Abstract
Conjugated equine estrogen (CEE) is the most commonly prescribed estrogen therapy, and is the estrogen used in the Women's Health Initiative study. While in-vitro studies suggest that CEE is neuroprotective, no study has evaluated CEE's effects on a cognitive battery and brain immunohistochemistry in an animal model. The current experiment tested whether CEE impacted: I) spatial learning, reference memory, working memory and long-term retention, as well as ability to handle mnemonic delay and interference challenges; and, II) the cholinergic system, via pharmacological challenge during memory testing and ChAT-immunoreactive cell counts in the basal forebrain. Middle-aged ovariectomized (Ovx) rats received chronic cyclic injections of either Oil (vehicle), CEE-Low (10 μg), CEE-Medium (20 μg) or CEE-High (30 μg) treatment. Relative to the Oil group, all three CEE groups showed less overnight forgetting on the spatial reference memory task, and the CEE-High group had enhanced platform localization during the probe trial. All CEE groups exhibited enhanced learning on the spatial working memory task, and CEE dose-dependently protected against scopolamine-induced amnesia with every rat receiving the highest CEE dose maintaining zero errors after scopolamine challenge. CEE also increased number of ChAT-immunoreactive neurons in the vertical diagonal band of the basal forebrain. Neither the ability to remember after a delay nor interference, nor long-term retention, was influenced by the CEE regimen used in this study. These findings are similar to those reported previously for 17 ß-estradiol, and suggest that CEE can provide cognitive benefits on spatial learning, reference and working memory, possibly through cholinergic mechanisms.
Keywords: estrogen, hormone replacement, learning, working memory, spatial memory, reference memory
Introduction
Conjugated equine estrogen (CEE; tradename Premarin) is the most widely used hormone therapy (HT) in the United States, given since 1942 (Stefanick, 2005), and was the estrogen used in the Women's Health Initiative (WHI) (Shumaker et al., 1998; 2004). In vitro, CEE increases neuronal growth in the basal forebrain, hippocampus and cortex (Diaz Brinton et al., 2000) and attenuates beta amyloid-induced cell death in hippocampus (Brinton et al., 2000). Clinical findings assessing CEE have been inconclusive. CEE-containing therapy improved memory via self-report (Campbell and Whitehead, 1977), case studies (Ohkura et al., 1995) and randomized psychometric evaluations (Kantor et al., 1973). Yet, findings evaluating global cognitive function in the large placebo-controlled WHI Memory Study (WHIMS), conducted by the National Institutes of Health, showed an increase in probable dementia risk and no effect on mild cognitive impairment in women 65+ years taking the combination therapy CEE+medroxyprogesterone (Shumaker et al., 2003). Unopposed treatment with CEE alone showed a trend for an increased incidence of probable dementia and mild cognitive impairment, although this did not reach statistical significance (Espeland et al., 2004; Shumaker et al., 2004). An ancillary study to the WHI testing more specific cognitive functions, the WHI Study of Cognitive Aging (WHISCA), reported that combination CEE+medroxyprogesterone therapy had a negative effect on verbal memory and a trend for positive effects on figural memory in women 65 and over free of probable dementia (Resnick et al., 2006). Together, the clinical studies indicate that CEE-containing therapy can result in both beneficial and detrimental actions on cognition in the aging brain.
Rodent models can provide insight into cognitive effects of HTs, and allow evaluation of correlative brain changes. Such studies enable excellent experimental control by obviating issues impacting HT use in women, such as socioeconomic status and education, as well as factors potentially affecting cognitive efficacy of HT such as time after ovarian hormone loss and age (Sherwin, 2006). The estrogen 17 ß-estradiol has been shown to benefit spatial working and reference memory in young rodents (Bimonte and Denenberg, 1999; Daniel et al., 1997; Daniel et al., 2005; El-Bakri et al., 2004; Fader et al., 1999; Feng et al., 2004; Hruska and Dohanich, 2007; Korol and Kolo, 2002; Luine and Rodriguez, 1994), as well as in middle-aged rodents (Bimonte-Nelson et al., 2006; Markham et al., 2002; Talboom et al., 2008).
Abundant evidence suggests that learning and memory is mediated, in part, by the cholinergic system. Supporting data include research showing that basal forebrain (BF) cholinergic lesions impaired acquisition of a delayed match-to-position (DMP) T-maze task (Gibbs, 2002, 2007), and that DMP performance correlated with increased choline acetyltransferase (ChAT) activity in the hippocampus and frontal cortex (Gibbs, 2002). This link between cholinergic neurons, neurochemistry and cognitive performance might be relevant to estradiol's effects during cognitive testing, as estradiol treatment potentiated increases in hippocampal acetylcholine levels during maze learning (Marriott and Korol, 2003), and estradiol induced memory improvements in animals with intact BF cholinergic neurons, but not with BF cholinergic lesions (Gibbs, 2002, 2007). Further, estradiol increases the number of ChAT-immunoreactive (IR) neurons in the BF medial septum (MS) and vertical diagonal band (VDB) in young Ovx rats (Gibbs, 1997; Gibbs and Pfaff, 1992), and increases the number and size of MS ChAT-IR neurons in middle-aged mice (Granholm et al., 2002). Estradiol administration also protects against the cholinergic challenge of scopolamine-induced amnesia in young and middle-aged rats (Dohanich et al., 1994; Fader et al., 1998; Fader et al., 1999; Packard and Teather, 1997; Savonenko and Markowska, 2003).
Estradiol is the primary estrogen used to test cognitive effects of HT in animal models; CEE has not been thoroughly evaluated. Estradiol is the most potent naturally-circulating estrogen, followed by estrone and estriol, in order of receptor affinity (Kuhl, 2005; Sitruk-Ware, 2002). CEE contains sulfates of at least ten estrogens. Only two of these, estrone and estriol, are found naturally in women; all others are unique to horses (Kuhl, 2005). CEE is over 50% estrone sulfate, and contains only trace amounts of estradiol; after metabolism, the resulting biologically active circulating hormones are primarily estrone and estradiol (Bhavnani, 2003; Sitruk-Ware, 2002). Because, after CEE treatment, other weaker estrogens are present that could alter efficacy of the resulting circulating levels of estradiol (Kuhl, 2005), the animal studies done thus far testing the cognitive and cholinergic effects of estradiol cannot be generalized to potential effects of CEE.
The goal of the current study was to determine whether CEE could positively impact cognition and the cholinergic system in middle-aged rodents, while controlling for factors possibly influencing HT outcome such as Ovx duration before treatment and age, and obviating clinical variables such as socioeconomic status and education. We evaluated whether CEE influenced learning, spatial reference and working memory, including after delay and interference challenges in order to better define parameters impacted by CEE-treatment. We also investigated whether CEE affects the cholinergic system by evaluating response to pharmacological challenge during memory testing, as well as assessing number of ChAT-IR neurons in the MS and the VDB of the BF. These endpoints were chosen because in the rat model estradiol treatment after Ovx impacts spatial working and reference memory maze performance (Bimonte-Nelson et al., 2006; Bimonte and Denenberg, 1999; Daniel et al., 1997; Daniel et al., 2005; El-Bakri et al., 2004; Fader et al., 1999; Feng et al., 2004; Hruska and Dohanich, 2007; Markham et al., 2002; Talboom et al., 2008), protects against pharmacological cholinergic challenge during maze testing (Dohanich et al., 1994; Fader et al., 1998; Fader et al., 1999; Gibbs et al., 1998; Packard and Teather, 1997; Savonenko and Markowska, 2003), and increases the number of ChAT-IR neurons in the basal forebrain (Gibbs, 1997; Gibbs and Pfaff, 1992; Granholm et al., 2002).
Materials and Methods
Subjects
At the start of the experiment there were 33 thirteen month old Fischer-344 female rats born and raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). Prior to surgery, rats were acclimated for several weeks, and were pair housed with an identical treatment assigned cage-mate. Animals had exposure to food and water ad-lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by the local IACUC committee and adhered to NIH standards.
Surgery and hormone administration
Forty-two±2 days before behavioral testing ensued, all rats received Ovx. Rats were anesthetized with an intraperitoneal injection of a cocktail of 70 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA)/and 6 mg/kg xylazine (Lloyd Laboratories, Shenandoah, IA, USA) and acepromazine 10mg/ml, Vedco Inc., St Joseph MO 64507. After bilateral dorsolateral incisions the ovaries and tips of uterine horns were ligatured and removed. The muscle was then sutured and the skin stapled. Twenty-five±3 days after surgery rats began hormone/vehicle administration after random assignment. One subcutaneous injection was given for two consecutive days followed by two days off, a pattern repeated throughout the study. Rats received either chronic cyclic treatment with vehicle injection (sesame oil, Ovx-Oil) or one of the following doses of injectable CEE prescribed to women (Wyeth Pharmaceuticals Inc., Philadelphia, PA) dissolved in sesame oil: 10 (Ovx-CEE Low), 20 (Ovx-CEE Medium) and 30 μg (OvxCEE High). The doses used in the current study were based on the daily 0.625 mg CEE dose commonly taken by women, and used in the WHIMS. Since no similar study had been done in the rodent, we extrapolated the dose to be used in our animal model as follows. We used the average female weight of 70 kg (www.halls.md) for calculations to determine mg drug/kg body weight for the 0.625 dose, which resulted in 0.00893 mg drug/kg body weight woman. Estimating dose based on a 220 gram rat, this resulted in approximately 20 micrograms daily CEE. This was used as the medium dose, with 10 micrograms lower and higher for the low and high groups, respectively. Behavioral testing began 18 days after hormone administration was initiated. The testing schedule is outlined in Table I. The last treatment injection was administered in the morning, 2 days prior to sacrifice.
Table I.
Behavioral testing timeline (info = information, OR = original room, NR = new room).
| Maze | Day of Experiment |
|
|---|---|---|
| Morris Maze plus probe trial | Days 1-5 | |
| Plus Maze | ||
| Initial learning (6 trials/day) | Days 6-10 | |
| 4-hour delay between trials 5-6 | Day 11 | |
| Baseline | Day 12 | |
| 6-hour delay between trials 5-6 | Day 13 | |
| 4-hour delay between trials 1-2 | Day 14 | |
| 6-hour delay between trials 1-2 | Day 15 | |
| 7-hour delay between trials 1-2 | Day 16 | |
| Probe trial | Day 17 | |
| Baseline | Day 18 | |
| First Interference Day: info trial OR, info trial NR, test trial OR, test trial NR |
Day 19 | |
| Second Interference Day info trial OR, info trial NR, test trial OR, 45-m delay, test trial NR |
Day 20 | |
| Third Interference Day 3 trials OR, 3 trials NR, test trial OR |
Day 21 | |
| Baseline in new room | Day 22-33 | |
| Scopolamine challenge in new room | Day 34 | |
| Baseline in new room | Day 35 | |
| Morris Maze retention | Day 36 | |
Vaginal smears and uterine weights
To confirm Ovx and CEE treatment, vaginal smears were taken daily for 17 days, beginning one day prior to the first CEE injection. Smears were classified as either proestrus, estrous, metestrus or diestrus (Goldman et al., 2007). Before CEE injection, all animals were in diestrus. Six days after the first CEE injection, all CEE-treated animals showed estrous smears. To examine CEE effects on uterine tissues, an additional 10 Ovx animals given injection were sacrificed at this same timepoint. The injection regimen was identical to that used in the behavior study. Six days after the initial injection of either oil (n=3), CEE-Medium (n=3) or CEE-High (n=4), uterine tissues were collected. After anesthesia, a ventral incision was made in the abdominal region, and the uterus was cut above the junction with the cervix and on the uterine horn below the ligature remaining from Ovx (Ashby et al., 1997). Uteri were trimmed of all visible fat and were immediately weighed to obtain wet weight.
Morris water maze
We tested spatial reference memory via the Morris water maze (MWM), a test we and others have shown to be enhanced by estradiol replacement given to older Ovx rats (Bimonte-Nelson et al., 2006; Markham et al., 2002). A video camera and tracking system (Ethovision, Noldus Instruments) tracked and analyzed each rat's path. The MWM consisted of a hidden platform (10 cm wide) in a round tub (188 cm diameter) filled with water made opaque with black non-toxic paint (Bimonte-Nelson et al., 2006; Morris et al., 1982). The rat was placed in the maze from any of four locations (North, South, East, or West) and had 60s to locate the platform which remained in a fixed location (Northeast quadrant). After 15s on the platform, the rat was placed into its heated cage until the next trial. Rats were given 5 trials a day/5 days. Animals were tested in squads (8-9 rats in each squad) so that trial 1 was completed for each rat in the group, then trial 2, etc., as done previously (Hyde et al., 2002; Schrott et al., 1992; Stavnezer et al., 2002). The inter-trial-interval (ITI) was 5-8m. Distance and Speed (Distance/Time) were the dependent variables.
To evaluate whether rats localized the platform to the spatial location, after the test trials a 60s probe trial was given whereby the platform was removed. Percent of total distance in the platform quadrant (NE, or target) was compared to the quadrant diagonally opposite (SW). To determine whether CEE impacted ability to locate the exact platform location in space, we also quantified platform crossings. Quantifying behavior by using these two measurements (quadrant preference and platform crossings) gives information about the animals' ability to navigate through the environment in search for the goal, which is located in a specific place in space. Evaluating localization to the platform quadrant is indicative of the ability to locate the general vicinity of the platform, while assessment of platform crossings yields insight into knowledge of the specific platform location. Furthermore, in this and other studies we have noted that during the initial portion of the probe trial some animals actively cross the platform location while others do not. As time commences, animals decrease platform crossings and swim elsewhere. To determine whether CEE impacts localization of the platform and behavior across trial time, we blocked platform crossings into the first half of the probe trial (0-30 seconds) and the second half of the probe trial (31-60 seconds).
After completion of all behavioral tests (5 weeks after initial MWM testing), rats were again tested on the MWM for retention and relearning of the maze. For this retest, animals received 7 trials within one maze session.
Delayed-match-to-sample plus maze
Two days after initial MWM testing concluded, we tested spatial working memory and short-term memory retention using a win-stay delayed-match-to-sample (DMS) place learning task in a new room. The black plexiglass maze (each arm was 38.1cm × 12.7cm) was filled with water made opaque with black non-toxic paint, and had a hidden platform at the end of one of the four arms. Start location varied across trials, and the platform location changed every day. Within a day, the platform remained in the spatial location. Rats received six consecutive trials within a daily session. Trial one was the information trial informing the animal where the platform was for that day's session, trial two was the working memory test trial, and trials three through six were recent memory test trials (Frick et al., 1995). Rats were given 90s to find the platform. Once on the platform, the rat remained on it for 15s, followed by placement into a heated cage for a 30s ITI. An arm entry was counted when the tip of a rat's snout reached a mark delineated on the outside of the arm and not visible from the inside of the maze (11 cm into the arm). Entry into any non-platformed arm was counted as an error. The total number of errors was analyzed for each trial.
Delay testing
After 5 days of testing at a 30s ITI, increased time delays were given (Table I). Four- and six- hour delays were initiated between trials 5 and 6, to assess retention of recent memory. Since these delays did not influence performance, we then gave delays between trials 1 and 2 after only one exposure to the correct platform location. Indeed, since the second trial is the first trial to test recall of the updated information (working memory), the next series of delays were given between trial 1 and trial 2 to determine whether the increasing delays impacted working memory. Using this procedure, delays of 4-, 6- and 7- hours were given on days 14, 15 and 16, respectively. On day 17, after trial 2, rats were given a probe trial whereby the platform was removed. This was initiated to confirm that animals localized the spatial location of the platform, and that an animal's choice was not due to platform visualization or another strategy.
Interference testing
Some animal research has imposed task-related interference on passive avoidance, operant behavior and visual discrimination tasks (Dunnett and Martel, 1990; Dunnett et al., 1990; Winocur, 1984, 1985, 1988). Classically, interference trials have been administered to people to examine processes of forgetting in short-term memory, which is driven by limited capacity (Underwood, 1957). We performed a series of interference manipulations to examine whether CEE influences performance after this mnemonic challenge (Table I). On days 19, 20 and 21, after the information trial in the original room, rats received interference trial/s in a new room on an identical maze, and were then brought back to the original room for the test trial. Performance on the test trial in the original room was a measure of retroactive interference. On days 19 and 20, each rat received an information trial in the original room, was immediately transported to the new room for one interference trial, and then was transported back to the original room for the test trial. The last interference test, on day 21, tested whether susceptibility to proactive interference, or to more retroactive interference trials, was influenced by CEE treatment. Rats were administered 3 consecutive trials in the original room (this was the proactive interference), followed by 3 consecutive trials in the new room (here the first trial was the “information trial” and the second trial was the proactive interference “test trial”). Next, the rat received the test trial in the original room (this was the retroactive interference “test trial”). Three trials were given in the new room so that there were three trials of retroactive interference (versus only one trial of retroactive interference on days 19 and 20) before the retroactive interference test trial in the original room.
Scopolamine challenge
After twelve days of baseline testing, when asymptotic performance was reached in the new room (Table I), rats received a 0.2 mg/kg IP dose of scopolamine (Sigma-Aldrich Inc., St Louis, MO) dissolved in saline 20 minutes prior to the information trial. To ensure drug clearance, baseline maze testing was re-established 24 hrs after scopolamine challenge.
Basal forebrain ChAT immunostaining and quantification
Animals were sacrificed 3 days after scopolamine challenge. All animals were sacrificed in the same day, with researchers blinded to treatment group assignment. Animals were decapitated under isofluorane anesthesia. While one researcher collected blood via cardiac puncture, the other rapidly blocked the anterior portion of the brain containing the BF. This large block of tissue was post fixed in 4% paraformaldehyde in phosphate-buffer solution (PB, pH 7.4). After 48 hours the brains were moved to PB until sectioning. Brains were sectioned (plates 1-25 Paxinos and Watson, 1998) on a Vibratome (Vibratome 3000) in phosphate-buffered saline (PBS; pH 7.4) at 40 microns throughout the BF and collected for immunohistochemistry (Granholm et al., 2002). Every 3rd section through the BF was selected for choline acetyl-transferase (ChAT) antibody stain and incubated for 15 minutes in a 0.03% Triton (Triton 100X) in PBS (PBST) to permeabilize the tissue. The tissue was then blocked, by incubating tissues at room temperature (RT) for 30 min in a blocking solution (BKS) containing 0.03% PBST and 0.03% heat inactivated horse serum (Fischer). Three PBS washes (3 min each) were then done. The primary antibody, goat Anti-ChAT (1:1000, Chemicon), was added to each well, and sections were incubated overnight at 4° C on a Rocker II (Boekel Scientific, Feasterville, PA). Next, sections were washed in PBS three times (3 min each) followed by immersion in the secondary antibody solution (1: 200 biotinylated Donkey anti-Goat IgG, Vector) and BKS for 45 min on a Titer Plate Shaker (Barnstead International, Dubuque, IO) at RT. Sections were washed three times in PBS (3 min each), and then placed into an 11% methanol and 1% H202 (Fischer) in PBS solution for 30 minutes on a Titer Plate Shaker to quench endogenous peroxidase activity. After three washes in PBS (3 min each), ABC reagent (Vector) was added to each well and incubated for 45 min at RT on a Titer Plate Shaker. Sections were washed three times in PBS (3 min each), and were then incubated with DAB solution (Vector). After the desired color was achieved (dark purple), brain sections were washed three times in PBS (3 min each), mounted on subbed slides, air dried, dehydrated and cover slipped with Permount. Each group was equally represented in each round of staining, to avoid group inter-variability in staining. Further, control procedures were run excluding primary and secondary antibodies. Exclusion of the primary antibody resulted in no cell staining, and exclusion of secondary antibody resulted in lack of DAB color development.
Basal forebrain ChAT-IR image analysis
ChAT immunohistochemically stained cells were quantified in the BF. One experimenter, blind to treatment group status of the animals, performed all image analysis procedures. Images were acquired using PictureFrame software (MicrobrightField, Burlington, VT) from a CX9000 camera (MicrobrightField, Burlington, VT) coupled to an Olympus BX51 microscope. A 2X objective was used to capture images. Captured images for each section where then manually counted using the Point Picker plugin under NIH ImageJ software (Rasband, 1997-2004). Cell counts were then exported to Excel (Microsoft, Redmond, WA) for analysis. Two sections per animal were quantified, corresponding to plates 13-17 from Paxinos and Watson (1998), similar to prior publications (Gibbs, 2002; Yamamoto et al., 2007). ChAT-IR cells were counted in the MS and the VDB. Counts from the two sections were averaged to yield one value per basal forebrain region per animal.
Hormone assays
Serum levels of estradiol and estrone were determined by liquid chromatography-Tandem Mass Spectrometry (LC/MSMS) according to previously published methods (Nelson et al., 2004). After dansyl chloride derivatization, samples were separated by fast gradient chromatography and then were injected in a tandem mass spectrometer after formation of positive ions with atmospheric pressure chemical ionization. Limits of quantification for estrone and estradiol was 0.2 and 0.5 pg/ml, respectively, with interassay CV's of 15% or less at the concentrations obtained for these steroids.
Statistical analyses
Unless otherwise noted, two-tailed tests were used and alpha was set at 0.05. For behavior assessments, data were analyzed separately for each maze, first with an omnibus repeated measures ANOVA with Treatment as the between variable and Days and/or Trials as the within variable, as appropriate for the specific maze test. This was done to allow interpretation of Days and/or Trials repeated measures effects in the context of potentially complex Treatment interactions. Since our interest was to determine whether each dose enhanced performance relative to the Ovx group, all of our follow-up two-group comparisons were planned. Additional analyses were run to address specific questions about how CEE influenced learning and memory, as follows. MWM overnight forgetting was tested since estradiol has been shown to aid in overnight retention on this task (Bimonte-Nelson et al., 2006; Markham et al., 2002). Overnight forgetting was analyzed by comparing trial 5 of one day to trial 1 of the next day, with these scores as repeated measures. Thus, the analysis had scores from day 1, trial 5 to day 2, trial 1 blocked; from day 2, trial 5 to day 3, trial 1 blocked; from day 3, trial 5 to day 4, trial 1 blocked. The analysis therefore consisted of a 1 between (Treatment), 2 within (overnight forgetting block and trial) repeated measures ANOVA. A significant increase in scores from trial 5 to trial 1 of the next day is indicative of forgetting. Because there were interactions with Treatment, follow up analyses were used analyzing each group alone (repeated measures ANOVA with overnight forgetting block and trial) to determine which group/s showed overnight forgetting. For MWM probe trial data, percent of total distance in the previously platformed (target) quadrant was compared to that in the diagonally opposite quadrant by using repeated measures (Quadrant) ANOVA. Rats that learned the platform location were expected to spend the greatest percent distance in the target vs. opposite quadrant. To evaluate whether CEE influenced search patterns, the probe trial was divided into Block 1 (first 30 seconds) and Block 2 (second 30 seconds), tested with a repeated measures (Block) ANOVA. To test MWM long-term retention, we compared distance on the final trial of testing (trial 5, day 5) to the retention trial (trial 1, day 36) using repeated measures (Trial) ANOVA.
Days 1-4 of the DMS plus maze were considered acquisition. All groups performed equally on DMS day 5; thus, this was considered baseline for delay and interference trials. Performance on the test trial after the delay or interference was compared to that same trial of the baseline score from day 5 using a repeated measures of Day (test trial on manipulation day vs. test trial at baseline day) ANOVA. For the DMS probe trial, entries into the arm that previously contained the platform were compared to average entries into the other 3 arms using a 2 within (arm entries into the platform arm versus other arms) repeated measures ANOVA. Performance after scopolamine was compared to baseline performance from the previous day (day 33).
Uterine weight and ChAT analyses were assessed using a 1 between (Treatment) ANOVAs. A priori planned comparisons using t-tests were conducted as follow-up analyses to determine significance of two group comparisons (Keppel and Wickens, 2004). After data transformation due to a skewed distribution (described below), trend analyses were used to analyze serum estradiol and estrone levels followed by planned t-tests for two-group comparisons.
We also ran a series of multiple regression analyses to examine the relationship between number of ChAT neurons in each basal forebrain region, serum levels of estradiol and estrone, and memory variables (alpha was set to < .01). Estradiol, estrone or number of VDB or MS ChAT-IR neurons were the predictor variables in the model, and the following served as outcome variables: MWM distance scores collapsed across all trials/days, MWM overnight forgetting scores, MWM block 1 probe trial platform crossings, DMS errors for baseline days, DMS working memory errors after each delay.
Results
Vaginal smears and uterine weights
Confirming complete Ovx, before CEE all animals showed diestrus smears, with overall few cells that were primarily leukocytes. By day 6 after CEE injections, all CEE-treated rats exhibited estrous smears as noted by an abundance of cornified cells. All Ovx-Oil rats continued diestrus smears. At the timepoint where all animals showed estrous smears due to CEE treatment (six days after CEE injections began), we sacrificed a subset of animals from the Ovx-CEE-Medium and Ovx-CEE-High groups to determine uterine weight, a reliable measure of peripheral estrogen stimulation. Mean±SE uterine weights were: Ovx-Oil (.17±.03), Ovx-CEE-Medium (.34±.04) and Ovx-CEE-High (.35±.03). The ANOVA analyzing the subset of animals yielded significant omnibus effects on uterine weight including all treatment groups [F(2,7)= 8.98; p< .01]. Further analyses revealed that uterine weight increased after medium-dose CEE treatment [Ovx-Oil vs. Ovx-CEE-Medium: F(1,4)=12.81, p < .05] and high-dose CEE treatment [Ovx-Oil vs. Ovx-CEE-High: F(1,5)=16.64, p < .01].
Morris water maze
MWM Distance
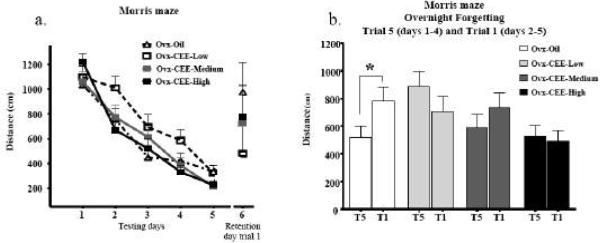
Figure 1a shows the mean distance to platform scores±SE for each treatment group across testing days. For the repeated measures omnibus ANOVA including all days and trials, there was no main effect of Treatment, nor did Treatment interact with Days or Trials. The overnight forgetting analysis with all groups showed a Treatment × Trial interaction [F(3,29)=4.25; P =.01]. The only group that showed significant forgetting was the Ovx-Oil group, as this was the only group that had a significant increase in swim distance from trial 5 to trial 1 of the next day [F(1,8)=6.96; p < .05] (Figure 1b).
Fig 1.
a) Mean ± SE distance to platform (cm) for each treatment group shown for the 5 testing days collapsed across trials, and for trial one of the retention day. b) Mean ± SE distance to the platform (cm) for each treatment group for trial 5 (days 1-4) to trial 1 of the next day (days 2-5). While the three CEE groups did not have a significant increase in distance scores from trial 5 to trial 1, the Ovx-Oil group did. Thus, all groups receiving CEE remembered the platform location overnight while the Ovx-Oil group did not. *p < 0.05.
MWM Swim Speed
For swim speed, there was no main effect of Treatment, while there was a Treatment × Day interaction [F(12,116)=1.96; < .05]. Each day was analyzed separately to further evaluate this interaction. There were no main effects or interactions with Treatment for any day, indicating no meaningful effect of Treatment on swim speed.
MWM Probe trial
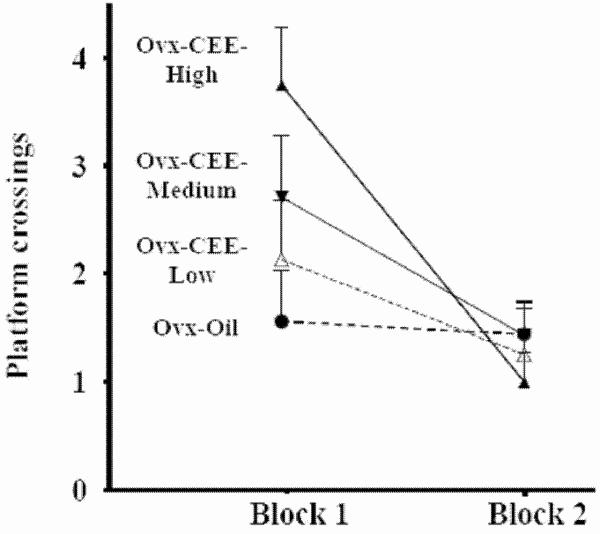
During the MWM probe trial, there was a Quadrant main effect [F(1,28)=214.81; p < .0001], and a null Treatment × Quadrant interaction (p=.88). Platform crossings yielded a Treatment × Block interaction [F(1,3)= 3.17; p< .05] with a CEE dose-dependent increase in platform crossings on Block 1, the first 30 seconds of the probe trial, and all groups making few crossings on Block 2 (Figure 2). On Block 1, the Ovx-CEE-High group made more platform crossings relative to the Ovx-Oil group [F(1,15)= 9.64; p< .01]; no other group differed from the Ovx-Oil group.
Fig 2.
Number of platform crossings±SE for each treatment group across the two 30 sec blocks during the probe trial. On Block 1 (0-30 seconds), the Ovx-CEE-High group made more platform crossings relative to the Ovx-Oil group. On Block 2 (31-60 seconds), there were no group differences.
Retention effects: Morris water maze
CEE treatment had no impact on retention or relearning. Indeed, for the retention test trial (trial 1 of the retest, Figure 1a) and relearning trials (trials 2-7, data not shown), there were no main effects of Treatment nor Treatment × Trial interactions. For the repeated measures ANOVA including the final trial of the original session and the first trial on the retention day, there was a Day main effect [F(1,28)= 15.07; p< .001]. The lack of Treatment × Day interaction indicated that all rats swam a greater distance on the retention day relative to the last test day, suggesting forgetting, regardless of treatment.
Delay-match-to-sample plus maze
Maze acquisition: Days 1-4
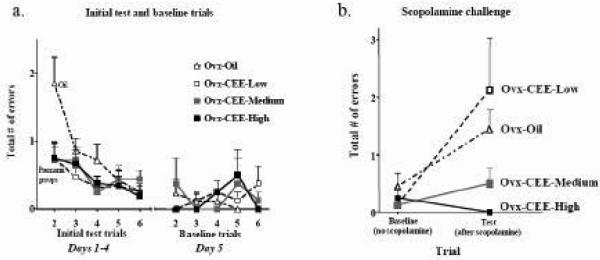
All three CEE doses enhanced performance on days 1-4, when the ITI was 30 seconds [ANOVA with all groups, Treatment × Trial interaction: F(15,145)= 2.15; p=.01; left half of Figure 3a]. For each Ovx-Oil vs CEE group comparison, there was a Treatment × Trial interaction [Ovx-Oil vs Ovx-CEE Low (F(5,75)= 3.72; p=.005), Ovx-Oil vs Ovx-CEE Med (F(5,75)= 2.29; p= .05) and Ovx-Oil vs Ovx-CEE High (F(5,75)= 3.98; p< .01)]. For each Ovx-Oil vs CEE group comparison, there was also a Treatment main effect for trial 2 for days 1-4, with the CEE-treated groups showing fewer errors on trial 2, the trial that represents spatial working memory performance [Treatment main effect: Ovx-Oil vs Ovx-CEE Low (F(1,15)= 4.89; p<.05), Ovx-Oil vs Ovx-CEE Med (F(1,15)= 5.79; p< .05) and Ovx-Oil vs Ovx-CEE High (F(1,15)= 4.51; p= .05); left half of Figure 3a]. Furthermore, after only one exposure to the new platform location, all three CEE-treated groups remembered the spatial location of the platform while the group receiving oil did not. Specifically, the Ovx-CEE-Low [F(1,7)= 50.73; p< .001], Ovx-CEE-Medium [F(1,7)= 16.45; p< .005], and Ovx-CEE-High [F(1,7)= 81.22; p< .0001] groups each decreased errors from the Information Trial (trial 1) to the Test Trial (trial 2), while the Ovx-Oil group did not (data not shown). By day 5, there was a null Treatment × Trial interaction (p=0.61), indicating that Ovx animals eventually learned to perform as well as the CEE treated rats, as shown for the Low [F(5,75)= 1.54; p= .19], Medium [F(5,75)= 0.21; p= .96] and High [F(5,75)= 0.67; p=.65] doses (right half of Figure 3a). Thus, performance on day 5 was utilized as the baseline score for delay and interference manipulations. In addition, for Days 1-4 and Day 5 there were no Treatment effects on the first daily exposure to the maze to gain information on where the platform was on that day, e.g. trial 1 (data not shown).
Fig 3.
a) Mean±SE total number of errors for each treatment group across the test trials (trials 2-6) for days 1-4 and baseline day 5. CEE-treated animals showed enhanced performance on trial 2 relative to Oil-treated animals for days 1-4. There were no group differences on baseline day 5. b) Mean±SE total number of errors for each treatment group after scopolamine (0.2 mg/kg, IP) challenge. The Ovx-CEE-High group made fewer errors compared to the Ovx-Oil group on the test trial after scopolamine challenge. The Ovx-Oil and Ovx-CEE-Low groups had increased errors after the scopolamine challenge relative to baseline. In contrast, the Ovx-CEE-Medium and Ovx-CEE-High groups showed no difference relative to baseline, suggesting maintained performance after cholinergic challenge. It is noteworthy that all Ovx-CEE-High animals made zero errors after scopolamine challenge.
DMS Delay Trials
CEE treatment did not influence retention over any delay manipulation. For both the 4-hour delays given either between trials 5 and 6 (on day 11) or between trials 1 and 2 (on day 14), there was a main effect of Day [Fs(1,29)= 5.12 and 6.26; ps < .05], respectively, indicating that rats made more errors on the trial after the delay as compared to that same trial on baseline day. There were no interactions with Treatment. There were no main effects or interactions for the 6-hr delay given between trials 5 and 6 or for the 6- or 7-hr delay given between trials 1 and 2.
DMS Interference Assessments
There were no significant Treatment × Day interactions for any interference manipulation, although there were Day main effects indicating an overall impact of interference (data not shown). Specifically, for the first interference test (day 19), one trial of interference given between the information and test trial impaired performance [Day effect: F(1,29)= 15.16; p< .0005]. For the second interference day (day 20), there were no significant main effects or interactions indicating all groups learned to handle this one-trial interference challenge. For the third interference day (day 21), three interference trials between trials 1 and 2 negatively impacted performance in the original room, suggesting effects of retroactive interference [Day effect: F(1,29)= 4.34; p< .05]. Three trials given before the information trial in the new room also negatively impacted test trial performance in the new room, suggesting effects of proactive interference [Day effect: F(1,29)=4.66; p < .05].
DMS Probe trial
During the probe trial, rats made more entries into the previously platformed arm vs the other 3 arms [Arm Choice main effect: F(1,29)= 12.95; p< .001; data not shown]. The null Trial × Treatment interaction indicated that rats located the correct spatial location and entered the arm corresponding to that location, even though the platform was not there, regardless of treatment group.
DMS Scopolamine challenge
The scopolamine challenge yielded a significant Treatment × Trial interaction for the omnibus ANOVA with all treatment groups [F(3,29)= 3.87; p< .05; Figure 3b]. This was a dose-dependent effect, with the low CEE dose yielding no protection, the medium dose yielding marginal protection, and the high dose showing complete protection against scopolamine-induced amnesia. Specifically, the Ovx-CEE High group made fewer errors after the scopolamine challenge on trial 2 as compared to the Ovx-Oil group [F(1,15)= 6.04; p< .05], suggesting that the high dose of CEE protected against the amnesic effects of scopolamine on working memory. Remarkably, every animal treated with high dose CEE remained at zero errors even after the scopolamine challenge. The effects of scopolamine were absent by the following day (day 35), as there were no main effects of Treatment nor a Treatment × Trial interaction.
ChAT positive neurons in the basal forebrain, and relationships with memory
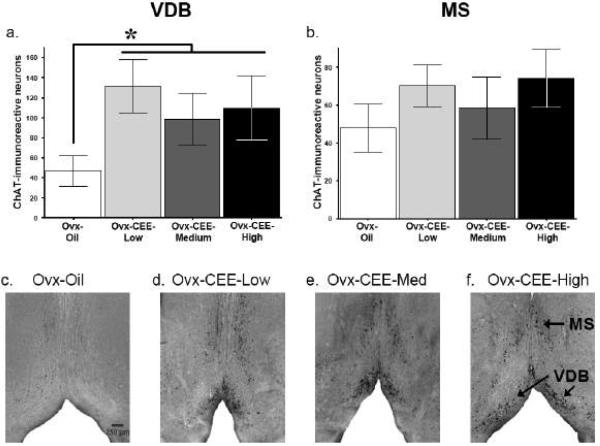
Mean±SE ChAT-immunoreactive neurons for the VDB and MS of the BF are shown in figures 4a and 4b, respectively, for each treatment group. Photomicrographs representing each group are also shown in figures 4c,d,e and f. For the ChAT-immunoreactive cell count analysis, all CEE-treated animals were collapsed and compared to the Ovx-Oil group since there were no group differences between CEE-treated groups. For the VDB, CEE-treated animals had a higher number of ChAT-immunoreactive neurons relative to the Ovx-Oil group [t(22)=2.37, p <.05]. No significant effects for the MS, nor significant regression analyses between ChAT-immunoreactive neurons and memory outcome or hormone variables, were found.
Fig 4.
Mean±SE ChAT-immunoreactive neurons in the VDB (a), and MS (b) of the BF. All CEE-treated groups were collapsed and compared to the Ovx-Oil group. CEE-treated animals had more ChAT-immunoreactive neurons relative to the Ovx-Oil group *ps < 0.05. Photomicrographs show ChAT-immunoreactive neurons in sections through the MS and VDB of the BF from animals in the Ovx-Oil (c), Ovx-CEE-Low (d), Ovx-CEE-Medium (e), Ovx-CEE-High (f).
Serum estradiol and estrone levels, and relationships with memory
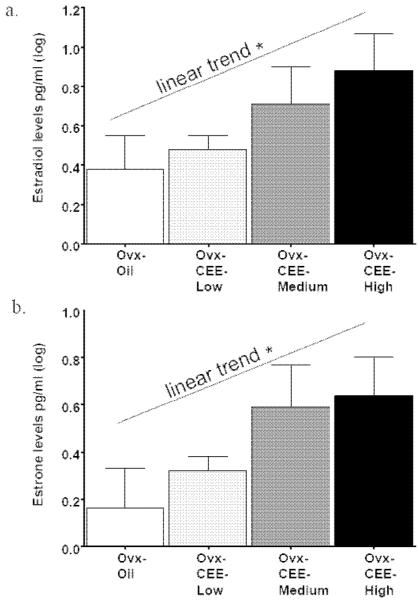
Blood was taken at sacrifice, two days after CEE injection. Mean±SE levels of estradiol and estrone, for each treatment group respectively, were: Ovx-Oil (4.14±2.28, 2.38±.81), Ovx-CEE-Low (3.30±.50, 2.25±.32), Ovx-CEE-Medium (9.51±5.56, 6.33±3.23) and Ovx-CEE-High (14.11±5.53, 7.67±3.66). Estradiol and estrone levels were positively skewed; thus, a log transformation was used to normalize scores prior to analysis (Tabachnick and Fidell, 2001). Linear trend analysis showed a dose-dependent increase in estradiol [F(1,28)=6.69; p = .015; Figure 5a] and estrone [F(1,28)=6.76; p = .015; Figure 5b] as CEE treatment progressed from none (Oil) to Low, Medium and High. The Ovx-CEE-High group had higher levels of both estradiol [t(14)=2.01; p < .05] and estrone [t(14)=1.94; p < .05], as compared to the Ovx-Oil group (one-tailed). There were no significant regression analyses between levels of estradiol or estrone and memory outcome variables.
Fig 5.
Mean±SE serum estradiol (a), and estrone (b), levels for each treatment group. Log transformation was performed due to a skewed distribution. A significant linear trend for both hormones was evident (*ps = .015), showing that estradiol and estrone levels increased with treatment. In addition, the Ovx-CEE High group had higher levels of both estradiol and estrone compard to the Ovx-Oil group (ps < 0.05).
Discussion
In the current study, CEE enhanced learning and memory, prevented scopolamine-induced amnesia and increased the number of ChAT-immunoreactive neurons in the VDB of the BF in middle-aged Ovx rodents. Mnemonic benefits of CEE-treatment spanned some, but not all, evaluated memory measures. Peripheral endocrine responsiveness and conversion to bioactive estrogens after CEE treatment was confirmed via vaginal smears, uterine weights and serum levels of estradiol and estrone.
CEE treatment enhances maze performance
For MWM, high-dose CEE decreased overnight forgetting of the platform location, corresponding to findings with estradiol replacement in Ovx young and middle-aged animals (Bimonte-Nelson et al., 2006; Markham et al., 2002; Talboom et al., 2008). MWM probe trial data indicated that although all groups localized to the platform quadrant, the Ovx-CEE-High group made more platform crossings relative to the Ovx-Oil group during the first 30 seconds, suggesting enhanced spatial localization of the platform in CEE-treated rats. Thus, while all groups remembered the general platform vicinity, the Ovx-CEE-High group had better recall of the exact platform location indicating a superior ability to efficiently navigate through the environment.
On DMS, all CEE-treatments enhanced acquisition. The effects were specific to trial 2, which was the only trial that needed information to be updated and therefore can be considered an assessment of working memory (Frick et al., 1995; Tulving and Craik, 2000). For the DMS task used in this study, animals could not use a response strategy to solve the task effectively; the start location was varied across trials while the goal arm remained in the same place in space on a given day. Thus, the observed findings of CEE enhancements on learning the DMS task could be due to an enhanced ability to use a place strategy, as observed previously with estradiol (Korol and Kolo, 2002). Importantly, the DMS probe trial confirmed that all groups in our study utilized spatial cues to solve the task. Place learning likely involves the cholinergic system. BF cholinergic lesions increased perseveration of a response strategy over a place strategy, impairing DMP acquisition in males and Ovx female rats (Fitz et al., 2008; Gibbs and Johnson, 2007). Additionally, hippocampal acetylcholine levels were greater prior to and after performing a reference memory task on a T-maze in animals that used a place strategy relative to animals that used a response strategy (McIntyre et al., 2003). On a spatial working memory task, animals showed increased hippocampal acetylcholine levels during initial testing blocks when a place strategy was used, yet animals showed decreased acetylcholine levels when they shifted to a response strategy (Pych et al., 2005). Based on these lines of evidence, it is tempting to speculate that CEE enhanced DMS acquisition by improving the ability to use a place strategy, and that these effects were at least partially mediated by enhanced cholinergic function resulting from an increased number of VDB neurons with levels of ChAT sufficient to immunohistochemically stain positive. Indeed, it is noteworthy that all three doses of CEE enhanced learning the DMS task, and that all three doses also increased number of ChAT positive neurons in the VDB of the BF. After task acquisition, CEE dose-dependently prevented scopolamine-induced amnesia, again linking the cholinergic system to CEE-induced mnemonic effects. Remarkably, all Ovx-CEE-High rats had zero errors after scopolamine, indicating complete protection from amnesia induced by muscarinic receptor antagonism. CEE-treatment did not protect against deficits due to time-delays nor retroactive or proactive interference. These findings concur with findings from the WHISCA showing that long-term CEE+medroxyprogesterone yielded null effects after interference, and only a trend for decreased performance after delays (Resnick et al., 2006).
Many animal studies have shown maze learning enhancements after estradiol treatment. In accordance with our findings using CEE, in middle-aged rats estradiol treatment prevented overnight forgetting on the spatial reference memory Morris water maze (Bimonte-Nelson et al., 2006; Markham et al., 2002; Talboom et al., 2008) and enhanced spatial working memory (Daniel et al., 2006). Similarly, estradiol-induced improvements were seen in young animals for spatial reference memory (El-Bakri et al., 2004; Feng et al., 2004) and working memory, including enhancements on spatial working memory task acquisition as we found herein (Bimonte and Denenberg, 1999; Daniel et al., 1997; Daniel et al., 2005; Fader et al., 1999; Hruska and Dohanich, 2007; Luine and Rodriguez, 1994). Thus, the current findings allow speculation that CEE may be effective, at least partially, due to estradiol increases. Further, a one-time CEE injection (approximately 125 micrograms/200 gram rat, 12x higher than the currently used lowest dose) enhanced non-spatial working memory object recognition (Walf and Frye, 2008). While it is unclear whether object recognition effects persist with lower doses, the findings indicate that CEE benefits might also translate to non-spatial tasks, in accordance with estradiol-induced benefits in object recognition (Luine et al., 2003; Scharfman et al., 2007).
Recent studies indicate that variables likely influencing estrogen's cognitive effects include time after ovarian hormone loss and age. Estradiol-treatment administered immediately, but not 5 months, after Ovx enhanced spatial working memory on a land radial-arm maze (Daniel et al., 2006), and estradiol-treatment given immediately or three months after Ovx, but not 10 months after Ovx, enhanced delayed match to position performance (Gibbs, 2000), suggesting estradiol treatment has a critical time-window for maximal cognitive efficacy. We have shown that aged Ovx rats were not responsive to the estradiol regimen that enhanced spatial reference memory in young and middle-aged Ovx rats, demonstrating age by estradiol interactions (Talboom et al., 2008), as seen by others (Foster et al., 2003). Age and a delay between menopause and CEE-treatment may have played a role in the lack of positive cognitive effects of CEE treatment in the WHIMS (see Sherwin and Henry, 2008, for discussion). Women in the WHIMS were 65-79-years old and many had not had hormone therapy previously, and thus likely had undergone ovarian hormone deprivation for many years before study initiation and CEE treatment began (Shumaker et al., 1998). While the current animal study controlled for age (all middle-aged) and time after Ovx before CEE treatment (treatment was initiated 25 days after Ovx), as these factors were held constant, there are likely complex interactions with CEE-treatment to be revealed in future studies. That we now report CEE can enhance multiple cognitive domains in the rodent model allows further manipulation of parameters governing the neural and functional effects of CEE treatment, as well as mechanism of these effects.
CEE treatment influences the cholinergic system: protection against scopolamine-induced amnesia and increases in number of VDB ChAT-IR neurons
Our study implicates the cholinergic system in CEE-induced memory enhancements. CEE protected against scopolamine-induced amnesia and increased the number of ChAT-IR neurons in the VDB of the BF. Studies testing estradiol have also implicated the cholinergic system as one mechanism of the effects. Estradiol protected against scopolamine-induced learning and memory in young (Dohanich et al., 1994; Fader et al., 1998; Fader et al., 1999; Gibbs et al., 1998; Packard and Teather, 1997) and middle-aged (Savonenko and Markowska, 2003) animals. Other studies have shown that estradiol treatment alters multiple markers of ChAT in middle-aged and aged animals, which appears to depend on duration of ovarian hormone deprivation before treatment as well as specific brain region and BF nuclei (Bohacek et al., 2008; Kompoliti et al., 2004; Singer et al., 1998). In young adult Ovx rats, DMP acquisition correlated with increased ChAT activity in two targets of BF cholinergic innervation, the hippocampus and frontal cortex (Gibbs, 2002), and high affinity choline uptake was increased in these regions (O'Malley et al., 1987; Singh et al., 1994). There is additional direct evidence that BF cholinergic neurons, and their projections to hippocampus and frontal cortex, are involved in estrogen's cognitive effects. Estradiol increased ChAT in the horizontal limb (Luine, 1985; Luine and McEwen, 1983; Singer et al., 1998) and in projection sites to hippocampus and cortex (Feng et al., 2004; Luine, 1985; Singh et al., 1994), and restored BF ChAT mRNA expression (Gibbs et al., 1994; McMillan et al., 1996). Also, BF lesions prevented estradiol-enhanced DMP acquisition (Gibbs, 2002, 2007). In the current study, high-dose CEE, specifically, enhanced DMS place learning and prevented anticholinergic-induced amnesia on the working memory trial of this task. The evidence above suggests that the BF may be involved in this process. In postmenopausal women, estradiol attenuated anticholinergic-induced impairment on attention and psychomotor tasks, processes mediated by frontal cortex (Dumas et al., 2006), supporting the tenet that the cholinergic system may be related to estrogenic effects on brain function in women as well. However, long-term treatment (2 years) with CEE alone did not alter BF or hippocampal ChAT or AChE activity in monkeys (Gibbs et al., 2002), similar to long-term estradiol effects (Gibbs, 1997). Further studies evaluating how timing, referring to both ovarian hormone deprivation and CEE-treatment duration, impacts efficacy will help determine replacement parameters for optimal brain function.
Concurrent with findings in aged monkeys that estradiol treatment increased number of ChAT-IR neurons in the VDB (Kompoliti et al., 2004), we found that CEE treatment increased number of ChAT-IR neurons in the VDB in middle-aged Ovx rats. These findings in the VDB concur with other studies giving estradiol treatment to young Ovx animals, although we failed to find significant increases in the MS that have also been reported (Gibbs, 1996, 1997; Gibbs and Pfaff, 1992; Gibbs et al., 1994). One reason for the current discrepant results could be the hormone regimen. Chronic cyclic administration of CEE might have produced fluctuating hormone levels throughout the regimen, and fluctuations in hormone levels have been shown to differentially alter ChAT mRNA in various regions of the BF (Gibbs, 1996). In cycling rats, the highest levels of ChAT mRNA in the MS and striatum occurred during diestrus 1 while highest levels were detected during diestrus 2 in the nucleus basalis magnocellularis (NBM) (Gibbs, 1996). In estradiol-treated rats, increases in the same regions were observed during time-points ranging from 5 to 72 hrs after injections that produced time-dependent fluctuations in estradiol levels (Gibbs, 1996). Estradiol treatment also produced region specific effects within the BF, which vary with dose and duration of treatment (Gibbs, 1997). In particular, in the MS increases in ChAT-IR were observed after one week of estradiol treatment at doses of 2, 10, and 25 μg given every other day, but not at a dose of 100μg or after 2 or 4 weeks of treatment (Gibbs, 1997); Yet, in the NBM, increases in ChAT-IR were only observed after administration of 10 μg of estradiol for 1 week or 2 μg of estradiol for 2 weeks (Gibbs, 1997). It is therefore possible that after over one month of treatment with CEE, as done in the current study, sensitivity to detect changes in the MS might have decreased.
CEE treatment induces changes in uterus, pituitary and blood
In the current study we confirmed CEE-induced peripheral endocrine responsiveness and CEE administration by finding positive vaginal smears, increases in uterine weight and dose-dependant elevations in serum estrone and estradiol levels. Both medium- and high- CEE doses increased uterine weights at the time point when estrous smears emerged. Blood was taken at sacrifice, two days after the second of a pair of CEE injections. There were dose-related increases in serum estrone and estradiol levels, with all CEE doses resulting in levels which were within physiological range for ovary-intact young and middle-aged rodents (Lerner et al., 1990; Page and Butcher, 1982).
CEE is largely estrone sulfate, which undergoes conversion to estrone in liver and fat via a desulfatase enzyme, and then gets converted to estradiol. Thus, that estradiol increases after CEE treatment corresponds with the expected sequence of steroid conversion, even though CEE itself contains only trace amounts of estradiol (Kronenberg et al., 2008). Women typically take CEE via daily oral administration, whereas we treated rats with CEE via parenteral subcutaneous administration. Oral CEE is subject to first pass and then a second hepatic metabolism, resulting in estrone levels that are higher than estradiol levels (Coelingh Bennink, 2004). Conversely, subcutaneous administration bypasses first pass hepatic metabolism, rendering higher levels of estradiol than estrone (Coelingh Bennink, 2004; Gleason et al., 2005, for discussion). The pattern we found of higher estradiol to estrone values after subcutaneous CEE administration were therefore expected, and also correspond with the pattern shown in women after parenteral CEE treatment (Gleason et al., 2005).
Notably, in the current study aside from all doses enhancing DMS task acquisition, the cognitive benefits of CEE were only seen with the highest dose. This dose yielded estradiol and estrone levels that were 14.1 and 7.6 pg/ml respectively. These levels are near or at the low physiological range for the ovary-intact young rat, with levels across the 4-5 estrous cycle ranging between 9 and 53 pg/ml for estradiol and 10 and 40 pg/ml for estrone (Page and Butcher, 1982). In women the circulating levels of estradiol and estrone resulting from the 0.625 mg and 1.25 mg CEE oral doses are within physiological range for the ovary-intact young woman. Indeed, across the 28 day menstrual cycle the ovary-intact young woman has levels between 40 and 500 pg/ml for estradiol and between 15 and 200 pg/ml for estrone; for oral CEE treatment in women, levels approximate 40 pg/ml for estradiol and 153 pg/ml for estrone for the 0.625 mg/day tablet, and 60 pg/ml estradiol and 200 pg/ml estrone for the 1.25 mg/day tablet (Gruber et al., 2002; O'Connell, 1995). Notably, an oral CEE regimen given to Ovx rats in future studies would likely yield an estradiol:estrone ratio more similar to oral treatment in women, with estrone levels higher than estradiol.
In conclusion, the current findings suggest that in the rodent model CEE can provide cognitive enhancements specific to particular domains of function. It benefits learning spatial working memory information, aids overnight memory retention, protects against cholinergic challenge, and enhances efficient navigation through the environment, possibly via cholinergic mechanisms. These findings are similar to those previously reported using estradiol.
Acknowledgements
This research was funded by grants awarded to HAB-N from the National Institute on Aging, Evelyn F. McKnight Brain Research Foundation, state of Arizona, ADHS, Alzheimer's Disease Core Center Pilot grant program, Institute for Mental Health Research and to JA from the American Psychological Association Diversity Program in Neuroscience Predoctoral Fellowship. We thank Dr. Matthew Quintero, Dr. Lei Lei, Elizabeth Engler, Ian Crain, Candy Tsang, Bronson Bowman, Therry The and Anthony Blome for excellent assistance, and Heidi Miers for her resourcefulness. We are also indebted to Dr. James Simpkins for guidance regarding the hormone doses, Dr. Gene Alexander and Dr. Clark Presson for discussion of the interference trials, as well as Dr. Jill Daniel and Dr. Gary Dohanich for advisement on the scopolamine dose. We are grateful to Dr. Ravinder Singh (Mayo Clinic, Rochester, MN) for performing the hormone assays by LC/MSMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ashby J, Odum J, Foster JR. Activity of raloxifene in immature and ovariectomized rat uterotrophic assays. Regul Toxicol Pharmacol. 1997;25:226–31. doi: 10.1006/rtph.1997.1108. [DOI] [PubMed] [Google Scholar]
- Bhavnani BR. Estrogens and menopause: pharmacology of conjugated equine estrogens and their potential role in the prevention of neurodegenerative diseases such as Alzheimer's. J Steroid Biochem Mol Biol. 2003;85:473–82. doi: 10.1016/s0960-0760(03)00220-6. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–42. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–73. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–7. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Chen S, Montoya M, Hsieh D, Minaya J. The estrogen replacement therapy of the Women's Health Initiative promotes the cellular mechanisms of memory and neuronal survival in neurons vulnerable to Alzheimer's disease. Maturitas. 2000;34(Suppl 2):S35–52. doi: 10.1016/s0378-5122(00)00107-9. [DOI] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clin Obstet Gynaecol. 1977;4:31–47. [PubMed] [Google Scholar]
- Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47:269–75. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–25. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Diaz Brinton R, Chen S, Montoya M, Hsieh D, Minaya J, Kim J, Chu HP. The women's health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol Aging. 2000;21:475–96. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behav Neurosci. 1994;108:988–92. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology. 2006;31:2065–78. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Martel FL. Proactive interference effects on short-term memory in rats: I. Basic parameters and drug effects. Behav Neurosci. 1990;104:655–65. doi: 10.1037//0735-7044.104.5.655. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Martel FL, Iversen SD. Proactive interference effects on short-term memory in rats: II. Effects in young and aged rats. Behav Neurosci. 1990;104:666–70. doi: 10.1037//0735-7044.104.5.666. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. J Cell Mol Med. 2004;8:537–44. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–68. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–40. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav. 1999;62:711–7. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. J Pineal Res. 2004;37:198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull. 2008 doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–52. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–60. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci. 1996;16:1049–55. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–6. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–57. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–9. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Burke AM, Johnson DA. Estrogen replacement attenuates effects of scopolamine and lorazepam on memory acquisition and retention. Horm Behav. 1998;34:112–25. doi: 10.1006/hbeh.1998.1452. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience. 2002;113:907–14. doi: 10.1016/s0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Wu D, Hersh LB, Pfaff DW. Effects of estrogen replacement on the relative levels of choline acetyltransferase, trkA, and nerve growth factor messenger RNAs in the basal forebrain and hippocampal formation of adult rats. Exp Neurol. 1994;129:70–80. doi: 10.1006/exnr.1994.1148. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Carlsson CM, Johnson S, Atwood C, Asthana S. Clinical pharmacology and differential cognitive efficacy of estrogen preparations. Ann N Y Acad Sci. 2005;1052:93–115. doi: 10.1196/annals.1347.007. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol Behav. 2002;77:371–85. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–52. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- Hruska Z, Dohanich GP. The effects of chronic estradiol treatment on working memory deficits induced by combined infusion of beta-amyloid (1-42) and ibotenic acid. Horm Behav. 2007;52:297–306. doi: 10.1016/j.yhbeh.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Stavnezer AJ, Bimonte HA, Sherman GF, Denenberg VH. Spatial and nonspatial Morris maze learning: impaired behavioral flexibility in mice with ectopias located in the prefrontal cortex. Behav Brain Res. 2002;133:247–59. doi: 10.1016/s0166-4328(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Kantor HI, Michael CM, Shore H. Estrogen for older women. Am J Obstet Gynecol. 1973;116:115–8. doi: 10.1016/0002-9378(73)90894-6. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD, editors. Design and analysis: a researcher's handbook. Pearson; Upper Saddle River, NJ: 2004. [Google Scholar]
- Kompoliti K, Chu Y, Polish A, Roberts J, McKay H, Mufson EJ, Leurgans S, Morrison JH, Kordower JH. Effects of estrogen replacement therapy on cholinergic basal forebrain neurons and cortical cholinergic innervation in young and aged ovariectomized rhesus monkeys. J Comp Neurol. 2004;472:193–207. doi: 10.1002/cne.20050. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–20. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM, Melmed S, Poloonsky KS, Larsen PR, editors. Williams textbook of endocrinology. Saunders Elsevier; Philadelphia, PA: 2008. [Google Scholar]
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric. 2005;8(Suppl 1):3–63. doi: 10.1080/13697130500148875. [DOI] [PubMed] [Google Scholar]
- Lerner SP, Meredith S, Thayne WV, Butcher RL. Age-related alterations in follicular development and hormonal profiles in rats with 4-day estrous cycles. Biol Reprod. 1990;42:633–8. doi: 10.1095/biolreprod42.4.633. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–6. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–90. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, McEwen BS. Sex differences in cholinergic enzymes of diagonal band nuclei in the rat preoptic area. Neuroendocrinology. 1983;36:475–82. doi: 10.1159/000123501. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–93. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Marriott LK, Gold PE. Patterns of brain acetylcholine release predict individual differences in preferred learning strategies in rats. Neurobiol Learn Mem. 2003;79:177–83. doi: 10.1016/s1074-7427(02)00014-x. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci. 1996;16:1860–5. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Grebe SK, DJ OK, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50:373–84. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- O'Connell MB. Pharmacokinetic and pharmacologic variation between different estrogen products. J Clin Pharmacol. 1995;35:18S–24S. doi: 10.1002/j.1552-4604.1995.tb04143.x. [DOI] [PubMed] [Google Scholar]
- O'Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–92. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Page RD, Butcher RL. Follicular and plasma patterns of steroids in young and old rats during normal and prolonged estrous cycles. Biol Reprod. 1982;27:383–92. doi: 10.1095/biolreprod27.2.383. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4 ed. Academic Press; New York: 1998. [Google Scholar]
- Pych JC, Chang Q, Colon-Rivera C, Gold PE. Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem. 2005;84:93–101. doi: 10.1016/j.nlm.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ. National Institute of Health; Bethesda, Maryland, USA: 19972004. http://rsb.info.nih.gov/ij/ [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–10. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–30. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Hintz TM, Gomez J, Stormes KA, Barouk S, Malthankar-Phatak GH, McCloskey DP, Luine VN, Maclusky NJ. Changes in hippocampal function of ovariectomized rats after sequential low doses of estradiol to simulate the preovulatory estrogen surge. Eur J Neurosci. 2007;26:2595–612. doi: 10.1111/j.1460-9568.2007.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrott LM, Denenberg VH, Sherman GF, Rosen GD, Galaburda AM. Lashley maze learning deficits in NZB mice. Physiol Behav. 1992;52:1085–9. doi: 10.1016/0031-9384(92)90463-c. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–6. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, Bowen D, Terrell T, Jones BN. The Women's Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–21. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Singer CA, McMillan PJ, Dobie DJ, Dorsa DM. Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Res. 1998;789:343–6. doi: 10.1016/s0006-8993(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R. Hormonal replacement therapy. Rev Endocr Metab Disord. 2002;3:243–56. doi: 10.1023/a:1020028510797. [DOI] [PubMed] [Google Scholar]
- Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133:261–70. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. Allyn and Bacon.; Boston, MA: 2001. [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–63. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Craik FIM, editors. Oxford Handbook of Memory. Oxford, NY: 2000. [Google Scholar]
- Underwood BJ. Interference and forgetting. Psychol Rev. 1957;64:49–60. doi: 10.1037/h0044616. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Conjugated equine estrogen enhances rats' cognitive, anxiety, and social behavior. Neuroreport. 2008;19:789–92. doi: 10.1097/WNR.0b013e3282fe209c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G. The effects of retroactive and proactive interference on learning and memory in old and young rats. Dev Psychobiol. 1984;17:537–45. doi: 10.1002/dev.420170510. [DOI] [PubMed] [Google Scholar]
- Winocur G. The hippocampus and thalamus: their roles in short- and long-term memory and the effects of interference. Behav Brain Res. 1985;16:135–52. doi: 10.1016/0166-4328(85)90088-9. [DOI] [PubMed] [Google Scholar]
- Winocur G. A neuropsychological analysis of memory loss with age. Neurobiol Aging. 1988;9:487–94. doi: 10.1016/s0197-4580(88)80102-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kitawaki J, Kikuchi N, Okubo T, Iwasa K, Kawata M, Honjo H. Effects of estrogens on cholinergic neurons in the rat basal nucleus. J Steroid Biochem Mol Biol. 2007;107:70–9. doi: 10.1016/j.jsbmb.2007.03.035. [DOI] [PubMed] [Google Scholar]