Abstract
Defining the molecular identity of stem cells may be critical for formulating a rational strategy for the therapeutic intervention of stem cell dysfunction. We find that high expression of Id1, a dominant-negative helix-loop-helix transcriptional regulator, identifies a rare population GFAP+ astrocytes with stem cell attributes among the subventricular astrocytes in the adult brain. The rare, long-lived, and relatively quiescent Id1high astrocytes with morphology characteristic of B1 type astrocytes self-renew and generate migratory neuroblasts that differentiate into olfactory bulb interneurons. Cultured Id1high neural stem cells can self-renew asymmetrically and generate a stem and a differentiated cell expressing progressively lower levels of Id1, revealing an Id1 gradient in unperturbed cells of subventricular neurogenic lineages. Moreover, Id genes are necessary to confer self-renewal capacity, a characteristic of stem cell identity. We suggest that high expression of a single transcriptional regulator, Id1, molecularly defines the long-sought-after B1 type adult neural stem cells.
Introduction
In songbirds, rodents, primates, and humans, neurogenesis continues in selected regions of the adult brain (Altman and Das, 1965; Eriksson et al., 1998; Goldman and Nottebohm, 1983; Kaplan et al., 1985; Kornack and Rakic, 2001). Adult neurogenesis generates new neurons that integrate into existing neural circuitry (Carleton et al., 2003; Paton and Nottebohm, 1984; van Praag et al., 2002). In rodents, at least two neurogenic niches–the subventricular zone (SVZ) of the striatum and subgranular zone of the hippocampal dentate gyrus–have been proposed to harbor NSC’s. The NSC's are thought to resemble astrocytes ultra-structurally and molecularly (reviewed in Ihrie and Alvarez-Buylla, 2008). Retroviral, adenoviral, and genetic lineage tracing experiments showed that at least some of the subventricular astrocytes, the B1 type astrocytes, continue to divide and produce cells that eventually differentiate into neurons and glia (Ahn and Joyner, 2005; Doetsch et al., 1999a; Merkle et al., 2007), supporting the central hypothesis that the adult brain harbors stem cells. Moreover, in vivo ablation experiments suggest that a morphologically distinct subpopulation of GFAP+ astrocytes may be required for the generation of neuroblasts and neurons (Garcia et al., 2004).
The incompletely defined population of NSC’s is thought to self-renew infrequently and is mostly quiescent or dormant. The cell cycle time of an endogenous NSC may be up to 28 days in vivo (Morshead et al., 1994). In the SVZ, the endogenous NSC’s, the B1 type astrocytes (Doetsch et al., 1999a), are estimated to constitute ~0.4% of subventricular cells (Morshead et al., 1998). These NSC's produce a cohort of Dlx2+ or Mash1+ C type transit-amplifying (TA) cells (Doetsch et al., 2002; Kuo et al., 2006) which in turn produce A type PSA-NCAM+ Doublecortin+ (DCX+) neuroblasts (Doetsch et al., 1999b; Nacher et al., 2001). The neuroblasts coalesce to form a homotypic chain, the rostral migratory stream (RMS), then travel rostrally into the olfactory bulb (OB) (Lois and Alvarez-Buylla, 1994). At the OB, the neuroblasts migrate radially and differentiate into granule neurons and periglomerular neurons (Altman, 1969; Luskin, 1993).
Although subventricular neurogenesis has been explored extensively, the precise identity of the adult NSC's has not yet been unequivocally established. For example, an ependymal identity for NSC’s (Coskun et al., 2008; Johansson et al., 1999) continues to stir controversy (Capela and Temple, 2002; Chiasson et al., 1999; Doetsch et al., 1999a; Laywell et al., 2000; Spassky et al., 2005). However, recent experiments suggest that ependymal cells contribute to neurogenesis only under pathologic conditions such as ischemic stroke (Carlen et al., 2009). Nevertheless, the defining molecular characteristics of B1 type adult NSC's and the mechanism by which the NSC's are maintained are incompletely understood.
Inhibitor of DNA binding (Id) genes encode dominant-negative antagonists of the basic-helix-loop-helix transcription factors such as E47 (Benezra et al., 1990). Id genes are necessary and sufficient for context-dependent regulation of cell cycle status and differentiation during embryogenesis (reviewed in Perk et al., 2005). Id genes are sufficient for maintenance of murine embryonic stem cell self-renewal and pluripotency in the absence of bone morphogenetic protein (Ying et al., 2003). Moreover, overexpression of Id4 is sufficient to drive postnatal astrocytes into a neural stem cell-like state (Jeon et al., 2008). We therefore hypothesized that Id1 may be a determinant of NSC identity.
In the work described below, we show that 1) the subventricular GFAP+ Id1high astrocytes are the rare B1 type adult NSC's, 2) that the Id1high NSC can self-renew asymmetrically to produce a stem and a differentiated cell expressing lower levels of Id1, recapitulating the gradient of Id1 levels evident in vivo, and 3) that the Id genes are necessary for self-renewal capacity, a characteristic of stem cell identity.
Results
A decreasing gradient of Id1 levels in the subventricular neurogenic lineages
Immunohistochemistry and tyramide signal amplification (TSA) immunofluorescence analyses of adult mouse brains with a highly specific rabbit monoclonal anti-Id1 antibody (Perk et al., 2006) revealed Id1 immunoreactivity (IR) in the parenchyma and in the subventricular region (data not shown). At least two morphologically distinguishable types of Id1+ nuclei, round and elongated, were evident.
Further TSA immunofluorescence and confocal microscopic analyses with the anti-Id1 and anti-GFAP antibody revealed predominantly round Id1 IR in the GFAP+subventricular region (Fig. 1A). Immunostaining of dissociated SVZ cells confirmed the expression of Id1 and GFAP in a single cell, indicating that some of the SVZ cells with the Id1+ nuclei are GFAP+ astrocytes (Fig. 1A, left inset).
Figure 1. A decreasing gradient of Id1 levels in the subventricular neurogenic lineages.
A. Round or elongated nuclear Id1 IR was apparent in GFAP+ subventricular region. Some of these round Id1+ cells were astrocytes, as rare single dissociated subventricular cells were Id1 and GFAP immunoreactive (left inset). LV, lateral ventricle. B. Some Id1+ cells were immunoreactive to Mash1, a TA cell marker (top inset). However, not all Id1+ cells co-expressed Mash1, as Id1+ Mash1− cells (bottom left inset) and Id1− Mash1+ cells (not shown) were also observed. Moreover, Mash1 IR was evident in cells expressing lower levels of Id1, the Id1int cells (compare bottom left inset, Id1hi Mash1lo cells and bottom right inset, Id1int Mash1int cell). Elongated Id1+ nuclei, of presumed endothelial cells, were located near the round Id1+ astrocyte nuclei (top inset, L, vessel lumen). C–E. Id1+ cells never co-expressed PSA-NCAM (C), NeuN (D), and S100b (E)-neuroblast, neuron, and ependymal cell marker, respectively. Notably, S100b+ parenchymal astrocytes were Id1−. F. CD31 IR confirmed the endothelial identity of the elongated Id1+ cells. G–I. Flow cytometric immunophenotyping of the anterior subventricular cells from Id1V/V mice confirmed and quantified the rare population of the Id1-expressing GFAP+ astrocytes (G) as well as the Mash1+ cells (H). PSA-NCAM+ cells did not express Id1 (I). Scale bar = 10 microns.
TSA immunofluorescence of Id1 and Mash1, a TA cell marker of the neuronal lineage, revealed co-localization of the two nuclear antigens in some SVZ cells (Fig. 1B, top inset). However, Mash1 IR was evident in only a minority of the Id1+ cells. Cells that were Id1+ Mash1− (Fig. 1B, bottom left inset), as well as Id1− Mash1+ (not shown), were evident. Interestingly, co-expression of Id1 and Mash1 appeared to be limited to cells expressing comparatively lower levels of Id1 (Fig. 1B, compare levels in Id1high cells at bottom left inset and in Id1intermediate cells at bottom right inset). Thus, a gradient of Id1 and Mash1 levels was apparent in the subventricular Id1-expressing cells. The cells expressing lower levels of Id1, Id1int cells, co-expressed Mash1, a marker of a more differentiated phenotype. Moreover, TSA immunofluorescence revealed similar results with Id1 and Olig2, a TA cell marker of the oligodendroglial lineage (Supplementary Figure 5).
Consistent with a gradually decreasing gradient of Id1 levels along the subventricular neurogenic lineages, PSA-NCAM+ neuroblasts or NeuN+ neurons never expressed Id1 (Fig. 1C, D). Moreover, S100b+ ependymal cells also never expressed Id1 (Fig. 1E). Importantly, S100b+ parenchymal astrocytes were Id1−, suggesting subventricular astrocyte-specific Id1 expression.
Finally, subventricular and parenchymal cells with elongated Id1+ nuclei were CD31+ endothelial cells (Fig. 1F and Supplementary Fig. 2C middle box and Supplementary Fig. 12). In the SVZ, the two morphologically discernable Id1+ cell types were often located nearby (Fig. 1B top inset), suggesting that the GFAP+ Id1high astrocytes reside in or near the perivascular stem cell niche (Shen et al., 2008; Tavazoie et al., 2008).
We further quantitated the Id1-expressing populations by flow cytometry using an Id1VenusYFP knock-in allele. In the Id1VenusYFP mouse, a fusion protein of Id1 and Venus fast-maturing yellow fluorescent protein is synthesized from the endogenous Id1 locus (Supplementary Fig. 1). Subventricular tissue from Id1V/V mice was microdissected from ~1 mm coronal slices (~0.7 mm to ~−0.3 mm relative to Bregma), dissociated with papain, and analyzed by flow cytometry (Supplementary Fig. 6). Flow cytometric quantitation of at least 20,000 cells indicated 3.1 ± 0.64% of the cells of the microdissected tissue expressed Id1 (mean ± standard deviation, n = 14, 3 mice per n). Of these Id1-expressing cells, 16% were GFAP+ (Fig. 1G). Thus, the Id1-expressing subventricular astrocytes constituted only 0.49% of the cells of the microdissected subventricular tissue, close to the 0.4% of subventricular cells estimated to be stem cells. Of the Id1-expressing cells, 24% were Mash1+ (Fig. 1H), though the majority of Mash1+ cells did not express Id1. Consistent with the immunohistological analyses, only one Id1high cell in ~20,000 subventricular cells dissociated from three mice expressed Mash1 (Fig. 1H inset). Moreover, most Id1intermediate-high cells expressed lower levels of Mash1 than Id1intermediate-low cells. Finally, PSA-NCAM+ cells did not express Id1 (Fig. 1I), consistent with the immunohistochemical analyses. In sum, of all subventricular Id1-expressing cells, 16% were GFAP+ astrocytes, 24% were Mash1+ cells, 10% were Olig2+ cells (data not shown), and 44% were CD31+ endothelial cells (Supplementary Fig. 12), with 6% unclassified.
Functional quiescence of the subventricular Id1high astrocytes
The analysis above identified two distinct neural cell types with round Id1+ nuclei: Id1high astrocytes and Id1int Mash1+ or Olig2+ TA cells. We characterized the cell cycle status of these cells with morphologically distinguishable round IR Id1+ nuclei, thereby excluding the endothelial cells. First, 68% of the neural Id1+ cells were Id1int Mash1+ or Olig2+ TA cells. Some of these TA cells are expected to be actively cycling in S-phase as identified by a one-hour pulse of thymidine analog. Indeed, 32% of the round Id1+ cells were EdU+ by immunofluorescence (Fig. 2A). The majority (95%) of the EdU+ S-phase cells, however, were Id1−. Ki67 and Mcm2 label a larger population of cycling cells than a one-hour pulse of thymidine analog: Ki67 labels cells in G1-, S-, G2-, and M-phase; Mcm2 specifically labels cells in G1-phase and as well as relatively quiescent cells (Maslov et al., 2004). Accordingly, 73% and 75% of the cells with round Id1+ nuclei were Ki67+ and Mcm2+, respectively (Fig. 2B, C). These results suggested that the 68% of Id1+ cells co-expressing TA cell markers, Mash1 or Olig2, are indeed actively cycling.
Figure 2. Id1high astrocytes are relatively quiescent but can be activated to enter cell cycle.
A–D. One hour after injection of thymidine analog EdU, 95% of all EdU+ (S-phase) cells were Id1− (A). 32% of round Id1+ cells were EdU+ (A insets). Only 13% of Ki67+ cells were Id1+ (B). 73% of round Id1+ cells were Ki67+ (B insets). Similarly, only 13% of Mcm2+ cells were Id1+ (C), but 75% of round Id1+ cells were Mcm2+ (C insets). Although a one-hour injection of EdU labeled only a fraction of Id1+ cells, a seven-day infusion of EdU via miniosmotic pump labeled the majority of Id1+ cells (D). E–G. A seven-day intraventricular infusion of Ara-C ablated all S-phase cells labeled by one-hour pulse of EdU (E). Id1+ GFAP+ cells persisted after the Ara-C infusion (E inset). One and two days after Ara-C infusion, EdU pulse revealed rare EdU+ S-phase cells (F, G). 63% of the EdU+ cells were Id1+ (F, G insets), though only 37% of round Id1+ cells were EdU+. Scale bars = 10 microns.
Second, 16% of neural Id1+ cells were Id1high GFAP+ astrocytes. Immunofluorescence analyses indicated a majority of the Id1high astrocytes are EdU− (Fig. 2A and see below), and thus not synthesizing DNA at detectable levels. Nevertheless, 73% of the Id1high astrocytes expressed Ki67. Based on Ki67 or Mcm2 expression but lack of detectable DNA synthesis, we suggest that Id1high astrocytes may be "paused" in various phases of the cell cycle, including G1. Consistent with the relative quiescence of Id1high astrocytes, a 7 d infusion of EdU by miniosmotic pump labeled a greater percentage of Id1high cells than a 1h pulse (Fig. 2D, compare to Fig. 2A).
The quantitation above suggested that some Id1high astrocytes may express proliferation markers Ki67 or Mcm2. Nevertheless these astrocytes are not actively synthesizing DNA and thus may persist after infusion of an antimitotic, Ara-C. This "functional quiescence" of the Id1high astrocytes was directly tested by 6 d infusion of Ara-C, which ablates rapidly dividing C and A cells but spares quiescent B cells and non-stem-cell astrocytes (Doetsch et al., 1999a). Indeed, the GFAP+ Id1high astrocytes persisted after Ara-C ablation (Fig. 2E inset), consistent with their relative quiescence. Concomitantly, the number of round Id1+ nuclei (i.e.neural Id1+ cells) decreased by 83% (compare Figs. 2E–G to 2A–D); most remaining Id1+ cells were Id1high (Fig. 2E–G). This reduction is roughly consistent with the quantitation in which 68% of the neural Id1+ cells were Mash1+ or Olig2+ Id1int TA cells (see above) and therefore expected to be ablated by Ara-C infusion. Thus, this experiment showed directly that the Id1high astrocytes are indeed functionally quiescent despite expression of Ki67 or Mcm2. At 12 and 48 hours after Ara-C infusion, EdU was injected to label the B cells activated to enter cell cycle. No EdU+ cells were observed immediately after Ara-C infusion (Fig. 2E). At 12 and 48 h after the infusion, the majority of the rare EdU+ cells were Id1+ (Id1+ EdU+ / all EdU+ = 63% at 48 h), although not all Id1+ cells were EdU+ (Id1+ EdU+ / all Id1+ = 37% at 48 h). Thus, the Id1high astrocytes are functionally quiescent but some entered S-phase upon activation. More than half of the cells that entered S-phase after Ara-C infusion were Id1high astrocytes.
Subventricular GFAP+ Id1high astrocytes are B1 type cells
That high Id1 expression defined a functionally quiescent population of subventricular astrocytes capable of entering S-phase raised the possibility that Id1high astrocytes are B1 type stem cells. Id1-expressing cells were fate-mapped using the Id1IRES-creERT2 and various reporter alleles (Supplementary Figs. 2, 3, and 8). In mice heterozygous for the Id1IRES-creERT2 allele, Id1-expressing cells express the tamoxifen-inducible cre recombinase, creERT2, from a bicistronic messenger RNA by means of an encephalomyocarditis virus internal ribosome entry site. Because of the low creERT2 expression level and thus activity in the Id1IRES-creERT2/+ mice, cre-mediated recombination most likely occurs in a small number of cells expressing comparatively higher levels of Id1. Fortuitously, this low-level creER activity enabled selective mapping of the Id1high cells (see below and Discussion). In Id1IRES-creERT2/+ mice, the creER activity was not leaky but tamoxifen inducible (Supplementary Fig. 9).
3 d after the last of three tamoxifen doses, X-gal histochemistry of Id1IRES-creERT2/+;StLa (Stop-tau Lac Z, Supplementary Fig. 3) mouse sections revealed rare single cells with astrocytic morphology in the SVZ (Supplementary Fig. 2 and Supplementary Fig. 10C, n = 3). In total, we examined 144 12-micron cryo-sections covering the anterior SVZ (from ~1.8 mm to ~−0.9 mm relative to Bregma), and found 10 single tau-β-gal+ subventricular astrocytic cells. A comparison of the number of X-gal+ cells to Id1-immunoreactive cells indicated <1% labeling efficiency in the brain at the three day time point, consistent with the notion that recombination occurred only in Id1high Mash1/Olig2− cells. These rare tau-β-gal+ cells in the SVZ with astrocytic morphology were GFAP+ astrocytes (Supplementary Fig. 10D). As expected, no X-gal+ cells with neuronal morphology nor tau-β-gal+ NeuN+ neurons were found in the OB at this time (Supplementary Fig. 10E, F).
At present no single marker specifically identifies the B1 type stem cells, with the possible exception of Tailless (Liu et al., 2008). Nevertheless, in the SVZ, the B1 type stem cells are organized in a "pinwheel" architecture and extend a characteristic long single basal process within the SVZ (Mirzadeh et al., 2008). Indeed, immunofluorescence of YFP+ cells in Id1IRES-creERT2/+;R26LSL-YFP (Rosa26 Lox-Stop-Lox YFP) mice revealed that YFP+ cells are Id1high astrocytes with round nuclei (Fig. 3A). Consistent with a B1 type identity of the genetically identified Id1high astrocytes, immunofluorescence of SVZ whole mounts from Id1IRES-creERT2/+;R26LSL-YFP mice 3 d post-tamoxifen revealed rare YFP+ GFAP+ cells with the characteristic long single GFAP+ basal process (Fig. 3B, n = 7). Moreover, the YFP+ astrocytes were located at the center of the pinwheel (Fig. 3C) and extended basal processes at least 90 µm long (Fig. 3D). B2 type astrocytes were never observed. Finally, all YFP+ astrocytes analyzed were Mash1− (and EdU−, see below), consistent with the data that the Id1high cells are Mash1−. Thus, the genetically labeled cells in these mice are indeed Id1high astrocytes, rather than the Id1int Mash1+ C type TA cells (n = 13 cells).
Figure 3. Subventricular GFAP+ Id1high astrocytes are B1 type astrocytes.
A. In the Id1IRES-creERT2/+;R26LSL-YFP mice three days post-tamoxifen, TSA immunofluorescence indicated high Id1 expression in the YFP+ astrocytes. Inset shows a z-stack projection. B. SVZ whole mount immunofluorescence revealed subventricular YFP+ GFAP+ astrocytes with a long single GFAP+ basal process, characteristic of B1 astrocytes. Inset shows YFP IR. The ventricular surface is shown en face. C.β-catenin staining revealed the pinwheel epithelial architecture, outlined by the dotted line. GFP IR of an Id1high astrocyte at the center of the pinwheel is shown. D. The basal processes of the YFP+ GFAP+ astrocytes extended at least 90 microns in these optical sections. E. In the Id1IRES-creERT2/+; GFAP∷LSL-GFP mice three days post-tamoxifen, genetically identified GFP+ (GFAP+ Id1high) astrocytes at the center of the pinwheels are shown. Two GFP+ astrocytes are in the field as well as two additional astrocytes in the insets. F–G. A GFP+ GFAP+ astrocyte with a long basal process is shown. Consistent with the cell cycle status of the Id1high astrocytes, these astrocytes did not incorporate a one-hour pulse of EdU (F), though EdU+ C type cells were observed elsewhere (F, inset). Scale bars = 10 microns.
Furthermore, combined with the Id1IRES-creER allele, the GFAP∷LSL-GFP transgenic mouse (Casper and McCarthy, 2006) enabled genetic identification of the GFAP-expressing Id1high astrocytes. Immunofluorescence of whole mounts from the Id1IREScreERT2/+; GFAP∷LSL-GFP mice 3 d post-tamoxifen revealed rare GFP+ (GFAP+ Id1high) subventricular astrocytes at the center of a pinwheel (Fig. 3E, n = 4). Moreover, immunofluorescence confirmed GFAP expression in these astrocytes and revealed a long basal process, consistent with the data above (Fig. 3F–G, n = 6). Consistent with the cell cycle status of most Id1high astrocytes, the GFP+ astrocytes did not incorporate EdU during a 1 h pulse (Fig. 3F), indicating that these cells are distinct from the Id1int Mash1+, Dlx2+, or Olig2+ C type TA cells in S phase. Finally, these astrocytes extended a single basal GFAP+ process and contacted the nearby vasculature (data not shown).
Subventricular GFAP+ Id1high B1 type astrocytes are neurogenic stem cells
We then examined whether the genetically identified GFAP+ Id1high B1 type astrocytes function as stem cells. 1 and 6 mo after the last tamoxifen dose, X-gal histochemistry of Id1IRES-creERT2/+;StLa and Id1IRES-creERT2/+;R26R mice sections revealed rare β-gal+ cells in the SVZ, RMS, and the OB (Supplementary Fig. 11). 6 mo after the last tamoxifen dose, in 72 12-micron sections examined, 4 single subventricular X-gal+ cells were found. X-gal+ cells were also found in the RMS and OB. Clusters of X-gal+ cells were never found, consistent with a cell division time longer than the time required for migration out of the SVZ. However, the number of X-gal+ cells in the SVZ increased by 1 mo post-tamoxifen (Supplementary Fig. 9).
Some tau-β-gal+ cells in the RMS and OB of the Id1IRES-creERT2/+;StLa mice at 1 mo post-tamoxifen were DCX+ neuroblasts with migratory morphology (Fig. 4A, B) and NeuN+ neurons (Fig. 4C), respectively. Thus, under normal physiologic conditions subventricular GFAP+ Id1high B1 type astrocytes gave rise to DCX+ neuroblasts that migrated to the OB and differentiated into NeuN+ neurons.
Figure 4. Subventricular GFAP+ Id1high B1 type astrocytes are neurogenic stem cells.
A–C. One month post-tamoxifen, single tau-β-gal+ DCX+ neuroblasts and tau-β-gal+ NeuN+ granular neurons were found in the RMS (A, B) and in the OB (C), respectively, in the Id1IRES-creERT2/+;StLa mice. A tau-β-gal+ endothelial cell (e) is also shown in (C). D. Six months post-tamoxifen, X-gal histochemistry revealed nuclear-β-gal+ nuclei of postmitotic neurons in the Id1IRES-creERT2/+;TauLSL-mGFP-IRES-nLacZ mice. X-gal+ nuclei were mapped from an overlay of eighteen 12-micron cryosections. Blue circles indicate labeled neurons. Images of X-gal+ nuclei in the granular cell layer (GCL) and glomerular layer (GL). E. The neurogenic output from the Id1high astrocytes increased over time. F. At 1 mo post-tamoxifen, immunofluorescence confirmed the neuronal morphology of GABA-immunoreactive mGFP+ neurons in the GCL. G. After Ara-C infusion, the Id1high astrocytes incorporated EdU, and gave rise to an EdU+ mGFP+ neuron in the GCL. In the inset, red circles indicate the location of EdU+ nuclei in a randomly selected section of the OB, demonstrating continued neurogenesis. H. 3 mo post-tamoxifen, whole mount immunofluorescence revealed β-gal+ GFAP+ astrocytes with B1 type morphology in the Id1IRES-creERT2/+;StLa mice. a, astrocyte. e, endothelial cell. I. Six months post-tamoxifen, YFP+ astrocytes (that once expressed high levels of Id1 and were thus labeled) expressed Id1 in the Id1IRES-creERT2/+;R26LSL-YFP mice.
As some of the genetically labeled cells in the Id1IRES-creERT2/+;StLa mouse brains were endothelial cells (Supplementary Figs. 2 and 12), we utilized a postmitotic neuron-specific reporter allele, TauLSL-mGFP-IRES-nLacZ (Hippenmeyer et al., 2005). In this mouse, the endogenous Tau (Mapt) promoter specifically labels the postmitotic neuronal progeny of the Id1high cells (Supplementary Fig. 8). This enabled facile identification of the neuronal progeny and flow cytometric quantitation of the neuronal output at the steady-state from subventricular Id1high B1 astrocytes to the OB's using X-gal histochemistry and flow cytometry.
X-gal histochemistry of OB's at six months post-tamoxifen revealed nuclear-β-gal+ postmitotic neurons in the granular cell layer and in the glomerular layer (Fig. 4D). Granular neurons were more numerous than periglomerular neurons. Flow cytometric quantitation at 2, 6, and 24 w post-tamoxifen indicated 6068 ± 2480, 28179 ± 4866, and 67195 ± 2087 GFP+ neurons, respectively (mean ± SEM, n = 3 per time point, Fig. 4E). The increase in neuronal output was statistically significant between 2 and 6 w (P < 0.05, unpaired two-tailed Student's t-test) and 6 and 24 w (P < 0.01, unpaired two-tailed Student's t-test). Immunofluorescence of OB sections from 1 mo post-tamoxifen confirmed the neuronal morphology of GFP+ cells in the granular cell layer (Fig. 4F) and GABA immunoreactivity (Fig. 4F inset).
We then directly demonstrated the quiescence, cell cycle entry, and neurogenic potential of the subventricular Id1high astrocytes with the Id1IRES-creERT2/+;TauLSL-mGFP mice. These mice were gavaged with tamoxifen, then infused with Ara-C. This infusion ablates the rapidly-dividing Id1int Mash1/Olig2int C type cells as well as the A type neuroblasts, while relatively quiescent Id1high B type cells persist (see Fig. 2F inset). 12 h after Ara-C infusion, when some of the normally quiescent B type cells enter cell cycle, EdU was injected to label B type cells in S-phase. Finally, after a 2 w chase, analyses of the sections revealed GFP+ EdU+ neurons in the olfactory bulb (Fig. 4G). This experiment directly demonstrated that 1) the genetically-identified Id1high B1 type astrocytes are functionally quiescent and thus can persist through the antimitotic treatment, 2) these genetically-identified astrocytes can enter S-phase, and 3) these genetically identified astrocytes are undifferentiated and give rise to postmitotic neurons whose identity is unambiguously reported by the TauLSL-mGFP knock-in allele.
Next, we examined whether the GFAP+ Id1high B1 type astrocytes persist in the niche. In Id1IRES-creERT2/+;StLa mice 3 mo after tamoxifen administration, whole mount immunofluorescence revealed tau- -gal+ GFAP+ astrocytes with B1 type morphology (Fig. 4H). Finally, in Id1IRES-creERT2/+;R26LSL-YFP mice 6 mo after tamoxifen administration, YFP+ cells (that once expressed high levels of Id1) continued to express Id1 (Fig. 4I).
Cultured subventricular Id1high stem cells can self-renew asymmetrically
The data described above strongly suggested that Id1high astrocytes are stem cells. Potentially, the Id1high B1 type stem cell could self-renew asymmetrically and produce an asymmetric pair of progeny cells in vivo, i.e., an Id1high B1 type stem cell and an Id1int Mash1int C type TA cell which differ in Id1 protein levels. Experiments indicated that the Id1 and Id1-Venus expression levels are heterogeneous in NS adherent adult SVZ cell cultures (Glaser et al., 2007; Pollard et al., 2006), as shown in Fig. 5A, B and Supplementary Fig. 7B). These adherent radial glia-like tripotent cells derived from adult SVZ neurospheres were ~100% nestin+, and importantly, free of non-neural cell types, e.g., endothelial cells (Supplementary Fig. 7).
Figure 5. Cultured subventricular Id1high stem cells can self-renew asymmetrically.
A. Confocal microscopy of Id1V/V NS cells revealed heterogeneous Id1-Venus fluorescence levels, consistent with the immunofluorescence analyses (Fig. 1B and Supplementary Fig. 7B). B. Flow cytometric measurements of the Id1-Venus levels also indicated heterogeneous populations in early passage NS cells. Four populations were arbitrarily gated. C. The four sorted populations were stained with sub-saturating amount of antibodies. The percentage of Mash1high or Olig2high cells was low in the Id1high population, peaked in the Id1intermediate-high population, and decreased again in the Id1intermediate-low and Id1low populations. D. In low density cultures, purified subventricular Id1high stem cells (solid line) generated Id1high, Id1int, and Id1low cells whereas purified Id1low neuroblasts (dashed line) generated only Id1low cells. Id1 gate and the percentage of Id1high and Id1low cells are shown. E. We define asymmetric self-renewal as an Id1high stem cell division that generates an Id1high stem and an Id1int transit amplifying cell. Modeling of cell cycle kinetics measurements of the Id1high and Id1low cells indicated population dynamics of the Id1high cells consistent with asymmetric self-renewal in at least ~50% of the Id1high cells (see Supplementary Information). F. Consistent with asymmetric self-renewal of the Id1high stem cells, the frequency of the Id1high cells gradually decreased during serial passages. G. Single FAC sorted Id1high cells gave rise to clusters of cells with high and lower Id1 levels.
Based on their in vitro functional activity (see below and Fig. 6) and transcriptional profile (see Supplementary Information), we surmised that the Id1high and Id1low NS cells are functionally and molecularly distinct B-like stem and A-like neuroblast cells, respectively. Most B or A cells do not express C cell markers, Mash1, and Olig2, and accordingly, these were not differentially expressed in Id1high B-like and Id1low A-like cells in microarray analyses. To determine whether Id1intermediate NS cells correspond to C-like cells, four populations were FAC sorted (Fig. 5B) and then stained with Mash1 or Olig2 antibodies. Indeed, the progression of Mash1 or Olig2in Id1high, Id1intermediate-high, Id1intermediate-low, to Id1low cells was consistent with that observed in B, C, to A cells in vivo, respectively (Fig. 5C). Thus, the Id1-Venus mouse and the adherent culture system provided a unique experimental system to examine the self-renewal behavior of unperturbed Id1high stem cells.
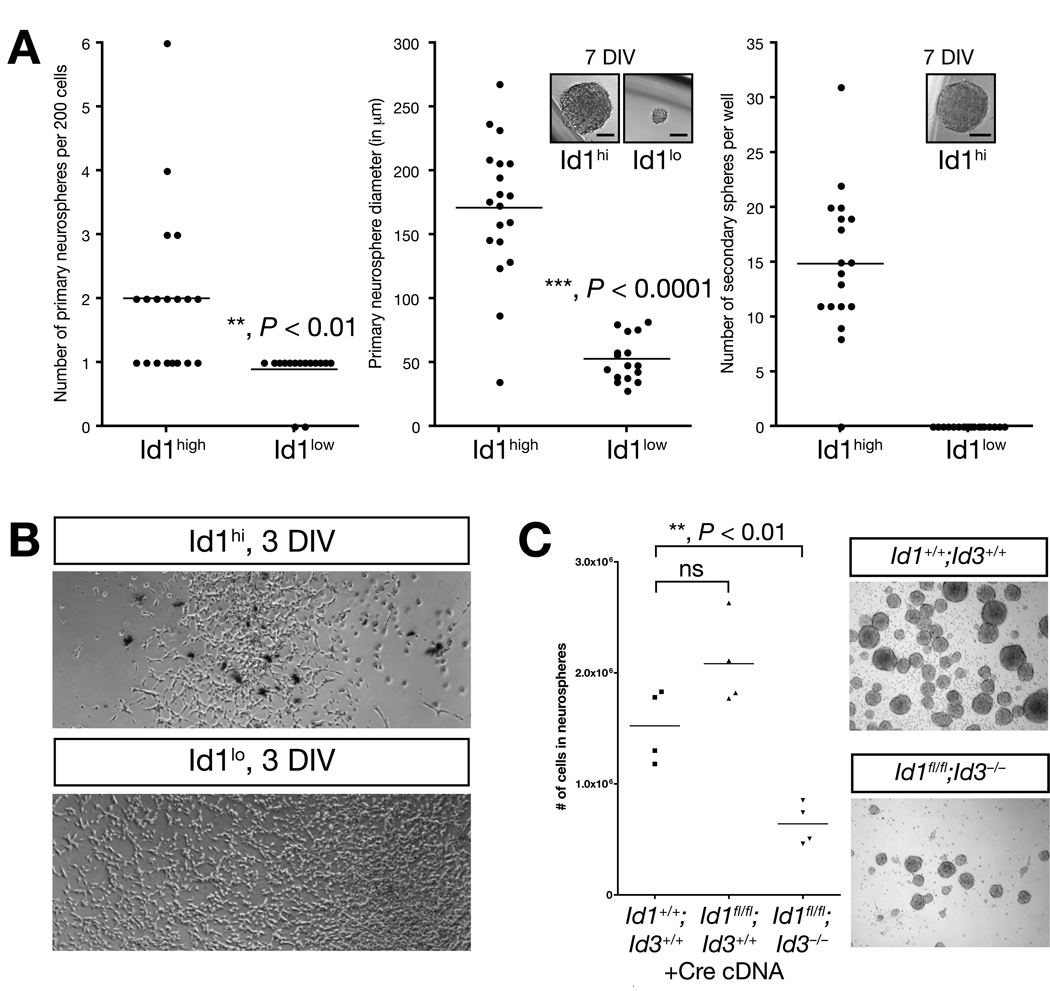
Figure 6. Id genes are necessary for self-renewal, a characteristic of stem cell identity, but dispensable for proliferation.
A. In clonal neurosphere assays, FAC-sorted Id1high cells formed larger and more numerous neurospheres that could be passaged while Id1low cells did not. Unpaired two-tailed Student’s t-test. DIV = days in vitro. Scale bar = 50 microns. B. Id1high and Id1low cells were FAC sorted and cultured as adherent monolayer. In this culture condition, Id1low cells proliferated more rapidly than the Id1high cells. C. In bulk neurosphere assays, Id1 and Id3, but not Id1 alone, were required for secondary neurosphere formation. ~50% efficiency nucleofection of cre cDNA (data not shown) resulted in ~50% reduction of secondary neurosphere cells. Neurosphere cells from Id1−/− and Id3−/− mice showed unimpaired secondary sphere formation (data not shown). Unpaired two-tailed Student’s t-test.
We examined the progeny of the Id1high B-like stem and Id1low A-like neuroblast cells in the adherent adult SVZ cell cultures by culturing the purified cells individually and measuring the percentage of Id1high, Id1int, and Id1low cells flow cytometrically. In serum-free medium supplemented with FGF-2 and EGF, the Id1high (Mash1− or Olig2−) stem cells generated Id1high (Mash1− or Olig2−) and Id1int (Mash1+ or Olig2+) cells (as evidenced by the leftward shift of the histogram concomitant with increase in cell density), but the Id1low neuroblast cells generated only Id1low cells and did not generate Id1high or Id1int cells (Fig. 5D).
Cell cycle parameters of the adherent Id1high and Id1low cells were measured by BrdU pulse-chase and flow cytometric quantitation (data not shown). When the experimental data (shown in Fig. 5D) were modeled with population dynamics based on experimentally obtained cell cycle parameters of the Id1high and Id1low populations, the data closely resembled the population dynamics predicted of asymmetric self-renewal in ~ 50% of the Id1high cells (Fig. 5E, also see Supplementary Information). This mathematical analysis was consistent with the notion that Id1high cells can self-renew asymmetrically in a population of stem/progenitor cells.
In support of the notion that the Id1high cells can self-renew asymmetrically during serial passages the percentage of the Id1high cells were diluted out by more rapidly proliferating Id1low cells (Fig. 5F). Despite the reduction in the frequency of Id1high cells, obvious morphological changes to the predominantly bipolar radial glia-like morphology were absent even after forty passages, or four months, in culture (data not shown). Finally, when Id1high cells were FAC sorted and cultured at clonal density, Id1high cells gave rise to clusters of cells expressing high and lower levels of Id1 (the latter undetectable by confocal microscopy) (Fig. 5G).
Id genes are necessary for self-renewal, a characteristic of stem cell identity
The experiments above suggested that in addition to identifying the B1 type astrocyte stem cells, high Id1 expression may be functionally important in neural stem cell identity. We thus assayed neurosphere-forming activity of Id1high and Id1low fractions in early and later passage Id1V/V adult adherent SVZ cell cultures. When Id1high and Id1low cells were FAC sorted and compared in clonal neurosphere assays, Id1high cells generated more and larger spheres (Fig. 6A, sphere number, ~2-fold increase, n = 19, P < 0.01; sphere size, ~3.5-fold increase, n = 19, P < 0.0001; unpaired two-tailed Student’s t-test). Upon passaging, only Id1high cell-derived neurospheres formed secondary neurospheres (n = 18). The Id1low cell-derived spheres did not form secondary spheres. When cultured as an adherent monolayer, Id1low cells proliferated more rapidly thanId1high cells (Figure 6B). Thus, only Id1high cells were capable of self-renewing anchorage-independently.
The analyses above suggested high levels of Id1 are required for self-renewal in unperturbed cells, a characteristic of stem cell identity. However, adult Id1−/− or Id3−/− mice are viable and fertile, and neurosphere self-renewal is normal (data not shown), suggesting redundancy or compensation. Thus, we asked whether both Id1 and Id3 are functionally required for neurosphere formation using the Id1-floxed conditional allele (Supplementary Fig. 4). Neurospheres of Id1+/+;Id3+/+, Id1fl/fl;Id3+/+, and Id1fl/fl;Id3−/− genotype were nucleofected with cre recombinase cDNA (~50% efficiency by flow cytometry). Ablation of Id1 and Id3, but not Id1 alone, was sufficient to reduce secondary neurosphere formation by ~50% (Fig. 6C), a number consistent with near complete absence of self-renewal in the transfected population. Genomic PCR detected the unrecombined floxed allele in neurospheres that formed (data not shown).
Subgranular GFAP+ Id1high astrocytes are also neurogenic stem cells
To determine whether high Id1 expression is a general characteristic of GFAP+ astrocyte stem cells, we examined Id1 expression and the progeny of Id1-expressing cells in the hippocampal dentate gyrus. Id1+ cells were evident in the subgranular layer, and these cells were GFAP+ (Fig. 7A), although a clear long apical GFAP+ process wasn't apparent for all Id1+ subgranular cells. These Id1+ cells also expressed nestin, a marker of neural stem/progenitor cells (Fig. 7B). Consistent with the Id1 gradient evident in the subventricular neurogenic lineages, subgranular Id1+ cells never expressed DCX and NeuN (Fig. 7C, D). Finally, in Id1IRES-creERT2/+;StLa mice one month post-tamoxifen, tau-β-gal+ NeuN+ cells with neuronal morphology were evident in the granuar cell layer (Fig. 7E).
Figure 7. Subgranular Id1high cells are also B type neural stem cells.
A. Id1 IR was apparent in the subgranular zone of the dentate gyrus. Often, the Id1+ cells were found in clusters. Some of these Id1+ cells were GFAP+ astrocytes with a single long apical process (arrows). B. The subgranular Id1+ cells also expressed nestin (arrows). C, D. None of the subgranular Id1+ cells co-expressed DCX nor NeuN. E. 1 mo post-tamoxifen, single tau-β-gal+ NeuN+ neurons with mature neuronal morphology were evident in the hippocampal granular cell layer of the Id1IRES-creERT2;StLa mice. The tau-β-gal reporter protein decorated the cell body, dendrites, and the axon extending into the hilus (arrowhead). Scale bars, 10 microns. F. We propose a gradient model of transcriptional regulators and transcription factorsin the subventricular neurogenic lineages.
Discussion
A window into the molecular identities of mammalian neural stem cells
We define and distinguish stem cells from progenitor cells as self-renewing and multipotent cells that persist throughout the adult life of an animal, in contrast to a brief window of developmental time. Our data strongly suggest that the GFAP+ Id1high B1 type astrocytes are the bona fide stem cells of the adult murine subventricular neurogenic lineages.
First, the Id1high B1 type astrocytes are rare. Previous estimation of the endogenous NSC frequency (Morshead et al., 1998) suggested merely 0.4% of the subventricular cells are stem cells, based on the number of cells that entered the cell cycle after high-dose 3H-thymidine kill. Flow cytometric quantitation of GFAP+ Id1high astrocytes in the anterior SVZ indicates that these cells constitute approximately 0.5% of the subventricular cells. Of note, a recent study showed that 2% of subventricular cells are GFAP+ EGFR+ activated stem cells (distinct from quiescent stem cells), suggesting the stem cell number to be somewhat higher than that in the Morshead et al. study (Pastrana et al., 2009). In contrast, an in vitro study utilizing a colony-forming assay suggested the quantitation in the Morshead et al. study to be an overestimation (Golmohammadi et al., 2008). Thus, a consensus on the NSC frequency is lacking. However, previous work in other organ systems, notably the hematopoietic system, suggest stem cells to be rare, with a frequency of less than 0.1–1% (e.g., (Kiel et al., 2005; Spangrude et al., 1988). Despite the greater cellular heterogeneity of the bone marrow, the low frequency of the HSC's suggest that stem cells in other niches may also be rare in comparison to differentiated cells.
Second, Id1high B1 type astrocytes are relatively quiescent at steady state but are capable of proliferation when stimulated. A majority of these cells are not actively synthesizing DNA at any given moment since they do not incorporate a thymidine analog at a detectable level in short pulses. However, many of the Id1high astrocytes express Ki67. Thus, we suggest that these astrocytes are paused in various stages of the cell cycle though the exact cell cycle phases are unclear. Nonetheless, these astrocytes are indeed capable of proliferation, as a week-long infusion of a thymidine analog labeled a greater fraction of the Id1high cells. In addition, Ara-C ablation activated more than half of these astrocytes to enter S-phase and give rise to neuronal progeny. Taken together, we suggest that the Id1high astrocytes are thus functionally quiescent in the steady-state. That is, these astrocytes are capable of cell division and express markers of a proliferative state, e.g., Ki67 or Mcm2, but are not actively dividing and thus can persist through Ara-C infusion. This result is consistent with the recent finding that the ventricle-contacting B1 type astrocytes are found in all phases of the cell cycle (Mirzadeh et al., 2008).
Third, the Id1high B1 type astrocytes are undifferentiated and give rise to differentiated neuronal progeny in vivo. The Id1high astrocytes give rise to both granule neurons and periglomerular neurons. In addition, we find β-gal+ Olig2+ cells with oligodendrocytic morphology throughout the parenchyma (H–s. N., unpublished observations). In vitro, Id1high cells also give rise to glial progeny. However, we cannot conclude whether a single subventricular Id1high astrocyte can give rise to the parenchymal oligodendrocytes as well as OB neurons, demonstrating multipotency of a single stem cell.
Fourth, the neuronal output from the Id1high B1 type astrocytes amplifies over time. This experiment quantitatively demonstrates the sustained neurogenic potential of the Id1high B1 type astrocytes. Previous studies indicate that when rapidly dividing cells are labeled by 3H-thymidine once per day for 4 d, neuronal output peaks at ~15 d then drops until ~45 d (Petreanu and Alvarez-Buylla, 2002). In contrast, when relatively quiescent stem cells are labeled by stereotaxic lentiviral injection, neuronal output continues to increase 2–6 w post-labeling, with a linear increase between 4–6 w (Reumers et al., 2008). The neuronal output from the Id1high astrocytes increases linearly between the 2 and 6 w time points. After 6 w, the neuronal output continues to increase and eventually plateaus at an approximately 2-fold increase relative to the 6 week time point. This neurogenic output curve is consistent with the Reumers et al. study. Thus, we suggest the neurogenic output is consistent with a stem cell identity of the Id1high B1 type astrocytes.
Fifth, the Id1high B1 type astrocytes maintain themselves and self-renew for at least 6 mo after labeling. At 3 mo post-tamoxifen, we find labeled astrocytes with B1 morphology in the SVZ. Moreover, the labeled Id1high B1 type astrocytes continue to express Id1. Since the Id1high astrocytes have functional characteristics of stem cells and the Id genes are critical for "stemness," a likely interpretation is that the Id1high B1 type astrocytes maintain themselves as stem cells for at least 6 mo after labeling. Future experiments should indicate whether the Id1high B1 type astrocytes maintain their ”stemness” and neurogenic potential during the entire lifespan of the mice.
Sixth, the Id1high B1 type astrocytes are capable of asymmetric self-renewal in vitro. Modeling of the flow cytometric quantitative data indicates that 50% of the Id1high cells in these cultures are self-renewing asymmetrically. Moreover, consistent with asymmetric self-renewal of the stem cells in these cultures, the frequency of the Id1high cells gradually decreases during long-term culture (up to 4 mo in these experiments). Finally, in clonal cultures, the Id1high (Mash− or Olig2−) stem cells give rise to Id1int (Mash1+ or Olig2+) TA and Id1low (Mash1− or Olig2−) neuroblast cells.
Taken together, these results strongly suggest that the subventricular GFAP+ Id1 high astrocytes are the long-sought-after B1 type neural stem cells. An outstanding question is whether these Id1high B1 type astrocyte stem cells represent all or a specific population of NSCs. The quantitation presented is consistent with the notion that these cells could constitute all of the subventricular stem cells based on previous quantitation in the Morshead et al. study. However, our experiments do not completely resolve this issue.
The distinct characteristics of Id1high, Id1intermediate, and Id1low cells
We propose a gradient model in which the cells of the subventricular neurogenic lineages express a gradually decreasing level of Id1 (Fig. 7F). That is, subventricular neurogenesis proceeds from GFAP+ Id1high B1 type stem cells to Id1int/low Mash1int/high C type TA cells to GFAP− Id1low Mash1low Pax6hi PSA-NCAM/DCX+ A type neuroblast cells.
This gradient is evident in vivo and in vitro. Fortuitously, the low frequency partial fate mapping of the subventricular Id1-expressing cells enabled genetic identification and fate mapping of the cells expressing comparatively higher levels of Id1, for the following reason. The probability of creERT2-mediated recombination depends upon its expression level (Kuhbandner et al., 2000). Even in Id1high cells, Id1 is expressed at a level lower than other gene products, e.g., GFAP (H–s. N., unpublished observations). The creER protein level is further lowered by translation initiated from the EMCV IRES (Bochkov and Palmenberg, 2006). As creER-mediated recombination activity depends upon its expression level, in cells expressing comparatively higher levels of Id1, i.e., the Id1high astrocytes, creER-mediated activation of the reporter gene is more likely to occur.
Indeed, YFP+ cells in Id1IRES-creERT2/+;R26LSL-YFP mice are Id1high at three days post-tamoxifen. Moreover, the GFP+ cells in Id1IRES-creERT2/+;GFAP∷LSL-GFP mice are EdU− and Mash1−, consistent with the immunofluorescence analyses in which most Id1high cells are EdU− and Mash1−.
Furthermore, in vitro experiments directly demonstrate the Id1 gradient in unperturbed cells of subventricular neurogenic lineages. In vitro, the Id1high B-like stem cells produce Id1high B-like stem and more differentiated Id1int C-like TA progeny cells, which in turn produce Id1low A-like neuroblast cells. Consistent with this, no difference in C cell markers, Dlx2, Mash1, or Olig2, is evident between the B-like Id1high and A-like Id1low populations. According to the model, the Id1int cells correspond to the C type TA cells. Indeed, when Id1high, Id1int, and Id1low cells are FAC sorted and analyzed with sub-saturating amount of anti-Mash1 or anti-Olig2 antibody, a C cell marker, the percentage of Mash1high cells and Olig2high cells is highest in the Id1int fraction and lower in the Id1high and Id1low fractions. Thus, for the first time to our knowledge, we directly demonstrate a gradient of transcriptional regulator, Id1, by a direct measurement and fractionation from the continuum of Id1-Venus fusion protein-expressing cells as they progress from stem to more differentiated precursor cells.
Importantly, these results suggest a threshold of Id1 levels for “stemness” (Fig. 7F). That is, distinct characteristics evident in Id1high, Id1int, and Id1low cells are expressions of subtle differences in the Id1 levels. These differences could contribute to the likelihood of a progeny cell altering its fate in concert with stochastic mechanism of cell fate determination, perhaps by altering the activity or expression of basic-helix-loop-helix transcription factors such as Mash1 or Olig2 (i.e., altering the balance of pro- and anti-neurogenic factors).
Towards a cell biological mechanism of stem cell self-renewal
In summary, our results suggest that high levels of Id1 expression define the B1 type NSC identity. Ongoing work shows that stem cells of another neural stem cell niche, e.g., olfactory epithelium, and a non-neural stem cell niche, e.g., intestinal crypt, express high levels of Id1 (H–s. N., unpublished observations), suggesting a broad and conserved role of Id1high stem cells and Id genes within and outside the central nervous system. Elucidating further the roles, contributions, and behavior of the Id gene products may ultimately shed light on the intricate molecular cell biological network process of adult stem cell self-renewal during normal and pathologic homeostasis.
Experimental Procedures
Gene targeting and mice
Bruce4 or CY2.4 ES cells were targeted and injected into wildtype or albino B6 or FVB blastocysts. Chimeras were bred with B6 or albino B6 mice. Following germline transmission, the FRT-Neo-FRT cassette was excised in vivo with B6 ACTB∷FLPE mice (Artemis Pharamaceuticals). Mice were backcrossed to B6 mice (The Jackson Laboratory) at least once before experiments. Thus, the mouse strains generated were on completely B6 genetic background. A. Joyner and S. Arber provided the R26LSL-YFP and TauLSL-mGFP-IRES-nLacZ mice. GFAP∷LSL-GFP mice were obtained from the MMRRC (B6(C3)-Tg(GFAP-lacZ, -EGFP)7Kdmc).
Histology and microscopy
Cryosections or whole mounts from 4% paraformaldehyde-fixed brains were stained and imaged with a confocal microscope (Leica) or Mirax scanner (Zeiss). All mice analyzed were ≥6-week-old.
Flow cytometry
Microdissected SVZ tissue was dissociated with papain (Worthington) then labeled with indicated antibodies. For phenotyping, ~20,000 cells were acquired with FACSCalibur or Aria cytometers (BD). Cells were sorted with a MoFlo sorter (DakoCytomation).
Ara-C infusion
A miniosmotic brain infusion kit (Alzet) was stereotaxically placed in coordinates of A/P: −1 mm and L: 1 mm. The pump delivered 0.5 ul of 4% Ara-C (Sigma) in artificial cerebrospinal fluid (Harvard Apparatus) for six days into the lateral ventricle.
Adherent neural stem/progenitor cell culture
Adult SVZ cells were cultured in Neurobasal-A (Invitrogen), B27 without vitamin A (Invitrogen), GlutaMax-1 (Invitrogen), Pen/Strep (Invitrogen), rmEGF (R&D), rmFGF-2 (R&D), and heparin (Sigma). For adherent culture, primary neurospheres were dissociated and plated in plates (Falcon) coated with poly-ornithine (Sigma) and laminin (Sigma). Thereafter the cells were maintained as adherent culture. Cells were dissociated with Accutase (Invitrogen) and passaged every ~2–3 d.
Supplementary Material
Acknowledgments
We thank K. Anderson, F. Doetsch, P. Mombaerts, L. Studer for helpful discussions; M. Khan for assistance with the generation of the Id1IRES-creERT2 allele; S. Anderson for critical review of the manuscript; S. Arber, M. Barbacid, A. Chenn, N. Copeland, R. DePinho, K. Hadjantonakis, and A. Joyner for reagents; C. Yang at the RU Gene Targeting Core; W. Mark at the Mouse Genetics Core; J. Hendrikx at the Flow Cytometry Core; and A. Viale at the Genomics Core. H.-S.N. designed and characterized the mice and performed the experiments. H.-S.N. and R.B. designed the experiments, interpreted the results, and wrote the manuscript. Work in this manuscript is the subject of a provisional US patent application entitled “Identification and Isolation of Adult Stem Cells and Related Methods of Use.” H.-S. N. is an NIGMS MSTP (T32GM07739), NIDCD Ruth L. Kirschstein (F30DC008707), and MSKCC Geoffrey Beene Fellow. Grants from the NIH (R.B.) and the Starr Foundation Tri-Institutional Stem Cell Initiative (H.-S.N. and R.B.) supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bochkov YA, Palmenberg AC. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques. 2006;41:283–284, 286, 288. doi: 10.2144/000112243. passim. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD. GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol Cell Neurosci. 2006;31:676–684. doi: 10.1016/j.mcn.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC, Jr., Fan G, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999a;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999b;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Glaser T, Pollard SM, Smith A, Brustle O. Tripotential differentiation of adherently expandable neural stem (NS) cells. PLoS ONE. 2007;2:e298. doi: 10.1371/journal.pone.0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS biology. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell and tissue research. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- Jeon HM, Jin X, Lee JS, Oh SY, Sohn YW, Park HJ, Joo KM, Park WY, Nam DH, DePinho RA, et al. Inhibitor of differentiation 4 drives brain tumor-initiating cell genesis through cyclin E and notch signaling. Genes Dev. 2008;22:2028–2033. doi: 10.1101/gad.1668708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, McNelly NA, Hinds JW. Population dynamics of adult-formed granule neurons of the rat olfactory bulb. J Comp Neurol. 1985;239:117–125. doi: 10.1002/cne.902390110. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc Natl Acad Sci U S A. 2001;98:4752–4757. doi: 10.1073/pnas.081074998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhbandner S, Brummer S, Metzger D, Chambon P, Hofmann F, Feil R. Temporally controlled somatic mutagenesis in smooth muscle. Genesis. 2000;28:15–22. doi: 10.1002/1526-968x(200009)28:1<15::aid-gene20>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci U S A. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HK, Belz T, Bock D, Takacs A, Wu H, Lichter P, Chai M, Schutz G. The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev. 2008;22:2473–2478. doi: 10.1101/gad.479308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Craig CG, van der Kooy D. In vivo clonal analyses reveal the properties of endogenous neural stem cell proliferation in the adult mammalian forebrain. Development. 1998;125:2251–2261. doi: 10.1242/dev.125.12.2251. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc Natl Acad Sci U S A. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Perk J, Gil-Bazo I, Chin Y, de Candia P, Chen JJ, Zhao Y, Chao S, Cheong W, Ke Y, Al-Ahmadie H, et al. Reassessment of id1 protein expression in human mammary, prostate, and bladder cancers using a monospecific rabbit monoclonal anti-id1 antibody. Cancer Res. 2006;66:10870–10877. doi: 10.1158/0008-5472.CAN-06-2643. [DOI] [PubMed] [Google Scholar]
- Perk J, Iavarone A, Benezra R. Id family of helix-loop-helix proteins in cancer. Nat Rev Cancer. 2005;5:603–614. doi: 10.1038/nrc1673. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SM, Conti L, Sun Y, Goffredo D, Smith A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16 Suppl 1:i112–i120. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. Estudios sobre la degeneracion y regeneracion del sistema nervioso. Madrid, Moya; 1913–14. [Google Scholar]
- Reumers V, Deroose CM, Krylyshkina O, Nuyts J, Geraerts M, Mortelmans L, Gijsbers R, Van den Haute C, Debyser Z, Baekelandt V. Stem cells. Vol. 26. Dayton, Ohio: 2008. Noninvasive and quantitative monitoring of adult neuronal stem cell migration in mouse brain using bioluminescence imaging; pp. 2382–2390. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.