Abstract
Myotonia congenita (MC) is a form of nondystrophic myotonia caused by a mutation of CLCN1, which encodes human skeletal muscle chloride channel (CLC-1). We performed sequence analysis of all coding regions of CLCN1 in patients clinically diagnosed with MC, and identified 10 unrelated Korean patients harboring mutations. Detailed clinical analysis was performed in these patients to identify their clinical characteristics in relation to their genotypes. The CLCN1 mutational analyses revealed nine different point mutations. Of these, six (p.M128I, p.S189C, p.M373L, p.P480S, p.G523D, and p.M609K) were novel and could be unique among Koreans. While some features including predominant lower extremity involvement and normal to slightly elevated creatine kinase levels were consistently observed, general clinical features were highly variable in terms of age of onset, clinical severity, aggravating factors, and response to treatment. Our study is the first systematic study of MC in Korea, and shows its expanding clinical and genetic spectrums.
Keywords: Myotonia Congenita, CLCN1, Clinical Features
INTRODUCTION
Myotonia congenita (MC) is a form of nondystrophic myotonia characterized by impaired relaxation of skeletal muscle after forceful voluntary contraction or following mechanical or electrical stimulation. MC can be inherited either in an autosomal dominant (Thomsen's disease, OMIM 160800) or an autosomal recessive manner (Becker's disease, OMIM 255700). Regardless of its inheritance pattern, most cases of MC are caused by mutations of CLCN1 encoding the human skeletal muscle chloride channel (CLC-1), which is important for the normal repolarization of muscle action potentials (1, 2). The functional defects caused by CLCN1 mutations impair CLC-1 functions and render the plasma membrane abnormally excitable, leading to clinical myotonia and myotonic discharges on the electromyography (2, 3).
CLCN1 is located on chromosome 7q35 and encompasses 35 kb in genomic DNA with 23 exons (4, 5). To date, more than 120 disease-causing mutations in CLCN1 have been reported, and none appears to have a particularly elevated frequency. They are almost evenly scattered through the gene and include small insertion/deletions, missense, nonsense, and splicing mutations (6). Some of these mutations have been characterized functionally. Many of these mutations are recessive and a compound heterozygous state is presumed in the majority of them, although it has been demonstrated in only a few cases (7).
In this study, we analyzed the DNA of 10 Korean MC patients by sequencing of whole exons of CLCN1 to identify their mutations. We also evaluated clinical features of these patients to understand the genotype-phenotype correlation of CLCN1 mutations.
MATERIALS AND METHODS
Patients
Ten unrelated Korean patients with MC harboring CLCN1 mutations were included in this study. All of them presented with sudden muscle stiffness accompanied by warm up with use, and showed electromyographic evidence of abnormal excitability of muscle fibers. Two hundred healthy Koreans were also studied as controls. The study was reviewed and approved by Pusan National University Hospital Institutional Review Board and all patients and controls provided written informed consent for DNA acquisition and analysis.
Mutational analysis of CLCN1
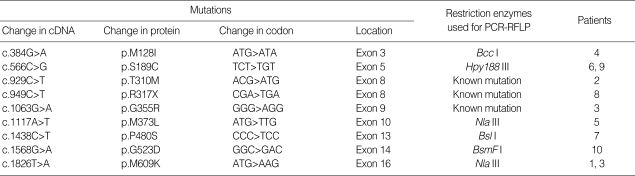
To identify the mutations in CLCN1, direct sequence analysis of whole coding regions of CLCN1 was performed. In some patients whose biopsied skeletal muscles were available for the study, reverse transcription-polymerase chain reaction (RT-PCR)-based sequence analysis was undertaken. For RT-PCR, total RNA was isolated from frozen muscle tissue of some patients and reverse transcribed to produce 1 µg of total DNA. The first strand cDNA was then PCR amplified by with the six overlapping cDNA primer pairs that were presently designed. In other patients, DNA was extracted from anticoagulated whole blood and sequence specific primer pairs covering the entire coding region of CLCN1 were used as described elsewhere (4) with minor modification. PCR amplified products were separated on 2% agarose gels, purified, cycle-sequenced with PCR primers using the BigDye™ Terminator Sequencing Kit (Applied Biosystems, Foster, CA, U.S.A.), and electrophoresed using an ABI PRISM® 3730XL DNA analyzer (Applied Biosystems). To confirm mutations identified in sequence analysis and to differentiate them from benign polymorphisms, PCR and restriction length polymorphism analysis (PCR-RFLP) was performed using DNA from patients and 200 normal controls. Restriction enzymes used in the PCR-RFLP analysis are listed in Table 1.
Table 1.
Mutations of CLCN1 identified in the study
PCR-RFLP, polymerase chain reaction and restriction length polymorphism analysis.
Clinical evaluation
Detailed clinical histories were acquired and standard neurological examinations were performed for all patients with CLCN1 mutations. Historical clinical parameters included age of onset, distribution of muscle stiffness, presence of muscle weakness, provoking factors, and family history. Physical examinations especially focused on the presence of muscle atrophy or hypertrophy, distribution of muscle weakness, status of deep tendon reflexes, and types of maneuvers provoking myotonia. Needle electromyography was performed on at least two different muscles in each extremity. Routine laboratory tests included complete blood count; liver, renal, and thyroid function tests; blood glucose, electrolytes; serum creatine kinase (CK) level; chest radiography; and electrocardiogram. A muscle biopsy was performed on five patients. Biopsied muscles were rapidly frozen in isopentane solution pre-chilled using liquid nitrogen, and were processed for routine histochemical staining reactions including hematoxylin-eosin, modified Gomori trichrome, NADH-tetrazolium reductase, periodic acid-Schiff (PAS), cytochrome oxidase (COX), and ATPase.
RESULTS
CLCN1 mutations
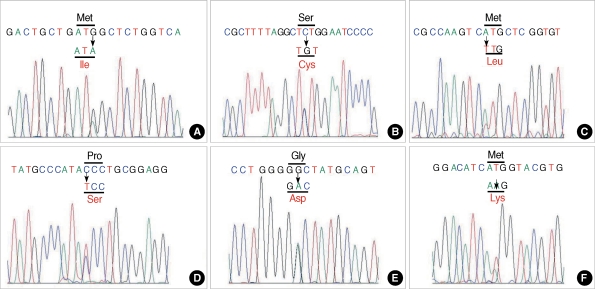
Nine different CLCN1 point mutations were identified; of these, six were novel and have not been reported elsewhere (Table 1, Figs. 1, 2). A single heterozygous mutation was identified in all patients except one (patient 3) who had two heterozygous mutations. All nine identified mutations were point mutations, either missense (n=8) or nonsense (n=1). Two new mutations were shared by two patients respectively: p.S189C in patients 14 and 18 (Fig. 1, panel C) and p.M609K in patients 4 and 10 (Fig. 1, panel F). PCR-RFLP screening of mutations listed in Table 1 did not reveal any pattern that was similar to those in the 200 normal controls.
Fig. 1.
Chromatograms of novel mutations identified in the study. (A) c.384G>A (p.M128I), (B) c.566C>G (p.S189C), (C) c.1117A>T (p.M373L), (D) c.1438C>T (p.480S), (E) c.1568G>A (p.G523D), (F) c.1826T>A (p.M609K).
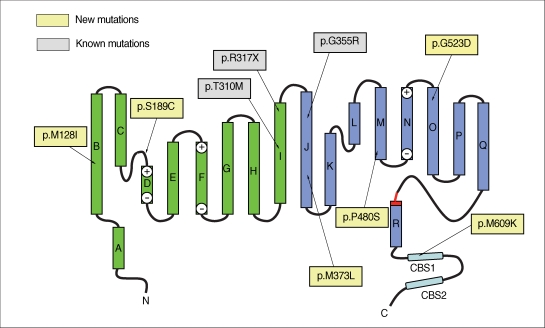
Fig. 2.
Two-dimensional representation of the structure of a CLC subunit (9) showing the locations of mutations identified in this study.
Clinical features
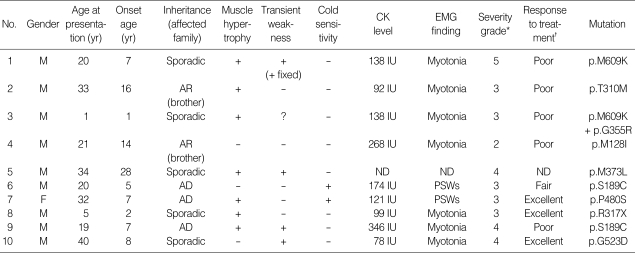
Clinical features are summarized in Table 2. The 10 MC patients with CLCN1 mutations included nine males and one female, whose ages at presentation and onset were 22.5 yr (range 1-40 yr) and 9.5 yr (range 1-28 yr), respectively. Family history was compatible with autosomal dominant inheritance in three patients. Another two patients had affected sibling(s), which was suggestive of autosomal recessive inheritance. In the other patients, there was no significant family history of any neuromuscular disease.
Table 2.
Clinical features of the patients with CLCN1 mutation
*Severity of myotonia congenita was graded as follows; (1) no symptoms, but unequivocal myotonia at examination, (2) mild (and/or fluctuating) symptoms, (3) pronounced myotonia, but no transient weakness, (4) pronounced myotonia with transient weakness,but without dystrophic features, and (5) pronounced myotonia, transient weakness, and dystrophic features (Colding-Jørgensen, 2005); †Response to treatment was judged as follows: excellent; complete or near-complete abolition of symptom, good; >70% reduction of frequency and duration of myotonic stiffness, fair; <70% and >30% reduction of frequency and duration of myotonic stiffness, poor; <30% reduction of frequency and duration of myotonic stiffness.
M, male; F, female; AR, autosomal recessive; AD, autosomal dominant; IU, international unit; ND, not done; PSWs, showers of positive sharp waves with or without complex repetitive discharges.
All patients complained of intermittent myotonic stiffness involving the limbs and trunk, which predominantly initially affected the lower extremities. The stiffness was always provoked by sudden initiation of movement after rest (such as grasping, walking, running, standing up, and lifting objects) and dissipated shortly after repeated use of the affected musculature. In three patients (patients 1, 8, and 9), myotonic stiffness affected facial musculature in addition to limbs and trunk, either as chewing difficulty, facial stiffness, speech disturbance, or eye opening difficulty in variable combinations. Two patients considered that there was a worsening of their symptoms by certain physiological factors including cold exposure (patient 6, p.S189C mutant and patient 7, p.P480S mutant) and fasting (patient 7). Myotonia could be provoked by tapping on skeletal muscles or following a firm hand grip in all patients. In five patients (patients 1, 3, 4, 5, and 9), myotonia was also provoked by tapping of the tongue as the 'napkin ring' sign. The degree of myotonia-induced muscle stiffness was highly variable, from mild discomfort caused by intermittent leg stiffness a few times a day (patient 4) to frequent and severe focal or generalized muscle stiffness provoked by virtually every motion attempted (patients 1 and 9). Patient 1 also complained of an accompanying myalgic pain during attacks of myotonic stiffness.
Four patients complained of muscle weakness in addition to the myotonic stiffness. Patient 1 (p.M609K mutant) initially experienced intermittent short lasting muscle weakness of limbs following myotonic stiffness beginning at 8-yr-of-age. His weakness became more frequent and longer-lasting, and constant muscle weakness began to develop from the age of 19. Initially, fixed weakness that arose in his hands and feet soon began to spread to other limb muscles. At age 20, his limb muscle power as graded by the modified Medical Research Council muscle power grading system was 4 in proximal/distal upper/lower limbs. His hand grip power as measured by a hand dynamometer was 9 kilograms on the right and 8 kilograms on the left. Patients 3 (p.M609K+p.G355R), 9 (p.S189C) and 10 (p.G523D) also had short-lasting muscle weakness following myotonic stiffness.
Seven patients (70.0%) showed muscular hypertrophy that varied in both degree and distribution. Among them, patients 1 and 9 showed the most prominent generalized form of muscular hypertrophy. A female patient (patient 7) also showed mild muscle hypertrophy.
Needle electromyography was performed in all patients except for patient 5, and all showed evidence of abnormal muscle fiber excitability, mostly in the form of myotonic discharges, except in patients 6 and 7, who experienced showers of positive sharp waves instead. Muscle biopsy was performed in five patients and only two of them showed mild myopathic patterns; patient 1 displayed a few regenerating fibers with type 1 fiber predominance, and patient 7 displayed mild type 2B fiber atrophy. Several drugs including mexiletine, phenytoin, and caramabazepine were tried and showed various responses (Table 2).
DISCUSSION
The present study identified six novel CLCN1 mutations from 10 unrelated Korean MC patients. The c.384G>A mutation located in the transmembrane region of helix B (Fig. 2) is expected to change the highly conserved amino acid methionine to isoleucine (p.M128I). A myotonia-associated mutation involving the same codon (M128V) has been reported previously (8). Another mutation, c.566C>G (S189C), present in two of the 10 patients (patients 6 and 9) is located in the highly conserved CLC-1 protein signature region (GSGIPE) (Fig. 2). Since this region is thought to be a chloride ion binding site, the introduction of the much larger sulfhydryl group of cysteine in place of the serine hydroxyl might be anticipated to have a significant effect on the conductance of the CLC-1 channel (9, 10). Since both of these patients showed an autosomal dominant inheritance pattern of disease, c.566C>G (p.S189C) is most likely associated with Thomsen's disease. Previously present, p.S189C had been evaluated as a site-directed mutant expressed in cultured tsA201 cells. However, the observation of a relatively normal chloride current fails to support a pathogenic role of the mutation in the development of myotonia (11).
The c.1117A>T mutation, which is located in the helix J transmembrane domain (Fig. 2), changes the codon for methionine to leucine (p.M373L). Since no pathogenic missense mutation has previously been found in helix J, this mutant will require functional study for the verification of its pathogenicity. Another new mutation, c.1438C>T (p.P480S), is located in the cytoplasmic loop connecting helices M and N (Fig. 2), where it changes the highly conserved amino acid proline to serine. Since three different missense mutations involving the same codon (p.P480T, p.P480L, and p.P480H) have been reported previously (12-14), its pathogenicity is almost certain. A novel mutation, c.1568G>A (p.G523D), is located in helix O (Fig. 2) at a glycine that is fairly conserved between different species. Since no pathogenic mutation has been identified in this area, its verification will also depend on functional study. Another novel mutation, c.1826T>A (p.M609K), which was present in two patients, is also notable. The mutated amino acid, methionine, is located at the CBS1 domain (Fig. 2) and is highly conserved between different CLC proteins as well as between different species. CBS (cystathione β-synthase) domains are sequence motifs of approximately 60 amino acids that occur in CBS and many other proteins in all organisms (15). Recently, the CBS domains of CLC-1 have been suggested to function as sensors of cellular energy status that act by binding ATP, which is crucial for channel opening (16). Another study also showed that the structure of CBS domains from CLC channels is highly conserved, and mutations in CBS domains were suggested to affect protein-protein interactions within CLC protein subunits as well as between the two subunits of CBS dimers, thereby influencing the voltage dependence of gating by acting on the common gate (17). Only one missense mutation affecting the CBS1 domain (p.A659V) has been reported so far, but has not yet been verified by a functional study (13). Thus, the functional study of the presently-reported novel mutation c.1826T>A (p.M609K) could be important to elucidate the role of the CBS1 domain in channel function. Other presently identified mutations c.929C>T (p.T310M), c.949C>T (p.R317X), and c.1063G>A (p.G355R) have been previously reported (18-20).
One of the interesting findings in our study was the apparent male predominance of MC in Korea. We had only one female patient and her disease was relatively mild. Although there is no direct evidence that males are more frequently affected than females, it seems clear that myotonia, both clinically and electrically, may be more pronounced in males, especially in the recessive form of disease (21-23). This gender difference in MC was not well-understood until recently, when the sex hormones testosterone, progesterone, and 17β-estradiol were implicated in gender differences and pregnancy-related symptoms of MC (24).
Our patients showed a broad spectrum of clinical severity, consistent with a previous study (21). Patient 1 showed the most severe symptoms of the disease: marked muscular hypertrophy, severe myotonia, and fixed weakness of the limbs leading to significant impediment to daily life. Unfortunately, we could not determine whether the mode of inheritance was dominant or recessive in this patient, since no other family member was affected and only a single mutant allele (p.M609K) was identified.
Patient 9 (p.S189C) is also noteworthy because of the exceptionally severe clinical features for an autosomal dominant MC: marked muscular hypertrophy, severe myotonia, and transient weakness. This might be related to the site of the mutation within the signature region of the channel. However, another patient with same mutation (patient 6) showed only a moderate degree of severity without muscular hypertrophy or transient weakness, suggesting that the type and location of the mutation is unlikely to be the sole determinant of clinical severity. Differential allelic expression might be responsible for this kind of phenotypic variability between individuals sharing same mutation (25).
Two of our patients complained of worsening of myotonia during cold exposure (patients 6, 7) and fasting (patient 7). So far, many stimuli within normal physiological range have been implicated as aggravating factors for myotonia, including cold temperature, pregnancy, emotional stress, fasting, and fatigue (8, 26). Cold sensitivity had been considered as one of the features differentiating paramyotonia congenita from other nondystrophic myotonias, but some clinical and electromyographic studies have confirmed that it is also common in patients with MC (8, 14, 27, 28). It has been suggested that the presence of cold sensitivity might be related to the type of CLCN1 mutation (14, 28). Our study further supports the growing consensus that cold sensitivity should not be considered as a clinical feature unique to paramyotonia congenita among different types of nondystrophic myotonias.
While many patients with MC can manage their lives without medication, treatment can be of help when myotonic stiffness interferes with everyday activity and is associated with pain. We tried various drugs including mexiletine, carbamazepine, or phenytoin in our patients, and the response was highly variable. Of the four patients treated with mexiletine, three patients showed dramatic improvement of myotonic stiffness while the improvement in one was moderate, while in other patients no treatment was effective. Although mexiletine is currently considered as a drug of choice in patients with MC (29, 30), it has no known direct effect on chloride channels and may simply attenuate myotonic stiffness by blocking, and thereby reducing the availability of, some sodium channels for action potential production (21).
In conclusion, we report six novel CLCN1 mutations in Korean patients with MC, and suggest their clinical features are quite variable even between patients sharing a same mutation.
ACKNOWLEDGMENT
We would like express our thanks to Professor Allan Bretag from the Sansom Institute, School of Pharmacy and Medical Sciences, University of South Australia, for his valuable comments on our new CLCN1 mutations.
Footnotes
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-331-E00201).
References
- 1.Koch MC, Steinmeyer K, Lorenz C, Ricker K, Wolf F, Otto M, Zoll B, Lehmann-Horn F, Grzeschik KH, Jentsch TJ. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- 2.George AL, Jr, Crackower MA, Abdalla JA, Hudson AJ, Ebers GC. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita) Nat Genet. 1993;3:305–310. doi: 10.1038/ng0493-305. [DOI] [PubMed] [Google Scholar]
- 3.Kubisch C, Schmidt-Rose T, Fontaine B, Bretag AH, Jentsch TJ. ClC-1 chloride channel mutations in myotonia congenita: variable penetrance of mutations shifting the voltage dependence. Hum Mol Genet. 1998;7:1753–1760. doi: 10.1093/hmg/7.11.1753. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz C, Meyer-Kleine C, Steinmeyer K, Koch MC, Jentsch TJ. Genomic organization of the human muscle chloride channel CIC-1 and analysis of novel mutations leading to Becker-type myotonia. Hum Mol Genet. 1994;3:941–946. doi: 10.1093/hmg/3.6.941. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann-Horn F, Rudel R. Molecular pathophysiology of voltage-gated ion channels. Rev Physiol Biochem Pharmacol. 1996;128:195–268. doi: 10.1007/3-540-61343-9_9. [DOI] [PubMed] [Google Scholar]
- 6.Lossin C, George AL., Jr Myotonia congenita. Adv Genet. 2008;63:25–55. doi: 10.1016/S0065-2660(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann-Horn F, Mailander V, Heine R, George AL. Myotonia levior is a chloride channel disorder. Hum Mol Genet. 1995;4:1397–1402. doi: 10.1093/hmg/4.8.1397. [DOI] [PubMed] [Google Scholar]
- 8.Colding-Jørgensen E, Dunø M, Schwartz M, Vissing J. Decrement of compound muscle action potential is related to mutation type in myotonia congenita. Muscle Nerve. 2003;27:449–455. doi: 10.1002/mus.10347. [DOI] [PubMed] [Google Scholar]
- 9.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 10.Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 11.Fahlke C, Desai RR, Gillani N, George AL., Jr Residues lining the inner pore vestibule of human muscle chloride channels. J Biol Chem. 2001;276:1759–1765. doi: 10.1074/jbc.M007649200. [DOI] [PubMed] [Google Scholar]
- 12.Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum Mutat. 2002;19:423–434. doi: 10.1002/humu.10063. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki R, Ichiyasu H, Ito N, Ikeda T, Takano H, Ikeuchi T, Kuzuhara S, Uchino M, Tsuji S, Uyama E. Novel chloride channel gene mutations in two unrelated Japanese families with Becker's autosomal recessive generalized myotonia. Neuromuscul Disord. 1999;9:587–592. doi: 10.1016/s0960-8966(99)00050-4. [DOI] [PubMed] [Google Scholar]
- 14.Fialho D, Schorge S, Pucovska U, Davies NP, Labrum R, Haworth A, Stanley E, Sud R, Wakeling W, Davis MB, Kullmann DM, Hanna MG. Chloride channel myotonia: exon 8 hot-spot for dominant-negative interactions. Brain. 2007;130:3265–3274. doi: 10.1093/brain/awm248. [DOI] [PubMed] [Google Scholar]
- 15.Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci. 1997;22:12–13. doi: 10.1016/s0968-0004(96)30046-7. [DOI] [PubMed] [Google Scholar]
- 16.Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estévez R, Pusch M, Ferrer-Costa C, Orozco M, Jentsch TJ. Functional and structural conservation of CBS domains from CLC chloride channels. J Physiol. 2004;557:363–378. doi: 10.1113/jphysiol.2003.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deymeer F, Cakirkaya S, Serdaroğlu P, Schleithoff L, Lehmann-Horn F, Rüdel R, Ozdemir C. Transient weakness and compound muscle action potential decrement in myotonia congenita. Muscle Nerve. 1998;21:1334–1337. doi: 10.1002/(sici)1097-4598(199810)21:10<1334::aid-mus16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Kleine C, Steinmeyer K, Ricker K, Jentsch TJ, Koch MC. Spectrum of mutations in the major human skeletal muscle chloride channel gene (CLCN1) leading to myotonia. Am J Hum Genet. 1995;57:1325–1334. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu FF, Ryan A, Devaney J, Warnstedt M, Korade-Mirnics Z, Poser B, Escriva MJ, Pegoraro E, Yee AS, Felice KJ, Giuliani MJ, Mayer RF, Mongini T, Palmucci L, Marino M, Rüdel R, Hoffman EP, Fahlke C. Novel CLCN1 mutations with unique clinical and electrophysiological consequences. Brain. 2002;125:2392–2407. doi: 10.1093/brain/awf246. [DOI] [PubMed] [Google Scholar]
- 21.Colding-Jørgensen E. Phenotypic variability in myotonia congenita. Muscle Nerve. 2005;32:19–34. doi: 10.1002/mus.20295. [DOI] [PubMed] [Google Scholar]
- 22.Mailänder V, Heine R, Deymeer F, Lehmann-Horn F. Novel muscle chloride channel mutations and their effects on heterozygous carriers. Am J Hum Genet. 1996;58:317–324. [PMC free article] [PubMed] [Google Scholar]
- 23.Deymeer F, Lehmann-Horn F, Serdaroğlu P, Çakirkaya S, Benz S, Rüdel R, Ozdemir C. Electrical myotonia in heterozygous carriers of recessive myotonia congenita. Muscle Nerve. 1999;22:123–125. doi: 10.1002/(sici)1097-4598(199901)22:1<123::aid-mus20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Fialho D, Kullmann DM, Hanna MG, Schorge S. Non-genomic effects of sex hormones on CLC-1 may contribute to gender differences in myotonia congenita. Neuromuscul Disord. 2008;18:869–872. doi: 10.1016/j.nmd.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Dunø M, Colding-Jørgensen E, Grunnet M, Jespersen T, Vissing J, Schwartz M. Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype. Eur J Hum Genet. 2004;12:738–743. doi: 10.1038/sj.ejhg.5201218. [DOI] [PubMed] [Google Scholar]
- 26.Hakim CA, Thomlinson J. Myotonia congenita in pregnancy. J Obstet Gynaecol Br Commonw. 1969;76:561–562. doi: 10.1111/j.1471-0528.1969.tb05883.x. [DOI] [PubMed] [Google Scholar]
- 27.Michel P, Sternberg D, Jeannet PY, Dunand M, Thonney F, Kress W, Fontaine B, Fournier E, Kuntzer T. Comparative efficacy of repetitive nerve stimulation, exercise, and cold in differentiating myotonic disorders. Muscle Nerve. 2007;36:643–650. doi: 10.1002/mus.20856. [DOI] [PubMed] [Google Scholar]
- 28.Fournier E, Viala K, Gervais H, Sternberg D, Arzel-Hézode M, Laforêf P, Eymard B, Tabti N, Willer JC, Vial C, Fontaine B. Cold extends electromyography distinction between ion channel mutations causing myotonia. Ann Neurol. 2006;60:356–365. doi: 10.1002/ana.20905. [DOI] [PubMed] [Google Scholar]
- 29.Rüdel R, Dengler R, Ricker A, Haass A, Emser W. Improved therapy of myotonia with the lidocaine derivative tocainide. J Neurol. 1980;222:275–278. doi: 10.1007/BF00313157. [DOI] [PubMed] [Google Scholar]
- 30.Meola G, Sansone V. Therapy in myotonic disorders and in muscle channelopathies. Neurol Sci. 2000;21(5 Suppl):S953–S961. doi: 10.1007/s100720070009. [DOI] [PubMed] [Google Scholar]