Abstract
Atopic myelitis is defined as myelitis with atopic diasthesis but the cause is still unknown. Toxocariasis is one of the common causes of hyperIgEaemia that may lead to neurologic manifestations. The purpose of this study was to evaluate the sero-prevalence of Toxocara specific IgG Ab among the atopic myelitis patients. We evaluated the medical records of 37 patients with atopic myelitis whose conditions were diagnosed between March 2001 and August 2007. Among them, the 33 sera were analyzed for specific serum IgG Ab to Toxocara excretory-secretory antigens (TES). All of 37 patients had hyperIgEaemia. Specific IgE to D. pteronyssinus and D. farinae was detected in 22 (64.7%) and 34 (100%) patients, respectively, of the 34 patients. Thirty-one of 33 patients (93.9%) were found to be positive by TES IgG enzyme-linked immunosorbent assay (ELISA). Based on the image findings of eosinophilic infiltrations in the lung and liver, 8 patients had positive results. These results inferred that the prevalence of toxocariasis was high in patients with atopic myelitis. Our results suggest that toxocariasis might be an important cause of atopic myelitis and Toxocara ELISA is essential for evaluating the causes of atopic myelitis.
Keywords: Myelitis, Atopy, Toxocariasis
INTRODUCTION
Atopic myelitis (myelitis with atopic diasthesis, AM) is a disease entity that is characterized by the association between allergic and central nervous system (CNS) disease, and it is usually defined as myelitis of an unknown cause with either 1) hyperIgEaemia and mite antigen-specific IgE positivity or 2) coexistent atopic disease (1). AM was first described by Kira et al. in 1997, and they reported 4 cervical myelitis patients with associated hyperIgEaemia and atopic dermatitis (2). AM has been reported in nearly 100 patients particularly in Japanese patients, and it is characterized by distinct features such as 1) stepwise progression and fluctuation of the clinical course, 2) paresthesia and/or dysesthesia as the predominant symptoms, 3) the relatively infrequent occurrence of muscle weakness, 4) T2-high signal intensity lesions on magnetic resonance imaging (MRI), 5) hyperIgEaemia, 6) mite antigen-specific IgE positivity, 7) mild peripheral blood eosinophilia, and 8) eosinophilic inflammation on spinal cord biopsy (1, 3, 4). Therefore, an allergic mechanism such as cross-reactivity between environmental allergens and CNS antigens is thought to be important for this condition (5). But the pathogenesis of the disease is still unclear.
Toxocariasis is an ubiquitous parasitic infection by Toxocara canis or T. cati in man, the accidental hosts. It is caused by ingesting eggs in soil or by eating uncooked/undercooked animal liver or meat containing the infective-stage larvae (6, 7). The larvae hatch in the proximal small intestine, penetrate the mucosa, migrate into the liver and lung, and then they enter the systemic circulation till their progress is impeded (8). They eventually penetrate the capillaries and migrate aimlessly into the host tissue. The migrating larvae leave tracks of hemorrhage, necrosis and inflammatory cells and they induce immune-mediated hypersensitivity reactions that may lead to clinical manifestations with peripheral blood eosinophilia and hyperIgEaemia (8). The diagnosis is made by serologic confirmation using the enzyme-linked immunosorbent assay (ELISA) with Toxocara excretory-secretory antigens (TES-Ag) (9, 10) or the diagnosis is made, on rare occasions, by tissue biopsy (11). The seroprevalence of toxocariasis in rural Korean adults was detected to be approximately 5% (12) although the seroprevalence of toxocariasis varies depending on the other country (13).
Myelitis due to T. canis is a rare disease. It has been reported in only about 20 patients (11, 14-26) although toxocariasis is a worldwide-occurring parasitic infection. One possible explanation is that the accurate diagnosis of toxocariasis is impossible because either the diagnostic methods are not available or there is a lack of awareness by medical doctors about toxocariasis. The characteristics of Toxocara myelitis are; 1) predominant sensory disturbances (Lhermitte's sign, paresthesia and hypesthesia) with rare severe motor weakness, 2) high signal intensities on T2-weighted MRI with relatively mild symptoms, 3) peripheral blood hyperIgEaemia, 4) peripheral blood eosinophilia, and 5) eosinophilic inflammation that is noted on tissue biopsy (15, 24).
Our previous study showed that the prevalence of toxocariasis was high (68%) in patients with unknown eosinophilia, the patients who had a history of raw liver eating had a higher incidence, and the patients with liver and/or lung involvement were common (6). In the paper, we suggested that the clinical triad of toxocariasis is unexplained eosinophilia, the liver or lung nodules on imaging studies, and a history of eating animal liver can support clinical diagnosis of toxocariasis. While studying the characteristics of AM patients in Korea, we found out that most of them had raw food intake histories which are known to be reservoirs of the encapsulated larva of T. canis (6). Similar neurological symptoms with predominant sensory disturbance and clinical signs such as peripheral blood eosinophilia, hyperIgEaemia, and the similar MRI findings between AM and Toxocara myelitis lead us to hypothesize that Toxocara myelitis might be an important cause of AM. The existence of such patients prompted us to study the prevalence of Toxocara specific IgG Ab among the AM patients to gain insight into a link between AM and Toxocara myelitis.
MATERIALS AND METHODS
Subjects
We retrospectively analyzed the medical records of 37 patients with AM who visited our clinic between March 2001 and August 2007. AM was defined as myelitis of unknown cause with either 1) hyperIgEaemia and mite antigen-specific IgE positivity, or 2) coexistent atopic disease such as atopic dermatitis, allergic rhinitis, bronchial asthma and food allergy as described by Osoegawa et al. (1). The study was approved by the research ethics committee at the Samsung Medical Center, and 33 of the subjects gave a written informed consent.
Methods
The medical records were reviewed for information related to the clinical data of the patients, including the age at onset, gender, a history of preceding atopic diseases, a history of raw food intake that included raw liver, raw meat or raw freshwater fish within 6 month of symptom development, mode of onset, the clinical course, the clinical symptoms and signs, the location of lesions (MRI lesions), other organ involvements including the lung and liver (by plain chest radiography, computed tomography, or hepatic sonography), stool examinations, laboratory findings of peripheral blood eosinophil counts, serum total IgE and allergen specific IgE levels, the eosinophilic cationic protein (ECP) levels, parasite ELISA, the Toxocara specific IgG levels, and the cell counts in cerebrospinal fluid (CSF).
The mode of onset was defined as either acute (reaching maximum intensity within 1 month) or chronic (reaching maximum intensity after 1 month). Eosinophilia was defined as more than 500 cells/µL in peripheral blood or more than 5% in CSF. The serum total IgE and two common mite antigens, Dermatophagoides pteronyssinus and Dermatophagoides farinae-specific IgE levels were measured using an ImmunoCAP-250 (Pharmacia & Upjohn Diagnostics AB, Uppsala, Sweden). The upper normal limits of the serum total IgE and the mite antigen-specific IgE levels are 250 U/mL and 0.34 U/mL, respectively.
Specific IgG antibodies to parasite (Paragonimus, Sparganum, Cysticercus, and Clonorchis) in the serum and the CSF were measured at the Department of Clinical and Laboratory Medicine of Yong-San Hospital, Chung-Ang University (Seoul, Korea). The serologic diagnosis of Toxocara infection was done by measuring the specific IgG antibody to T. canis with a Toxocara ELISA kit (Bordier affinity products, Crissier, Switzerland) as described previously (7). Total 33 sera were kept at -70℃ until use. The result was positive when the absorbance of the sample was higher than the absorbance of the weak positive control, which meant that the IgG antibody concentration against TES Ag was clinically significant. The immunodiagnosis of toxocariasis by TES IgE Western blot analysis of the serum was also performed to eliminate possible cross-reactions. The TES Ag were kindly supplied from Dr. Sun Huh (Department of Parasitology, College of Medicine, Hallym University, Chuncheon, Korea).
All patients had supportive therapy with systemic corticosteroid and some had additive albendazole therapy (800 mg/day for 28 days). The outcome score was determined according to a score system (0, complete resolution; 1, near complete resolution; 2, marked improvement; 3, moderate improvement; 4, minimal improvement; 5, relapse and/or aggravation). Patients with outcome score 5 were defined as non-responders and others were defined as responders.
Statistical analysis
The data was analyzed by SAS. The relationships among the eosinophil counts, the laboratory data and organ involvement were analyzed by Mann-Whitney U test. We performed Pearson's correlation analysis, and Spearman's correlation analysis for the non-parametric distributions.
RESULTS
Patient characteristics and clinical findings
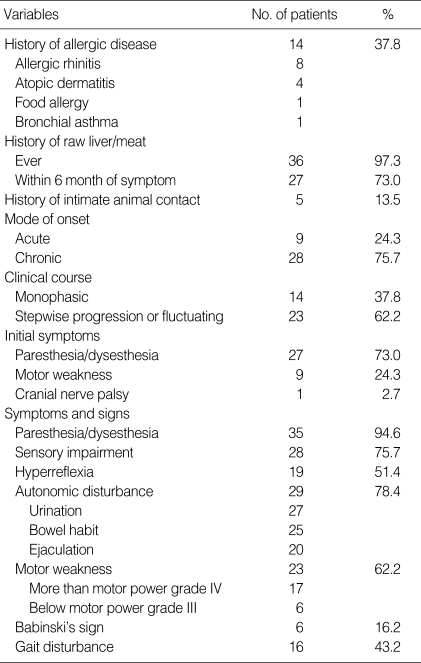
The clinical data is summarized in Table 1. The male/female ratio was 36:1. The age at onset was 47±13 (Mean±SD) yr old on average, although it varied from 23 to 74 (median=45 yr), and 20 (55.6%) of the patients were between 20 and 49 yr old. The mean follow-up period was 570±337 days (Mean±SD, range 133-1,425 days, median 433 days). Out of the 37 subjects, 36 patients (97.3%) had a history of raw food intake history and 27 patients (73.0%) had eaten raw foods within 6 months of developing symptoms. For the 33 Toxocara ELISA-available patients, all the patients had a history of raw food intake and 25 patients (75.8%) had a history of raw food intake within 6 month of symptom development. Five patients (13.5%) had a history of close contact with pets. Atopic diseases were present in 14 (37.8%) of the 37 patients. The most frequent atopic diseases were allergic rhinitis (57.0%), atopic dermatitis (28.6%), food allergy (7.2%), and bronchial asthma (7.2%) in that order. The remaining 23 (62.1%) patients with both hyperIgEaemia and mite antigen-specific IgE were considered to have atopic diasthesis. There were no differences of the clinical data between the patients with atopic disease and the patients with atopic diasthesis.
Table 1.
Summary of clinical data from 37 patients with atopic myelitis
The mode of onset was acute in 9 (24.3%) and chronic in 28 (75.7%) of the 37 patients. Stepwise progression and/or fluctuation of symptoms were seen in 23 (62.2%) and monophasic features were seen in 14 (37.8%) of the 37 AM patients. The most common initial symptoms were paresthesia and/or dysesthesia (73.0%) followed by muscle weakness (24.3%). Paresthesia and/or dysesthesia (94.6%) were also the most frequently observed symptoms/signs throughout the entire clinical course and this was followed by sensory impairment (75.7%), muscle weakness (62.2%), hyperreflexia (51.4%) and gait disturbance (43.2%) although severe muscle weakness (motor power below Grade III) or pathologic reflexes were infrequent (n=6, 16.2% and n=6, 16.2%, respectively). Autonomic dysfunctions were frequently observed in 29 (78.4%) of the 37 patients with bladder (93.1%), bowel (86.2%) and sexual dysfunction (70.0%).
Laboratory findings
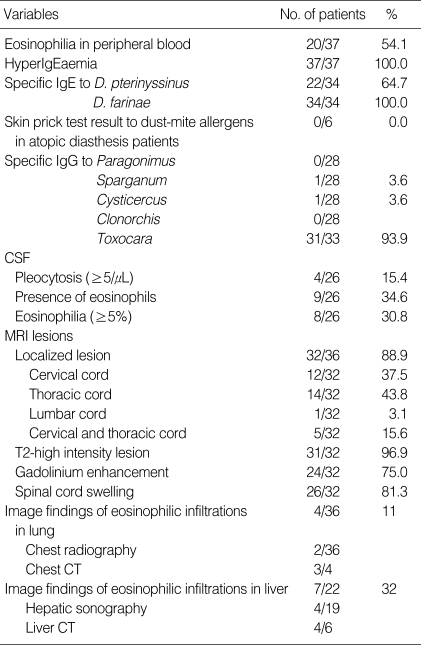
The laboratory findings are summarized in Table 2. The initial peripheral blood white blood cell counts were ranged from 4,640 to 18,050/µL (median: 7,445/mL) and leukocytosis (>10,000/µL) was seen in 7 patients (18.9%). The peak peripheral blood eosinophil counts were ranged from 0 to 8,484/µL (median: 511/mL). Peripheral blood eosinophilia (>500/µL) was detected in 20 (54.1%) of 37 patients and the degree of eosinophilia was median 915/µL in these 20 patients. Of the 31 Toxocara sero-positive patients, peripheral blood eosinophilia was detected in 17 (56.7%) patients and the degree of eosinophilia was median 1,073/µL (692/µL, 1,661/µL) although 7 of them pretreated with systemic corticosteroids. Of the 26 patients with CSF analyses, only 4 patients (15.4%) showed pleocytosis (≥5/µL), 9 patients (34.6%) showed the presence of eosinophils, and 8 patients (30.8%) showed CSF eosinophilia (≥5%). All 37 patients had hyperIgEaemia and the median level of serum IgE was 937 IU/mL (523 IU/mL, 2,302 IU/mL). Of the 31 Toxocara sero-positive patients, all had hyperIgEaemia and the median level of serum IgE was 1,226 IU/mL (534 IU/mL, 3,031 IU/mL).
Table 2.
Summary of laboratory data from 37 patients with atopic myelitis
CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; CT, computed tomography.
Parasite and Toxocara serologic findings
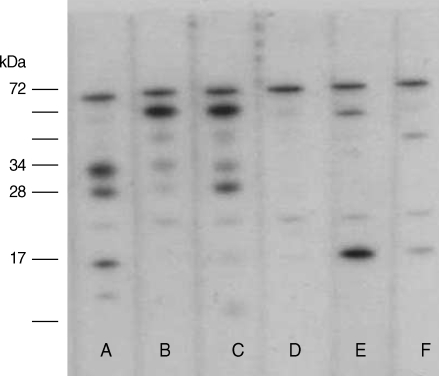
For the 33 patients who underwent serum TES IgG ELISA, 31 patients (93.9%) had positive values (Mean±SD of absorbance, 2.02±0.67). Among the 31 Toxocara-seropositive patients, all had specific IgE to D. farinae and only two of them (6.5%) showed a positive reaction against cysticercus and sparganum antigen, respectively. There was significant correlation between the Toxocara-specific IgG values and the serum IgE levels (ρ=0.632, P<0.001). Positive ELISA results for Toxocara were confirmed by IgE western blot with a typical pattern of Toxocara specific bands in the low molecular weight fractions in 8 of 11 patients (Fig. 1). Of the 10 patients who underwent CSF TES IgG ELISA, all had positive values (Mean±SD of absorbance, 1.90±0.85).
Fig. 1.
IgE binding to TES Antigens on blotting strips. Serum of A, B were obtained from toxocariasis patients with liver abscess. Serum C was obtained from AM patient with Toxocara-IgG positivity. Serum of D, E, and F were obtained from D. farinae specific allergic rhinitis patients. Lane A, B, and C revealed a typical pattern of Toxocara specific bands in low molecular weight fractions.
Radiologic findings
Regarding the spinal cord MRI, 2 patients showed normal finding and another 2 patients showed bulging discs. Localized spinal cord lesions were detected in 32 of 36 patients (88.6%) and the thoracic cord (43.8%) was the most frequently observed lesion, followed by the cervical (37.5%), both the cervical and thoracic (15.6%) and the lumbar cord (3.1%) in that order. Thirty-one of 32 patients (96.9%) showed high signal intensity lesions on the T2-weighted images and 78% of them showed involvement of more than 2 segments (range, 1-8; median, 2). Twenty-six patients (81.3%) had spinal cord swelling and 24 patients (75.0%) showed nodular or patchy contrast enhancement (Fig. 2).
Fig. 2.
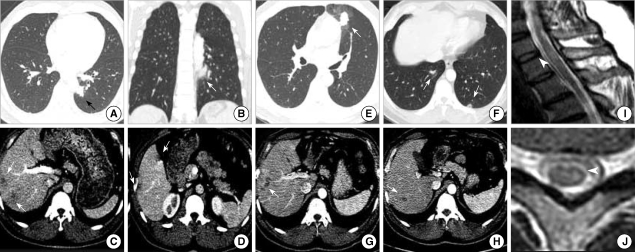
Radiologic images from 45-yr-old man whose first symptoms were chest tightness and progressive lower extremities weakness. He used to eat uncooked cow liver one or two times a month. The peripheral blood showed leukocytosis (11,090/µL) with 33% eosinophils and hyperIgEaemia (2,724 U/mL). The values of specific IgE to D. pteronyssinus and D. farinae were 1.4 U/mL and 4.98 U/mL, respectively although he showed negative skin-prick test results to common aeroallergens. The ELISA test for Toxocara canis was strongly positive (absorbance 2.195). (A-D) Initial transverse CT scan showing a lung nodule with halo sign and multiple low attenuating nodules in the liver. (E-H) One month follow up transverse CT scan showing migrating nodules in the lung and liver. (I, J) T2-weighted MRI images with high signal intensity lesions at the cervical spinal cord.
Based on image findings of eosinophilic infiltrations in the lung, four of 36 patients (11.1%) had positive results. Among the 4 patients with positive lung findings, two of 36 patients who underwent chest radiography showed evidence on the imaging findings of eosinophilic infiltration while 3 of 4 patients who underwent chest computed tomography (CT) showed positive results. Based on image findings of eosinophilic infiltrations in the liver, 7 of 22 patients (31.8%) had positive results. Among the 7 patients with positive liver findings, 4 of 19 patients who underwent hepatic sonography showed eosinophilic liver abscess findings while 4 of 6 patients who underwent liver CT showed positive results.
Treatment response
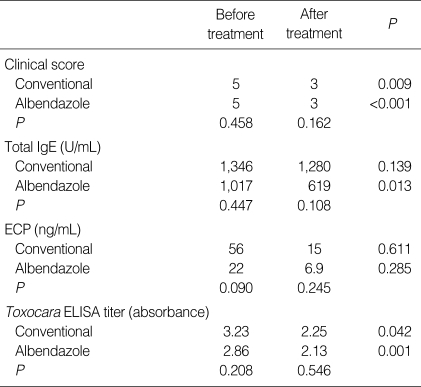
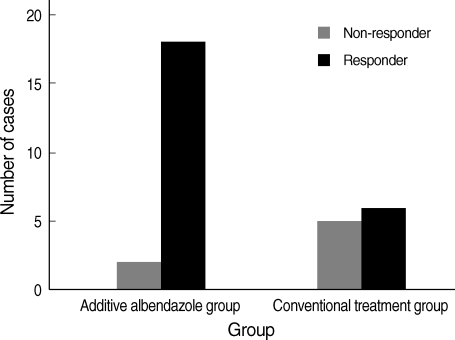
The treatment outcome data are summarized in Table 3. The post-treatment follow up period was 450±273 days (Mean±SD). Out of 31 Toxocara myelitis patients, 20 had additive albendazole treatment. There were no demographic and biological differences between conventional corticosteroid treatment group and additive albendazole treatment group. Analysis of the results showed a significant decrease of the clinical score in both groups after treatment but additional albendazole treatment was more efficient when it was compared as responder and non-responder (P=0.032) (Fig. 3). Moreover, early albendazole treatment subgroup (within 1 yr from symptom development, n=13) had better response in the clinical score (P=0.015) than late albendazole treatment subgroup. Serum Toxocara-specific IgG levels were decreased in both group (each, P=0.001, P=0.042) while serum total IgE levels were significantly decreased only in the additional albendazole treatment group (each, P=0.013, P=0.139). But, serum ECP levels were not significantly decreased after treatment in both groups.
Table 3.
Summary of treatment response from 31 patients with Toxocara myelitis
ECP, eosinophilic cationic protein; ELISA, enzyme-linked immunosorbent assay.
Fig. 3.
Treatment response. All patients had conventional supportive therapy with systemic corticosteroid and some had additive albendazole therapy. The outcome score was determined according to a score system and patients with outcome score 5 were defined as non-responders. Two of 20 patients in additive albendazole group and 5 of 11 patients in corticosteroid group were non-responders (P=0.032).
Improvement of spinal MRI findings (resolution or decrease of high signal intensities on T2WI were observed in 1 of 3 conventional corticosteroid group and 3 of 5 additional albendazole treatment group.
DISCUSSION
AM is one of the idiopathic transverse myelitis associated with atopic disease or diasthesis. The stepwise progression and fluctuation of the symptoms are frequent in AM but the pathomechanism is still unknown (1). Toxocariasis is one of the most prevalent parasitic infections in the world, caused by larval stage of Toxocara spp. It is usually non-fatal disease, but the larvae can migrate through the CNS system and may cause such neurologic manifestations in AM. The frequency and localization of Toxocara larvae in the spinal cord in humans are unknown. Following a high dose and repeated infections by T. canis, some larvae can migrate to the spinal cord and induce local inflammation with immediate-type and delayed-type hypersensitivity, along with clinical symptoms. Accurate diagnosis of toxocariasis is not possible sometimes, because the diagnostic method is not available worldwide although increased awareness of this disease. We could infer that many patients who were diagnosed as having AM might have Toxocara myelitis.
Our clinical and MRI imaging findings were similar with those of previously reported in AM except for the difference in the male preponderance (1). Many Koreans, and especially the males, have a custom of eating raw animal tissue in their social life based on the belief that is health-promoting food, and so the chances of being infected by Toxocara larvae might be more likely increased in Korean males (6).
The data presented herein suggested that the Toxocara-seroprevalence in AM patients was approximately 94% and the neuropathological mechanism of AM might be due to neural involvement by Toxocara larvae if one takes into account: 1) the positive histories of ingesting raw liver/meat, which is known to be the main risk factor for toxocariasis in adults (6), 2) the accompanied eosinophilic infiltration in the lung and liver (27), 3) the positive serology in both sera and CSF with IgG anti-Toxocara, and some of the patients had this confirmed by IgE western blotting using TES antigen, 4) the relatively higher Toxocara ELISA values (absorbance >1.5) that are associated with active or recent clinical toxocariasis (9, 10), 5) the peripheral blood eosinophilia and an increase in the concentration of serum total IgE (>500 IU/mL), which are further evidence of recent Toxocara infection (12), 6) the improvement of signs and symptoms after treatment with albendazole in several cases in our preliminary study and better treatment responses after early additional albendazole treatment in sub-group analysis.
A diagnosis of toxocariasis is made by detection of antibody by using ELISA because the histological proof of Toxocara larvae in tissue is extremely difficult and there is little serologic cross-reaction between toxocariasis and other helminthiases (12). The diagnostic sensitivity is superior to 90% and specificity of the reaction is also high. However, an ELISA kit for this disease is not widely available and it is not capable of distinguishing between current and past infection. So when interpreting a serologic result and selecting candidates for anthelmintics therapy, we feel that the immunologic test should be accompanied by full history taking and laboratory tests. Therefore, the food habits of suspected patients should be assessed and Toxocara myelitis should be suspected in those AM patients with a positive history of raw animal tissue ingestion. Moreover, all our patients had hyperIgEaemia, about 60% of them had peripheral blood eosinophilia, and about 30% of them had eosinophilic liver abscess (27), and these findings might give us additional help to diagnose toxocariasis, although long-standing toxocariasis with no or minor systemic involvement except CNS infection might explain the lack of a specific immune response in blood or other organs.
In our study, out of total 11 patients with positive ELISA results for Toxocara, only 8 patients were confirmed by IgE western blot with a typical pattern of Toxocara specific bands in the low molecular weight antigen of 24, 28, 30, and 35 kDa. This discrepancy could have been due either to crossreactivity due to helminthic disease other than toxocariasis or to some patients' selective protein antigen components destroyed during the boiling process. The value of additional Western blotting for corroborating serological results cannot be overstated, but it might be more helpful in cases in which the TES-ELISA values lie under the cut-off or to rule out cross-reactivity due to other helminthic diseases as Magnaval et al. suggested in their study (28).
Further investigations needed to determine the association of Toxocara specific antibody and mite specific antibody. One possible explanation is that the false positivity of mite specific antibody due to increased production of the serum total IgE. This is supported by 1) the negative skin prick test results in the available 8 AM patients with atopic diasthesis who showed Toxocara sero-positivity, 2) the relatively low serum value of specific IgE to D. farinae, in 87% of the 23 atopic diasthesis patients, less than 10 IU/mL. Allergenic cross-reactivity between the nematode T. canis and the dust mite D. farinae might also be a possible explanation. Several studies have suggested there is allergenic cross-reactivity between Anisakis. simplex and other nematode (29). Allergenic crossreactivity between A. simplex and four different dust-mite species has also been reported (30). Therefore, we investigated a possible allergenic cross-reactivity between T. canis and D. farinae. We performed western blotting using TES antigens and whole body extracts of D. farinae in some selected patients with both atopic diasthesis and Toxocara sero-positivity. In case of western blotting using whole body extracts of D. farinae, none of 6 patients showed a typical pattern of D. farinae specific bands as shown in positive controls with mite-specific allergic rhinitis. In Toxocara-ELISA inhibition tests using extracts of D. farinae varying from 1, 10, 100, to 1,000 µg/mL of concentration, no inhibition was observed in any of the 2 selected sera. So we concluded that mite-antigen specific IgE positivity in AM patients with Toxocara seropositivity is false positive results with increased production of total IgE, rather than allergenic cross-reactivity between whole body extracts of D. farinae and TES antigens although the results are not shown here.
In summary, our study reveals that there is a high prevalence of toxocariasis in patients with atopic myelitis. Taking a good history and performing Toxocara ELISA should be done for evaluating the causes of atopic myelitis. Liver or lung nodules seen on imaging studies can support diagnosis of Toxocara myelitis.
References
- 1.Osoegawa M, Ochi H, Minohara M, Murai H, Umehara F, Furuya H, Yamada T, Kira J. Myelitis with atopic diathesis: a nationwide survey of 79 cases in Japan. J Neurol Sci. 2003;209:5–11. doi: 10.1016/s0022-510x(02)00441-0. [DOI] [PubMed] [Google Scholar]
- 2.Kira J, Yamasaki K, Kawano Y, Kobayashi T. Acute myelitis associated with hyperIgEemia and atopic dermatitis. J Neurol Sci. 1997;148:199–203. doi: 10.1016/s0022-510x(97)05369-0. [DOI] [PubMed] [Google Scholar]
- 3.Kira J, Horiuchi I, Suzuki J, Osoegawa M, Tobimatsu S, Murai H, Minohara M, Furue M, Ochi H. Myelitis associated with atopic disorders in Japan: a retrospective clinical study of the past 20 years. Intern Med. 2001;40:613–619. doi: 10.2169/internalmedicine.40.613. [DOI] [PubMed] [Google Scholar]
- 4.Osoegawa M, Ochi H, Kikuchi H, Shirabe S, Nagashima T, Tsumoto T, Tamura Y, Yamabe K, Takahashi H, Iwaki T, Kira J. Eosinophilic myelitis associated with atopic diathesis: a combined neuroimaging and histopathological study. Acta Neuropathol. 2003;105:289–295. doi: 10.1007/s00401-002-0645-2. [DOI] [PubMed] [Google Scholar]
- 5.Kira J. Atopy and neural damage. Intern Med. 2002;41:169–174. doi: 10.2169/internalmedicine.41.169. [DOI] [PubMed] [Google Scholar]
- 6.Kwon NH, Oh MJ, Lee SP, Lee BJ, Choi DC. The prevalence and diagnostic value of toxocariasis in unknown eosinophilia. Ann Hematol. 2006;85:233–238. doi: 10.1007/s00277-005-0069-x. [DOI] [PubMed] [Google Scholar]
- 7.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39:1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- 9.de Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis by enzyme immunoassay. J Clin Pathol. 1979;32:284–288. doi: 10.1136/jcp.32.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquier P, Gottstein B, Stingelin Y, Eckert J. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J Clin Microbiol. 1991;29:1831–1835. doi: 10.1128/jcm.29.9.1831-1835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Huang CY, Chan PH, Preston P, Chau PY. Transverse myelitis associated with larva migrans: finding of larva in cerebrospinal fluid. Lancet. 1983;1:423. doi: 10.1016/s0140-6736(83)91544-1. [DOI] [PubMed] [Google Scholar]
- 12.Park HY, Lee SU, Huh S, Kong Y, Magnaval JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002;40:113–117. doi: 10.3347/kjp.2002.40.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magnaval JF, Galindo V, Glickman LT, Clanet M. Human Toxocara infection of the central nervous system and neurological disorders: a case-control study. Parasitology. 1997;115:537–543. doi: 10.1017/s0031182097001558. [DOI] [PubMed] [Google Scholar]
- 14.Strupp M, Pfister HW, Eichenlaub S, Arbusow V. Meningomyelitis in a case of toxocariasis with markedly isolated CSF eosinophilia and an MRI-documented thoracic cord lesion. J Neurol. 1999;246:741–744. doi: 10.1007/s004150050446. [DOI] [PubMed] [Google Scholar]
- 15.Eberhardt O, Bialek R, Nagele T, Dichgans J. Eosinophilic meningomyelitis in toxocariasis: case report and review of the literature. Clin Neurol Neurosurg. 2005;107:432–438. doi: 10.1016/j.clineuro.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Kumar J, Kimm J. MR in Toxocara canis myelopathy. AJNR Am J Neuroradiol. 1994;15:1918–1920. [PMC free article] [PubMed] [Google Scholar]
- 17.Russegger L, Schmutzhard E. Spinal toxocaral abscess. Lancet. 1989;2:398. doi: 10.1016/s0140-6736(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 18.Engel H, Spieckermann DA, Tismer R, Mobius W. Acute meningomyelitis due to Toxocara larvae. Dtsch Med Wochenschr. 1971;96:1498. doi: 10.1055/s-0028-1110168. [DOI] [PubMed] [Google Scholar]
- 19.Muller-Jensen A, Schall J, Weisner B, Lamina J. Eosinophilic meningoencephalomyelitis and visceral syndrome caused by ascaridia larvae in adults. Dtsch Med Wochenschr. 1973;98:1175–1177. doi: 10.1055/s-0028-1106989. [DOI] [PubMed] [Google Scholar]
- 20.Sellal F, Picard F, Mutschler V, Marescaux C, Collard M, Magnaval JF. Myelitis caused by Toxocara canis (larva migrans) Rev Neurol (Paris) 1992;148:53–55. [PubMed] [Google Scholar]
- 21.Ota S, Komiyama A, Johkura K, Hasegawa O, Kondo K. Eosinophilic meningo-encephalo-myelitis due to Toxocara canis. Rinsho Shinkeigaku. 1994;34:1148–1152. [PubMed] [Google Scholar]
- 22.Duprez TP, Bigaignon G, Delgrange E, Desfontaines P, Hermans M, Vervoort T, Sindic CJ, Buysschaert M. MRI of cervical cord lesions and their resolution in Toxocara canis myelopathy. Neuroradiology. 1996;38:792–795. doi: 10.1007/s002340050350. [DOI] [PubMed] [Google Scholar]
- 23.Goffette S, Jeanjean AP, Duprez TP, Bigaignon G, Sindic CJ. Eosinophilic pleocytosis and myelitis related to Toxocara canis infection. Eur J Neurol. 2000;7:703–706. doi: 10.1046/j.1468-1331.2000.00123.x. [DOI] [PubMed] [Google Scholar]
- 24.Osoegawa M. Diagnosis and treatment of CNS parasite infection with special reference to parasitic myelitis. Rinsho Shinkeigaku. 2004;44:961–964. [PubMed] [Google Scholar]
- 25.Dauriac-Le Masson V, Chochon F, Demeret S, Pierrot-Deseilligny C. Toxocara canis meningomyelitis. J Neurol. 2005;252:1267–1268. doi: 10.1007/s00415-005-0688-0. [DOI] [PubMed] [Google Scholar]
- 26.Moreira-Silva SF, Rodrigues MG, Pimenta JL, Gomes CP, Freire LH, Pereira FE. Toxocariasis of the central nervous system: with report of two cases. Rev Soc Bras Med Trop. 2004;37:169–174. doi: 10.1590/s0037-86822004000200011. [DOI] [PubMed] [Google Scholar]
- 27.Chang S, Lim JH, Choi D, Park CK, Kwon NH, Cho SY, Choi DC. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. AJR Am J Roentgenol. 2006;187:W622–W629. doi: 10.2214/AJR.05.1416. [DOI] [PubMed] [Google Scholar]
- 28.Magnaval JF, Fabre R, Maurieres P, Charlet JP, de Larrard B. Application of the western blotting procedure for the immunodiagnosis of human toxocariasis. Parasitol Res. 1991;77:697–702. doi: 10.1007/BF00928685. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy MW, Tierney J, Ye P, McMonagle FA, McIntosh A, Mc-Laughlin D, Smith JW. The secreted and somatic antigens of the third stage larva of Anisakis simplex, and antigenic relationship with Ascaris suum, Ascaris lumbricoides, and Toxocara canis. Mol Biochem Parasitol. 1988;31:35–46. doi: 10.1016/0166-6851(88)90143-0. [DOI] [PubMed] [Google Scholar]
- 30.Johansson E, Aponno M, Lundberg M, van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001;56:660–666. doi: 10.1034/j.1398-9995.2001.00798.x. [DOI] [PubMed] [Google Scholar]