Abstract
Although androgens induce numerous actions in brain, relatively little is known about which cell signaling pathways androgens activate in neurons. Recent work in our laboratory showed that the androgens testosterone and dihydrotestosterone (DHT) activate androgen receptor (AR)-dependent mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling. Since the transcription factor cyclic AMP response element binding protein (CREB) is a downstream effector of MAPK/ERK and androgens activate and CREB in non-neuronal cells, we investigated whether androgens activate CREB signaling in neurons. First, we observed that DHT rapidly activates CREB in cultured hippocampal neurons, as evidenced by CREB phosphorylation. Further, we observed that DHT-induced CREB phosphorylation is AR-dependent, as it occurs in PC12 cells stably transfected with AR but in neither wild-type nor empty vector-transfected cells. Next, we sought to identify the signal transduction pathways upstream of CREB phosphorylation using pharmacological inhibitors. DHT-induced CREB phosphorylation in neurons was found to be dependent upon protein kinase C (PKC) signaling but independent of MAPK/ERK, phosphatidylinositol 3-kinase, protein kinase A, and Ca2+/calmodulin-dependent protein kinase IV. These results demonstrate that DHT induces PKC-dependent CREB signaling, which may contribute to androgen-mediated neural functions.
Keywords: Androgen receptor, dihydrotestosterone, protein kinase C, signal transduction, testosterone
1. INTRODUCTION
A large body of data documents numerous androgen actions in brain [14,81,96]. As the nervous system develops, testosterone, either directly through androgen-specific pathways and or indirectly (via aromatization) through estrogen-specific pathways, regulates apoptosis in sexually dimorphic regions of brain [51,76,103]. In addition, testosterone exerts a range of neurotrophic effects including cell differentiation [129], neurite growth [59,65], hippocampal excitability [89], neurogenesis [104,128], and development of hippocampal [75], motor [42,49,67], and autonomic [51] neurons. Further, testosterone can regulate glial activity [20,78] in ways that promote neuron survival.
Increasing evidence indicates a key role of testosterone and related androgens in regulation of neuron loss due to disease-related insults [88]. In some cases, androgens can promote neuron loss [74,124,125]. In addition, androgens can also promote neuron survival against cell death induced by β-amyloid protein (Aβ) [72,86,130], serum withdrawal [36], pro-oxidants [3], and excitotoxins [91]. Androgen neuroprotection is dependent at least in part on androgen receptors (AR), as evidenced by inhibition of protective effects by AR antagonists [3,36,130]. Consistent with these findings, we observed that androgens reduce cell death in cultured hippocampal neurons challenged with Aβ toxicity [72], an apoptotic insult with relevance to Alzheimer’s disease. Furthermore, we found androgen neuroprotection in a PC12 cell line stably transfected with AR but in neither wild-type nor empty vector-transfected cells [72].
Downstream of AR, the mechanisms underlying the neurotrophic actions of androgens are unclear. We reported that androgen neuroprotection involves AR-dependent activation of a mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK), leading to activation of p90 ribosomal S6 kinase (Rsk) and subsequent inactivation of Bcl-2 associated death protein (Bad) [72]. An important downstream transcriptional effector of MAPK/ERK is the cyclic AMP response element binding protein (CREB) [23,31,112,114].
CREB activation is known to regulate a variety of neurotrophic effects, including neuroprotection [18,29,52]. Interestingly, androgens activate CREB signaling in non-neural cells [30,111,116]. Further, androgen-induced, AR-dependent MAPK/ERK-CREB signaling in prostate cancer cells attenuates apoptosis [56]. In addition to MAPK/ERK, several other cell signaling pathways modulate CREB activity, including phosphatidylinositol 3-kinase (PI3K)/Akt [82], protein kinase A (PKA) [43,113], Ca2+/calmodulin-dependent protein kinase (CaMK) IV [21,93], and protein kinase C (PKC) [94,131]. In this study, we investigated the ability of the potent endogenous androgen dihydrotestosterone to activate CREB in neurons, and identified upstream signaling pathways regulating this action.
2. RESULTS
Dihydrotestosterone induces CREB phosphorylation in cultured hippocampal neurons
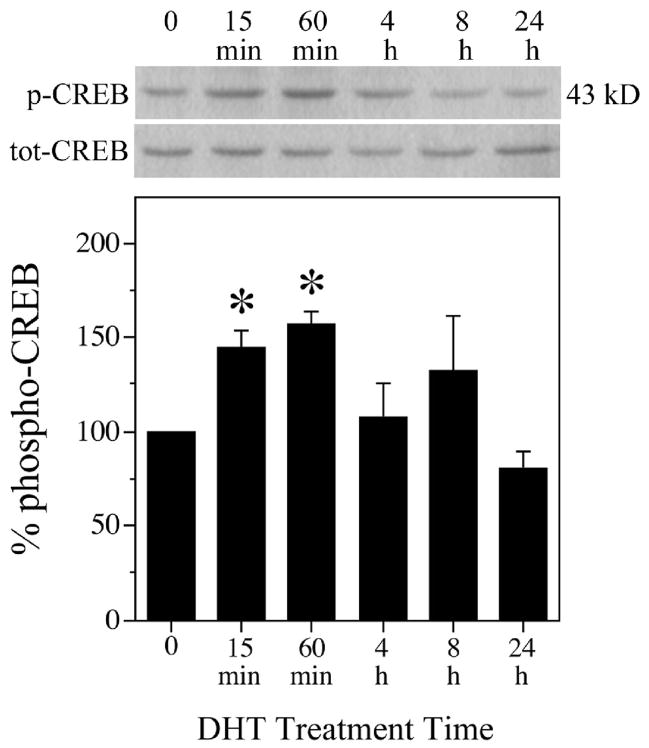
To investigate whether androgens activate CREB signaling in neurons, we tested the ability of dihydrotestosterone (DHT), a non-aromatizable metabolite of testosterone, to phosphorylate CREB in cultured hippocampal neurons. Cultures were treated for increasing amounts of time between 0 to 24 h with 10 nM DHT, a concentration previously determined to exert significant neuroprotective activity in this culture system [86], and then processed for western blot using phospho-specific and total CREB antibodies. In hippocampal neuron cultures treated with DHT, CREB phosphorylation significantly increased within 15 min, peaked by 60 min, decreased at 4 h to 8 h after DHT, and returned to basal levels by 24 h (Fig. 1) in comparison to vehicle-treated control conditions. Reprobing the immunoblot with a pan CREB antibody showed that total CREB levels were maintained across conditions, verifying an effect on CREB phosphorylation state rather than expression levels. Thus, androgen activation of CREB occurs rapidly and in a time-dependent manner.
Fig. 1.
Androgens activate CREB in primary hippocampal neurons. Cultures were treated with 10 nM DHT or vehicle for the indicated times, and then examined by western blot using phosphorylated (p-CREB) and total (tot-CREB) CREB (43 kDa) antibodies. Percent phospho-CREB is expressed as a ratio of phosphorylated to total CREB, normalized to the vehicle-treated control condition (bottom panel). Immunoblots pictured are of representative experiments and data shown are means (± SEM) of the combined experiments (n = 3), [F (5,11) = 4.5; P = 0.018]. * p < 0.05 relative to the vehicle-treated control condition.
DHT-induced CREB phosphorylation is AR-dependent
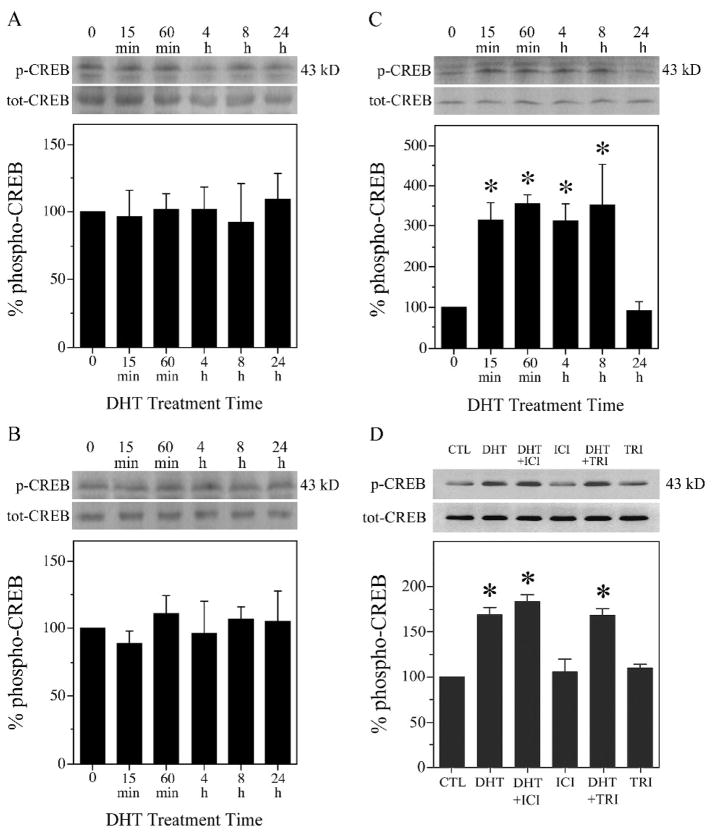
To determine if androgen-induced CREB activation requires AR, we compared DHT effects on levels of CREB phosphorylation in wild-type PC12 cells, pcDNA3-ctl (empty vector) cells, and pcDNA3-AR (AR-transfected) cells. PC12 cell cultures were switched to serum-free medium for 24 h, treated with 10 nM DHT ranging in times from 0 to 24 h, and then processed for western blot using phospho-specific and total CREB antibodies. DHT treatment did not significantly affect CREB phosphorylation in wild-type PC12 (Fig. 2A) or pcDNA3-ctl (Fig. 2B) cells, which lack AR expression [72]. However, in AR-transfected pcDNA-AR cells, DHT resulted in levels of CREB phosphorylation that were significantly increased within 15 min of treatment, remained elevated through 8 h, and returned to basal levels by 24 h (Fig. 2C).
Fig. 2.
Androgen-induced CREB activation is AR-dependent. Western blots using phosphorylated (p-CREB) and total (tot-CREB) CREB (43 kDa) antibodies show that exposure to 10 nM DHT did not affect levels of phosphorylated CREB in (A) wild-type (wt) [F (5,11) = 0.02; P = 0.999] and (B) empty vector-transfected (pcDNA-ctl) [F (5,11) = 0.23; P = 0.942] PC12 cells. C, In PC12 cells stably transfected with AR (pcDNA-AR), 10 nM DHT significantly increased CREB phosphorylation [F (5,11) = 6.9; P = 0.004]. D, Hippocampal neuron cultures were pretreated for 2 h with ER antagonist 1 μM ICI 182,780 (ICI), 3β-hydroxysteroid dehydrogenase inhibitor 10 μM trilostane (TRI), or vehicle, followed by exposure to DHT for 2 h. DHT-induced CREB phosphorylation was not blocked by ICI 182,780 or trilostane [F (5,11) = 30.5; P < 0.0001]. Percent phospho-CREB is expressed as a ratio of phosphorylated to total CREB, normalized to the vehicle-treated control condition (bottom panels). Immunoblots are from representative experiments and data show means (± SEM) of the combined experiments (n = 3). *p < 0.05 relative to the vehicle-treated control condition.
DHT acts as a potent agonist of AR but is also metabolized into androgens that act independently of AR. DHT is converted in brain by 3β-hydroxysteroid dehydrogenase into the androgen 5α-androstan-3β,17β-diol (3β-diol), which can activate estrogen receptor β (ERβ) [62,77,119,120]. Because ER activation can induce CREB phosphorylation in neurons [1,11,100,109,132], we investigated the possibility that DHT-induced CREB activation may result from conversion to 3β-diol and subsequent activation of ERβ. First, cultured hippocampal neurons were pretreated for 1 h with 10 μM trilostane, which effectively inhibits 3β-hydroxysteroid dehydrogenase activity at this concentration [6,101]. Following trilostane pretreatment, cultures were exposed to 10 nM DHT for 2 h, and then probed by western blot for levels of CREB phosphorylation. Trilostane treatment had no effect on basal levels of CREB phosphorylation and did not significantly alter the DHT-induced increase in CREB phosphorylation (Fig. 2D). In these experiments, we also evaluated the effects of 1 μM ICI 182,780, an ER antagonist [115] previously demonstrated to block ER actions in neuron cultures at this concentration [127]. We found that ICI 182,780 altered neither basal levels nor the DHT-induced increase in CREB phosphorylation (Fig. 2D).
DHT-induced CREB phosphorylation is mediated by neither MAPK/ERK, PI3K/Akt, PKA, nor CaMKIV signaling pathways
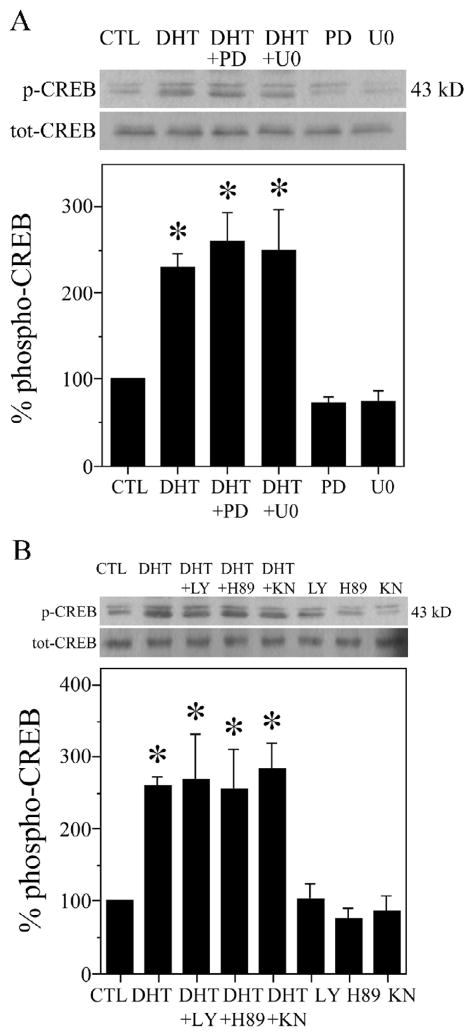
Next, we evaluated cell signaling pathways that may contribute to the observed AR-dependent CREB activation. One key upstream regulator of CREB activation is MAPK/ERK [10,11], which we previously found to be activated by androgens in neurons [72]. To determine if MAPK/ERK signaling mediates the activation of CREB in our neuronal paradigm, we compared CREB phosphorylation in the presence and absence of MEK inhibitors PD98059 and U0126 [19], which interrupt the MAPK/ERK pathway at a point just upstream of ERK. Hippocampal neuron cultures were treated with 50 μM PD98059 [19,24,79] or 10 μM U0126 [19,22,27] for 2 h, followed by exposure to DHT for 2 h, and then collected for western blot. Though both MEK inhibitors blocked the DHT-induced increases in ERK, Rsk, and Bad phosphorylation [72], they did not block the androgen-induced increase in CREB phosphorylation (Fig. 3A). Thus, inhibiting upstream MEK does not prevent androgen-induced CREB activation.
Fig. 3.
MAPK/ERK, PI3K/Akt, PKA, and CaMKIV do not contribute to androgen-induced CREB activation in hippocampal neuron cultures. DHT-induced CREB phosphorylation was significantly affected by neither (A) inhibitors of MAPK/ERK [F (5,11) = 5.3; P = 0.010] nor (B) inhibitors of PI3K/Akt, PKA, and CaMKIV [F (7,15) = 3.4; P = 0.023]. Cultures were pretreated for 2 h with kinase inhibitors 50 μM PD98059 (PD; MEK), 10 μM U0126 (U0; MEK), 10 μM LY294002 (LY; PI3K/Akt), 1 μM H89 (PKA), 10 μM KN93 (KN; CaMKIV), or vehicle, followed by exposure to 10 nM DHT for 2 h, and then were assessed by western blot using phosphorylated (p-CREB) and total (tot-CREB) CREB antibodies (43 kDa). Percent phospho-CREB is expressed as a ratio of phosphorylated to total CREB, normalized to the vehicle-treated control condition (bottom panels). Immunoblots are from representative experiments and data show means (± SEM) of the combined experiments (n = 3). *p < 0.05 relative to the vehicle-treated control condition.
We then evaluated alternative upstream effectors of CREB activation, including PI3K/Akt, which androgens activate in non-neuronal cells [7,50,54], PKA, and CaMKIV. To determine if these signaling pathways underlie androgen-induced CREB activation, we used the specific kinase inhibitors LY294002 (PI3K/Akt) [12,45,126], H89 (PKA) [15,19,28], and KN93 (CaMKIV) [26,60,64], and assessed their effects on CREB phosphorylation. We treated hippocampal neuron cultures with 10 μM LY294002, 1 μM H89, or 10 μM KN93 for 2 h, followed by exposure to DHT. Similar to findings with MEK inhibitors, the pharmacological inhibitors of PI3K/Akt, PKA, and CaMKIV did not block the DHT-induced CREB phosphorylation (Fig. 3B). Thus, inhibiting PI3K/Akt, PKA, or CaMKIV signaling does not prevent the androgen activation of CREB.
PKC contributes to DHT-induced CREB phosphorylation
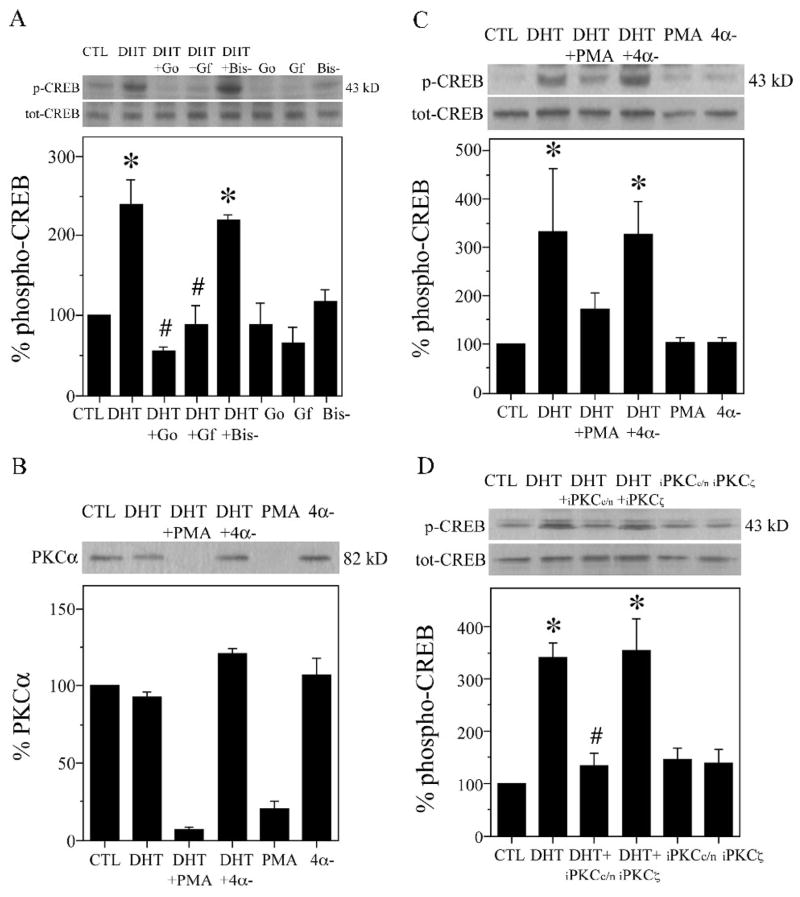
Emerging data suggest a role for PKC in regulation of CREB activity [94,131]. To test whether PKC mediates androgen-induced CREB activation, we first evaluated the efficacies of specific PKC inhibitors GF109203X (2 μM) and Gö6983 (2 μM) on CREB phosphorylation. At the concentration used, Gö6983 inhibits activities of all PKC isoforms except PKCμ [34,107,117], whereas GF109203X has high affinity for conventional (α, β, γ) and novel (ζ, ε) PKC isoforms [33,55,110]. We found that 2 h pretreatment of hippocampal neuron cultures with GF109203X or Gö6983 resulted in complete attenuation of DHT-induced CREB phosphorylation (Fig. 4A). In contrast, bisindolylmaleimide V (2 μM, 2 h), an inactive structural analog of GF109203X and Gö6983 [63,102], had no effect on CREB phosphorylation (Fig. 4A). These findings provide pharmacological evidence of PKC involvement in androgen activation of CREB.
Fig. 4.
PKC contributes to androgen-induced CREB activation in hippocampal neuron cultures. A, Hippocampal neuron cultures were pretreated for 2 h with PKC inhibitor 2 μM GF109203X (GF), Gö6983 (Go), the inactive analog 2 μM bisindolylmaleimide V (Bis-), or vehicle, followed by 2 h exposure to 10 nM DHT, and then assessed by western blot using phosphorylated (p-CREB) and total (tot-CREB) CREB antibodies (43 kDa). Percent phospho-CREB is expressed as a ratio of phosphorylated to total CREB, normalized to the vehicle-treated control condition (bottom panels), [F (7,15) = 6.0; P = 0.002]. B–C, Hippocampal neuron cultures were pretreated with 100 ng/ml PMA, inactive 4α-phorbol (4α-), or vehicle, followed 24 h later by application of 10 nM DHT for 2 h, and then immunoblots were probed for (B) PKCα (82 kDa) and (C) phosphorylated and total CREB [F (5,11) = 2.7; P = 0.075]. D, Neuron cultures were pretreated with 1 μM peptide inhibitors of conventional and novel (iPKCc/n) and atypical (iPKCα) PKC or vehicle for 2 h, and then exposed to 10 nM DHT for 2 h [F (5,11) = 3.6; P = 0.036]. Immunoblots are from representative experiments and data show means (± SEM) of the combined experiments (n = 3). *p < 0.05 relative to the vehicle-treated control condition. # p < relative to the paired DHT condition.
One unique property of phorbol esters such as PMA is that when applied chronically, they down-regulate expression of conventional and novel PKC isoforms [4,16,108]. Using this approach of chronic PMA exposure, we assessed androgen-induced CREB activation in PKC-depleted hippocampal neuron cultures. Similar to findings with specific PKC inhibitors (Fig. 4A), we observed that a 24 h exposure to 100 ng/ml PMA reduced the DHT-induced increase in CREB phosphorylation (Fig. 4C). In contrast, the same concentration of an inactive structural analog of PMA, 4α-phorbol, did not affect phosphorylation of CREB protein (Fig. 4C). As a control, we verified that chronic PMA exposure depleted PKC by stripping and reprobing the immunoblot for conventional PKCα. While chronic treatment with PMA down-regulated PKCα expression, the inactive analog 4α-phorbol had no effect on PKCα levels (Fig. 4B). Neither PMA nor 4α-phorbol had a significant effect on total CREB levels (Fig. 4C).
To further investigate the role of PKC in androgen-induced CREB activation, we used a specific peptide inhibitor of conventional and novel PKC isoforms (iPKCc/n) [25,37,118] and a specific inhibitor for atypical PKCζ (iPKCζ) [105,106]. These inhibitors differ from GF109203X and Gö6983 in that they are cell-permeable peptides that specifically bind to the pseudosubstrate region of PKCα and PKCβ (iPKCc/n) or PKCζ (iPKCζ), thereby blocking entry to enzyme substrate [25,37]. We found that the DHT-induced increase in CREB phosphorylation in hippocampal neuron cultures was significantly attenuated by 2 h pretreatment with 1 μM iPKCc/n but not with 1 μM iPKCζ (Fig. 4D), suggesting involvement of conventional and or novel PKC but not atypical PKCζ in androgen activation of CREB.
3. DISCUSSION
Our investigation of androgen cell signaling yields several novel findings. We found that the androgen DHT rapidly increases CREB phosphorylation in neurons. This DHT-induced CREB activation is AR-dependent. In addition, pharmacological inhibition of upstream MAPK/ERK, PI3K/Akt, PKA, or CaMKIV did not prevent DHT-induced phosphorylation of CREB, while inhibition or depletion of PKC largely blocked the phosphorylation. Taken together, these results identify an AR- and PKC-dependent pathway of DHT-induced CREB activation that may contribute to androgen actions in neurons.
The primary male androgen testosterone mediates its effects not only by directly activating AR but also by functioning as a prohormone that is metabolized to other active hormones. In particular, testosterone is converted by i) aromatase to the estrogen 17β-estradiol, which acts on estrogen receptors ERα and ERβ, and ii) 5α-reductase to the androgen DHT, which is a potent agonist for AR [14]. DHT is a substrate for additional metabolizing enzymes in brain, including 3α-hydroxysteroid dehydrogenase that reversibly forms 5α-androstan-3α,17β-diol and 3β-hydroxysteroid dehydrogenase that irreversibly yields 3β-diol [66]. Recent evidence demonstrated that 3β-diol is an agonist for ERβ [119,120] and that some DHT actions in brain are mediated by conversion to 3β-diol and subsequent ERβ activation [62,77]. Our observations are not consistent with a 3β-diol/ERβ pathway since DHT-induced CREB phosphorylation was inhibited by neither ER antagonism nor pharmacological inhibition of 3β-hydroxysteroid dehydrogenase. Rather, our data with AR-transfected PC12 cells suggests that CREB phosphorylation is dependent on AR activation.
Our observation of androgen-mediated CREB phosphorylation in neurons is consistent with the actions of sex steroid hormones in neurons and other cell types. For example, estrogen [1,11,100,109,132] and in some circumstances progesterone [35] increase CREB phosphorylation in neurons. Although an effect of androgens on neuronal CREB activation has not been previously demonstrated, neonatal male rat pups exhibit higher levels of CREB phosphorylation than female pups in several brain regions including hippocampus [5]. Also consistent with our observations are findings in non-neural cells of a similarly rapid and AR-dependent increase in CREB phosphorylation in prostate cancer cells [111] and Sertoli cells [30,116]. Delayed CREB activity has also been observed 1–4 days after androgen treatment in rat granular convoluted tubules/submandibular gland [53].
The initial goal of our study was to elucidate non-classical, indirect genomic mechanisms of androgen signaling downstream of MAPK/ERK activity [2]. In previous work, we identified a non-genomic mechanism of androgen protection against Aβ toxicity involving AR-dependent ERK-Rsk-Bad signaling in hippocampal neurons [72]. The MAPK/ERK cascade not only mediates phosphorylation-dependent, non-genomic changes in protein activity [47,98], but also regulates gene transcription [39,112]. The transcription factor CREB was among the first documented downstream effectors of MAPK/ERK signaling [10,31,70]. MAPK/ERK activation of CREB regulates many neuronal actions, including cell proliferation, differentiation, long-term potentiation, and survival [31]. For example, Bonni and colleagues [10] found that suppression of ERK-CREB activity (and synthesis of viability proteins) facilitates apoptosis in cerebellar granule cells, while ERK-induced inactivation of Bad (a death protein) facilitates cell survival against apoptosis. Despite in vivo [70,80] and in vitro [10,97] evidence that links the MAPK/ERK pathway to CREB, in our paradigm, androgen-induced CREB activation appears to be independent of MAPK/ERK activity since it is not reduced by MEK inhibitors. These data are consistent with reports that CREB can function independently of ERK signaling in neuronal paradigms of differentiation [103], synapse activity [44], and gene regulation [48].
CREB transcriptional regulation underlies many gene-dependent neuronal functions induced by several signaling pathways [29,83]. Four well-characterized signaling pathways in neurons apart from MAPK/ERK [10,112,123] are PI3K/Akt [82], PKA [43,113], CaMKIV [21,93], and PKC [94,131]. These kinases can localize to the nucleus to activate CREB and other transcription factors [58,68,69]. Studies indicate that CREB at serine 133 may be activated specifically by one pathway (e.g., PI3K/Akt) [41,58], two converging pathways (e.g., PKA and PKC) [94], or irrespective of pathway (e.g., MAPK/ERK or CaMKIV) [57]. There is significant crosstalk between pathways, which can vary according to stimulus intensity (e.g., toxic or non-toxic), and pattern of kinase activation (i.e., rapid and transient or delayed and prolonged) [40,57,97]. Since MAPK/ERK is not involved in our androgen-induced CREB activation, we assessed the contribution of other protein kinases. We found that androgen-induced CREB activation is independent of PI3K/Akt, PKA, and CaMKIV, as inhibitors of these kinases do not reduce the DHT-induced increase in CREB phosphorylation. To a certain extent, our results are surprising, as androgens activate upstream signaling kinases such as PI3K/Akt in prostate cancer cells [54,84], osteoblasts [50], and epithelial cells [7], with direct relevance to inhibition of apoptosis [54].
Our data suggest that androgen-mediated CREB activation is dependent upon PKC. Prior studies have also reported PKC-dependent CREB phosphorylation in neurons [94,131]. However, to our knowledge, this study provides the first evidence of PKC involvement in androgen activation of CREB, in either neuronal or non-neuronal cells. Our observations support previous findings by Peterziel and colleagues indicating that PKC contributes to androgen signaling in prostate cancer cells [84]. Involvement of PKC in androgen actions is consistent with prior observations that androgens can act via second messengers such as cyclic AMP [95], inositol trisphosphate [61], phospholipase C [9], diacylglycerol (DAG) [61], and calcium (Ca2+) [122], which in turn can result in PKC-dependent CREB phosphorylation [13,38].
PKC isoforms belong to conventional (α, βI, βII, γ), novel (δ, ε, η/Δ, θ, μ), or atypical (ζ, λ/ι) classes [90]. Conventional isoforms need Ca2+ and DAG for activation, while novel isoforms need only DAG [76]. Ca2+ and DAG are not necessary for activation of atypical isoforms [71]. Two lines of evidence implicate conventional and or novel PKC isoforms in androgen activation of CREB. First, we observed that CREB activation is blocked by chronic exposure to PMA, which acts on the DAG binding region of conventional and novel PKC isoforms [73]. Second, both general (Gö6983) and conventional/novel-specific (GF109203X) PKC inhibitors block activation. The same CREB inhibition appears using conventional/novel-specific (iPKCc/n) but not atypical (iPKCζ) PKC peptide inhibitors. Interestingly, our previous data implicates conventional PKC isoforms in the mechanism of estrogen neuroprotection [17].
To our knowledge, our observation that androgens induce CREB activation in primary hippocampal neuron cultures is the first demonstration of this androgen pathway in neurons. Prior studies have reported an androgen effect on CREB in non-neuronal prostate cancer cells [111], Sertoli cells [30,116], and granular convoluted tubule cells [53]. Interestingly, though these studies found that androgen-induced CREB activation occurs downstream of AR-dependent MAPK/ERK signaling, we did not observe such effect. Excluding other upstream effectors such as (PI3K)/Akt [82], PKA [43,113], and CaMKIV [21,93], we found that androgens induce CREB activation via regulation by PKC signaling. CREB activation is a mechanism of cell survival in neuron cultures [11,32,92,114] against insults such as ischemia/hypoxia [46,99], excitotoxicity [57], and Aβ [8], as well as underlying many beneficial brain actions, including proliferation [83], differentiation [5], axon growth [132], and synaptic plasticity [52,121]. Our observations that androgens induce CREB activation may be relevant to functions important for cell growth, maintenance, and resilience.
4. EXPERIMENTAL PROCEDURE
Materials
Dihydrotestosterone (DHT) and trilostane were purchased from Steraloids (Newport, RI). PD98059, U0126, LY294002, H89, KN93, phorbol-12-myristate-13-acetate (PMA), 4α-phorbol-12, 13-didecanoate (4α-phorbol), GF109203X (bisindolylmaleimide I), Gö6983, bisindolylmaleimide V, cell-permeable (myristoylated) pseudosubstrate peptide inhibitor of conventional and novel protein kinase C (iPKCc/n), and myristoylated PKCζ (iPKCζ) pseudosubstrate peptide inhibitor were obtained from Calbiochem-Novabiochem (La Jolla, CA). ICI 182,780 was purchased from Tocris (Ellisville, MO). Mouse monoclonal phosphorylated cyclic AMP response element binding protein (CREB; serine 133) and rabbit polyclonal total CREB antibodies were purchased from Cell Signaling Technology (Beverly, MA). Mouse monoclonal PKCα was acquired from Transduction Laboratories (Lexington, KY). Horseradish peroxidase (HRP)-conjugated anti-mouse was from Jackson ImmunoResearch Laboratories (West Grove, PA), and HRP-conjugated anti-rabbit was acquired from Pierce Chemical (Rockford, IL).
Neuron culture
Hippocampal neurons were obtained from embryonic day 18 Sprague-Dawley rats (n ≥ 6 per preparation) and cultured according to a previously described technique that yields cultures that are ~ 95% neuronal based upon differential immunoreactivity with neuron-specific (NeuN, catalog #MAB377; Chemicon, Billerica, MA) and astrocyte-specific (GFAP, catalog #Z0334; Dako, Carpinteria, CA) antibodies [72,87]. In brief, we dissected the hippocampi of pups, dissociated cells enzymatically with 0.125% trypsin-EDTA for 10 min at 37°C and mechanically with a flamed-tipped glass pipette, and then filtered the cell solution through a 40 μm strainer (Falcon, Franklin Lakes, NJ). We diluted the single cell solution in serum-free Dulbecco’s modified Eagle medium with 20 mM HEPES added, and supplemented the medium with 100 μg/ml transferrin, 5 μg/ml insulin, 100 μM putrescine, and 30 nM selenium. Cells were then plated onto poly-L-lysine-coated 12-well plates at densities of 1 × 105 cells/cm2. For 3–4 days, cell cultures were stored in a humidified incubator (room air/5% CO2, 37°C) before experimentation.
PC12 culture
Wild-type PC12 cells and clones stably transfected with full-length rat AR (pcDNA3-AR) or empty vector (pcDNA-ctl) have been described previously [72]. Wild-type and cell lines were grown in poly-L-lysine (0.05 mg/mL)-treated 75 cm2 flasks (Fisher Scientific, Tustin, CA) containing RPMI 1640, 20 mM HEPES, 10 ml/L penicillin-streptomycin, 10% horse serum/5% fetal bovine serum, and 100 μg/ml G418 (except for wild-type). Cells were plated at a density of 5 × 104 cells/cm2 in 12-well plates. After 24 h, cultures were switched to serum-free medium, and maintained at 37°C in a humidified incubator with room air/5% CO2 for another 24 h before experimentation.
Experimental treatment of cultures
Cultures were treated with 10 nM DHT or ethanol vehicle for 15 min, 60 min, 4 h, 8 h, or 24 h. DHT was solubilized in 100% ethanol and diluted in culture medium to a final ethanol concentration of ≤ 0.1%; vehicle controls for DHT consisted of 0.1% ethanol. In experiments with an ER antagonist (1 μM ICI 182,780) and specific active or inactive inhibitors of MAPK/ERK kinase (MEK; 50 μM PD98059 or 10 μM U0126), PI3K/Akt (10 μM LY294002), PKA (1 μM H89), CaMKIV (10 μM KN93), PKC (2 μM GF109203X/bisindolylmaleimide I, 2 μM Gö6983, 2 μM bisindolylmaleimide V, 1 μM iPKCc/n, or 1 μM iPKCζ), and 3β-hydroxysteroid dehydrogenase (10 μM trilostane), the inhibitor or an equal amount of the final concentration of dimethyl sulfoxide (DMSO) vehicle was added 1 or 2 h before steroid treatment. Chronic PMA exposure consisted of 100 ng/ml PMA, inactive 4α-phorbol, or an equal concentration of 100% ethanol vehicle added 24 h before steroid treatment. All of the inhibitors, except for PKC peptide inhibitors (dissolved in sterile double deionized water), were solubilized in DMSO and diluted in culture medium to a final DMSO concentration of ≤ 0.1%. PMA was solubilized in 100% ethanol, and diluted in culture medium to a final ethanol concentration of ≤ 0.1%.
Western blot
Cell lysates were processed for phospho-specific and total levels of CREB or PKCα proteins using a standard western blot protocol [72,85]. In brief, protein samples were loaded into 12% polyacrylamide gels, electrophoresed for 2 h at constant 120 V, and then transferred onto PVDF membranes (Millipore, Bedford, MA) for 1 h at constant 100 V. After blocking (10 nM Tris, 100 nM NaCl, 0.1% Tween, 3% bovine serum albumin), immunoblots were incubated with phospho-CREB (1:1000) or PKCα (1:1000) antibodies overnight at 2–6°C, and then visualized with chemiluminescence detection (Amersham Pharmacia Biotech, Piscataway, NJ). To assure equal amounts of total proteins across conditions, immunoblots were stripped (15 min in 100 mM glycine, pH 2.5, followed by 15 min in 2% SDS, 0.7% 2β-mercaptoethanol, 62.5 mM Tris-HCl, pH 6.7, 60°C) and reprobed with a pan antibody for CREB (1:1000). Blots were quantified by band densitometry of scanned films using NIH Image 1.61 software. Phosphorylation of CREB is expressed as a ratio of phosphorylated to total proteins. Raw data of the ratios of phosphorylated to total proteins were statistically analyzed using a split-plot ANOVA design, followed by pairwise comparisons using the Student’s t test (significance indicated by p < 0.05). Three independent preparations were included for each experiment. For graphical presentation, phosphorylated CREB or PKCα is expressed as a percentage of control values with denotation of significance obtained from statistical analyses of raw data.
Acknowledgments
This study was supported by a grant to CJP from the National Institute on Aging (AG23739).
Abbreviations
- AR
Androgen receptor
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CREB
cyclic AMP response element binding protein
- DHT
dihydrotestosterone
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- PMA
phorbol 12-myristate 13-acetate
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE REFERENCES
- 1.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams JP, Roberson ED, English JD, Selcher JC, Sweatt JD. MAPK regulation of gene expression in the central nervous system. Acta Neurobiol Exp (Wars) 2000;60:377–394. doi: 10.55782/ane-2000-1357. [DOI] [PubMed] [Google Scholar]
- 3.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 4.Ahnadi CE, Giguère P, Gravel S, Gagné D, Goulet AC, Fülöp TJ, Payet MD, Dupuis G. Chronic PMA treatment of Jurkat T lymphocytes results in decreased protein tyrosine phosphorylation and inhibition of CD3- but not Ti-dependent antibody-triggered Ca2+ signaling. J Leukoc Biol. 2000;68:293–300. [PubMed] [Google Scholar]
- 5.Auger AP, Hexter DP, McCarthy MM. Sex difference in the phosphorylation of cAMP response element binding protein (CREB) in neonatal rat brain. Brain Res. 2001;890:110–7. doi: 10.1016/s0006-8993(00)03151-6. [DOI] [PubMed] [Google Scholar]
- 6.Barker S, Malouitre SDM, Glover HR, Puddefoot JR, Vinson GP. Comparison of effects of 4-hydroxy tamoxifen and trilostane on oestrogen-regulated gene expression in MCF-7 cells: Up-regulation of oestrogen receptor beta. J Steroid Biochem Mol Biol. 2006;100:141–151. doi: 10.1016/j.jsbmb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–14586. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 8.Bayatti N, Zschocke J, Behl C. Brain region-specific neuroprotective action and signaling of corticotropin-releasing hormone in primary neurons. Endocrinology. 2003;144:4051–4060. doi: 10.1210/en.2003-0168. [DOI] [PubMed] [Google Scholar]
- 9.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. Faseb J. 1999;13:123–33. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 10.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–62. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 11.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 13.Cato AC, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE. 2002;2002:RE9. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- 14.Celotti F, Melcangi RC, Martini L. The 5 alpha-reductase in the brain: molecular aspects and relation to brain function. Front Neuroendocrinol. 1992;13:163–215. [PubMed] [Google Scholar]
- 15.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 16.Cordey M, Gundimeda U, Gopalakrishna R, Pike CJ. Estrogen activates protein kinase C in neurons: role in neuroprotection. J Neurochem. 2003;84:1340–8. doi: 10.1046/j.1471-4159.2003.01631.x. [DOI] [PubMed] [Google Scholar]
- 17.Cordey M, Pike CJ. Conventional protein kinase C isoforms mediate neuroprotection induced by phorbol ester and estrogen. J Neurochem. 2006;96:204–217. doi: 10.1111/j.1471-4159.2005.03545.x. [DOI] [PubMed] [Google Scholar]
- 18.Crino P, Khodakhah K, Becker K, Ginsberg S, Hemby S, Eberwine J. Presence and phosphorylation of transcription factors in developing dendrites. Proc Natl Acad Sci U S A. 1998;95:2313–2318. doi: 10.1073/pnas.95.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day JR, Laping NJ, Lampert-Etchells M, Brown SA, O’Callaghan JP, McNeill TH, Finch CE. Gonadal steroids regulate the expression of glial fibrillary acidic protein in the adult male rat hippocampus. Neuroscience. 1993;55:435–443. doi: 10.1016/0306-4522(93)90512-e. [DOI] [PubMed] [Google Scholar]
- 21.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 22.DeSilva DR, Jones EA, Favata MF, Jaffee BD, Magolda RL, Trzaskos JM, Scherle PA. Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy. J Immunol. 1998;160:4175–4181. [PubMed] [Google Scholar]
- 23.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–9. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 24.Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci U S A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichholtz T, de Bont DB, de Widt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- 26.Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol. 1999;56:39–45. doi: 10.1124/mol.56.1.39. [DOI] [PubMed] [Google Scholar]
- 27.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 28.Findik D, Song Q, Hidaka H, Lavin M. Protein kinase A inhibitors enhance radiation-induced apoptosis. J Cell Biochem. 1995;57:12–21. doi: 10.1002/jcb.240570103. [DOI] [PubMed] [Google Scholar]
- 29.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 30.Fix C, Jordan C, Cano P, Walker WH. Testosterone activates mitogen-activated protein kinase and the cAMP response element binding protein transcription factor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:10919–10924. doi: 10.1073/pnas.0404278101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 32.Gao C, Chen L, Tao Y, Chen J, Xu X, Zhang G, Chi Z. Colocalization of phosphorylated CREB with calcium/calmodulin-dependent protein kinase IV in hippocampal neurons induced by ohmfentanyl stereoisomers. Brain Res. 2004;1024:25–33. doi: 10.1016/j.brainres.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 33.Gekeler V, Boer R, Uberall F, Ise W, Schubert C, Utz I, Hofmann J, Sanders KH, Schächtele C, Klemm K, Grunicke H. Effects of the selective bisindolylmaleimide protein kinase C inhibitor GF 109203X on P-glycoprotein-mediated multidrug resistance. Br J Cancer. 1996;74:897–905. doi: 10.1038/bjc.1996.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 35.Gu G, Rojo AA. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–26. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 37.Harris TE, Persaud SJ, Saermark T, Jones PM. A myristoylated pseudosubstrate peptide inhibitor of protein kinase C: effects on glucose- and carbachol-induced insulin secretion. Mol Cell Endocrinol. 1996;121:133–141. doi: 10.1016/0303-7207(96)03858-0. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi S, Ueyama T, Kajimoto T, Yagi K, Kohmura E, Saito N. Involvement of gamma protein kinase C in estrogen-induced neuroprotection against focal brain ischemia through G protein-coupled estrogen receptor. J Neurochem. 2005;93:883–891. doi: 10.1111/j.1471-4159.2005.03080.x. [DOI] [PubMed] [Google Scholar]
- 39.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–7. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 40.Herdegen T, Blume A, Buschmann T, Georgakopoulos E, Winter C, Schmid W, Hsieh TF, Zimmermann M, Gass P. Expression of activating transcription factor-2, serum response factor and cAMP/Ca response element binding protein in the adult rat brain following generalized seizures, nerve fibre lesion and ultraviolet irradiation. Neuroscience. 1997;81:199–212. doi: 10.1016/s0306-4522(97)00170-x. [DOI] [PubMed] [Google Scholar]
- 41.Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- 42.Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci. 2005;25:4004–4013. doi: 10.1523/JNEUROSCI.5279-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 44.Inglefield JR, Mundy WR, Meacham CA, Shafer TJ. Identification of calcium-dependent and -independent signaling pathways involved in polychlorinated biphenyl-induced cyclic AMP-responsive element-binding protein phosphorylation in developing cortical neurons. Neuroscience. 2002;115:559–573. doi: 10.1016/s0306-4522(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 45.Izzard RA, Jackson SP, Smith GC. Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res. 1999;59:2581–2586. [PubMed] [Google Scholar]
- 46.Jin K, Mao XO, Simon RP, Greenberg DA. Cyclic AMP response element binding protein (CREB) and CREB binding protein (CBP) in global cerebral ischemia. J Mol Neurosci. 2001;16:49–56. doi: 10.1385/JMN:16:1:49. [DOI] [PubMed] [Google Scholar]
- 47.Jin K, Mao XO, Zhu Y, Greenberg DA. MEK and ERK protect hypoxic cortical neurons via phosphorylation of Bad. J Neurochem. 2002;80:119–25. doi: 10.1046/j.0022-3042.2001.00678.x. [DOI] [PubMed] [Google Scholar]
- 48.Johnson CM, Hill CS, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distinct pathways that can function independently of the Ras/mitogen-activated protein kinases (ERKs) signaling cascade. J Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones KJ, Brown TJ, Damaser M. Neuroprotective effects of gonadal steroids on regenerating peripheral motoneurons. Brain Res Rev. 2001;37:372–382. doi: 10.1016/s0165-0173(01)00107-2. [DOI] [PubMed] [Google Scholar]
- 50.Kang HY, Cho CL, Huang KL, Wang JC, Hu YC, Lin HK, Chang C, Huang KE. Nongenomic androgen activation of phosphatidylinositol 3-kinase/Akt signaling pathway in MC3T3-E1 osteoblasts. J Bone Miner Res. 2004;19:1181–1190. doi: 10.1359/JBMR.040306. [DOI] [PubMed] [Google Scholar]
- 51.Keast JR, Saunders RJ. Testosterone has potent, selective effects on the morphology of pelvic autonomic neurons which control the bladder, lower bowel and internal reproductive organs of the male rat. Neuroscience. 1998;85:543–556. doi: 10.1016/s0306-4522(97)00631-3. [DOI] [PubMed] [Google Scholar]
- 52.Kelly A, Mullany PM, Lynch MA. Protein synthesis in entorhinal cortex and long-term potentiation in dentate gyrus. Hippocampus. 2000;10:431–437. doi: 10.1002/1098-1063(2000)10:4<431::AID-HIPO9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 53.Kim J, Amano O, Wakayama T, Takahagi H, Iseki S. The role of cyclic AMP response element-binding protein in testosterone-induced differentiation of granular convoluted tubule cells in the rat submandibular gland. Arch Oral Biol. 2001;46:495–507. doi: 10.1016/s0003-9969(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 54.Kimura K, Markowski M, Bowen C, Gelmann EP. Androgen blocks apoptosis of hormone-dependent prostate cancer cells. Cancer Res. 2001;61:5611–5618. [PubMed] [Google Scholar]
- 55.Kiss Z, Tomono M. Compound D609 inhibits phorbol ester-stimulated phospholipase D activity and phospholipase C-mediated phosphatidylethanolamine hydrolysis. Biochim Biophys Acta. 1995;1259:105–108. doi: 10.1016/0005-2760(95)00148-6. [DOI] [PubMed] [Google Scholar]
- 56.Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leinninger GM, Backus C, Uhler MD, Lentz SI. and Feldman, E.L., Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J. 2004;18:1544–1546. doi: 10.1096/fj.04-1581fje. [DOI] [PubMed] [Google Scholar]
- 59.Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24:495–9. doi: 10.1523/JNEUROSCI.4516-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li PM, Fukazawa H, Mizuno S, Uehara Y. Evaluation of protein kinase inhibitors in an assay system containing multiple protein kinase activities. Anticancer Res. 1993;13:1957–1964. [PubMed] [Google Scholar]
- 61.Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J Biol Chem. 1994;269:7217–23. [PubMed] [Google Scholar]
- 62.Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lynch K, Fernandez G, Pappalardo A, Peluso JJ. Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium levels through a protein kinase Cdelta-dependent pathway. Endocrinology. 2000;141:4209–4217. doi: 10.1210/endo.141.11.7742. [DOI] [PubMed] [Google Scholar]
- 64.Mamiya N, Goldenring JR, Tsunoda Y, Modlin IM, Yasui K, Usuda N, Ishikawa T, Natsume A, Hidaka H. Inhibition of acid secretion in gastric parietal cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-93. Biochem Biophys Res Commun. 1993;195:608–615. doi: 10.1006/bbrc.1993.2089. [DOI] [PubMed] [Google Scholar]
- 65.Marron TU, Guerini V, Rusmini P, Sau D, Brevini TA, Martini L, Poletti A. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92:10–20. doi: 10.1111/j.1471-4159.2004.02836.x. [DOI] [PubMed] [Google Scholar]
- 66.Martini L. The 5alpha-reduction of testosterone in the neuroendocrine structures. Biochemical and physiological implications. Endocr Rev. 1982;3:1–25. doi: 10.1210/edrv-3-1-1. [DOI] [PubMed] [Google Scholar]
- 67.Matsumoto A. Hormonally induced neuronal plasticity in the adult motoneurons. Brain Res Bull. 1997;44:539–47. doi: 10.1016/s0361-9230(97)00240-2. [DOI] [PubMed] [Google Scholar]
- 68.Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR. Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem. 1993;127–128:179–186. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- 69.Mirandola P, Ponti C, Gobbi G, Sponzilli I, Melloni E, Vitale M. The response of human natural killer cells to interleukin-2. J Endocrinol Invest. 2004;27:146–150. [PubMed] [Google Scholar]
- 70.Morikawa Y, Ueyama E, Senba E. Fasting-induced activation of mitogen-activated protein kinases (ERK/p38) in the mouse hypothalamus. J Neuroendocrinol. 2004;16:105–112. doi: 10.1111/j.0953-8194.2004.01135.x. [DOI] [PubMed] [Google Scholar]
- 71.Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: Role in neuroprotection. J Neurochem. 2005;94:1639–1651. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- 73.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 74.Nuñez JL, McCarthy MM. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Exp Neurol. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nuñez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing Gamma-Aminobutyric Acid and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2009;158:623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohno S, Akita Y, Hata A, Osada S, Kubo K, Konno Y, Akimoto K, Mizuno K, Saido T, Kuroki T. Structural and functional diversities of a family of signal transducing protein kinases, protein kinase C family; two distinct classes of PKC, conventional cPKC and novel nPKC. Adv Enzyme Regul. 1991;31:287–303. doi: 10.1016/0065-2571(91)90018-h. [DOI] [PubMed] [Google Scholar]
- 77.Pak TR, Chung WC, Hinds LR, Handa RJ. Estrogen receptor-beta mediates dihydrotestosterone-induced stimulation of the arginine vasopressin promoter in neuronal cells. Endocrinology. 2007;148:3371–3382. doi: 10.1210/en.2007-0086. [DOI] [PubMed] [Google Scholar]
- 78.Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 79.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 80.Park EM, Joh TH, Volpe BT, Chu CK, Song G, Cho S. A neuroprotective role of extracellular signal-regulated kinase in N-acetyl-O-methyldopamine-treated hippocampal neurons after exposure to in vitro and in vivo ischemia. Neuroscience. 2004;123:147–154. doi: 10.1016/j.neuroscience.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 81.Patchev VK, Schroeder J, Goetz F, Rohde W, Patchev AV. Neurotropic action of androgens: principles, mechanisms and novel targets. Exp Gerontol. 2004;39:1651–1660. doi: 10.1016/j.exger.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 82.Perkinton MS, Sihra TS, Williams RJ. Ca(2+)-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Persengiev SP, Green MR. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis. 2003;8:225–228. doi: 10.1023/a:1023633704132. [DOI] [PubMed] [Google Scholar]
- 84.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene. 1999;18:6322–9. doi: 10.1038/sj.onc.1203032. [DOI] [PubMed] [Google Scholar]
- 85.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer’s disease. J Neurochem. 1999;72:1552–63. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 86.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–5. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 87.Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–87. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pouliot WA, Handa RJ, Beck SG. Androgen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse. 1996;23:10–19. doi: 10.1002/(SICI)1098-2396(199605)23:1<10::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 90.Quest AF. Regulation of protein kinase C: a tale of lipids and proteins. Enzyme Protein. 1996;49:231–261. doi: 10.1159/000468635. [DOI] [PubMed] [Google Scholar]
- 91.Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–8. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 92.Rani CS, Qiang M, Ticku MK. Potential role of cAMP response element-binding protein in ethanol-induced N-methyl-D-aspartate receptor 2B subunit gene transcription in fetal mouse cortical cells. Mol Pharmacol. 2005;67:2126–2136. doi: 10.1124/mol.104.007872. [DOI] [PubMed] [Google Scholar]
- 93.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 94.Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rubin JM, Hidalgo A, Bordallo C, Cantabrana B, Sanchez M. Positive inotropism induced by androgens in isolated left atrium of rat: evidence for a cAMP-dependent transcriptional mechanism. Life Sci. 1999;65:1035–45. doi: 10.1016/s0024-3205(99)00334-3. [DOI] [PubMed] [Google Scholar]
- 96.Rubinow DR, Schmidt PJ. Androgens, brain, and behavior. Am J Psych. 1996;153:974–84. doi: 10.1176/ajp.153.8.974. [DOI] [PubMed] [Google Scholar]
- 97.Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–36. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–13. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- 99.Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol Appl Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- 100.Sharma K, Mehra RD, Dhar P, Vij U. Chronic exposure to estrogen and tamoxifen regulates synaptophysin and phosphorylated cAMP response element-binding (CREB) protein expression in CA1 of ovariectomized rat hippocampus. Brain Res. 2007;1132:10–19. doi: 10.1016/j.brainres.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 101.Shirakawa H, Katsuki H, Kume T, Kaneko S, Akaike A. Aminoglutethimide prevents excitotoxic and ischemic injuries in cortical neurons. Br J Pharmacol. 2006;147:729–736. doi: 10.1038/sj.bjp.0706636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soliakov L, Wonnacott S. Involvement of protein kinase C in the presynaptic nicotinic modulation of [(3)H]-dopamine release from rat striatal synaptosomes. Br J Pharmacol. 2001;132:785–791. doi: 10.1038/sj.bjp.0703873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Son H, Kim KO, Kim JS, Chang MY, Lee SH, Lee YS. Pairing of forskolin and KCl increases differentiation of immortalized hippocampal neurons in a CREB Serine 133 phosphorylation-dependent and extracellular-regulated protein kinase-independent manner. Neurosci Lett. 2001;308:37–40. doi: 10.1016/s0304-3940(01)01984-x. [DOI] [PubMed] [Google Scholar]
- 104.Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- 105.Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- 106.Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 107.Stempka L, Girod A, Müller HJ, Rincke G, Marks F, Gschwendt M, Bossemeyer D. Phosphorylation of protein kinase Cdelta (PKCdelta) at threonine 505 is not a prerequisite for enzymatic activity. Expression of rat PKCdelta and an alanine 505 mutant in bacteria in a functional form. J Biol Chem. 1997;272:6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- 108.Szallasi Z, Smith CB, Pettit GR, Blumberg PM. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J Biol Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- 109.Szego EM, Barabas K, Balog J, Szilagyi N, Korach KS, Juhasz G, Abraham IM. Estogen induces estrogen receptor alpha-dependent cAMP response element-binding protein phosphorylation via mitogen activated protein kinase pathway in basal forebrain cholinergic neurons in vivo. J Neurosci. 2006;26:4104–4110. doi: 10.1523/JNEUROSCI.0222-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- 111.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, Marcelli M. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 112.Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–46. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Voulalas PJ, Holtzclaw L, Wolstenholme J, Russell JT, Hyman SE. Metabotropic glutamate receptors and dopamine receptors cooperate to enhance extracellular signal-regulated kinase phosphorylation in striatal neurons. J Neurosci. 2005;25:3763–3773. doi: 10.1523/JNEUROSCI.4574-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 116.Walker WH. Nongenomic actions of androgen in Sertoli cells. Curr Top Dev Biol. 2003;56:25–53. doi: 10.1016/s0070-2153(03)01006-8. [DOI] [PubMed] [Google Scholar]
- 117.Wang D, Yu X, Brecher P. Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem. 1998;273:33027–33034. doi: 10.1074/jbc.273.49.33027. [DOI] [PubMed] [Google Scholar]
- 118.Ward NE, O’Brian CA. Inhibition of protein kinase C by N-myristoylated peptide substrate analogs. Biochemistry. 1993;33:11903–11909. doi: 10.1021/bi00095a020. [DOI] [PubMed] [Google Scholar]
- 119.Weihua Z, Lathe R, Warner M, Gustafsson J. An endrocrine pathway in the prostate, ERβ, AR, 5α-androstane-3β, 17β-diol, and CYP7B1, regulates prostrate growth. Proc Natl Acad Sci USA. 2002;99:13589–13594. doi: 10.1073/pnas.162477299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weihua Z, Makela S, Andersson LC, Salmi S, Saji S, Webster JI, Jensen EV, Nilsson S, Warner M, Gustafsson J. A role for estrogen receptor β in the regulation of growth of the ventral prostate. Proc Natl Acad Sci USA. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, Sekeris CE, Mossmann H. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–8. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 123.Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–63. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- 124.Yang SH, Liu R, Wen Y, Perez E, Cutright J, Brun-Zinkernagel AM, Singh M, Day AL, Simpkins JW. Neuroendocrine mechanism for tolerance to cerebral ischemia-reperfusion injury in male rats. J Neurobiol. 2005;62:341–351. doi: 10.1002/neu.20103. [DOI] [PubMed] [Google Scholar]
- 125.Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 126.Yano H, Agatsuma T, Nakanishi S, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Biochemical and pharmacological studies with KT7692 and LY294002 on the role of phosphatidylinositol 3-kinase in Fc epsilon RI-mediated signal transduction. Biochem J. 1995;312:145–150. doi: 10.1042/bj3120145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yao M, Nguyen TV, Pike CJ. Estrogen regulates Bcl-w and Bim expression: role in protection against beta-amyloid peptide-induced neuronal death. J Neurosci. 2007;27:1422–1433. doi: 10.1523/JNEUROSCI.2382-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang JM, Konkle AT, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang L, Chang YH, Barker JL, Hu Q, Maric D, Li BS, Rubinow DR. Testosterone and estrogen affect neuronal differentiation but not proliferation in early embryonic cortex of the rat: the possible roles of androgen and estrogen receptors. Neurosci Lett. 2000;281:57–60. doi: 10.1016/s0304-3940(99)00942-8. [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1–42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–21. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao L, Brinton RD. Vasopressin-induced cytoplasmic and nuclear calcium signaling in embryonic cortical astrocytes: dynamics of calcium and calcium-dependent kinase translocation. J Neurosci. 2003;23:4228–4239. doi: 10.1523/JNEUROSCI.23-10-04228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhao L, Chen S, Ming Wang J, Brinton RD. 17beta-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience. 2005;132:299–311. doi: 10.1016/j.neuroscience.2004.11.054. [DOI] [PubMed] [Google Scholar]