Abstract
Mammalian sterile 20-like kinase 1 (Mst1) plays an important role in mediating apoptosis and inhibiting hypertrophy in the heart. Since Hippo, a Drosophila homologue of Mst1, forms a signaling complex with Warts, a serine/threonine kinase, which in turn stimulates cell death and inhibits cell proliferation, mammalian homologs of Warts, termed Lats1 and Lats2, may mediate the function of Mst1. We here show that Lats2, but not Lats1, dose dependently increased apoptosis in cultured cardiac myocytes. Lats2 also dose-dependently reduced [3H]phenylalanine incorporation and cardiac myocyte size, whereas dominant negative Lats2 (DN-Lats2) increased them, suggesting that endogenous Lats2 negatively regulates myocyte growth. DN-Lats2 significantly attenuated induction of apoptosis and inhibition of hypertrophy by Mst1, indicating that Lats2 mediates the function of Mst1 in cardiac myocytes. Cardiac specific overexpression of Lats2 in transgenic mice significantly reduced the size of left and right ventricles, whereas that of DN-Lats2 caused hypertrophy in both ventricles. Overexpression of Lats2 reduced left ventricular systolic and diastolic function without affecting baseline levels of myocardial apoptosis. Expression of endogenous Lats2 was significantly upregulated in response to transverse aortic constriction (TAC). Overexpression of DN-Lats2 significantly enhanced cardiac hypertrophy and inhibited cardiac myocyte apoptosis induced by TAC. These results suggest that Lats2 is necessary and sufficient for negatively regulating ventricular mass in the heart. Although Lats2 is required for cardiac myocyte apoptosis in response to pressure overload, it was not sufficient to induce apoptosis at baseline. In conclusion, Lats2 affects both growth and death of cardiac myocytes, but it primarily regulates the size of the heart and acts as an endogenous negative regulator of cardiac hypertrophy.
Keywords: Apoptosis, Cardiac hypertrophy, Heart failure, Signal transduction
Introduction
The size of organs is controlled by the intricate coordination between cell growth and death 1. Increasing lines of evidence suggest that the heart is a renewing organ 2. However, hypertrophy of existing cardiac myocytes and cell death, including apoptosis, are still major, if not sole, determinants of the heart size. Although the signaling mechanisms controlling hypertrophy and apoptosis have been extensively studied, only a few mechanisms reported thus far can regulate both growth and death of cardiac myocytes in a coordinated fashion.
Mammalian sterile 20-like kinase 1 (Mst1) is a serine threonine kinase, which is activated by pro-apoptotic stimuli and potently induces apoptosis in cardiac myocytes. Interestingly, Mst1 not only induces apoptosis but also negatively regulates hypertrophy of cardiac myocytes 3. Stimulation of apoptosis and inhibition of compensatory hypertrophy could be detrimental for the heart because they could lead to progressive wall thinning and elevated wall stress, which further increases myocardial cell death and initiates a malignant spiral of heart failure 4.
In Drosophila, hippo (Hpo), a homologue of human Mst1, also regulates the size of organs, such as eyes and wings 5, 6. Using genetic approaches, a series of molecules associating with Hpo have been identified. Hpo physically interacts with Salvador (Sav), a scaffold protein, which in turn binds to Warts (Wts also known as lats) 7, 8. Hpo phosphorylates Wts and triggers cell cycle arrest and apoptosis 7, 9, 10.
In mammals, the lats tumor suppressor family encodes Ser/Thr protein kinases 11. One family member, Lats2, negatively regulates cell growth in NIH3T3/v-ras and HeLa cells 12, 13. Reduced expression of Lats2 has been shown to enhance the tumorigenesis 14, 15. Lats2 initiates a positive feedback mechanism with p53, thereby acting as a checkpoint mechanism for the maintenance of the proper chromosome number 16. Lats2 also regulates the mitotic machinery, thereby playing an important role in coordinating the mitotic fidelity and the genomic stability 17, 18. Recent reports have shown that the effects of Lats2 may be mediated by Yki and YAP, nuclear transcription co-factors, in Drosophila and mammals, respectively 19, 20.
Northern blot analyses showed ubiquitous expression of Lats2 mRNA in several human tissues with the highest expression in the heart 21. Lats2 KO mice are embryonic lethal and display atrial and ventricular defects, pericardial distension, and blood pooling, suggesting that Lats2 may play an important role in mediating cardiac development 17. Judging from the prominent proapoptotic and anti-growth functions of Mst1 in the heart 3, we expect that Lats2 should mediate some of the functions of Mst1 in the heart. However, the role of Lats2 in mediating growth and death of cardiac myocytes is not well understood.
Thus, the purposes of this study were 1) to clarify the cardiac function of Lats2 and 2) to determine whether the pro-apoptotic and anti-hypertrophic actions of Mst1 in the heart are mimicked by Lats2.
Material and Methods
Detailed methods are provided in the online data supplement at http://circres.ahajournals.org.
cDNA
Dominant-negative form of Mst1 (Mst1K59R) was described 3. A dominant negative form of Lats2 (DN-Lats2) was generated by mutating Lys-697 to alanine.
Antibodies
Mouse monoclonal antibody against N-terminal portion of human Lats2 (amino acid from 78 to 256) and rabbit polyclonal antibody against N-terminal portion of human Lats2 (amino acid from 79 to 257) have been described 18, 21. Rat monoclonal antibody against human Lats1 has been described 22. Other antibodies include monoclonal anti-Myc 9E10 (Santa Cruz Biotechnology); anti-Mst1, anti-Bcl-2, anti-Bcl-xL (Pharmingen); anti-α-actin (Sigma); polyclonal anti-cleaved-caspase 3 (Cell Signaling) antibodies.
Transgenic mice
Lats2 and DN-Lats2 transgenic mice (Tg-Lats2 and Tg-DN-Lats2) were generated using the α-myosin heavy chain promoter (courtesy of J. Robbins, University of Cincinnati, Cincinnati, Ohio, USA) to achieve cardiac-specific expression of transgene on an FVB background. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey.
Statistics
Statistical analyses between groups were done by one-way ANOVA, and differences among group means were evaluated using Fisher's project least significant difference post test procedure for group data with a P value less than 0.05 considered significant.
Results
Lats2 induces apoptosis in cardiac myocytes
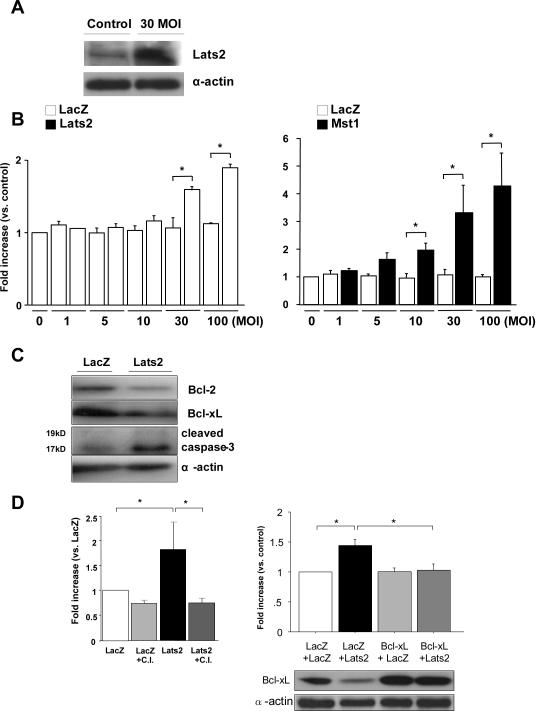
To examine the effect of Lats2 expression on growth and death of cardiac myocytes, cultured myocytes were transduced with Ad-Lats2 or Ad-LacZ. After 2 days, expression of Lats2 was significantly increased (Fig. 1A). Due to differences in amino acid sequence in Lats2 between mice/rats and humans, antibodies raised against human proteins detected endogenous Lats2 in rat cardiac myocytes with lower efficiencies than the exogenous Lats2 encoded by human cDNAs, which did not allow us to precisely evaluate the extent of overexpression by adenovirus transduction. In any event, Ad-Lats2 dose-dependently increased cytoplasmic accumulation of mono- and oligo-nucleosomes, a sensitive indicator of apoptosis in cardiac myocytes, whereas Ad-LacZ failed to cause an increase (Fig. 1B). Ad-Lats1 failed to induce accumulation of mono- and oligo-nucleosomes (suppl. Fig. I).
Figure 1. Lats2 induces apoptosis in cardiac myocytes.
(A) Neonatal rat cardiac myocytes (NRCMs) were transduced with Ad-LacZ (control) or Ad-Lats2 at 30 MOI. Immunoblot analyses were performed using anti-Lats2 antibody.. (B) NRCMs were transduced with Ad-LacZ, Ad-Lats2 or Ad-Mst1. Cytoplasmic accumulation of mono- and oligo-nucleosomes was quantitated. Values are mean ± SEM obtained from 3 experiments. *p<0.05. (C) NRCMs were transduced with Ad-LacZ and Ad-Lats2 at 30 MOI. The result shown is representative of 3 experiments.. (D) (Left) NRCMs were transduced with Ad-LacZ and Ad-Lats2 at 30 MOI in the presence or absence of caspase inhibitor (C.I.) (100μM, ApoBlock, BD Biosciences). (Right) NRCMs were transduced with Ad-LacZ or Ad-Bcl-xL at 10 MOI. Twenty-four hours after transduction, NRCMs were transduced with Ad-LacZ or Ad-Lats2 at 10 MOI. Forty-eight hours after the second transduction, cytoplasmic accumulation of mono- and oligo-nucleosomes was quantitated. Values are mean ± SEM obtained from 3 experiments. *p<0.05. Cell lysates were subjected to immunoblots for detection of Bcl-xL and α-actin.
The increase in the index of DNA fragmentation by Lats2 was accompanied by significant downregulation of Bcl-2/Bcl-xL and an increase in cleaved caspase 3 (Fig. 1C) and it was abolished in the presence of a general caspase inhibitor or by inhibiting downregulation of Bcl-xL (Fig. 1D). Expression of p53 was not significantly affected by Lats2 (suppl. Fig. II). Taken together, these data suggest that Lats2 induces apoptosis in cardiac myocytes, possibly through downregulation of Bcl-xL.
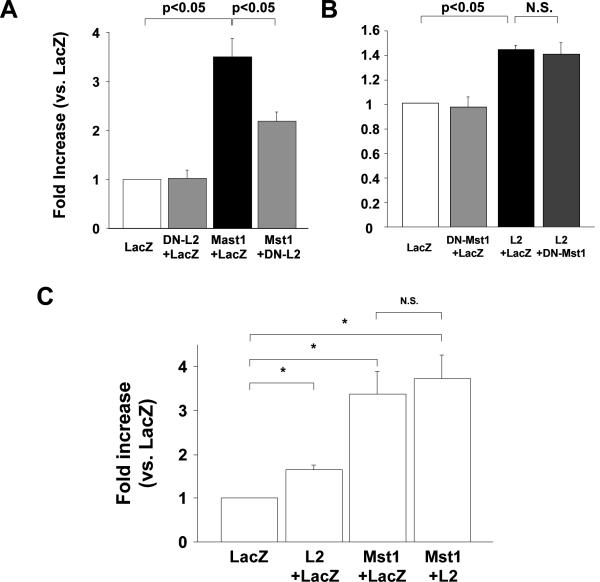
We next examined whether Lats2 mediates Mst1-induced apoptosis in cardiac myocytes 3. To this end, we used Ad-DN-Lats2. We have shown recently that DN-Lats2 inhibits Mst1-induced increases in phosphorylation of YAP in cardiac myocytes, suggesting that DN-Lats2 inhibits endogenous Lats2 23. Although Ad-DN-Lats2 alone did not affect cell death, it significantly reduced Mst1-induced increases in apoptosis as determined by cytoplasmic accumulation of mono- and oligo-nucleosomes (Fig. 2A). Downregulation of Lats2 by shRNA-Lats2 also inhibited Mst1-induced apoptosis (suppl. Fig. III). On the other hand, increases in apoptosis by Ad-Lats2 were not inhibited by Ad-DN-Mst1 (Fig. 2B). Ad-Lats2 did not exhibit additive effects on the induction of cell death by Ad-Mst1 (Fig. 2C). Taken together, Lats2 partially mediates Mst1-induced increases in cardiac myocyte apoptosis.
Figure 2. Lats2 partly mediates Mst1-induced apoptosis.
(A) NRCMs were transduced with Ad-LacZ or Ad-DN-Lats2 (DN-L2) at 30 MOI. Twenty-four hours after transduction, myocytes were transduced with Ad-Mst1 at 30MOI. (B) NRCMs were transduced with Ad-LacZ and Ad-DN-Mst1 at 30 MOI. Twenty-four hours after transduction, myocytes were transduced with Ad-Lats2 (L2) at 30 MOI. In A and B, 48 hours after the second transduction, cytoplasmic accumulation of mono- and oligo-nucleosomes was quantitated. Values are mean ± SEM obtained from 3 experiments. N.S., not significant. (C) NRCMs were transduced with Ad-LacZ, Ad-Lats2 or Ad-Mst1 with different combinations (total 60 MOI). Forty-eight hours after the transduction, cytoplasmic accumulation of mono- and oligo-nucleosomes was quantitated. Values are mean ± SEM obtained from 3 experiments. *p<0.05.
Lats2 negatively regulates cardiac myocyte size in vitro
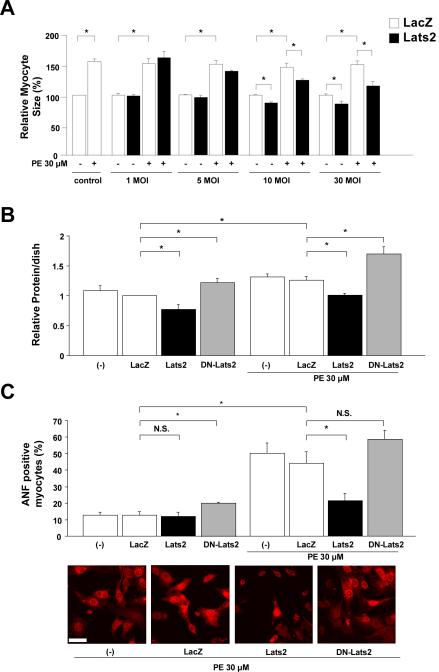
Since the Drosophila Hpo pathway regulates both cell death and the organ size 6, we next examined the effect of Lats2 upon cardiac myocyte size. Ad-Lats2 dose-dependently decreased the size of cardiac myocytes at baseline and in the presence of phenylephrine (PE), an agonist for the α1 adrenergic receptor (Fig. 3A and suppl. Fig. IVA). The significant reduction in the myocyte size by Ad-Lats2 was observed at 10 MOI, which was lower than the dose required for the induction of apoptosis (See Fig. 1B). A similar result was obtained regarding the effect of Ad-Lats2 upon total protein content (Fig. 3B) and [3H]phenylalanine incorporation (suppl. Fig. IVB) in cardiac myocytes. Lats2-induced decreases in cardiac myocyte size were observed even when apoptosis was completely suppressed by Bcl-xL, suggesting that Lats2 negatively regulates cell size independently of apoptosis (suppl. Fig. V). Ad-DN-Lats2 significantly increased the total protein content (Fig. 3B) and [3H]phenylalanine incorporation (suppl. Fig. IVC) at baseline and in response to PE, suggesting that endogenous Lats2 acts as a negative regulator of cardiac hypertrophy. Similar results were obtained in the presence of Ad-shRNA-Lats2 (suppl. Fig. VI). Ad-Lats2 also significantly reduced PE-induced increases in expression of ANF, a protein upregulated in many forms of cardiac hypertrophy (Fig. 3C). On the other hand, Ad-DN-Lats2 significantly increased ANF expression at baseline (Fig. 3C and suppl. Fig. VIIA). Expression of Lats2 dose-dependently decreased, whereas that of DN-Lats2 dose-dependently increased, transcription of ANF, as determined by ANF-Luc assays (Fig. VIIB). Lats2 significantly reduced phosphorylation of Akt and p70S6K, positive mediators of cardiac hypertrophy, whereas it increased phosphorylation of eEF2, a negative regulator of hypertrophy (suppl. Fig. VIII). Conversely, DN-Lats2 increased phosphorylation of Akt (suppl. Fig. IX). These results are consistent with the notion that Lats2 negatively regulates cardiac hypertrophy at least partially through suppression of protein synthesis.
Figure 3. LATS2 inhibits cardiac hypertrophy.
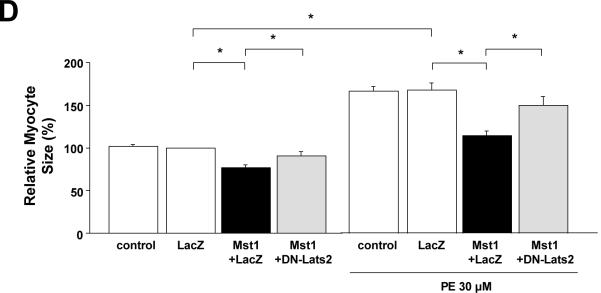
(A-C) NRCMs were transduced with Ad-LacZ, Ad-Lats2 or Ad-DN-Lats2. Forty-eight hours after transduction, myocytes were stimulated with or without phenylephrine (PE, 30 μM) for 48 hours. In A, myocyte surface area was determined. The cell-surface area without PE was designated as 100%. Values are mean ± SEM obtained from 3 experiments. *p<0.05. In B, total protein content was determined. The protein content in myocytes transduced with Ad-LacZ without PE was designated as 1. Values are mean ± SEM obtained from 3 experiments. *p<0.05. In C, myocytes were stained with anti-ANF antibody. The percent of myocytes expressing ANF, as determined by typical perinuclear staining, was counted. Values are mean ± SEM obtained from 3 experiments. *p<0.05. N.S, not significant. Bar = 20 μm. (D) NRCMs were transduced with or without Ad-LacZ (60MOI), Ad-Mst1 (30MOI) + Ad-LacZ (30MOI) and Ad-Mst1 (30MOI) + Ad-DNLats2 (30MOI). Fort-eight hours after transduction, myocytes were stimulated with or without PE (30 μM) for 48 hours and then myocyte surface measured. Experimental values in myocytes transduced with Ad-LacZ without PE were designated as 1. Values are mean ± SEM obtained from 3 experiments. * p<0.05
Expression of Mst1 decreases the cell size and total protein content in cardiac myocytes at baseline and in the presence of PE. Co-expression of DN-Lats2 reversed Mst1-induced decreases in the cell size and total protein content at baseline and in the presence of PE (Fig. 3E and suppl. Fig. X). These results suggest that Lats2 plays a significant role in mediating the anti-hypertrophic effect of Mst1.
Lats2 negatively regulates ventricular chamber size in vivo
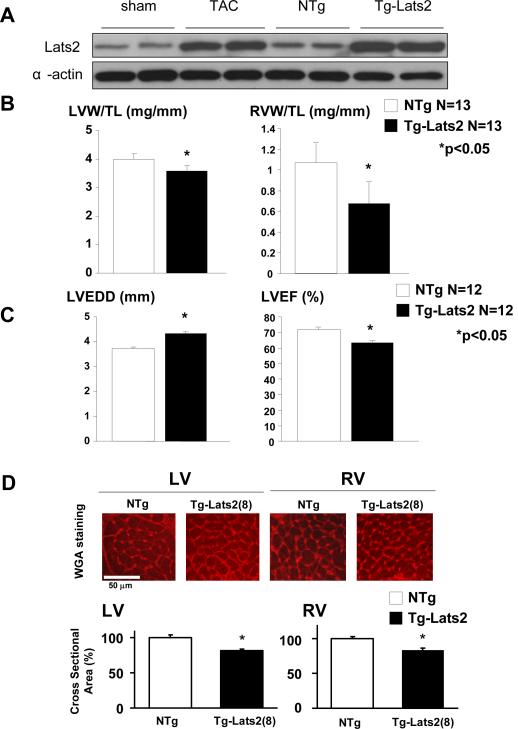
We next examined the function of Lats2 in the heart in vivo. Although expression of Lats2 in the mouse heart was greater in the neonatal period than in the adult, Lats2 was expressed in the adult heart (suppl. Fig. XI). Both mRNA and protein expression of Lats2 were significantly upregulated (6 and 4.5 fold, respectively) by pressure overload in the adult mouse heart (Fig. 4A and suppl. Fig. XII). In order to examine the function of Lats2 in vivo, we generated transgenic mice with cardiac specific overexpression of Lats2 (Tg-Lats2). We made 2 lines and confirmed expression of the Lats2 transgene in the heart, where Lats2 was overexpressed up to 7 fold in line 8 and up to 5 fold in line 20 (Fig. 4A and not shown). The level of transgene expression seems overestimated because the antibody detected human Lats2 with a greater sensitivity compared to endogenous Lats2. Thus, Lats2 expression in Tg-Lats2 (lines 8 and 20) appears to be comparable to that of endogenous Lats2 after pressure overload. In line 8, many mice exhibited enlargement of the right atrium (RA) (suppl. Fig. XIII). At 5 months of age, both left ventricular weight (LVW)/TL and right ventricular weight (RVW)/TL were significantly smaller in Tg-Lats2 than in NTg mice (Fig. 4B). RVW/TL was also significantly smaller in Tg-Lats2 (line 20) than in NTg mice (suppl. Table I).
Figure 4. Cardiac Phenotype of Tg-Lats2 mice.
(A) Heart homogenates were prepared from normal mice subjected to either sham operation (sham) or transverse aortic constriction for 2 weeks (TAC), Tg-Lats2 (line 20) or non-transgenic (NTg) littermates, and then immunoblotted with anti-Lats2 and anti-α-actin antibodies. (B) Postmortem measurements of left ventricular weight/tibial length (LVW/TL, left) and right ventricular weight/tibial length (RVW/TL, right) in Tg-Lats2 (line 8) and NTg. Values are mean ± SD. (C) Echocardiographically measured LV end diastolic dimension (LVEDD) and LV ejection fraction (LVEF) in Tg-Lats2 (line 8) and NTg mice. Values are mean ± SEM. (D) Representative WGA staining of the LV and RV myocardium obtained from Tg-Lats2 (line 8) and NTg mice (upper panel) and the relative myocyte cross sectional area (lower panel). Values are mean ± SEM obtained from 5 experiments. *p<0.05.
Echocardiography showed that LV end diastolic dimension (LVEDD) was significantly greater and LV ejection fraction (LVEF) was slightly lower in Tg-Lats2 (line 8) than in NTg (Fig. 4E and Table 1). Invasive hemodynamic measurements, using pressure-volume loop analysis, showed that LV end-systolic volume (LVESV) is significantly higher, but LV dP/dtmax and the slope of the end-systolic pressure-volume relationship (end systolic elastance) are significantly lower in Tg-Lats2 (line 20) than in NTg, indicating that Tg-Lats2 mice have significantly reduced LV systolic function (Table 2). The time constant of isovolumic pressure decay (Tau) was higher in Tg-Lats2 but did not reach statistical significance. LV dP/dtmin was significantly lower in Tg-Lats2, and the slope of the end-diastolic pressure-volume relationship was significantly higher in Tg-Lats2 than in NTg (Table 2). These data suggest that Tg-Lats2 mice also have LV diastolic dysfunction. The indices of LV wall stress (LV end diastolic wall stress, and LV end diastolic pressure/end diastolic dimension) were greater in Tg-Lats2 than in NTg, suggesting that LV wall stress was elevated in Tg-Lats2 (suppl. Fig. XIV).
Table 1.
Echocardiographic Analysis of Tg-Lats2 (Line 8) at 5 Months of Age
| NTg | Tg | |
|---|---|---|
| n | 12 | 12 |
| DSEP WT (mm) | 0.84±0.03 | 0.88±0.03 |
| LVEDD (mm) | 3.73±0.07 | 4.32±0.09** |
| DPW WT (mm) | 0.81±0.03 | 0.86±0.03 |
| SSEP WT (mm) | 1.27±0.03 | 1.27±0.04 |
| LVESD (mm) | 2.44±0.05 | 3.09±0.07** |
| SPW WT (mm) | 1.12±0.03 | 1.13±0.03 |
| %FS | 34.6±0.5 | 28.6±0.7** |
| LVEF(%) | 71.9±1.2 | 63.4±1.3** |
| HR (/min) | 463±13 | 452±13 |
| Echocardiographic Analysis of Tg-Lats2 (Line 20) at 5 Months of Age | ||
|---|---|---|
| NTg | Tg | |
| n | 16 | 16 |
| DSEP WT (mm) | 0.81±0.02 | 0.80±0.01 |
| LVEDD (mm) | 3.73±0.05 | 3.73±0.07 |
| DPW WT (mm) | 0.78±0.02 | 0.75±0.02 |
| SSEP WT (mm) | 0.87±0.02 | 0.85±0.02 |
| LVESD (mm) | 2.46±0.04 | 2.57±0.05 |
| SPW WT (mm) | 0.86±0.02 | 0.85±0.02 |
| %FS | 34.2±0.4 | 31.1±0.5** |
| LVEF(%) | 71.5±1.2 | 67.3±1.2** |
| HR (/min) | 427±16 | 468±12 |
p<0.01 vs NTg
Table 2.
PV-loop measurements of Tg-Lats2 (line 20)
| NTg | Tg-Lats2 | |
|---|---|---|
| N | 4 | 4 |
| Heart rate(bpm) | 512.5±10.4 | 529.0±36.7 |
| ESV(μL) | 10.95±2.9 | 22.0±2.3* |
| EDV(μL) | 37.95±2.7 | 43.54±1.7 |
| ESP(mmHg) | 89.8±3.4 | 77.0±11.4 |
| EDP(mmHg) | 6.2±0.9 | 8.3±2.1 |
| CO(μL/min) | 17141.4±327.6 | 14019.5±1054.0* |
| dPdt max(mmHg/s) | 10646±724.1 | 4671.7±738.2** |
| dPdt min(mmHg/s) | -7108.0±492.8 | -5012.7±714.3* |
| Tau (ms) | 7.5±0.6 | 11.4±2.3 |
| ESPVR Slope | 2.6±0.3 | 1.3±0.3* |
| EDPVR Slope | 0.07±0.02 | 0.16±0.04* |
P<0.05 vs. NTg.
P<0.01 vs NTg
The total myocyte number estimated by histological analyses was not significantly different between Tg-Lats2 and NTg (suppl. Fig. XVA). Myocyte cross sectional area in LV and RV was significantly smaller in Tg-Lats2 (line 8) than in NTg. These results suggest that Lats2 decreases the size of ventricular myocytes (Fig. 4D). Histologically determined fibrosis in the LV was significantly greater in Tg-Lats2 (both lines 8 and 20) than in NTg (suppl. Fig. XVB and not shown). Neither Tg-Lats2 line 8 nor 20 had increased numbers of TUNEL positive cardiac myocytes at baseline (not shown here but see suppl. Fig. XX).
Biochemical analysis showed that expression of L-type Ca2+ channels and sarcoplasmic reticulum Ca2+-ATPase (SERCA)2a, and phosphorylated phospholamban are significantly reduced in Tg-Lats2 compared to NTg, whereas expression of Na+/Ca2+ exchanger did not differ significantly (suppl. Fig. XVI).
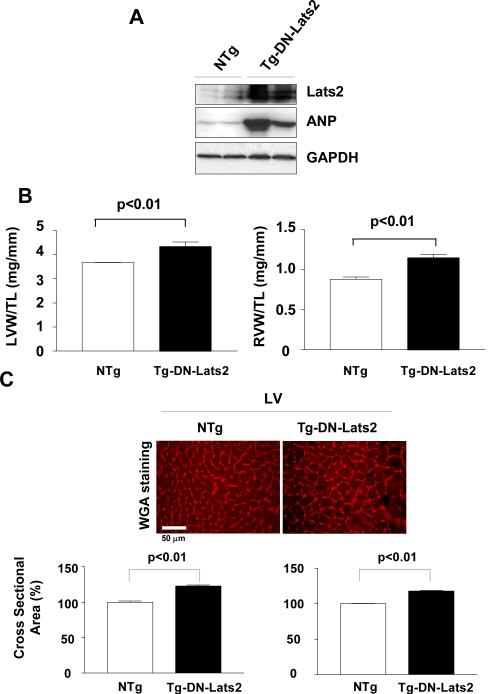
Tg-DN-Lats2 mice exhibit bi-ventricular hypertrophy at baseline
In order to examine the function of endogenous Lats2, we generated two lines (27 and 31) of transgenic mice with cardiac specific overexpression of dominant negative Lats2 (Tg-DN-Lats2). Overexpression of DN-Lats2 in the heart was confirmed by immunoblot analyses (up to 9 fold in line 27 and 11 fold in line 31) (Fig. 5A and not shown). Both LVW/TL and RVW/TL were significantly greater in Tg-DN-Lats2 (line 31) than in NTg mice (Table 2). LVW/BW and RVW/BW were significantly greater in Tg-DN-Lats2 than in NTg mice in both lines 27 and 31 (Fig. 5B and suppl. Table II). LV and RV myocyte cross sectional area was significantly greater in Tg-DN-Lats2 than in NTg (Fig. 5C). These results suggest that inhibition of endogenous Lats2 induces hypertrophy in both RV and LV cardiac myocytes. Expression of ANF was elevated in Tg-DN-Lats2 hearts (Fig. 5A). Taken together, Tg-DN-Lats2 hearts exhibited all features of biventricular hypertrophy at baseline. Echocardigraphically determined chamber size and LV function were normal in Tg-DN-Lats2 at baseline except that LV wall thickness was significantly greater in Tg-DN-Lats2 (line 31) than in NTg (suppl. Table III).
Fig. 5. Cardiac phenotype of Tg-DN-Lats2 mice.
(A) Immunoblot analyses of heart homogenates from Tg-DN-Lats2 (line 31) and NTg mice with anti-Lats2, anti-ANP, and anti-α actin antibodies. (B) Postmortem measurements of left ventricular weight/tibial length (LVW/TL, mg/mm, left) and right ventricular weight/tibial length (RVW/TL, mg/mm, right) in Tg-DN-Lats2 (line 31) and NTg. Values are mean ± SD. N=12-16. (C) Representative WGA staining of the LV and RV myocardium obtained from Tg-DN-Lats2 and NTg mice and the relative myocyte cross sectional area (lower panel). Values are mean ± SEM obtained from 5 experiments.
Endogenous Lats2 is a negative regulator of pathological hypertrophy in response to pressure overload
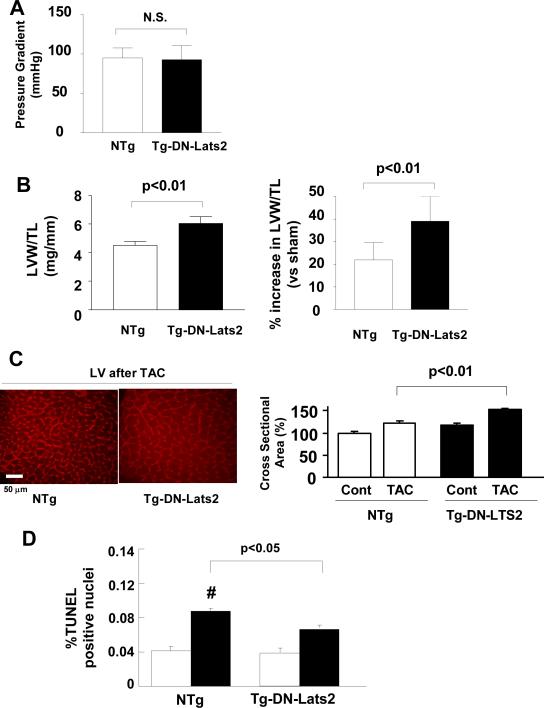
Since expression of Lats2 is increased in response to pressure overload, we examined whether upregulation of endogenous Lats2 during pressure overload works as a negative feedback mechanism for cardiac hypertrophy. To this end, Tg-DN-Lats2 mice were subjected to TAC. After 2 weeks of TAC, the pressure gradient was similar between NTg and Tg-DN-Lats2 (Fig. 6A). Tg-DN-Lats2 mice exhibited significantly greater LVW/TL than NTg after TAC (Fig. 6B and suppl. Table IV). Increases in LVW/TL after TAC (compared with mean LVW/TL in sham operated mice in each group) were significantly greater in Tg-DN-Lats2 than in NTg (+37% vs 27% p<0.05). Increases in LV cross sectional area after TAC were also greater in Tg-DN-Lats2 than in NTg (+25% vs +16%, p<0.05) (Fig. 6C). TUNEL analyses indicated that TAC-induced increases in LV apoptosis were significantly smaller in Tg-DN-Lats2 than in NTg (Fig. 6D). These results suggest that endogenous Lats2 negatively regulates LV hypertrophy and apoptosis by pressure overload.
Fig. 6. Effects of TAC on cardiac hypertrophy in Tg-DN-Lats2 mice.
Tg-DN-Lats2 (line 31) and NTg were subjected to either TAC (2 weeks) or sham operation. (A) The pressure gradient was measured after 2 weeks of TAC. (B) (left) Postmortem measurements of left ventricular weight/tibial length (LVW/TL, mg/mm, left) after TAC. (right) Percent increase in LVW/TL after TAC compared with the mean value of sham operated mice. Values are mean ± SD. N=7. (C) Representative WGA staining of the LV obtained from Tg-DN-Lats2 and NTg mice after TAC (left panel) and the relative myocyte cross sectional area (right panel). Values are mean ± SEM obtained from 5 experiments. (D) Percent TUNEL positive myocytes. Values are mean ± SEM obtained from 5 experiments. #p<0.05 vs NTg sham.
Discussion
Appropriate cell numbers and the organ size in multicellular organisms are determined by coordination of cell growth, proliferation, and apoptosis 1. A recent explosion in studies in Drosophila has demonstrated that a novel and evolutionarily conserved signaling cascade termed the “Hpo pathway” critically controls the size of cells and organs 6. Wts/Lats protein kinase is a key component of the pathway, which controls the coordination between cell proliferation and apoptosis 8-10. We demonstrated here that Mst1 and Lats2, the mammalian counterparts of the Hpo pathway components, play an important role in mediating both apoptosis and inhibition of growth of cardiac myocytes, thereby controlling the size of left and right ventricles in the adult mouse heart.
Previous reports demonstrated that both Drosophila Wts and mammalian Lats1 induce apoptosis 9, 24-26. However, overexpression of Lats1 failed to induce apoptosis in cardiac myocytes (Suppl Fig. I). Although Lats1 is phosphorylated and activated by Mst2, no stable interaction could be observed between Lats1 and hWW45, a mammalian homologue of Sav and an adaptor protein in the Hpo pathway, or Mst2 27. On the other hand, Lats2, but not Lats1, interacts with hWW45, forms a complex with Mst1 through hWW45 in cardiac myocytes, and is phosphorylated by Mst1 in COS-7 cells (suppl. Figs. XVIII and XIX). These results suggest that the function of Lats1 and Lats2 may be cell type-dependent in mammalian cells.
Our results suggest that Lats2 induces apoptosis in cardiac myocytes in vitro. Furthermore, Lats2 plays an important role in mediating Mst1-induced apoptosis. Downregulation of Bcl-2 or Bcl-xL might be partially responsible for Lats2-induced apoptosis in cardiac myocytes. Lats2 binds to Mdm2 and upregulates p53, which in turn upregulates Lats2, thereby constituting a positive feedback loop for p53 activation in HEK293 cells 16. However, Lats2 did not upregulate p53 in cardiac myocytes. The effect of Lats2 upon apoptosis may be modulated by YAP, a co-factor of nuclear transcription factors, whose phosphorylation and nuclear localization are regulated by Lats2 28. YAP mediates c-Jun-induced apoptosis through a p73-dependent mechanism in MEF 29, whereas it inhibits apoptosis through activation of Tead, a transcription factor in embryonic tissues 30. The effect of YAP upon cell death in the heart remains to be elucidated.
Tg-Lats2 mice displayed modest but significant LV dysfunction at baseline (Table 1) and in response to pressure overload (Suppl Table V). Apoptosis may not be responsible for basal LV dysfunction, because increases in TUNEL positive cells were not significant in Tg-Lats2 at baseline. Downregulation of Ca2+ handling proteins, such as L-type Ca2+ channels and SERCA, and reduction of phosphorylated phospholamban levels, possibly through both transcriptional and posttranslational modifications initiated by increased activity of Lats2, may contribute to baseline LV dysfunction in Tg-Lats2. On the other hand, since TUNEL positive myocytes were significantly greater in Tg-Lats2 than in NTg under pressure overload (Suppl. Fig. XX) and because increases in apoptosis by pressure overload are partially inhibited by DN-Lats2, Lats2 may sensitize myocytes to apoptosis under stress.
Our results from both gain and loss of function experiments suggest that endogenous Lats2 acts as a negative regulator of the size of both left and right ventricles and cardiac myocytes therein in vivo. Although left and right ventricles in Tg-Lats2 were significantly smaller at baseline, the enhancement of apoptosis was observed only under stress. Thus, one of the most prominent functions of Lats2 in the heart is similar to that of Drosophila Wts, namely the regulation of the organ size 6. Furthermore, Mst1-induced decreases in cardiac myocyte size were significantly reduced by DN-Lats2 in vitro, suggesting that Lats2 plays an important role in mediating the anti-hypertrophic effects of Mst1.
Although Tg-Lats2 mice exhibited dilated hearts at baseline, the wall thickness was not increased, suggesting that the LV wall stress is elevated. Suppression of compensatory hypertrophy might have contributed to LV dysfunction in Tg-Lats2 hearts. Since the lack of compensatory hypertrophy despite LV dilation was also observed in Tg-Mst1 3, these results are consistent with the notion that Lats2 partly mediates the detrimental function of Mst1 in the adult heart. We speculate that upregulation of Lats2 observed during pressure overload is detrimental because it not only increases apoptosis but also prevents compensatory hypertrophy.
In Drosophila Hpo pathway, Wts negatively regulates cyclin E and diap, a homolog of XIAP and an antiapoptotic molecule 7. Lats2 induced significant reduction in expression of cyclin E in cardiac myocytes in vitro (suppl. Fig. XXI). Although it is generally believed that postnatal cardiac myocytes are terminally differentiated and its growth is regulated primarily by hypertrophy rather than proliferation, recent evidence suggests that cyclins, mediators of cell proliferation, also play an important role in mediating hypertrophy 31. At present, the significance of cyclin E in the regulation of cardiac myocyte growth remains to be elucidated. Wts regulates nuclear localization of Yki 20, thereby regulating its transcriptional targets, such as microRNA bantam 32. Whether or not Lats2 regulates gene transcription through YAP and, if so, which genes are the targets of YAP would be important issues to address in the future. Recent evidence suggests that miRNA-327 and -373 downregulate Lats2, thereby neutralizing p53-mediated CDK inhibition in human testicular germ cell tumors 33. Our results suggest that Lats2 negatively regulates protein synthesis in cardiac myocytes. It remains to be elucidated how activation of Lats2 modulates the activity of signaling molecules, which regulate cardiac protein synthesis, such as Akt, p70S6K and eEF2.
In conclusion, our results suggest that Lats2 regulates both growth and death of cardiac myocytes, thereby mimicking the cellular function of Mst1 (Fig. 6E). Although Lats2 partly mediates cardiac myocyte apoptosis in response to pressure overload in vivo, Lats2 has more prominent effects upon regulation of the heart size and plays an essential role in inhibiting hypertrophy at baseline and in response to pressure overload.
Supplementary Material
Acknowledgments
Sources of Funding This work was in part supported by U.S. Public Health Service Grants HL 59139, HL67724, HL67727, HL69020, and HL 73048 and by American Heart Association 0340123N.
Footnotes
Disclosures: None.
References
- 1.Stanger BZ. Organ size determination and the limits of regulation. Cell Cycle. 2008;7:318–324. doi: 10.4161/cc.7.3.5348. [DOI] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, Molina CA, Yatani A, Vatner DE, Vatner SF, Sadoshima J. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morisco C, Sadoshima J, Trimarco B, Arora R, Vatner DE, Vatner SF. Is treating cardiac hypertrophy salutary or detrimental: the two faces of Janus. Am J Physiol (Heart Circ Physiol) 2003;284:H1043–H1047. doi: 10.1152/ajpheart.00990.2002. [DOI] [PubMed] [Google Scholar]
- 5.Saucedo LJ, Edgar BA. Filling out the Hippo pathway. Nat Rev Mol Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 6.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 7.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 8.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 9.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 11.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Pei J, Xia H, Ke H, Wang H, Tao W. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene. 2003;22:4398–4405. doi: 10.1038/sj.onc.1206603. [DOI] [PubMed] [Google Scholar]
- 13.Kamikubo Y, Takaori-Kondo A, Uchiyama T, Hori T. Inhibition of cell growth by conditional expression of kpm, a human homologue of Drosophila warts/lats tumor suppressor. J Biol Chem. 2003;278:17609–17614. doi: 10.1074/jbc.M211974200. [DOI] [PubMed] [Google Scholar]
- 14.Dijkman R, van Doorn R, Szuhai K, Willemze R, Vermeer MH, Tensen CP. Gene-expression profiling and array-based CGH classify CD4+CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109:1720–1727. doi: 10.1182/blood-2006-04-018143. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi Y, Miyoshi Y, Takahata C, Irahara N, Taguchi T, Tamaki Y, Noguchi S. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–1385. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 16.Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McPherson JP, Tamblyn L, Elia A, Migon E, Shehabeldin A, Matysiak-Zablocki E, Lemmers B, Salmena L, Hakem A, Fish J, Kassam F, Squire J, Bruneau BG, Hande MP, Hakem R. Lats2/Kpm is required for embryonic development, proliferation control and genomic integrity. Embo J. 2004;23:3677–3688. doi: 10.1038/sj.emboj.7600371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yabuta N, Okada N, Ito A, Hosomi T, Nishihara S, Sasayama Y, Fujimori A, Okuzaki D, Zhao H, Ikawa M, Okabe M, Nojima H. Lats2 is an essential mitotic regulator required for the coordination of cell division. J Biol Chem. 2007;282:19259–19271. doi: 10.1074/jbc.M608562200. [DOI] [PubMed] [Google Scholar]
- 19.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Yabuta N, Fujii T, Copeland NG, Gilbert DJ, Jenkins NA, Nishiguchi H, Endo Y, Toji S, Tanaka H, Nishimune Y, Nojima H. Structure, expression, and chromosome mapping of LATS2, a mammalian homologue of the Drosophila tumor suppressor gene lats/warts. Genomics. 2000;63:263–270. doi: 10.1006/geno.1999.6065. [DOI] [PubMed] [Google Scholar]
- 22.Tao W, Zhang S, Turenchalk GS, Stewart RA, John MA, St, Chen W, Xu T. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21:177–181. doi: 10.1038/5960. [DOI] [PubMed] [Google Scholar]
- 23.Nakano N, Matsui Y, Sadoshima J. Yap is a nuclear target of Mst1/Lats2 regulating cell size in cardiac myocytes. Circulation. 2007;116(Suppl II):II–128. (abstract) [Google Scholar]
- 24.Kuninaka S, Nomura M, Hirota T, Iida S, Hara T, Honda S, Kunitoku N, Sasayama T, Arima Y, Marumoto T, Koja K, Yonehara S, Saya H. The tumor suppressor WARTS activates the Omi/HtrA2-dependent pathway of cell death. Oncogene. 2005;24:5287–5298. doi: 10.1038/sj.onc.1208682. [DOI] [PubMed] [Google Scholar]
- 25.Xia H, Qi H, Li Y, Pei J, Barton J, Blackstad M, Xu T, Tao W. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene. 2002;21:1233–1241. doi: 10.1038/sj.onc.1205174. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Li DM, Chen W, Xu T. Human homologue of Drosophila lats, LATS1, negatively regulate growth by inducing G(2)/M arrest or apoptosis. Oncogene. 2001;20:6516–6523. doi: 10.1038/sj.onc.1204817. [DOI] [PubMed] [Google Scholar]
- 27.Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 28.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes-Associated Protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008;15:217–219. doi: 10.1038/sj.cdd.4402226. [DOI] [PubMed] [Google Scholar]
- 30.Sawada A, Kiyonari H, Ukita K, Nishioka N, Imuta Y, Sasaki H. Redundant roles of Tead1 and Tead2 in notochord development and the regulation of cell proliferation and survival. Mol Cell Biol. 2008;28:3177–3189. doi: 10.1128/MCB.01759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. Embo J. 2006;25:3869–3879. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, Zlotorynski E, Yabuta N, De Vita G, Nojima H, Looijenga LH, Agami R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.