Abstract
Success of hematopoietic-cell transplantation (HCT) can vary by race, but the impact of socioeconomic-status (SES) is not known. To evaluate the role of race and SES, we studied 6207 unrelated-donor myeloablative HCT recipients transplanted between 1995–2004 for acute or chronic leukemia or myelodysplastic syndrome. Patients were reported by transplant center to be White (n=5253), African-American (n=368), Asian/Pacific-Islander (n=141), or Hispanic (n=445). Patient income was estimated from residential ZIP Code at time of HCT. Cox-regression analysis adjusting for other significant factors showed that African-American (but not Asian or Hispanic) recipients had worse overall survival (OS) (relative-risk [RR] 1.47 (95% CI 1.29–1.68), P<0.001) compared to Whites. Treatment-related mortality (TRM) was higher in African-Americans (RR 1.56, (1.34–1.83), P<0.001) and in Hispanics (RR 1.30, (1.11–1.51), P=0.001). Across all racial groups, patients with median incomes in the lowest quartile (<$34,700) had worse OS (RR 1.15 (1.04–1.26), P=0.005) and higher risks of TRM (RR 1.21 (1.07–1.36), P=0.002). Inferior outcomes among African-Americans are not fully explained by transplant-related factors or SES. Potential other mechanisms such as genetic polymorphisms that impact drug metabolism or unmeasured co-morbidities, socioeconomic factors and health behaviors may be important. Low SES, regardless of race, has a negative impact on unrelated donor HCT outcomes.
Keywords: Allogeneic hematopoietic cell transplantation, unrelated donor, race, socioeconomic status, survival
INTRODUCTION
The use of hematopoietic cell transplantation (HCT) is increasing worldwide. The prognostic impact of patient and donor specific demographic factors has been well described, but there are limited data regarding the impact of race and socioeconomic status (SES) on outcome of HCT. Many studies have described racial differences in tumor presentation, histology, stage at diagnosis and response to therapy in cancer patients, including prostate cancer1–3, carcinoma of the breast4,5, colon4, oral cancers5, acute myeloid leukemia (AML)6 and Hodgkin lymphoma7. Possible explanations for these differences might include cultural attitudes in seeking medical care, treatment variability as well as potential lack of access to primary care (and subsequently delayed diagnosis). SES has also been considered as a contributing factor to racial differences in outcome for cancer patients. However, even controlling for stage of disease, most studies suggest that SES alone cannot explain a racial difference in outcome8–11.
Until recently, differences in the outcome of ethnic minorities undergoing HCT have not been described in detail. A previous study from the Center for International Blood and Marrow Transplant Research (CIBMTR) compared trends in survival rates in ethnic minorities after HCT from human leukocyte antigen (HLA)-identical sibling donors12. The study found that Hispanics had lower 1-year and 3-year survival rates compared with Whites, while no differences were identified between Whites and African-Americans or Asians12. A follow-up study found that the decrease in overall survival among Hispanics was primarily related to higher risks of treatment failure (death or relapse) and higher risk of overall mortality13. Mielcarek et al, in a cohort of sibling and unrelated donor HCT recipients, have also reported a significantly higher risk of mortality among African-American HCT recipients compared to White recipients.14.
Increased genetic disparity at both the HLA locus and at minor transplantation antigens may importantly influence the outcome of HCT. In addition, polymorphism in cytokine genes can also influence HCT outcomes15,16. These genetic factors might be expected to vary between ethnicities and may contribute to disparate outcomes. A previous CIBMTR study among recipients of sibling donor HCT, performed in collaboration with transplant registries in Japan, Scandinavia and Ireland, showed reduced risk of graft-versus-host disease (GVHD) in the less genetically diverse Japanese and Scandinavian populations compared with White-American and African-American populations17. However, the risks of GVHD were similar between the Irish and the White-American and African-American populations. The study concluded that the etiology of ethnic disparities in GVHD are complex and may include differences in HLA and minor antigen diversity, frequencies of cytokine polymorphisms, and non-genetic variables such as diet, environment and differences in GVHD diagnosis and management.
Available studies investigating race in HCT are largely limited to recipients of sibling donor HCT and suggest important and as yet unexplained racial differences. Also, the impact of socio-cultural factors on outcomes of HCT has not been well described14. In the current study we explore the association of race and SES with outcomes in unrelated HCT recipients.
METHODS
Data source
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR) and the National Marrow Donor Program (NMDP) that comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic SCT to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally, with yearly follow-up. The overall follow-up of the cohort was 100% at 1 year and 95% overall and did not differ significantly among the various racial categories. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done so with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Participants
The study included patients who received an unrelated donor allogeneic HCT with a myeloablative preparative regimen using either a bone marrow or peripheral blood stem-cell source for AML, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML) or myelodysplastic syndrome (MDS) between 1995 and 2004. Only patients transplanted in a center in the USA and with available residential postal ZIP Codes were eligible for this analysis; 8 patients with missing ZIP Code information were excluded. Patients who received unrelated umbilical cord blood as donor source (n=163) or had previously undergone HCT (n=516) were also excluded from the study. Information about patient race was reported by transplant centers and was categorized according to the US Office of Management and Budget classification as White, African-American, Hispanic or Asian/Pacific-Islander. Patient income was estimated by the mean household income of their ZIP Code from the 2004 US Census. Distances between the center of a patient’s residence and the transplant center were approximated using the Haversine approximation on the latitude and longitude of the ZIP Code18. The package “ZIP Code deluxe”19 was used to obtain income and location data from the ZIP Code. All surviving recipients included in this analysis were retrospectively contacted and provided informed consent for participation in the National Marrow Donor Program (NMDP) research program. Informed consent was waived by the NMDP Institutional Review Board for all deceased recipients. Approximately 10% of surviving patients would not provide consent for use of research data. To adjust for the potential bias introduced by exclusion of non-consenting surviving patients, a corrective action plan modeling process randomly excluded appropriately the same percentage of deceased patients (n=532) using a biased coin randomization with exclusion probabilities based on characteristics associated with not providing consent for use of the data in survivors20. The final study cohort consisted of 6207 patients (Table 1). The followup completeness index from time of HCT, which is the ratio of total observed person-time and the potential person-time of followup in a study,21 was 98% at 1-year and 90% at 5-years post-HCT.
Table 1.
Characteristics of patients transplanted at US centers who received unrelated donor myeloablative hematopoietic cell transplants for AML, ALL, CML, and MDS from 1995–2004 by race
| Variable | White N (%) |
African- American N (%) |
Asian/ Pacific Islander N (%) |
Hispanic N (%) |
P Value |
|---|---|---|---|---|---|
| Number of patients | 5253 | 368 | 141 | 445 | |
| Number of centers | 119 | 87 | 40 | 80 | |
| Age, median (range), years | 35 (<1–70) | 25 (<1–58) | 30 (<1–56) | 22 (<1–62) | <0.001 |
| Age at transplant, years | <0.001 | ||||
| < 10 | 535 (10) | 64 (17) | 22 (16) | 99 (22) | |
| 10–19 | 683 (13) | 83 (23) | 24 (17) | 92 (21) | |
| 20–29 | 846 (16) | 70 (19) | 23 (16) | 97 (22) | |
| 30–39 | 1091 (21) | 65 (18) | 35 (25) | 81 (18) | |
| 40–49 | 1291 (25) | 69 (19) | 28 (20) | 54 (12) | |
| ≥ 50 | 807 (15) | 17 (5) | 9 (6) | 22 (5) | |
| Male gender | 2979 (57) | 214 (58) | 83 (59) | 270 (61) | 0.39 |
| Median income, 2000 a | $44,776 | $36,275 | $50,991 | $40,111 | <0.001 |
| Missing | 524 | 39 | 12 | 60 | |
| Distance to transplant center | <0.001 | ||||
| < 17 Miles | 1109 (21) | 159 (43) | 64 (46) | 181 (41) | |
| 17–55 Miles | 1321 (25) | 69 (19) | 34 (24) | 113 (25) | |
| 55–150 Miles | 1398 (27) | 76 (21) | 17 (12) | 74 (17) | |
| > 150 Miles | 1364 (26) | 62 (17) | 23 (16) | 74 (17) | |
| Missing ZIP Code | 61 (1) | 2 (<1) | 3 (2) | 3 (<1) | |
| Karnofsky status | 0.06 | ||||
| ≥ 90 | 3574 (68) | 268 (73) | 92 (65) | 323 (73) | |
| < 90 | 1352 (26) | 75 (20) | 43 (30) | 94 (21) | |
| Missing | 327 (6) | 25 (7) | 6 (4) | 28 (6) | |
| Comorbid conditions | 0.01 | ||||
| 0–1 conditions | 4792 (91) | 342 (93) | 136 (96) | 420 (94) | |
| ≥ 2 conditions | 461 (9) | 26 (7) | 5 (4) | 25 (6) | |
| Body mass index, kg/m2 | 25 (11–44) | 24 (11–42) | 21 (13–41) | 23 (11–44) | <0.001 |
| Disease | <0.001 | ||||
| AML | 1825 (35) | 108 (29) | 39 (28) | 98 (22) | |
| ALL | 1261 (24) | 90 (24) | 48 (34) | 193 (43) | |
| CML | 1531 (29) | 153 (42) | 39 (28) | 121 (27) | |
| MDS | 636 (12) | 17 (5) | 15 (11) | 33 (7) | |
| Disease status at transplant | <0.001 | ||||
| Early | 2067 (39) | 120 (33) | 47 (33) | 158 (36) | |
| Intermediate | 1528 (29) | 163 (44) | 49 (35) | 182 (41) | |
| Advanced | 1490 (28) | 80 (22) | 38 (27) | 99 (22) | |
| Unknown | 168 (3) | 5 (1) | 7 (5) | 6 (1) | |
| HLA match status | <0.001 | ||||
| Well-matched | 2421 (46) | 81 (22) | 41 (29) | 122 (27) | |
| Partially matched | 1939 (37) | 135 (37) | 48 (34) | 160 (36) | |
| Mismatched | 893 (17) | 152 (41) | 52 (37) | 163 (37) | |
| Race match (donor/recipient) | <0.001 | ||||
| Match | 4138 (78) | 251 (68) | 116 (82) | 211 (47) | |
| Mismatch | 431 (9) | 110 (30) | 21 (15) | 205 (46) | |
| Unknown | 684 (13) | 7 (2) | 4 (3) | 29 (7) | |
| Donor age, median (range), years | 35 (18–61) | 36 (19–60) | 34 (18–59) | 34 (19–59) | 0.09 |
| Age at transplant, years | 0.11 | ||||
| 18–19 | 38 (1) | 3 (1) | 2 (1) | 5 (1) | |
| 20–29 | 1475 (28) | 102 (28) | 46 (33) | 125 (28) | |
| 30–39 | 2029 (39) | 115 (31) | 51 (36) | 184 (41) | |
| 40–49 | 1354 (26) | 120 (33) | 32 (23) | 100 (22) | |
| ≥ 50 | 357 (7) | 28 (8) | 10 (7) | 31 (7) | |
| Gender match (donor/recipient) | <0.001 | ||||
| Male/male | 2050 (39) | 103 (28) | 42 (30) | 146 (33) | |
| Male/female | 1262 (24) | 72 (20) | 34 (24) | 81 (18) | |
| Female/male | 929 (18) | 111 (30) | 41 (29) | 124 (28) | |
| Female/female | 1012 (19) | 82 (22) | 24 (17) | 94 (21) | |
| CMV match (donor/recipient) | <0.001 | ||||
| Negative/negative | 1945 (37) | 63 (17) | 8 (6) | 59 (13) | |
| Negative/positive | 1514 (29) | 94 (26) | 36 (26) | 131 (29) | |
| Positive/negative | 820 (16) | 69 (19) | 20 (14) | 72 (16) | |
| Positive/positive | 887 (17) | 133 (36) | 75 (53) | 176 (40) | |
| Unknown | 87 (2) | 9 (2) | 2 (1) | 7 (2) | |
| Year of transplant | <0.001 | ||||
| 1995–1999 | 2716 (52) | 181 (49) | 71 (50) | 177 (40) | |
| 2000–2004 | 2537 (48) | 187 (51) | 70 (50) | 268 (60) | |
| Conditioning regimen | <0.001 | ||||
| Bu + Cy ± other | 1066 (20) | 50 (14) | 16 (11) | 52 (12) | |
| Cy + TBI ± other | 3720 (71) | 279 (76) | 120 (85) | 336 (76) | |
| TBI ± other | 183 (3) | 10 (3) | 5 (4) | 26 (6) | |
| Other | 284 (6) | 29 (8) | 0 | 31 (7) | |
| GVHD prophylaxis b | |||||
| CsA + MTX ± other | 2681 (51) | 167 (45) | 86 (61) | 187 (42) | |
| Tacrolimus + MTX ± other | 1200 (23) | 85 (23) | 25 (18) | 144 (32) | |
| T-cell depletion ± other | 941 (18) | 88 (24) | 16 (11) | 73 (16) | |
| CsA or tacrolimus ± other | 371 (7) | 27 (7) | 13 (9) | 37 (8) | |
| Other | 60 (1) | 1 (<1) | 1 (<1) | 4 (<1) | |
| Graft type | 0.02 | ||||
| Bone marrow | 4298 (82) | 321 (87) | 109 (77) | 358 (80) | |
| Peripheral blood | 955 (18) | 47 (13) | 32 (23) | 87 (20) | |
| Infused cell dose b | |||||
| BM > 2 × 108 | 2659 (62) | 173 (54) | 61 (56) | 219 (61) | |
| BM ≤ 2 × 108 | 1601 (37) | 146 (45) | 43 (39) | 139 (39) | |
| BM missing | 38 (<1) | 2 (<1) | 5 (5) | 0 | |
| PB > 5 × 108 | 592 (62) | 28 (60) | 18 (56) | 62 (71) | |
| PB ≤ 5 × 108 | 292 (31) | 19 (40) | 12 (38) | 22 (25) | |
| PB missing | 71 (7) | 0 | 2 (6) | 3 (3) | |
| Time from diagnosis to transplant, median (range), months |
10 (1–309) | 15 (2–273) | 13 (2–179) | 16 (2–170) | <0.001 |
| Donor search time, median (range), months c |
|||||
| Diagnosis to preliminary search |
3 (<1–303) | 5 (<1–268) | 5 (<1–134) | 5 (<1–164) | <0.001 |
| Preliminary search to formal search |
<1 (<1–116) | <1 (<1–51) | <1 (<1–85) | <1 (<1–41) | 0.03 |
| Formal search to transplant | 3 (<1–125) | 4 (1–91) | 3 (<1–89) | 4 (1–58) | <0.001 |
| Follow-up of survivors, median (range), months |
66 (3–138) | 48 (3–130) | 64 (11–127) | 48 (11–132) | <0.001 |
Abbreviations: AML, Acute myeloid leukemia; ALL, Acute lymphoblastic leukemia; MDS, Myelodysplastic syndrome; CML, Chronic myeloid leukemia; HLA, human-leukocyte antigen; CMV, cytomegalovirus; Cy, Cyclophosphamide; Bu, Busulfan; TBI, Total body radiation; CsA, Cyclosporine; MTX, Methotrexate; GVHD, graft-versus-host disease; BM, bone marrow; PB, peripheral blood
Based on 2004 Census tract data linking income to residential ZIP Code.
Univariate comparison not done due to small cell counts
Preliminary search provides a list of potential donors at a given time but does not initiate contact with nor further testing of the donors. If the transplant center decides to proceed with unrelated donor HCT, formal search is initiated on behalf of the patient. This includes confirmatory HLA typing of the patient and donor and confirmation of the availability of the donor for obtaining hematopoietic stem cells.
Outcomes and study definitions
The primary objective of this study was to determine the impact of race and household income on overall survival (OS), disease-free survival (DFS), relapse, and treatment-related mortality (TRM). DFS was defined as survival in complete remission after HCT. For OS, death from any cause was considered an event. Relapse was defined as disease recurrence at any site. TRM was defined as death in complete remission. OS, DFS, relapse and TRM were assessed from the date of HCT. All patients were assessed for acute and chronic GVHD by standard criteria22,23.
Based on previous CIBMTR publications of disease specific outcomes and differential outcomes in ethnic minorities with related donor transplants,12,13,24–27 disease status was classified as early, intermediate or advanced. Early disease included AML and ALL in first complete remission, CML in first chronic phase, and MDS with refractory anemia or refractory anemia with ringed sideroblasts. AML and ALL in second or greater remission or CML in accelerated phase or second or greater chronic phase was categorized as intermediate disease. Patients with advanced disease had AML and ALL in relapse or primary induction failure, CML in blast phase, or MDS with refractory anemia with excess blasts or excess blasts in transformation.
The NMDP classification of HLA matching status that allows adequate adjustment for donor-recipient HLA compatibility while accounting for best available resolution of typing was used to categorize HLA matching status as well-matched, partially-matched or mismatched25. Briefly, well-matched patients had no identified mismatches at HLA-A, -B, -C and -DRB1 with low/intermediate or high resolution data available at HLA-A, -B and high resolution -DRB1. Partially-matched patients had a single locus mismatch at any of the 4 loci and/or missing HLA-C data. Mismatched patients had 2 or more allele or antigen mismatches.
Statistical analysis
Patient, disease and HCT related characteristics were compared by Chi-square statistic for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of OS and DFS were calculated using the Kaplan-Meier method. Probabilities of TRM, relapse, neutrophil engraftment and acute and chronic GVHD were calculated by the cumulative-incidence function method.
To adjust for differences in baseline characteristics, multivariate Cox proportional-hazards regression models were used. Household income was correlated with outcome using a series of threshold models in the Cox model framework. These models were then constructed to find cut points that best described impact of income on outcome by picking the model with the largest partial likelihood28. Associations between each outcome and potential prognostic variables (Table 2) were evaluated using a stepwise approach. Variables significantly associated with each outcome event (P<0.05) were included as covariate factors in subsequent comparisons. The assumption of proportional hazards was tested in a time-dependent covariate fashion. Results were expressed as relative risks (RR) of each outcome. The models for OS, DFS, relapse and TRM were stratified on Karnofsky Score. The models for acute GVHD and chronic GVHD were stratified on patient age. For engraftment, logistic regression was used to model the chance of neutrophil recovery at day 28. A similar analysis to the Cox analysis was performed; the results are reported as the odds in favor of engraftment. For each outcome the main effects of race and household income were tested for an interaction with each of the other covariates (including gender) that entered the models. This was done for each outcome. None of these were found to be significant at a 5% significance level.
Table 2.
Variables tested in multivariate analysis
| Main effect variable |
| Race/ethnicity: White* vs. African-American vs. Asian/Pacific Islander vs. Hispanic |
| Patient-related variables |
| Age: ≤ 10* vs. 11–20 vs. 21–30 vs. 31–40 vs. 41–50 vs. > 50 |
| Gender: Male* vs. female |
| Karnofsky performance status at transplant: < 90% vs. ≥ 90%* vs. missing |
| Median income: above* vs. below median vs. missing |
| Co-morbid medical conditions: 0–1* vs. ≥ 2 comorbidities |
| Distance to transplant center: above* vs. below median vs. missing |
| Disease-related variables |
| Disease: AML* vs. ALL vs. CML vs. MDS |
| Disease status: early* vs. intermediate vs. advanced disease |
| Time from diagnosis to transplant: continuous |
| Transplant-related variables |
| Source of stem cells: bone marrow* vs. peripheral blood |
| HLA match: well-matched* vs. partially matched vs. mismatched |
| Donor age: 18–20* vs. 21–30 vs. 31–40 vs. 41–50 vs. > 50 |
| Donor-recipient gender match: F-M vs. M-F vs. M-M* vs. F-F |
| Donor-recipient CMV status: −/−* vs. −/+ vs. +/− vs. +/+ vs. unknown |
| Year of transplant: 1995–1999 vs. 2000–2004* |
| Conditioning regimen: Bu + Cy ± others vs. Cy + TBI ± others * vs. TBI ± others vs. other |
| Donor-recipient race match: same ethnicity* vs. disparate ethnicity vs. unknown |
| Infused cell dose: ≤ 2 × 108 vs. > 2 × 108* nucleated cells/kg for bone marrow and ≤ 5 × 108 vs. > 5 × 108 * nucleated cells/kg for peripheral blood |
| Donor search time (months) (diagnosis to preliminary search, preliminary search to formal search and formal search to transplant): above* vs. below median |
Abbreviations: AML, Acute myeloid leukemia; ALL, Acute lymphoblastic leukemia; MDS, Myelodysplastic syndrome; CML, Chronic myeloid leukemia; HLA, human-leukocyte antigen; F, female; M, male; CMV, cytomegalovirus; Cy, Cyclophosphamide; Bu, Busulfan; TBI, Total body radiation
Reference group
The analysis found a significant transplant center effect using a random effects test on the survival times29. To adjust for center, a stepwise regression model was used which included at each step the main effects as well as all covariates adjusted for in the model for the given event. The centers found to enter and stay in the model at a 5% significance level were included in the final model.
To examine the robustness of our results in patients with high-resolution HLA typing, a subset analysis limited to patients with allele-level typing at the HLA-A, -B, -C and -DRB1 loci (n=3864) was performed for the endpoints of OS, DFS, relapse and TRM.
All p-values are two-sided and, to account for multiple comparisons, a p-value <0.01 was considered to be significant. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Table 1 describes patient, disease and treatment characteristics by race. Asian/Pacific Islanders had the highest median household income ($50,991) while African-Americans had the lowest ($36,275).
Table 3 describes our cohort’s characteristics by socioeconomic status. A greater proportion of African-Americans (45%) belonged to the lowest income quartile (median income <$34,700), compared to Hispanics (35%), Whites (21%) or Asian/Pacific-Islanders (9%).
Table 3.
Characteristics of patients transplanted at US centers who received unrelated donor myeloablative hematopoietic cell transplants for AML, ALL, CML, and MDS from 1995–2004 by socioeconomic status
| Variable | Median Income a | ||||
|---|---|---|---|---|---|
| <$34,700 N (%) |
$34,700– 43,600 N (%) |
$43,600– 56,300 N (%) |
>$56,300 N (%) |
P Value | |
| Number of patients | 1414 | 1541 | 1517 | 1559 | |
| Number of centers | 107 | 116 | 111 | 107 | |
| Age, median (range), years | 31 (<1–65) | 33 (<1–67) | 34 (<1–70) | 35 (<1–67) | <0.001 |
| Age at transplant, years | <0.001 | ||||
| < 10 | 172 (12) | 189 (12) | 167 (11) | 177 (11) | |
| 10–19 | 234 (17) | 215 (14) | 216 (14) | 191 (12) | |
| 20–29 | 264 (19) | 264 (17) | 245 (16) | 233 (15) | |
| 30–39 | 298 (21) | 316 (21) | 303 (20) | 316 (20) | |
| 40–49 | 293 (21) | 351 (23) | 370 (24) | 388 (25) | |
| ≥ 50 | 153 (11) | 206 (13) | 216 (14) | 254 (16) | |
| Male gender | 814 (58) | 889 (58) | 849 (56) | 885 (57) | 0.76 |
| Race | <0.001 | ||||
| White | 1091 (77) | 1308 (85) | 1321 (87) | 1383 (89) | |
| African-American | 160 (11) | 89 (6) | 53 (3) | 54 (3) | |
| Asian/Pacific Islander | 12 (1) | 37 (2) | 28 (2) | 57 (4) | |
| Hispanic | 151 (11) | 107 (7) | 115 (8) | 65 (4) | |
| Distance to transplant center | <0.001 | ||||
| < 20 Miles | 274 (19) | 312 (20) | 414 (27) | 500 (32) | |
| 20–70 Miles | 157 (11) | 331 (21) | 464 (31) | 558 (36) | |
| 70–175 Miles | 509 (36) | 505 (33) | 353 (23) | 169 (11) | |
| > 175 Miles | 474 (34) | 393 (26) | 286 (19) | 332 (21) | |
| Karnofsky status | 0.29 | ||||
| ≥ 90 | 959 (68) | 1070 (69) | 1047 (69) | 1063 (68) | |
| < 90 | 378 (27) | 386 (25) | 371 (24) | 384 (25) | |
| Missing | 77 (5) | 85 (6) | 99 (7) | 112 (7) | |
| Comorbid conditions | 0.21 | ||||
| 0–1 conditions | 1292 (91) | 1414 (92) | 1378 (91) | 1448 (93) | |
| ≥ 2 conditions | 122 (9) | 127 (8) | 139 (9) | 111 (7) | |
| Body mass index, kg/m2 | 24 (11–44) | 25 (12–44) | 25 (13–44) | 24 (13–43) | 0.008 |
| Disease | 0.91 | ||||
| AML | 471 (33) | 508 (33) | 511 (34) | 520 (33) | |
| ALL | 382 (27) | 390 (25) | 384 (25) | 391 (25) | |
| CML | 417 (29) | 461 (30) | 443 (29) | 467 (30) | |
| MDS | 144 (10) | 182 (12) | 179 (12) | 181 (12) | |
| Disease status at transplant | 0.37 | ||||
| Early | 515 (36) | 588 (38) | 602 (40) | 608 (39) | |
| Intermediate | 464 (33) | 482 (31) | 445 (29) | 477 (31) | |
| Advanced | 403 (29) | 424 (28) | 417 (27) | 424 (27) | |
| Unknown | 32 (2) | 47 (3) | 53 (3) | 50 (3) | |
| HLA match status | 0.009 | ||||
| Well-matched | 582 (41) | 630 (41) | 675 (44) | 705 (45) | |
| Partially matched | 523 (37) | 584 (38) | 531 (35) | 586 (38) | |
| Mismatched | 309 (22) | 327 (21) | 311 (21) | 268 (17) | |
| Race match (donor/recipient) | 0.54 | ||||
| Match | 187 (13) | 180 (12) | 188 (12) | 190 (12) | |
| Mismatch | 1069 (76) | 1184 (77) | 1163 (77) | 1168 (75) | |
| Unknown | 158 (11) | 177 (11) | 166 (11) | 201 (13) | |
| Donor age, median (range), years | 35 (18–60) | 35 (18–60) | 36 (18–60) | 35 (18–61) | 0.011 |
| Gender match (donor/recipient) | 0.89 | ||||
| Male/male | 535 (38) | 573 (37) | 573 (38) | 592 (38) | |
| Male/female | 321 (23) | 354 (23) | 364 (24) | 379 (24) | |
| Female/male | 279 (20) | 316 (21) | 276 (18) | 293 (19) | |
| Female/female | 279 (20) | 298 (19) | 304 (20) | 295 (19) | |
| CMV match (donor/recipient) | 0.002 | ||||
| Negative/negative | 417 (29) | 511 (33) | 543 (36) | 546 (35) | |
| Negative/positive | 456 (32) | 412 (27) | 416 (27) | 434 (28) | |
| Positive/negative | 207 (15) | 250 (16) | 228 (15) | 267 (17) | |
| Positive/positive | 308 (22) | 342 (22) | 304 (20) | 288 (18) | |
| Unknown | 26 (2) | 26 (2) | 26 (2) | 24 (2) | |
| Year of transplant | 0.19 | ||||
| 1995–1999 | 741 (52) | 761 (49) | 783 (52) | 766 (49) | |
| 2000–2004 | 673 (48) | 780 (51) | 734 (48) | 793 (51) | |
| Conditioning regimen | 0.003 | ||||
| Bu + Cy ± other | 234 (17) | 304 (20) | 337 (22) | 288 (18) | |
| Cy + TBI ± other | 1022 (72) | 1118 (73) | 1045 (69) | 1129 (72) | |
| TBI ± other | 72 (5) | 47 (3) | 51 (3) | 50 (3) | |
| Other | 86 (6) | 72 (5) | 84 (6) | 92 (6) | |
| GVHD prophylaxis | 0.10 | ||||
| CsA + MTX ± other | 713 (50) | 777 (50) | 783 (52) | 770 (49) | |
| Tacrolimus + MTX ± other | 300 (21) | 356 (23) | 342 (23) | 404 (26) | |
| T-cell depletion ± other | 279 (20) | 289 (19) | 263 (17) | 259 (17) | |
| CsA or tacrolimus ± other | 112 (8) | 101 (6) | 114 (7) | 107 (7) | |
| Other | 10 (1) | 18 (1) | 14 (1) | 19 (2) | |
| Graft type | 0.007 | ||||
| Bone marrow | 1201 (85) | 1262 (82) | 1232 (81) | 1251 (80) | |
| Peripheral blood | 213 (15) | 279 (18) | 285 (19) | 308 (20) | |
| Infused cell dose | |||||
| BM > 2 × 108 | 744 (62) | 748 (59) | 756 (61) | 786 (63) | |
| BM ≤ 2 × 108 | 445 (37) | 501 (40) | 466 (38) | 456 (36) | |
| BM missing | 12 (1) | 13 (1) | 10 (1) | 9 (1) | |
| PB > 5 × 108 | 132 (62) | 181 (65) | 178 (62) | 190 (62) | 0.36 |
| PB ≤ 5 × 108 | 68 (32) | 86 (31) | 85 (30) | 89 (29) | |
| PB missing | 13 (6) | 12 (4) | 22 (8) | 29 (9) | |
| Time from diagnosis to transplant, median (range), months Donor search time, median (range), months |
12 (2–309) | 11 (1–309) | 11 (2–242) | 10 (<1–232) | 0.04 |
| Diagnosis to
preliminary search |
4 (<1–300) | 3 (<1–303) | 3 (<1–231) | 3 (<1–192) | 0.06 |
| Preliminary search to
formal search |
<1 (<1–69) | <1 (<1–77) | <1 (<1–116) | <1 (<1–106) | 0.33 |
| Formal search to transplant | 3 (<1–81) | 3 (<1–94) | 3 (<1–121) | 3 (<1–125) | 0.81 |
| Follow-up of survivors, median (range), months |
60 (5–133) | 60 (5–135) | 69 (3–138) | 64 (3–137) | <0.001 |
Abbreviations: AML, Acute myeloid leukemia; ALL, Acute lymphoblastic leukemia; MDS, Myelodysplastic syndrome; CML, Chronic myeloid leukemia; HLA, human-leukocyte antigen; CMV, cytomegalovirus; Cy, Cyclophosphamide; Bu, Busulfan; TBI, Total body radiation; CsA, Cyclosporine; MTX, Methotrexate; GVHD, graft-versus-host disease; BM, bone marrow; PB, peripheral blood
Based on 2004 Census tract data linking income to residential ZIP Code.
Race and outcomes
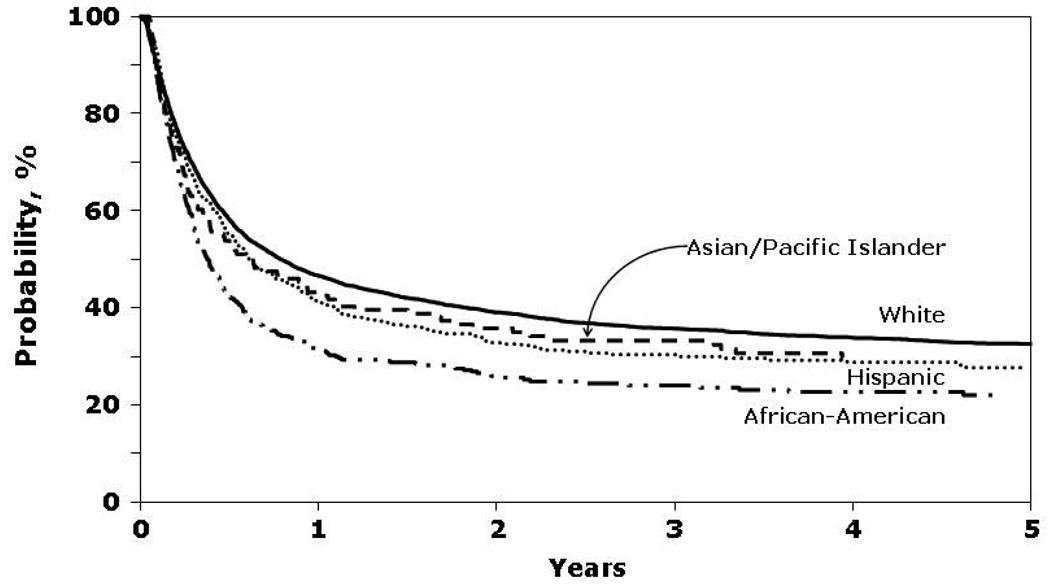
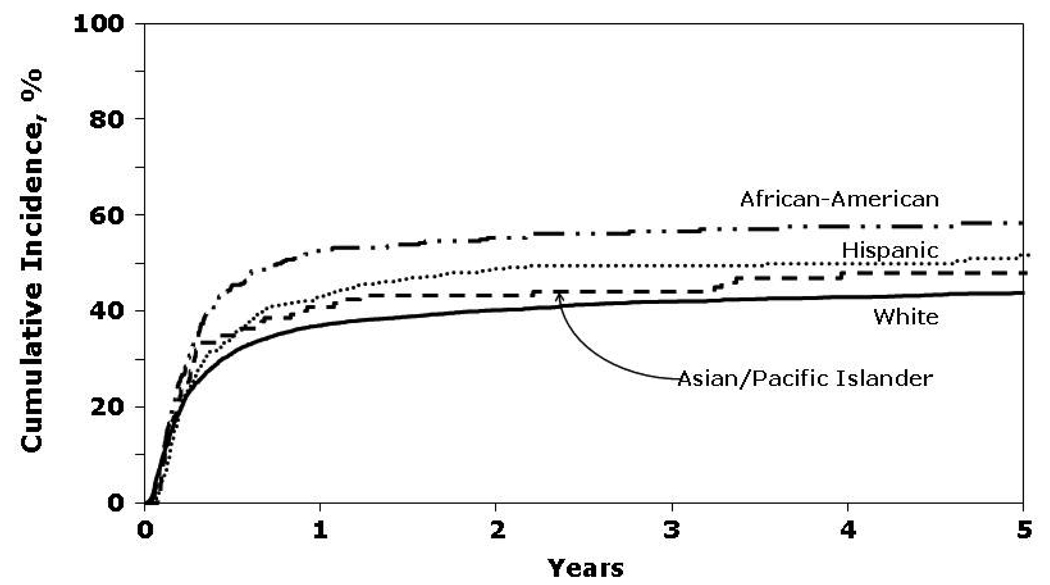
In univariate analyses, African-Americans had the lowest probability of OS and DFS and the highest rates of TRM (Figure 1 and Figure 2). Cumulative incidence of relapse was similar among all four racial groups.
Figure 1.
Probability of overall survival by race.
Figure 2.
Cumulative incidence of treatment-related mortality by race.
In a multivariate analysis adjusting for other prognostic variables (including annual income), African-American race was associated with significantly worse OS and DFS and higher TRM than Whites (Table 4). Risk of TRM was also increased in Hispanics, but OS and DFS was comparable. The risk for relapse was similar among the four racial groups. Race had no impact on neutrophil engraftment or risks of grades 2–4 acute GVHD. The RR of grade 3–4 acute GVHD was slightly higher in African-Americans (1.26 [1.03–1.54], P=NS) while that for chronic GVHD was higher in African-Americans (1.34 [1.10–1.63], P=0.003) and Hispanics (1.25 [1.06–1.48], P=0.008). A subset analysis limited to 3,864 recipients with allele-level typing at the HLA-A, -B, -C and -DRB1 loci showed risks for OS, DFS, relapse and TRM similar to those observed in analyses that included the whole cohort (data not shown).
Table 4.
Multivariate analysis for overall survival, disease-free survival, relapse and transplant-related mortality
| Variable | Relative risk (95% confidence intervals) a |
|||
|---|---|---|---|---|
| Overall survival | Disease-free survival |
Relapse | Transplant- related mortality |
|
| Race | ||||
| White b | 1.00 | 1.00 | 1.00 | 1.00 |
| African-American | 1.47 (1.29–1.68) d | 1.48 (1.30–1.69) d | 1.32 (1.03–1.68) | 1.56 (1.34–1.83) d |
| Asian/Pacific Islander | 0.96 (0.76–1.20) | 1.04 (0.83–1.31) | 1.13 (0.80–1.61) | 0.99 (0.75–1.32) |
| Hispanic | 1.15 (1.01–1.30) | 1.14 (1.00–1.29) | 0.87 (0.70–1.09) | 1.30 (1.11–1.51) d |
| Income c | ||||
| > $56,300 b | 1.00 | 1.00 | 1.00 | 1.00 |
| $43,600–56,300 | 1.06 (0.97–1.16) | 1.03 (0.94–1.13) | 0.94 (0.81–1.10) | 1.11 (0.99–1.24) |
| $34,700–43,600 | 1.06 (0.97–1.16) | 1.02 (0.93–1.12) | 0.97 (0.83–1.13) | 1.11 (0.99–1.24) |
| <$34,700 | 1.15 (1.04–1.26) d | 1.12 (1.01–1.23) | 1.07 (0.92–1.26) | 1.21 (1.07–1.36) d |
| Missing | 1.10 (0.86–1.39) | 1.08 (0.85–1.38) | 0.97 (0.63–1.48) | 1.16 (0.87–1.55) |
Models were stratified on Karnofsky performance status prior to hematopoietic cell transplant
Reference group; Table 2 lists variables tested in multivariate analysis
Based on 2004 Census data linking income to residential ZIP Code
P<0.01
Income and outcomes
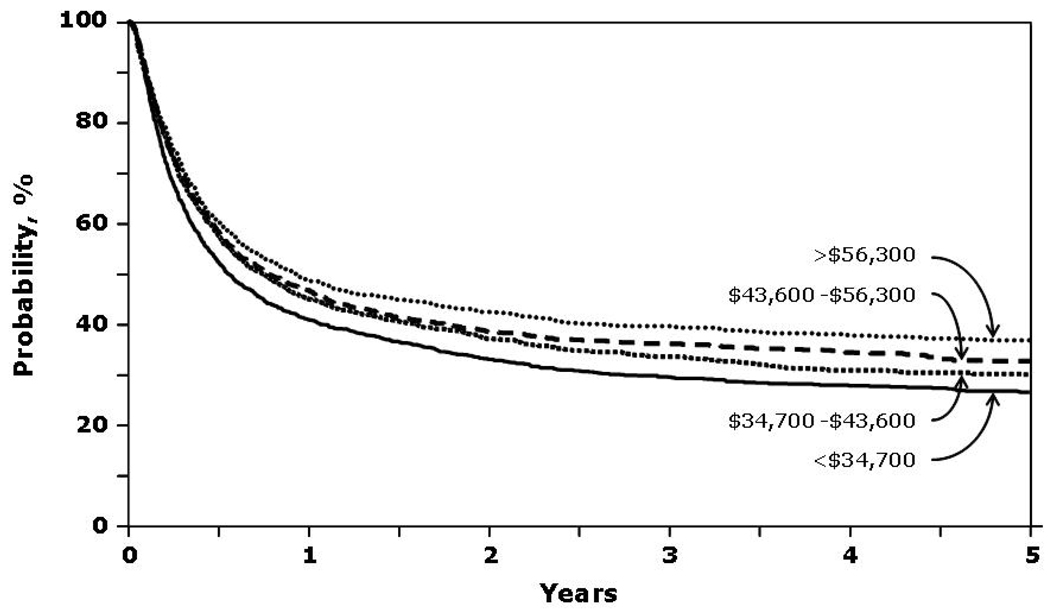
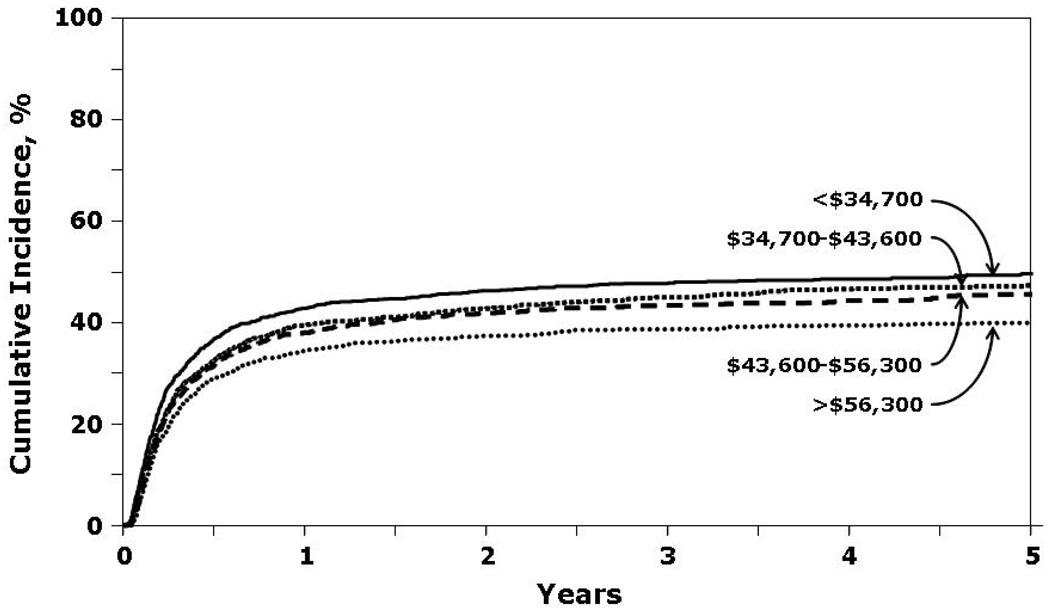
Patients with a median annual income in the lowest quartile (<$37,400) had lower probability of OS and DFS and higher rates of TRM (Figure 3 and Figure 4). Relapse was similar in all income categories.
Figure 3.
Probability of overall survival by income.
Figure 4.
Cumulative incidence of treatment-related mortality by income.
In a multivariate analysis adjusting for other prognostic variables (including race), patients with incomes in the lowest quartile (<$37,400) had significantly worse OS (RR 1.15 [1.04–1.26], P=0.005) and TRM (RR 1.21 [1.07–1.36], P=0.002) than those with incomes in the highest quartile (>$56,000) (Table 4). Household income had no impact on risks of DFS, relapse, acute GVHD or chronic GVHD. A subset analysis limited to patients with allele-level typing again showed risks similar to those observed for the whole cohort (data not shown).
Causes of death
For the whole cohort, the principal causes of death included recurrence of primary malignancy (25%), infection (20%), organ failure (18%) acute or chronic GVHD (14%) and interstitial pneumonitis (12%), and these did not differ when compared by race or SES. Table 5 describes the causes of death within and after 100 days following transplantation by race and SES.
Table 5.
Important causes of death by race and socioeconomic status
| Variable | Total deaths |
Cause of death |
|||
|---|---|---|---|---|---|
| Relapse | Infection | GVHD | Organ toxicity a | ||
| Causes of death < 100 days | |||||
| Race | |||||
| White | 1531 | 167 (11%) | 339 (22%) | 236 (15%) | 593 (39%) |
| African-American | 147 | 10 (7%) | 24 (16%) | 28 (19%) | 57 (39%) |
| Asian/Pacific Islander | 51 | 2 (4%) | 10 (20%) | 8 (16%) | 25 (49%) |
| Hispanic | 139 | 8 (6%) | 32 (23%) | 31 (22%) | 51 (37%) |
| Income | |||||
| > $56,300 | 417 | 47 (11%) | 95 (23%) | 69 (17%) | 151 (36%) |
| $43,600–56,300 | 438 | 43 (10%) | 84 (19%) | 75 (17%) | 180 (41%) |
| $34,700–43,600 | 458 | 44 (10%) | 100 (22%) | 66 (14%) | 188 (41%) |
| < $34,700 | 490 | 50 (10%) | 108 (22%) | 80 (16%) | 182 (37%) |
| Causes of death ≥100 days | |||||
| Race | |||||
| White | 1969 | 735 (37%) | 357 (18%) | 266 (14%) | 457 (23%) |
| African-American | 132 | 40 (30%) | 30 (23%) | 22 (17%) | 27 (21%) |
| Asian/Pacific Islander | 46 | 21 (46%) | 8 (17%) | 6 (13%) | 10 (22%) |
| Hispanic | 174 | 60 (35%) | 31 (18%) | 25 (14%) | 40 (23%) |
| Income | |||||
| > $56,300 | 550 | 213 (39%) | 83 (15%) | 77 (14%) | 142 (26%) |
| $43,600–56,300 | 580 | 207 (36%) | 113 (20%) | 77 (13%) | 131 (23%) |
| $34,700–43,600 | 598 | 221 (37%) | 116 (19%) | 83 (14%) | 125 (21%) |
| <$34,700 | 528 | 193 (37%) | 103 (20%) | 67 (13%) | 124 (24%) |
Includes patients with cause of death reported as organ failure or interstitial pneumonitis
DISCUSSION
Our study shows notably decreased survival in African-American unrelated donor HCT recipients compared with White, Asian and Hispanic recipients, despite adjustment in multivariate analysis for HLA matching, disease status, family income and other variables likely to influence outcome. Reduced survival was due to increased TRM in African-American recipients with no increased risk of acute GVHD or relapse detected. There are many possible reasons for reduced survival in African-American HCT recipients, including biology (e.g. polymorphism at non-HLA loci), access to care including post-HCT follow-up, disparities in treatment or follow-up practices, environmental factors (that may also be influenced by SES), and health behaviors. Additionally there were several differences in baseline patient characteristics between the groups. However, the analysis was adjusted for the potential patient, disease, and center characteristics that differ between the groups. If the survival differences found were caused by these baseline differences, this adjustment would actually tend to lower, not increase, the reported effect of race. Additionally, there was no evidence of an interaction between these factors in the model with race or income.
Biological factors, such as increased genetic polymorphism in African-American recipients, could impact outcomes. It is clear that improved HLA matching improves HCT outcome, and it is known that there is greater diversity at HLA loci in the African-American population compared with other races and ethnicities17. In our study, however, outcomes were inferior in African-American recipients even after adjustment for HLA matching with the donor. This raises the possibility that genetic variation at other loci may be modifying outcomes. At a population level, African-Americans show significantly higher levels of nucleotide heterozygosity compared with Americans of European origin30. Polymorphisms that modify expression of cytokine genes have been shown to modify a number of HCT endpoints and it is possible that less favorable alleles may occur more frequently in African-American HCT recipients12,15,16,31. In agreement with our study, reduced graft survival and OS have been reported in African-American recipients of renal transplants32, with a similar finding reported after liver transplantation in African-American recipients secondary to chronic rejection33. In cancer patients, pharmacogenetic variation in drug metabolism has been reported for cyclophosphamide, methotrexate and busulfan, all of which impacted HCT outcomes34–38. Frequencies of many pharmacogenetic variants influencing metabolism of these drugs vary by race, and future pharmacogenetic studies, which would likely need to be multi-center to achieve adequate sample size, might determine whether this is an important variable, as personalized drug dosing might improve outcomes39–41.
Racial disparities exist in health care access and outcomes and are related to SES. It has been reported that African-American patients with a myocardial infarction and heart failure receive less intensive and poorer-quality care Whites42,43–45. In a study of Medicare beneficiaries, who are presumed to have no financial obstacle to care, treatment and outcomes of heart failure were similar among White and African-American patients46. All of the patients in our study received HCT so therefore had access to high-cost, technologically demanding care, although it is unknown whether their overall insurance coverage was similar. Despite this, survival was lower in those with the lowest income, even after adjustment for race and measured co-morbidities, and the excess mortality was treatment related It is still possible though that reduced access to or utilization of post-HCT follow-up care might contribute to the inferior outcomes seen in our study.
The mechanism for the excess mortality seen in African-American recipients seems likely to be complex. Additional contributors may include unmeasured co-morbidities such as poor nutrition, inability to comply with medication regimens, and poor access to follow-up care. In addition, the National Health and Nutrition Examination Survey reports increased frequency of high blood pressure, high body mass index, physical inactivity and diabetes in African-American women, all of which, as unmeasured co-morbidities, might contribute to increased late mortality after HCT47. Addressing and improving such issues is challenging, as some are societal rather than medical issues. However, awareness of the problem is an important first step so that care providers can consider the issues and perhaps provide specific resources for this high-risk population after leaving the transplant center.
Similar to the findings in our study, low SES, assessed independently of race, has been reported to have an adverse impact on outcomes of many diseases, including cancer, chronic renal disease and solid organ transplantation38,48,49. Mackillop et al. studied the impact of SES on outcome of treatment of cancer in Ontario, Canada, where the heath system is designed to provide equitable access to healthcare for all50. Their study demonstrated higher mortality rates from cancer among poorer communities compared with wealthier areas. Recipients of renal and liver transplants with private insurance have been reported to have better survival than Medicare recipients, but SES measured by census tract was not associated with outcome.51 The difference in these observations may reflect the dominant influence of the quality of the surgical procedure and inpatient hospital care on long-term outcomes of solid organ transplantation, in contrast to HCT where the period of immune reconstitution is long (lasting months to years) and the incidence of late mortality after the patient has left the transplant center is significant51. Clearly, low SES has a significant negative impact on the unrelated donor HCT outcomes and transplant centers need to carefully examine and optimize the resources available to these individuals during the peri-HCT time period as well as during ongoing long-term follow-up.
Despite the large size of this study and the meaningful cohorts of racial minority groups, there are limitations that should be considered. Our study only included patients who actually received HCT and thus we could not address the impact of race or SES on access to transplantation. A challenge for our study, in common with all studies of race, is a lack of precision in the definition of race. In our study race was reported by the transplant center, and may not reflect accurately persons of mixed heritage. While the relative proportions of minority racial groups in this study do not match that of the US population we feel this is less of an issue related to access to care, or to misclassification of race, but rather due to the fact that minorities are underrepresented in the unrelated donor pool making it more difficult to obtain a donor for minority patients. Additionally, cancer incidence rates for acute leukemia have been shown to vary by race with a higher incidence being found in Whites compared to Blacks.52 Also, we do not have data to reflect the insurance status of patients represented in the study. Also, our study could not address other important factors that may impact access to transplant and access to and quality of post-transplant care. Some of these factors, for instance insurance coverage, lack of adequate support services and cultural biases, are potentially modifiable. In addition, socioeconomic status was estimated from Zip code of residence and was not self-reported by patients. Despite these limitations, we believe our data indicate importantly inferior outcomes in African-American unrelated donor HCT recipients that are not explicable by reduced family income, and should lead to future biological, sociological and epidemiological studies to address and improve this disparity. In addition, we show that reduced family income reduced survival in recipients of all races, indicating the need for careful support and follow-up of such patients.
Acknowledgements
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Annual Meeting of the American Society of Hematology, 2007
References
- 1.Asbell SO, Vijayakumar S. Racial differences in prostate-specific antigen levels in patients with local-regional prostate cancer. Prostate. 1997;31:42–46. doi: 10.1002/(sici)1097-0045(19970401)31:1<42::aid-pros7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Iselin CE, Box JW, Vollmer RT, Layfield LJ, Robertson JE, Paulson DF. Surgical control of clinically localized prostate carcinoma is equivalent in African-American and white males. Cancer. 1998;83:2353–2360. doi: 10.1002/(sici)1097-0142(19981201)83:11<2353::aid-cncr15>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Kim JA, Kuban DA, el-Mahdi AM, Schellhammer PF. Carcinoma of the prostate: race as a prognostic indicator in definitive radiation therapy. Radiology. 1995;194:545–549. doi: 10.1148/radiology.194.2.7529936. [DOI] [PubMed] [Google Scholar]
- 4.Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995;87:1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 5.Arbes SJ, Slade GD. Racial differences in stage at diagnosis of screenable oral cancers in North Carolina. J Public Health Dent. 1996;56:352–354. doi: 10.1111/j.1752-7325.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 6.Rubnitz JE, Lensing S, Razzouk BI, Pounds S, Pui CH, Ribeiro RC. Effect of race on outcome of white and black children with acute myeloid leukemia: the St. Jude experience. Pediatr Blood Cancer. 2007;48:10–15. doi: 10.1002/pbc.20878. [DOI] [PubMed] [Google Scholar]
- 7.Metzger ML, Castellino SM, Hudson MM, et al. Effect of race on the outcome of pediatric patients with Hodgkin's lymphoma. J Clin Oncol. 2008;26:1282–1288. doi: 10.1200/JCO.2007.14.0699. [DOI] [PubMed] [Google Scholar]
- 8.Bain RP, Greenberg RS, Whitaker JP. Racial differences in survival of women with breast cancer. J Chronic Dis. 1986;39:631–642. doi: 10.1016/0021-9681(86)90188-8. [DOI] [PubMed] [Google Scholar]
- 9.Moul JW, Douglas TH, McCarthy WF, McLeod DG. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol. 1996;155:1667–1673. [PubMed] [Google Scholar]
- 10.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. Jama. 1998;279:1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 11.Franzini L, Williams AF, Franklin J, Singletary SE, Theriault RL. Effects of race and socioeconomic status on survival of 1,332 black, Hispanic, and white women with breast cancer. Ann Surg Oncol. 1997;4:111–118. doi: 10.1007/BF02303792. [DOI] [PubMed] [Google Scholar]
- 12.Serna DS, Lee SJ, Zhang MJ, et al. Trends in survival rates after allogeneic hematopoietic stem-cell transplantation for acute and chronic leukemia by ethnicity in the United States and Canada. J Clin Oncol. 2003;21:3754–3760. doi: 10.1200/JCO.2003.03.133. [DOI] [PubMed] [Google Scholar]
- 13.Baker KS, Loberiza FR, Jr, Yu H, et al. Outcome of ethnic minorities with acute or chronic leukemia treated with hematopoietic stem-cell transplantation in the United States. J Clin Oncol. 2005;23:7032–7042. doi: 10.1200/JCO.2005.01.7269. [DOI] [PubMed] [Google Scholar]
- 14.Mielcarek M, Gooley T, Martin PJ, et al. Effects of race on survival after stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:231–239. doi: 10.1016/j.bbmt.2004.12.327. [DOI] [PubMed] [Google Scholar]
- 15.Cavet J, Middleton PG, Segall M, Noreen H, Davies SM, Dickinson AM. Recipient tumor necrosis factor-alpha and interleukin-10 gene polymorphisms associate with early mortality and acute graft-versus-host disease severity in HLA-matched sibling bone marrow transplants. Blood. 1999;94:3941–3946. [PubMed] [Google Scholar]
- 16.Dickinson AM, Harrold JL, Cullup H. Haematopoietic stem cell transplantation: can our genes predict clinical outcome? Expert Rev Mol Med. 2007;9:1–19. doi: 10.1017/S1462399407000488. [DOI] [PubMed] [Google Scholar]
- 17.Oh H, Loberiza FR, Jr, Zhang MJ, et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–1416. doi: 10.1182/blood-2004-06-2385. [DOI] [PubMed] [Google Scholar]
- 18.Sinnott R. Virtures of the Haversine. Sky and Telescope. 1984:159. [Google Scholar]
- 19.Zip-Codes.com. Zip Code Database-Deluxe Database specifications [Google Scholar]
- 20.Farag SSBA, Eapen M, Hurley C, Dupont B, Caligiuri MA, Boudreau C, Nelson G, Oudshoorn M, van Rood J, Velardi A, Maiers M, Setterholm M, Confer D, Posch PE, Anasetti C, Kamani N, Miller JS, Weisdorf D, Davies SM KIR Study Group. Center for International Blood and Marrow Transplantation Research. The Effect of KIR Ligand Incompatibility on the Outcome of Unrelated Donor Transplants: A report from the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry and the Dutch Registry. Biology of Blood and Marrow Transplantation. 2006;12:876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Clark TG, Altman DG, De Stavola BL. Quantification of the completeness of follow-up. Lancet. 2002;359:1309–1310. doi: 10.1016/s0140-6736(02)08272-7. [DOI] [PubMed] [Google Scholar]
- 22.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 23.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–259. [PubMed] [Google Scholar]
- 24.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 27.Pasquini M, Wang Z. CIBMTR summary slides - 2007 (Part 2) CIBMTR Newsletter. 2008;14:6–13. [Google Scholar]
- 28.Klein JaW JT. Handbook of Statistics. Advances in Survival Analysis. Vol. 25. Elsevier Science; 2004. Chapter 2 Discretizing a Continuous Covariate in Survival Studies. [Google Scholar]
- 29.Andersen CA. Score Tests Of Homogeneity For Survival Data. Lifetime Data Analysis. 1:145–156. doi: 10.1007/BF00985764. [DOI] [PubMed] [Google Scholar]
- 30.Lohmueller KE, Indap AR, Schmidt S, et al. Proportionally more deleterious genetic variation in European than in African populations. Nature. 2008;451:994–997. doi: 10.1038/nature06611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DH, Park JY, Sohn SK, Lee NY, Suh JS, Lee KB. The association between multidrug resistance-1 gene polymorphisms and outcomes of allogeneic HLA-identical stem cell transplantation. Haematologica. 2006;91:848–851. [PubMed] [Google Scholar]
- 32.Chesney RW, Wyatt RJ. Racial disparities in renal transplantation in children. Pediatrics. 2003;112:409–411. doi: 10.1542/peds.112.2.409. [DOI] [PubMed] [Google Scholar]
- 33.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002;359:287–293. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 34.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 35.Robien K, Schubert MM, Bruemmer B, Lloid ME, Potter JD, Ulrich CM. Predictors of oral mucositis in patients receiving hematopoietic cell transplants for chronic myelogenous leukemia. J Clin Oncol. 2004;22:1268–1275. doi: 10.1200/JCO.2004.05.147. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava A, Poonkuzhali B, Shaji RV, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- 37.Robien K, Bigler J, Yasui Y, et al. Methylenetetrahydrofolate reductase and thymidylate synthase genotypes and risk of acute graft-versus-host disease following hematopoietic cell transplantation for chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2006;12:973–980. doi: 10.1016/j.bbmt.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 38.McCune JS, Batchelder A, Deeg HJ, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Suarez-Kurtz G, Pena SD. Pharmacogenomics in the Americas: the impact of genetic admixture. Curr Drug Targets. 2006;7:1649–1658. doi: 10.2174/138945006779025392. [DOI] [PubMed] [Google Scholar]
- 40.Spielman RS, Bastone LA, Burdick JT, Morley M, Ewens WJ, Cheung VG. Common genetic variants account for differences in gene expression among ethnic groups. Nat Genet. 2007;39:226–231. doi: 10.1038/ng1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Duan S, Kistner EO, et al. Evaluation of genetic variation contributing to differences in gene expression between populations. Am J Hum Genet. 2008;82:631–640. doi: 10.1016/j.ajhg.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popescu I, Vaughan-Sarrazin MS, Rosenthal GE. Differences in mortality and use of revascularization in black and white patients with acute MI admitted to hospitals with and without revascularization services. Jama. 2007;297:2489–2495. doi: 10.1001/jama.297.22.2489. [DOI] [PubMed] [Google Scholar]
- 43.Stafford RS, Saglam D, Blumenthal D. National patterns of angiotensin-converting enzyme inhibitor use in congestive heart failure. Arch Intern Med. 1997;157:2460–2464. [PubMed] [Google Scholar]
- 44.Ayanian JZ, Weissman JS, Chasan-Taber S, Epstein AM. Quality of care by race and gender for congestive heart failure and pneumonia. Med Care. 1999;37:1260–1269. doi: 10.1097/00005650-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Kahn KL, Pearson ML, Harrison ER, et al. Health care for black and poor hospitalized Medicare patients. Jama. 1994;271:1169–1174. [PubMed] [Google Scholar]
- 46.Rathore SS, Foody JM, Wang Y, et al. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. Jama. 2003;289:2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- 47.Winkleby MA, Kraemer HC, Ahn DK, Varady AN. Ethnic and socioeconomic differences in cardiovascular disease risk factors: findings for women from the Third National Health and Nutrition Examination Survey, 1988–1994. Jama. 1998;280:356–362. doi: 10.1001/jama.280.4.356. [DOI] [PubMed] [Google Scholar]
- 48.Zell JA, Cinar P, Mobasher M, Ziogas A, Meyskens FL, Jr, Anton-Culver H. Survival for patients with invasive cutaneous melanoma among ethnic groups: the effects of socioeconomic status and treatment. J Clin Oncol. 2008;26:66–75. doi: 10.1200/JCO.2007.12.3604. [DOI] [PubMed] [Google Scholar]
- 49.Zell JA, Rhee JM, Ziogas A, Lipkin SM, Anton-Culver H. Race, socioeconomic status, treatment, and survival time among pancreatic cancer cases in California. Cancer Epidemiol Biomarkers Prev. 2007;16:546–552. doi: 10.1158/1055-9965.EPI-06-0893. [DOI] [PubMed] [Google Scholar]
- 50.Mackillop WJ, Zhang-Salomons J, Groome PA, Paszat L, Holowaty E. Socioeconomic status and cancer survival in Ontario. J Clin Oncol. 1997;15:1680–1689. doi: 10.1200/JCO.1997.15.4.1680. [DOI] [PubMed] [Google Scholar]
- 51.Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. 2004;10:235–243. doi: 10.1002/lt.20069. [DOI] [PubMed] [Google Scholar]
- 52. [accessed 07-21-2009];SEER Cancer Statistics Review. 1975–2006 http://www.seer.cancer.gov/csr/1975_2006/browse_csr.php?section=13&page=sect_13_table.07.html.