC isolated from embryos (data not shown) or from 1 day-old mice (Figures 3B and S5A) formed primary neurospheres with the same frequency as FoxO3+/+ NSC. In contrast, FoxO3−/− NSC isolated from both young adult mice and middle-aged mice formed neurospheres at a significantly lower frequency than FoxO3+/+ NSC (Figures 3B and S5B). These results indicate that the absence of FoxO3 leads to defects in the NSC pool that are only evident in adult mice. As FoxO3 is constitutively deleted in FoxO3−/− mice, it is not possible to distinguish if FoxO3 only regulates the NSC pool in adults or if FoxO3 also acts in NSC during embryonic and/or post-natal development. As previously reported (Molofsky et al., 2006), the frequency of primary neurospheres formed in culture decreased significantly with the age of the mice (Figure 3B). The absence of FoxO3 did not have a greater impact on NSC from middle-aged mice than in NSC from young adult mice (Figure 3B), which might be due to a progressive inactivation of FoxO3 function during aging.
FoxO3 is necessary for NSC self-renewal
We tested if FoxO3 is necessary for NSC self-renewal. Primary neurospheres can be dissociated to generate secondary neurospheres, and the number of secondary neurospheres formed can serve as a measure of NSC self-renewal (Kippin et al., 2005). We dissociated FoxO3−/− and FoxO3+/+ primary neurospheres and tested the ability of the dissociated cells to form secondary neurospheres. Whereas FoxO3−/− NSC from neonates formed secondary neurospheres at the same frequency as FoxO3+/+ NSC, FoxO3−/− NSC isolated from young and middle-aged adults generated fewer secondary neurospheres than FoxO3+/+ NSC (Figures 3C, S5C-S5E). This result suggests that FoxO3 regulates NSC self-renewal. The ability of adult FoxO3−/− NSC to form neurospheres at later passages in vitro was not significantly different from that of FoxO3+/+ NSC (Figure S5F), perhaps because other FoxO family members compensate for the lack of FoxO3 after several passages in culture. Concomitant deletion of FoxO1, FoxO3, and FoxO4 results in defects in neurosphere formation upon serial passage in culture (Paik et al., 2009 [this issue of Cell stem Cell]), confirming the functional redundancy among FoxO family members in cultured NSC. These results suggest that FoxO3 prevents the premature exhaustion of NSC by preserving their self-renewal capacity.
The ability of NSC to generate different neural lineages is defective in the absence of FoxO3
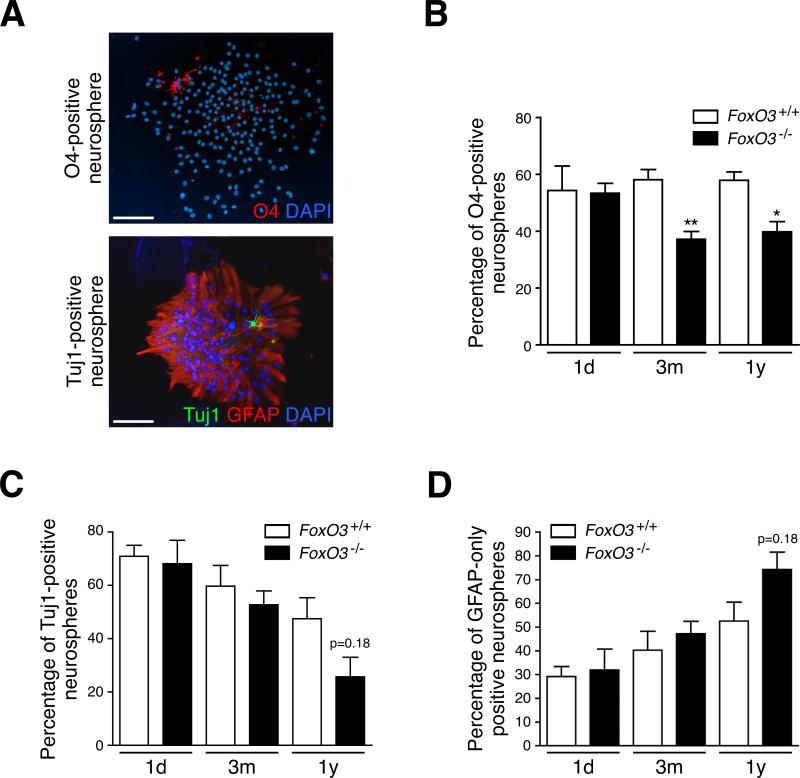
NSC give rise to three types of progeny, neurons, astrocytes, and oligodendrocytes, and the proper balance between the three fates is pivotal for the functionality of the NSC pool. The ability of NSC to generate progeny can be measured by assessing cellular fates in secondary neurospheres, as each neurosphere derives from a single NSC (Kippin et al., 2005). We examined the progeny of FoxO3−/− and FoxO3+/+ secondary neurospheres after seven days in differentiation conditions. Neurospheres generated from adult NSC contained mainly astrocytes (GFAP-positive), as well as fewer numbers of immature neurons (Tuj1-positive) or immature oligodendrocytes (O4-positive) (Figure 4A), indicating that these neurospheres arose from at least a bipotent NSC. To measure NSC functionality, we scored the number of differentiated secondary neurospheres formed at low cell density that contain at least one immature neuron (Tuj1-positive) or one immature oligodendrocyte (O4-positive) in addition to astrocytes. We found that NSC isolated from neonate FoxO3−/− and FoxO3+/+ mice formed similar numbers of bipotent neurospheres (Figures 4B and 4C). In contrast, NSC isolated from young or middle-aged adult FoxO3−/− mice formed significantly fewer secondary neurospheres containing oligodendrocytes than those isolated from FoxO3+/+ mice (Figure 4B). FoxO3−/− NSC from middle-aged mice tended to form fewer neurospheres containing both neurons and astrocytes and more neurospheres containing only astrocytes compared to FoxO3+/+ NSC (Figures 4C and 4D). Similar results were obtained when differentiated neurospheres were stained for all three cell fates simultaneously (Figure S6). These findings indicate that FoxO3 loss results in a deficiency in the ability of NSC to generate different neural lineages that is evident only in adulthood. Our observations further suggest that in the absence of FoxO3, NSC become more similar to progenitors (i.e. less able to self-renew and more committed to a specific lineage). NSC lacking FoxO3 also resemble in vivo NSC from older animals, which are less able to self-renew and more skewed towards astrocytes (Bondolfi et al., 2004).
Figure 4. FoxO3 controls the ability of adult NSC to give rise to different lineages.
(A) Adult NSC give rise to at least bipotent neurospheres. Astrocytes, neurons, and oligodendrocytes present in whole neurospheres after 7 days of differentiation were stained with antibodies to GFAP, Tuj1, and O4 respectively. Scale bars: 100 μm.
(B) FoxO3−/− NSC from adult mice, but not from neonates, give rise to fewer oligodendrocyte-containing neurospheres than FoxO3+/+ NSC. FoxO3+/+ and FoxO3−/− NSC from mice at different ages (1 day-old, 1d; 3 month-old, 3m; 1 year-old, 1y) were grown as secondary neurospheres at low density. The neurospheres were differentiated for a week and stained with antibodies to O4. Neurospheres that contained oligodendrocytes were counted. Values represent mean ± SEM from 2 independent experiments with 2 littermates (1d), 5 littermates (3m), and 3-5 littermates (1y) for each genotype. Student's t-test, *: p<0.05; **: p<0.01.
(C-D) FoxO3−/− NSC from middle-aged mice tend to give rise to fewer neuron-containing neurospheres than FoxO3+/+ NSC. Neurospheres were grown as described in B and stained with antibodies to Tuj1 and GFAP. Neurospheres that contained neurons and astrocytes were counted. Values represent mean ± SEM from 2 independent experiments with 2 littermates (1d), 5 littermates (3m) and 3-5 littermates (1y) for each genotype. Student's t-test, p=0.18. (C) Neurospheres containing at least one Tuj-1 positive cell; (D) Neurospheres containing only GFAP-positive cells.
FoxO3 acts in the nervous system to regulate the NSC pool
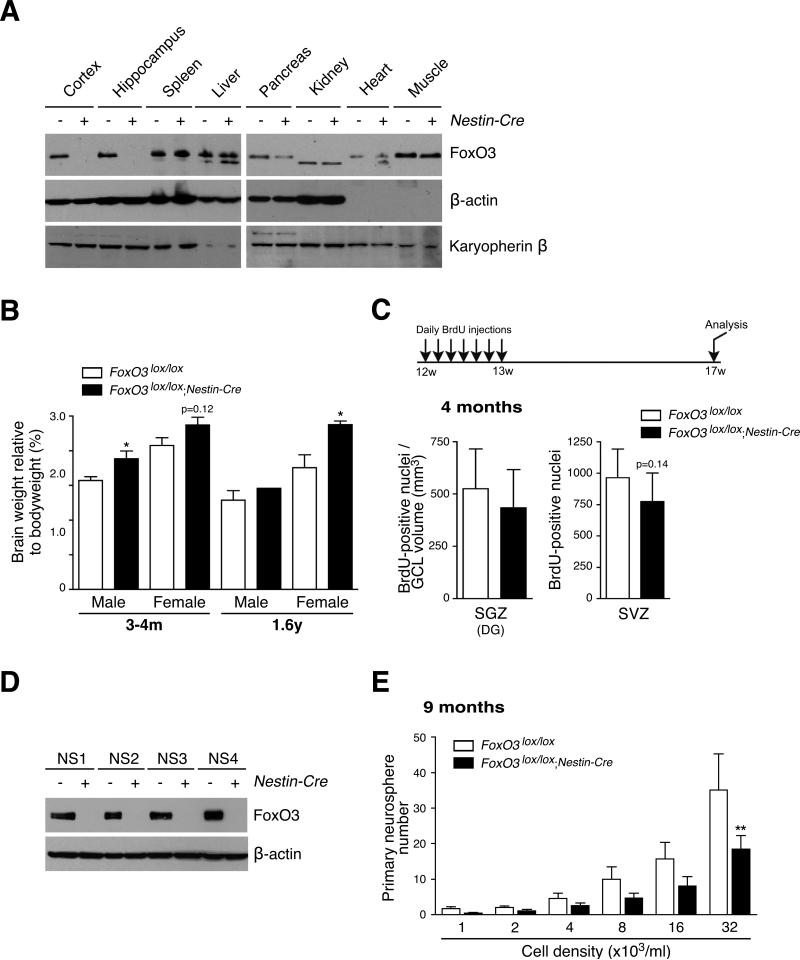
FoxO3 is expressed in a number of tissues, raising the question of whether FoxO3 regulates the NSC pool by acting in the brain or in other tissues, which could in turn affect NSC. To address this question, we crossed FoxO3lox/lox mice with Nestin-Cre transgenic mice, which express the Cre recombinase in NSC/progenitors from embryonic day 10.5 (Tronche et al., 1999). As expected, FoxO3lox/lox;Nestin-Cre mice displayed an ablation of the FoxO3 protein in the brain, but not in the majority of other tissues (Figure 5A). Interestingly, young and middle-aged adult FoxO3lox/lox;Nestin-Cre mice had significantly heavier brains than their control siblings (Figure 5B). These results suggest that FoxO3 regulates brain weight by acting in the nervous system, rather than in other tissues.
Figure 5. Consequences of FoxO3 loss in the nervous system on brain weight and the NSC pool.
(A) Expression of FoxO3 in the tissues of FoxO3lox/lox;Nestin-Cre mice. FoxO3 expression in different tissues of 2 month-old FoxO3lox/lox;Nestin-Cre mice was determined by Western blot with antibodies to FoxO3 (‘NFL’) and antibodies to β-actin and karyopherin β. Note that FoxO3 was partially deleted in the pancreas.
(B) Brain weights of FoxO3lox/lox;Nestin-Cre and FoxO3lox/lox mice. Brain weights were measured after perfusion in 3 to 4 month-old mice (3-4m) and 1.6 year-old mice (1.6y). Values represent mean ± SEM from 9-10 males and 9-10 females (3-4m), and 1-3 males and 5-8 females (1.6y). One male and 2 females (3-4m) and 3 females (1.6y) of the FoxO3lox/+ genotype were included in the control group (FoxO3lox/lox).
(C) Quantification of label-retaining NSC in FoxO3lox/lox;Nestin-Cre and FoxO3lox/lox animals in vivo. Number of BrdU-positive cells in the SGZ (left panel) and the SVZ (right panel) in 3 month-old FoxO3lox/lox;Nestin-Cre and FoxO3lox/lox littermates injected daily with BrdU for 7 days and sacrificed one month after the last BrdU injection. The number of BrdU-positive cells in the SGZ was normalized to the volume of the granular cell layer (GCL). Values represent mean ± SEM (left panel) and mean ± SD (right panel) from 8 FoxO3lox/lox;Nestin-Cre mice and 11 FoxO3lox/lox control littermates. Two FoxO3lox/+ littermates were included in the control group (FoxO3lox/lox). Mann-Whitney test, p=0.27 (left panel) and p=0.14 (right panel).
(D) Ablation of the FoxO3 protein in NSC from FoxO3lox/lox;Nestin-Cre mice. FoxO3 expression in NSC isolated from 3 month-old FoxO3lox/lox mice (−) or FoxO3lox/lox;Nestin-Cre mice (+) seven days after isolation (NS1) or at 3 consecutive passages (NS2 to 4) was determined by Western blot with antibodies to FoxO3 (‘NFL’) and antibodies to β-actin.
(E) Ablation of FoxO3 in the brain impairs primary neurosphere formation. NSC isolated from 9 month-old FoxO3lox/lox mice (control) or FoxO3lox/lox;Nestin-Cre mice were seeded at low density. The number of neurospheres formed after one week was counted. Values represent mean ± SEM from triplicates from 4 independent experiments conducted with 3 littermates for each genotype. Twoway ANOVA, p<0.01 for the genotype variable, Bonferroni post-tests, **: p<0.01.
To test if FoxO3 regulates the NSC pool by acting in the nervous system, we performed BrdU long-term retention experiments in vivo in FoxO3lox/lox;Nestin-Cre mice and FoxO3lox/lox siblings (Figure 5C). We found that young adult FoxO3lox/lox;Nestin-Cre mice tended to have fewer label-retaining NSC in the SVZ and in the SGZ than control siblings, though this did not reach statistical significance (Figure 5C). The effects of FoxO3 loss in vivo were less pronounced in FoxO3lox/lox;Nestin-Cre mice than in FoxO3−/− mice, which could suggest that FoxO3 regulates NSC in part by acting in tissues other than the nervous system. However, the difference in magnitude in long-term BrdU retention between FoxO3lox/lox;Nestin-Cre and FoxO3−/− mice is likely due to the different genetic backgrounds of these mice and/or to the different ages of the mice when the NSC pools were examined.
To independently test if FoxO3 acts in the nervous system to regulate NSC, we isolated NSC from FoxO3lox/lox;Nestin-Cre mice and control mice and assessed their ability to form primary neurospheres. NSC isolated from FoxO3lox/lox;Nestin-Cre mice had no FoxO3 protein expression for up to four passages (Figure 5D), indicating that the deletion of the FoxO3 gene was efficient. Interestingly, NSC isolated from 9 month-old FoxO3lox/lox;Nestin-Cre mice displayed significant defects in their ability to form primary neurospheres compared to NSC isolated from control FoxO3lox/lox littermates (Figure 5E). Together, these results suggest that FoxO3 acts in the nervous system to regulate adult brain weight and NSC homeostasis in vivo. Because FoxO3 is deleted from embryonic day 10.5 in FoxO3lox/lox;Nestin-Cre mice, the effects of FoxO3 loss on brain weight and NSC may be a result of FoxO3's action in the embryonic and/or post-natal brain. It is also possible that FoxO3 regulates NSC in part by acting in other tissues, e.g. blood vessels that form the NSC niche or metabolic tissues that would then affect the systemic milieu. However, two independent ways of deleting FoxO factors in the nervous system — Nestin-Cre (this study) or GFAP-cre (Paik et al., 2009 [this issue of Cell stem Cell]) — both result in similar NSC phenotypes, supporting the notion that FoxO factors act in the nervous system to regulate NSC.
FoxO3 regulates the quiescence and survival of NSC
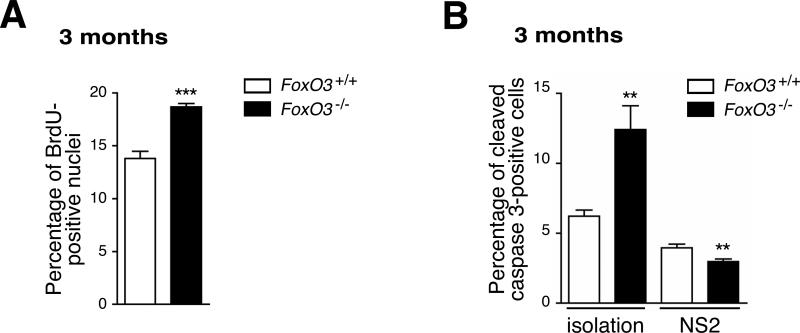
To determine the cellular mechanisms by which FoxO3 regulates NSC, we tested if the absence of FoxO3 affects cell proliferation in neurospheres. Cells dissociated from secondary neurospheres from young adult FoxO3−/− mice displayed a significant increase in BrdU incorporation in culture compared to cells from FoxO3+/+ mice (Figure 6A). There was no difference in the proportion of cells in G2/M phases between FoxO3+/+ and FoxO3−/− NSC (data not shown), suggesting that the primary effect of FoxO3 loss is at the G1/S transition. Thus, the absence of FoxO3 leads to the proliferation of NSC/progenitors cells in neurospheres. Without FoxO3, NSC may lose their ability to re-enter a state of relative quiescence after they divide, which may lead to the amplification of progenitors and the exhaustion of the pool of NSC in vivo.
Figure 6. FoxO3 is necessary for maintaining NSC/neural progenitor quiescence.
(A) Increased cell proliferation in FoxO3−/− neurospheres. Cells dissociated from secondary neurospheres from 3 month-old FoxO3+/+ or FoxO3−/− mice were plated on poly-D-lysine and incubated for 1 hour with BrdU. Cells were immunostained with antibodies to BrdU. BrdU-positive nuclei were counted. Values represent mean ± SEM from 2 independent experiments with 5 littermates for each genotype. Student's t-test, ***: p<0.001.
(B) Apoptosis in FoxO3−/− NSC compared to FoxO3+/+ NSC. Freshly isolated NSC or cells dissociated from secondary neurospheres isolated from 3 month-old FoxO3+/+ or FoxO3−/− mice were stained with antibodies to cleaved caspase 3. Cleaved caspase 3-positive cells were counted. Values represent mean ± SEM from 2 independent experiments with 5 littermates for each genotype. Student's t-test, **: p<0.01.
We also quantified apoptotic cell death in FoxO3−/− and FoxO3+/+ NSC. Freshly isolated NSC from adult FoxO3−/− mice displayed an increased number of cleaved caspase 3-positive cells compared to FoxO3+/+ NSC (Figure 6B, left panel), which might contribute to the decreased self-renewal of FoxO3−/− NSC. In contrast, the level of apoptosis for cells dissociated from secondary FoxO3−/− neurospheres was lower than for cells dissociated from FoxO3+/+ neurospheres (Figure 6B, right panel). These findings, coupled with the observation that FoxO3−/− mice have increased brain weight compared to FoxO3+/+ littermates (Figure 2F), suggests that cell death is not a major consequence of FoxO3 loss and that loss of quiescence may play a more important role in the depletion of NSC in vivo in FoxO3−/− mice.
FoxO3 coordinates the expression of a specific program of genes in NSC
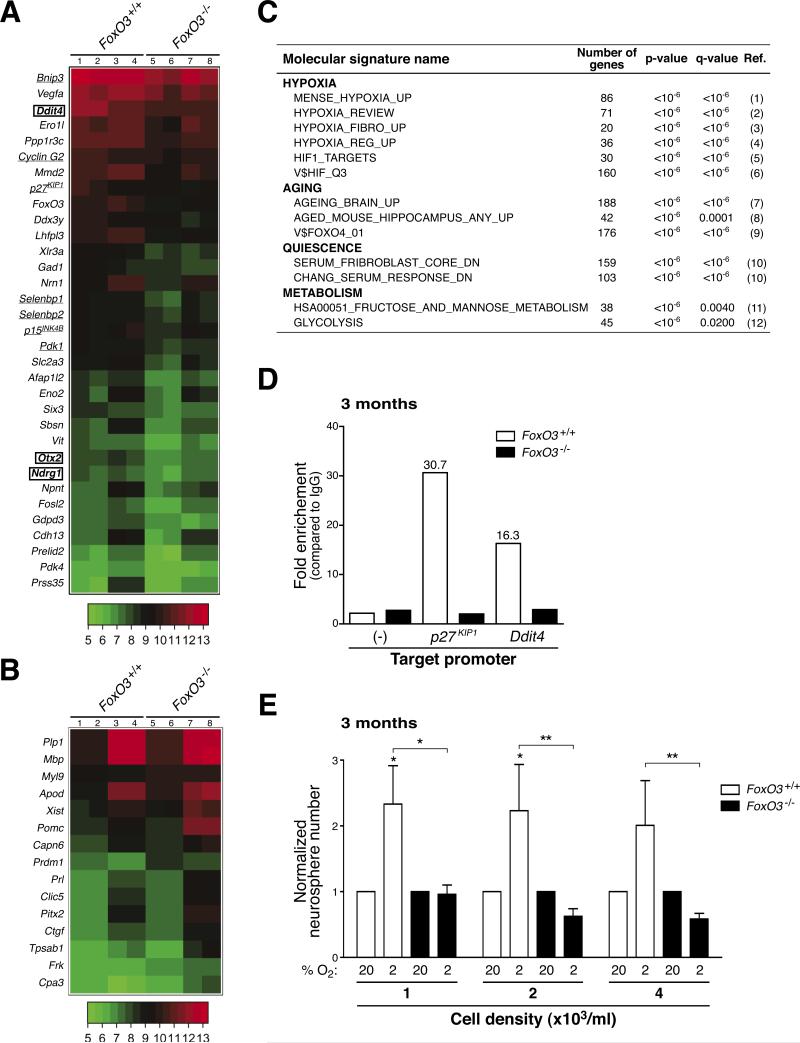
To gain insight into the molecular mechanisms by which FoxO3 regulates NSC, we determined the program of genes regulated by FoxO3 in NSC. We performed a genome-wide microarray analysis on RNA isolated from two independent biological replicates, each in duplicate, of FoxO3−/− and FoxO3+/+ secondary neurospheres from young adult mice. We used secondary neurospheres because FoxO3−/− and FoxO3+/+ neurospheres displayed a significant difference in self-renewal ability. Analysis of the microarray data revealed that the expression of a specific subset of genes is decreased in FoxO3−/− neurospheres compared to FoxO3+/+ neurospheres (Figures 7A, 7B, and Table S1). The changes in expression levels of a number of these genes were validated by reverse transcription followed by quantitative PCR (Figure S7).
Figure 7. FoxO3 is necessary for the expression of a program of genes that coordinately regulates NSC homeostasis.
(A-B) Differentially regulated genes in FoxO3−/− NSC compared to FoxO3+/+ NSC. Whole genome microarray data obtained from two independent biological replicates of RNA from duplicates of FoxO3−/− and FoxO3+/+ secondary neurospheres isolated from 3 month-old mice (5 littermates for each genotype). Heat-map of selected genes down-regulated (A) and up-regulated (B) more than 1.5 fold in FoxO3−/− NSC compared to FoxO3+/+ NSC with a false discovery rate less than 5%. Lanes 1-2 and 5-6: duplicates from one experiment. Lanes 3-4 and 7-8: duplicates from a second independent experiment. Underlined: known FoxO target genes. Surrounded by a square: genes containing FoxO binding motifs in their regulatory regions (Table S1).
(C) FoxO3-regulated genes in NSC are involved in quiescence, hypoxia, aging, and glucose metabolism. Selected publicly available molecular signatures highly enriched for genes down-regulated in FoxO3−/− NSC as provided by GSEA (Gene Set Enrichment Analysis). References (Ref.) 1-12 are included in Supplemental Data.
(D) FoxO3 is recruited to the promoters of p27KIP1 and Ddit4 in NSC. ChIP of FoxO3 from NSC isolated from 3 month-old mice shows significant recruitment of FoxO3 at the promoters of p27KIP1 and Ddit4. FoxO3 recruitment was not found at control regions that did not have FoxO binding sites (−) and did not occur in FoxO3−/− neurospheres. Depicted is a representative ChIP analysis. Similar results were obtained in independent experiments (Figure S10C).
(E) FoxO3−/− NSC form fewer neurospheres than FoxO3+/+ NSC in low oxygen conditions. Normalized frequency of neurospheres formed from NSC at low-density in 20% oxygen (20) or in 2% oxygen (2). NSC were dissociated from secondary neurospheres generated from 3 month-old FoxO3−/− and FoxO3+/+ mice. Values represent mean ± SEM from 4 independent experiments with 3-5 littermates for each genotype. The normalization was done by dividing the triplicate average from each experiment in 2% oxygen by the triplicate average from the same experiment in 20% oxygen. Two-way ANOVA, Bonferroni post-tests, *: p<0.05; **: p<0.01.
The analysis of our microarray data revealed that FoxO3 is required for the expression of genes involved in cell quiescence, as was previously shown in other cell types (Salih and Brunet, 2008). For example, FoxO3 is necessary for the expression of p27KIP1 and Cyclin G2 (Figures 7A and S7). The comparison of our microarray data with available molecular signatures revealed that FoxO3-regulated genes were significantly enriched for genes that form a molecular signature for quiescence (Figures 7C, S8A, and S8B). These results support the notion that FoxO3 regulates the homeostasis of the NSC pool by preventing the premature overproliferation of progenitors and preserving the relative quiescence of NSC. We also found that FoxO3 was necessary for the expression of genes involved in oxidative stress resistance (e.g. selenbp1) (Figures 7A and S7) and in glucose metabolism and transport (e.g. Pdk1, Slc2a3) (Figures 7A, 7C, and S7), consistent with the known role of the FoxO family in stress resistance and cellular metabolism (Accili and Arden, 2004). The ability of FoxO3 to coordinate a program maintaining quiescence, stress resistance, and glucose metabolism in adult NSC may be critical for preserving the stemness of these cells.
Interestingly, we also identified genes that were not previously known to be upregulated by FoxO factors. First, FoxO3 was necessary for the upregulation of genes involved in early neurogenesis (e.g. Otx2) (Figures 7A and S7), which may contribute to defect in multipotency of FoxO3−/− neurospheres (Figure S6). Surprisingly, the comparison between our microarray data and publicly available molecular signatures revealed that FoxO3 was necessary for the expression of genes upregulated in hypoxic brains and other hypoxic tissues, including Ddit4, Ndrg1, Ero1l, and Vegfa (Figures 7A, 7C, S7, S8C, S8D, and Table S1). These findings raise the possibility that FoxO3 is necessary for the response of NSC to low oxygen. The ability of FoxO3 to control oxygen metabolism in NSC may participate in the regulation of NSC in vivo.
FoxO3 is also required for the repression of specific genes in neurospheres (Figure 7B). For example, genes expressed in mature oligodendrocytes, including Myelin Basic Protein, Plp1, and Apod, were upregulated in FoxO3−/− NSC compared to FoxO3+/+ NSC (Figures 7B, S7, and Table S1). These results suggest that FoxO3 normally prevents premature oligodendrocyte differentiation. Consistent with these findings, we observed that the corpus callosum area, a brain structure that contains mature oligodendrocytes, is increased in adult FoxO3−/− mice compared to FoxO3+/+ littermates (Figure S9). The inhibition of the premature differentiation of NSC by FoxO3 may contribute to the functionality of NSC in vivo.
Finally, the comparison of our microarray data with genes that are known to change during aging in human and mouse brains revealed a correlation between FoxO3-regulated genes and genes that are regulated during aging in the brain (Figures 7C, S8E, and S8F). These observations are consistent with the possibility that FoxO3, a gene that belongs to a family that promotes longevity in invertebrates, regulates genes that are important to counteract the aging process in mammalian adult stem cells.
Identification of FoxO3 direct targets in NSC
FoxO3-regulated genes are enriched for the presence of a FoxO binding motif in their regulatory regions (Figure 7A, Table S1), raising the possibility that a subset of FoxO3-regulated genes might be direct FoxO3 target genes in NSC. To identify direct targets of FoxO3, we performed Electrophoretic Mobility Shift Assays (EMSA) and chromatin immunoprecipitation (ChIP) in NSC. EMSA experiments revealed that FoxO3 could bind in vitro to FoxO binding sites present in the regulatory regions of the Ddit4, Ndrg1, and Otx2, suggesting that these genes are direct targets of FoxO3 (Figures S10A and S10B). ChIP experiments revealed that FoxO3 is recruited to the FoxO binding sites in the promoters of the p27KIP1 and Ddit4 genes (Figure 7D and S10C). These results indicate that p27KIP1, a cell cycle inhibitor involved in cell quiescence and Ddit4, a known target of hypoxia-inducible factor 1 (HIF1), are direct target genes of FoxO3 in NSC. In contrast, FoxO3 was not bound at the promoters of Otx2 and Ndrg1 by ChIP (data not shown), suggesting that these two genes may not be direct FoxO3 targets in NSC.
The response of FoxO3−/− NSC to low oxygen is impaired in vitro
As a subset of FoxO3-regulated genes is involved in the cellular response to hypoxia, we compared the ability of FoxO3+/+ and FoxO3−/− NSC to form neurospheres in low oxygen (2%) versus atmospheric oxygen (20%) conditions (Figure 7E). Consistent with published findings for embryonic NSC/progenitors (Studer et al., 2000), adult NSC showed an increased ability to form neurospheres in low oxygen compared to atmospheric oxygen (Figure 7E). In contrast, FoxO3−/− NSC did not display an increased ability to form neurospheres in low oxygen compared to atmospheric oxygen conditions (Figure 7E). These results suggest that the response of FoxO3−/− NSC to low oxygen is impaired in vitro. It is also possible that 2% oxygen, which mimics in vivo physiological oxygen concentrations in the mammalian brain (Zhu et al., 2005), helps reveal differences between FoxO3−/− and FoxO3+/+ NSC. Together, these results indicate that FoxO3 is necessary for the expression of a program of genes that coordinate cell quiescence, inhibition of premature differentiation, and oxygen metabolism, which may all contribute to the ability of FoxO3 to regulate the homeostasis of the NSC pool.
Discussion
Our results indicate that FoxO3 regulates processes and pathways relevant to NSC homeostasis both in vitro and in vivo. In the absence of FoxO3, NSC display a decreased ability to self-renew and to give rise to different neural lineages, which may result in the depletion of NSC in vivo. Our findings also suggest that FoxO3 regulates NSC homeostasis by controlling a program of genes that precisely governs cell cycle re-entry and promotes a state of glucose and oxygen metabolism compatible with optimal self-renewal. As the NSC pool is a limited reserve that needs to be maintained throughout life, preserving this pool may be an important factor in the healthspan of long-lived organisms.
The effects of FoxO3 loss on NSC only manifest themselves in adult animals. This observation raises the possibility that FoxO3 plays a more important role in adult NSC than in embryonic or neonatal NSC. It is also possible that FoxO3 acts to alter embryonic or post-natal NSC biology or the NSC niche, which would ultimately result in the depletion of adult NSC later in life. Alternatively, FoxO3 loss may be better compensated by other FoxO family members in embryonic and post-natal NSC than in adult NSC. Precisely studying the timing of action of FoxO3 in NSC will require crossing FoxO3lox/lox mice with mice expressing a tamoxifen-inducible form of Cre (CreER) driven from promoters that are active in NSC, such as Nestin, GFAP, Glast, or TLX (Johnson et al., 2009).
Our experiments indicate that FoxO3 exerts at least part of its action in the nervous system to regulate brain weight and the NSC pool. FoxO3 may also act in part in the stem cell niche or systemically to regulate NSC. FoxO3 is expressed in neurons, astrocytes, and oligodendrocytes, as well as in endothelial cells of the vasculature, all of which may contribute to the NSC niche. As FoxO3 has been shown to play an important role in specific endothelial cells (Paik et al., 2007), a portion of the defect observed in the brain of FoxO3−/− mice might come from FoxO3 loss in blood vessels. A rigorous demonstration of the cell-autonomy of FoxO3 will require the generation of mouse models in which FoxO3 is specifically deleted in NSC. Nevertheless, the observation that FoxO3lox/lox;Nestin-Cre mice (this study) and FoxO1/3/4lox/lox;GFAP-Cre mice (Paik et al., 2009 [this issue of Cell stem Cell]), which both lead to FoxO deletion in NSC, have similar NSC phenotypes both in vivo and in vitro, suggests that FoxO factors act cell-autonomously in NSC. This notion is further supported by the observation that in vitro deletion of FoxO in NSC leads to self-renewal defects and by the functional validation of direct FoxO targets in NSC (Paik et al., 2009 [this issue of Cell stem Cell]). FoxO factors may in fact provide a pivotal link between extrinsic and intrinsic signals by integrating a variety of external stimuli, including growth factors and oxidative stress (Salih and Brunet, 2008).
FoxO factors are necessary for the maintenance and self-renewal of adult hematopoietic stem cells (HSC) (Miyamoto et al., 2007; Tothova et al., 2007). HSC continuously regenerate the hematopoietic tissue whereas NSC have lower regenerative capacity, Interestingly, the program of genes controlled by FoxO3 in NSC reveals little overlap with the program of genes controlled by FoxO factors in HSC (Miyamoto et al., 2007; Tothova et al., 2007). FoxO factors may regulate different stem cell types by inducing specific gene expression programs tailored for the unique biology of these types of stem cells. Identifying the programs induced by FoxO3 in different types of stem cells should help uncover differences between stem cells from rapidly regenerating versus slowly regenerating tissues.
FoxO factors are inhibited by the PI3K-Akt pathway and activated by the PTEN phosphatase. Increased PI3K-Akt signaling promotes the self-renewal of cortical progenitors and adult SVZ cells (Groszer et al., 2006; Li et al., 2002; Sinor and Lillien, 2004). Whether increased PI3K-Akt signaling promotes the short-term expansion of progenitors at the expense of the NSC pool in vivo is unknown. The deletion of the PTEN gene in HSC leads to the long-term depletion of HSC, which is rescued by rapamycin, an mTOR inhibitor (Yilmaz et al., 2006). It will be interesting to determine the importance of the PI3K-Akt pathway and downstream pathways (FoxO versus mTOR) in the long-term regulation of the NSC pool.
Our findings suggest that FoxO3 is necessary for the expression of hypoxia-dependent genes in NSC, several of which are known to be targets of the hypoxia inducible factor, HIF1. In C. elegans, the FoxO transcription factor DAF-16 and HIF1 also share common target genes (McElwee et al., 2004). However, in other mammalian cells, FoxO3 inhibits hypoxia-dependent genes in an indirect manner (Bakker et al., 2007; Emerling et al., 2008). Thus, FoxO3 may both directly regulate some HIF1 target genes and indirectly inhibit HIF1-dependent transcription. The balance between these two functions of FoxO3 could be altered by variations in the levels of oxygen. Alternatively, the regulation of the hypoxic program may differ depending on the types of cells or tissues. The finding that FoxO3 is required for the expression of genes that mediate the response to hypoxia in NSC is consistent with the observation that hypoxia and HIF1 promote self-renewal and multipotency in stem cells (Keith and Simon, 2007). As NSC are located in close proximity to blood vessels in vivo and may be subjected to varying levels of oxygen (Mirzadeh et al., 2008; Shen et al., 2008; Tavazoie et al., 2008), the ability of FoxO3 to regulate oxygen metabolism in NSC might also play a role in the protection of these cells in vivo.
In conclusion, our findings suggest that a gene identified based on its function in regulating lifespan in invertebrates may have evolved to play a critical role in regulating NSC homeostasis in mammals. Because NSC have been shown to be important for learning, memory, and mood regulation, our findings could give insight into the decline in cognitive function that occurs during aging.
Experimental Procedures
Materials and other experimental procedures are described in the Supplemental Data.
Quantification of BrdU-positive NSC and progenitors in vivo
Mice were injected intraperitoneally with 50 mg/kg of BrdU (EMD Biosciences) once a day for 7 days and sacrificed either 1 day or 1 month later. Mice were anesthetized and perfused transcardially with PBS containing 5 U/ml of Heparin, then with 4% paraformaldehyde (PFA). Brains were fixed in 4% PFA for 4 hrs at 4°C, then in 30% Sucrose/4% PFA overnight at 4°C, and embedded in Tissue-Tek (Sakura) at −80°C. Coronal sections (40 μm) were cut using a microtome. Sections were incubated in 3% H2O2 for 30 min, in 2 N HCl solution for 30 min at 37°C, followed by washes in Boric Acid buffer, pH 8.4. Sections were incubated with the anti-BrdU antibody (AbD serotec, 1:500) overnight at 4°C, and then with the secondary antibody (biotinylated donkey anti-rat, Jackson ImmunoResearch, 1:500) overnight at 4°C. The sections were then incubated in the ABC solution (Vector Laboratories) for 90 min, and with DAB (Sigma, 0.05%) containing 0.15% H2O2 for 2-10 min. The sections were dried, dehydrated on slides, and mounted using Permount (Fisher Scientific). BrdU staining and quantification was performed on every sixth section from Bregma +1.54 mm to −1.34 mm (SVZ) and from Bregma −0.94 mm to −3.88 mm (DG). BrdU-positive cells were counted in a blinded manner. The total number of BrdU-positive cells in each region was obtained by multiplying the number of BrdU-positive nuclei by 6. In the DG, the number of BrdU-positive cells was normalized by the granular cell layer (GCL) volume (in mm3). The GCL volume was quantified on every sixth section by calculating the area of the GCL on both sides using Metamorph (Molecular Devices). The total GCL volume was estimated by multiplying these areas by 6 and then by 40 μm.
Mouse NSC cultures
Isolation of mouse NSC/progenitors was done as described previously (Palmer et al., 1997). Briefly, the brains were dissected to remove the olfactory bulbs, cerebellum, and brainstem. The forebrains were finely minced, digested for 30 min at 37°C in HBSS media containing 2.5 U/ml Papain (Worthington), 1U/ml Dispase (Roche), and 250 U/ml DNase I (Sigma) and mechanically dissociated. NSC/progenitors were purified using two Percoll gradients (25% then 65%) (Amersham) and plated at a density of 105 cells/cm2 in NeuroBasal-A medium with penicillin-streptomycin-glutamine (Invitrogen), B27 supplement (Invitrogen, 2%), bFGF (Peprotech, 20 ng/ml), and EGF (Peprotech, 20 ng/ml) (self-renewal/proliferation media). Cells were incubated at 37°C in 5% CO2 and 20% oxygen at 95% humidity.
Primary and secondary neurosphere assays
At isolation, NSC were plated in triplicate at low density (1 to 32,000 cells/ml) into a 24-well plate in self-renewal/proliferation media and the number of primary neurospheres formed was assessed in a blinded manner after 7 days. Primary neurospheres were dissociated with Accutase (Chemicon) and plated in triplicate at low density (1 to 4,000 cells/ml) into a 24-well plate in self-renewal/proliferation media and the number of secondary neurospheres formed was assessed after 7 days. For moderate hypoxia (2%), cell culture dishes were placed into an Invivo2 humidified hypoxia workstation (Ruskinn Technologies, Bridgend, UK).
NSC ability to generate different neural lineages
Secondary neurospheres from self-renewal assays were plated onto acid-treated glass coverslips (Bellco) coated with poly-D-lysine (Sigma, 50 μg/ml) and incubated in differentiation media (NeuroBasal-A medium supplemented with penicillin-streptomycin-glutamine, B27 supplement and 1% Fetal Bovine Serum (Invitrogen)). Differentiated neurospheres were fixed in 4% PFA and then stained with antibodies to Tuj1 (Covance, 1:1,000), or to GFAP (guinea pig, Advanced Immunochemicals Inc., 1:1,000). Alternatively, neurospheres were incubated with the antibody to O4 (a gift from Ben Barres, 1:1 in 10% goat serum/1% BSA/0.1M L-lysine) before fixation. Secondary antibodies (Molecular Probes) were used at a dilution of 1:400. For quantification, three coverslips containing 50 whole neurospheres per coverslip were counted in a blinded manner.
Microarray analysis
FoxO3−/− and FoxO3+/+ secondary neurospheres were collected 6 days after initial plating at 5x104 cells/ml. Total RNA was extracted using the mirVana kit (Ambion). Microarray hybridization was performed at Stanford microarray facility using oligonucleotide arrays (Affymetrix, Mouse Genome 430 2.0 Array). Background adjustment and normalization with RMA (robust multi-array analysis) was performed. The RankProd implementation of the method of Rank Products was used to determine the differentially expressed probes.
Supplementary Material
Acknowledgements
Work supported by an NIH/NIA grant R01 AG026648, a CIRM grant, a Brain Tumor Society grant, a Klingenstein Fellowship and an AFAR grant (A.B.), a Stanford University Dean's fellowship (V.M.R), a NSF graduate fellowship (V.A.R), an NIH/NRSA 5T32 CA09302 (A.E.W), the Lucile Packard Foundation for Children's Health and NLM grant R01 LM009719 (A.J.B), and NLM grant T15 LM007033 (A.M.). We thank Kimmi Hoang for help with mouse colony management, Michael Stadler for help with Luxol blue staining, Ben Barres for the generous gift of the O4 antibody, Brandon Cord (T.D.P.'s lab) for help in NSC cultures, and Nathan Woodling (Katrin Andreasson's lab) for help with the BrdU protocol. We thank Ioanna Papandreou (N.C.D.'s lab) for her help with low oxygen experiments. We thank Ron DePinho for the generous gift of the FoxO3−/− and FoxO3lox/lox mice and for discussing results pre-publication. We thank Steve Artandi, Michael Greenberg, Tom Rando, Julien Sage, and members of the Brunet lab for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ACCESSION NUMBER
Microarray data were deposited at the Gene Expression Omnibus (GEO) database under the accession number XXXX.
SUPPLEMENTAL DATA
Supplemental Data includes one table, ten figures, additional references, and Experimental Procedures and can be found with this article online at http://www.cell.com/cell-stem-cell/.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. [PubMed] [Google Scholar]
- Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25:333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci USA. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheusi G, Cremer H, McLean H, Chazal G, Vincent JD, Lledo PM. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Ables JL, Eisch AJ. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–259. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19:756–767. doi: 10.1101/gad.1272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu F, Salmonsen RA, Turner TK, Litofsky NS, Di Cristofano A, Pandolfi PP, Jones SN, Recht LD, Ross AH. PTEN in neural precursor cells: regulation of migration, apoptosis, and proliferation. Mol Cell Neurosci. 2002;20:21–29. doi: 10.1006/mcne.2002.1115. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Araki KA, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zhaeng H, Ying H, Mahoney J, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. doi: 10.1016/j.stem.2009.09.013. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres--reevaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Salih DA, Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr Opin Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Evans MS, Hsieh J, Panici J, Bartke A. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endocrinol. 2005;146:1138–1144. doi: 10.1210/en.2004-1115. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17:7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Makaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005;31:231–242. doi: 10.1385/MN:31:1-3:231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.