Abstract
Long-term survivors of hematopoietic stem cell transplantation (HSCT) during childhood and adolescence are at risk of developing endocrine complications. The purpose of this study was to evaluate the long-term endocrine complications and their associated risk factors among such patients. We reviewed the data from 111 patients (59 males and 52 females) who underwent HSCT at the mean age of 8.3±4.1 yr. Thirty patients (27.0%) had growth impairment, and seven (21.2%) out of 33 patients who attained final height reached final height below 2 standard deviation (SD). The final height SD score of the patients conditioned with total body irradiation (TBI) was significantly lower than that of the patients conditioned without TBI (-1.18±1.14 vs. -0.19±0.78, P=0.011). Thirteen patients (11.7%) developed hypothyroidism (11 subclinical, 2 central) 3.8±1.8 (range 1.6-6.2) yr after HSCT. Nineteen (65.5%) out of 29 females had evidence of gonadal dysfunction, and 18 (64.3%) out of 28 males had evidence of gonadal dysfunction. The risk for gonadal dysfunction was significantly higher in females conditioned with busulfan/cyclophosphamide (P=0.003). These results suggest that the majority of patients treated with HSCT during childhood and adolescence have one or more endocrine complications. Therefore, multiple endocrine functions should be monitored periodically after HSCT until they reach adult age.
Keywords: Endocrine Complications, Transplantation, Childhood
INTRODUCTION
Indications for hematopoietic stem cell transplantation (HSCT) in childhood and adolescence have expanded and include hematological malignancies, aplastic anemia, nonhematological malignancies and inherited metabolic disorders (1, 2). With the cumulative experiences in HSCT and advances in supportive care, the number of long-term survivors after HSCT has increased. However, graft versus host disease (GvHD) following HSCT can affect multiple organs chronically and requires prolonged treatment. In addition, conditioning regimens for HSCT including high dose chemotherapy and total body irradiation (TBI) cause long-term complications. Among the late effects, endocrine complications are most prevalent (3, 4) and have significant impacts on physical and mental health. The endocrine organs are highly sensitive to chemotherapy and irradiation because they contain a relatively high proportion of growing cells that are more susceptible to the deleterious effects of these treatment modalities.
The purpose of this study was to investigate the long-term effects of HSCT on various endocrine functions in children and adolescents and to evaluate the associated risk factors for endocrine complications.
MATERIALS AND METHODS
Study populations
The subjects of this study were patients who underwent a HSCT between 1998 and 2006 at The Catholic University of Korea St. Mary's Hospital, and were in prolonged remission for at least two years or longer. Clinical information was obtained by a review of medical records and laboratory evaluations including pituitary hormones, thyroid function and gonadal function. Data included the initial diagnosis, gender, type of HSCT, age at HSCT, conditioning regimens (chemotherapeutic agents and TBI) and sexual maturity rating. The study protocol was approved by the Ethics Committee of The Catholic University of Korea St. Mary's Hospital, and informed consent was obtained from all the parents of the patients.
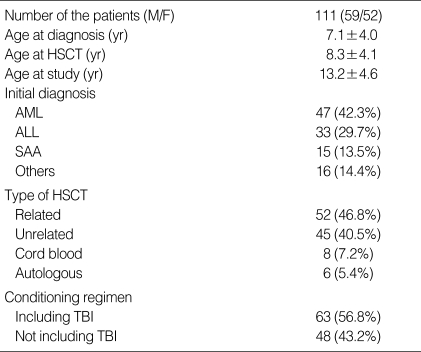
A total of 114 subjects (61 males and 53 females) were included in this study (Table 1). The mean age at HSCT was 8.3±4.1 (range 0.8-15.0) yr, and the mean time from the HSCT to the last evaluation was 4.9±2.0 yr. The mean age at the time of the last evaluation was 13.2±4.6 yr. The initial diagnoses included 47 cases (42.3%) of acute myelogenous leukemia (AML), 33 cases (29.7%) of acute lymphoblastic leukemia (ALL), and 15 cases (13.5%) of severe aplastic anemia (SAA). The other patients were diagnosed as Fanconi anemia, chronic myelogenous leukemia (CML), malignant lymphoma, hemophagocytic lymphohistiocytosis, endodermal sinus tumor and pure red cell anemia. Preparation for the HSCT included TBI in 63 subjects (56.8%), while a conditioning regimen without TBI was used for the other patients (43.2%). All TBI was delivered with fractionation, and the mean dose of TBI was 1,073.0±241.8 (maximum 1,200) cGy.
Table 1.
Clinical characteristics of the patients
HSCT, hematopoietic stem cell transplantation; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; SAA, severe aplastic anemia; TBI, total body irradiation.
Endocrine disorders at the time of HSCT or gonadal involvement of the original disease were predefined exclusion criteria, but none of the patients had such conditions.
Height measurement and assessment of pubertal status
Height was measured using a Harpenden stadiometer (Holtain Ltd., Crymych, U.K.) at the time of the diagnosis, at the time of the HSCT and every six months thereafter. The growth status was evaluated with reference to the most recent growth charts for Korean children and adolescents (5). The pubertal status was determined by the method of Tanner and Whitehouse (6).
Endocrine function tests
For the determination of endocrine function, thyroid hormones, insulin-like growth factor I (IGF-I), gonadotropins, testosterone in males and estradiol in females were measured. The following assay methods were used: immunoradiometric assay using TSH-CTK-3 (Diasorin SpA, Saluggia, Italy) for thyroid stimulating hormone (TSH); radioimmunoassay using fT4-CTK (Diasorin SpA) for free T4; radioimmunoassay using T3-CTK (Diasorin SpA) for T3; radioimmunoassay (Biosource Technologies, Inc., Nivelles, Belgium) for IGF-I; immunoradiometric assay using LH-CTK-4 (Diasorin SpA) for luteinizing hormone (LH); immunoradiometric assay using FSH-CTK-4 (Diasorin SpA) for follicle stimulating hormone (FSH); radioimmunoassay using Testosterone RIA CT (RaDIM SpA, Rome, Italy) for testosterone; radioimmunoassay using Estradiol MAIA kit (Adaltis Italia, Reno, Italy) for estradiol. Bone age was determined by the Greulich Pyle method (7). Pelvic ultrasonogram was performed in the selected female patients with delayed puberty.
The patients who met one out of the following two criteria were categorized as having growth impairment: 1) height below 2 standard deviation (SD); or 2) growth velocity below 2 SD (4 cm/yr). Growth hormone (GH) deficiency was defined by decreased peak GH concentrations (<10 ng/mL) in two or more GH stimulation tests while fulfilling criteria for growth impairment (8). Hypothyroidism was defined as free T4 level below 0.8 ng/dL, and subclinical (compensated) hypothyroidism as free T4 level above 0.8 ng/dL with TSH level above 6.4 mIU/L (9). The final height was defined as a height velocity of less than 0.5 cm/yr and/or adult bone age. Obesity was defined as body mass index (BMI) for age and gender higher than the 95th percentile. Dyslipidemia (abnormal lipid profiles) was defined as triglyceride ≥110 mg/dL or HDL ≤40 mg/dL measured on two or more separate serum samples after fasting (10).
Gonadal function was determined in female patients who were 13 yr or older and in male patients 14 yr or older. Gonadal dysfunction in females was defined as elevated levels of FSH or LH or the use of estrogen therapy (8). Gonadal dysfunction in males was defined as elevated levels of FSH or LH or the use of androgen replacement (8). Abnormally elevated gonadotropin levels in female subjects were defined as FSH levels ≥15 IU/mL, and LH levels ≥20 IU/mL. Abnormally elevated gonadotropin levels in male subjects were defined as FSH levels ≥15 IU/mL, and LH levels ≥10 IU/mL. These cutoff levels were chosen according to the values used at the St. Mary's Hospital laboratory and reference values from the medical literatures (11).
Statistical analysis
The data was expressed as mean±standard deviation. Laboratory and clinical variables were compared using the t-test. The differences in categorical factors between groups were analyzed by the Pearson's chi-square test and Fisher's exact test as appropriate. Comparison of the final height and midparental height for each patient was performed using paired t-test and Wilcoxon signed rank test. The statistical analyses were performed using SPSS statistical software package (SPSS Inc., Chicago, IL, U.S.A.). A P value <0.05 was considered to be statistically significant.
RESULTS
Growth impairment and final height
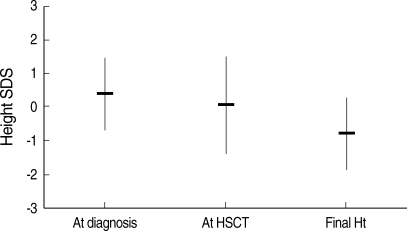
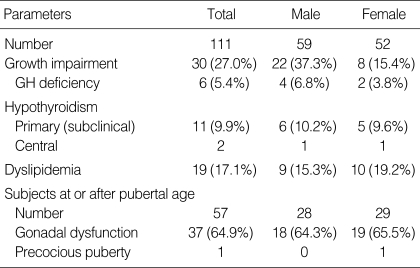
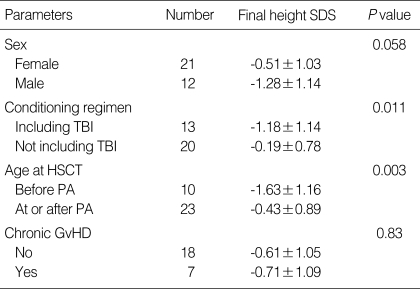
The height SD score at diagnosis and at HSCT was 0.41±1.07 and 0.07±1.45, respectively (Fig. 1). Thirty patients (27.0%) were categorized as having growth impairment, and six of them had GH deficiency (Table 2). A total of 33 patients (21 females and 12 males) attained their final height by criteria at the time of the study. The final height SD score in the female and male patients was -0.51±1.03 and -1.28±1.14, respectively (P=0.058, Table 3). The final height SD score of the patients who were conditioned with TBI was significantly lower than that of the patients who were treated without TBI (-1.18±1.14 vs. -0.19±0.78, P=0.011). The final height SD score of the patients who underwent HSCT before pubertal age (10 yr in females and 12 yr in males, defined arbitrarily) was significantly lower than that of the patients who underwent HSCT at or after pubertal age (-1.63±1.16 vs. -0.43±0.89, P=0.003). The final height SD score was not influenced by initial diagnosis, type of HSCT or development of chronic GvHD.
Fig. 1.
The final height (Ht) standard deviation score (SDS) of the patients showed a decreasing tendency compared to the height SDS at diagnosis or at hematopoietic stem cell transplantation (HSCT).
Table 2.
Endocrine complications in the patients treated with hematopoietic stem cell transplantation
GH, growth hormone.
Table 3.
Final height standard deviation (SD) score according to various clinical factors
TBI, total body irradiation; PA, pubertal age (≥10 yr for female, ≥12 yr for male); HSCT, hematopoietic stem cell transplantation; GvHD, graft versus host disease; SDS, standard deviation score.
Among the 33 patients who attained final height, seven (4 females, 3 males) (21.2%) reached final height lower than -2.0 SD. The final height SD score was -2.65±0.74 for females and -2.17±0.22 for males. Their initial diagnoses included 3 ALL, 2 SAA, one AML and one CML. The age at HSCT was 10.6±4.4 yr for females and 10.9±3.7 yr for males. The midparental height was 158.1±6.1 cm for females and 171.2±2.8 cm for males.
Thyroid dysfunction
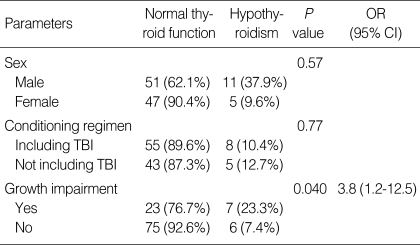
Thirteen patients (11.7%) developed hypothyroidism during the follow-up period. Eleven of them were categorized as subclinical hypothyroidism, and the others had central (pituitary or hypothalamic) hypothyroidism (Table 2). The mean duration from HSCT to diagnosis of hypothyroidism was 3.8±1.8 yr (range 1.6-6.2 yr). The risk of thyroid dysfunction was not associated with gender, conditioning regimen with TBI, initial diagnosis, type of HSCT or chronic GvHD. The incidence of hypothyroidism in the patients with growth impairment was higher than that of the patients without growth impairment (23.3% vs. 7.4%, P=0.040, OR=3.8 [1.2-12.5]) (Table 4).
Table 4.
Risk of thyroid dysfunction after hematopoietic stem cell transplantation
TBI, total body irradiation; OR, odds ratio; CI, confidence interval.
Gonadal dysfunction
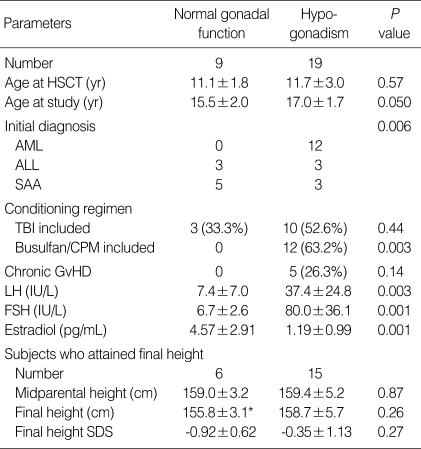
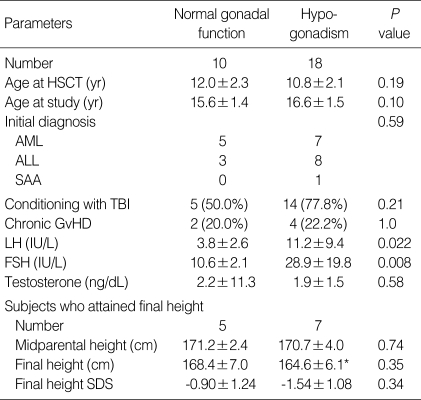
Nineteen (65.5%) out of 29 females had evidence of gonadal dysfunction, and one female patient was diagnosed as precocious puberty (Table 2). Eighteen (64.3%) out of 28 males had evidence of gonadal dysfunction. The incidence of gonadal dysfunction in female patients conditioned with TBI was similar to that of females treated without TBI (Table 5). All 12 female patients conditioned with busulfan/cyclophosphamide developed gonadal dysfunction. The risk for gonadal dysfunction in this group was significantly higher than that of the patients who were not conditioned with busulfan/cyclophosphamide (100% vs. 43.8%, P=0.003). The incidence of gonadal dysfunction was not influenced by the age at HSCT, conditioning regimen or chronic GvHD in male patients (Table 6). The final height and final height SD score of hypogonadic females were not different from those of the female patients with normal gonadal function (Table 5). In the female patients with normal gonadal function, the final height (155.8±3.1 cm) was significantly lower than the midparental height (159.0±3.2 cm) (P=0.038). By contrast, the final height of hypogonadic females was not different from their midparental height. The final height and final height SD score of hypogonadic males were not different from those of the male patients with normal gonadal function (Table 6). In the hypogonadic male patients, the final height (164.6±6.1 cm) was significantly lower than the midparental height (170.7±4.0 cm) (P=0.012). By contrast, the final height of the male patients with normal gonadal function was not different from their midparental height.
Table 5.
Clinical characteristics of female patients according to gonadal dysfunction
*P=0.038 compared to midparental height of females with normal gonadal function.
HSCT, hematopoietic stem cell transplantation; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; SAA, severe aplastic anemia; TBI, total body irradiation; CPM, cyclophosphamide; GvHD, graft versus host disease; SDS, standard deviation score.
Table 6.
Clinical characteristics of male patients according to gonadal dysfunction
*P=0.012 compared to midparental height of hypogonadic males.
HSCT, hematopoietic stem cell transplantation; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; SAA, severe aplastic anemia; TBI, total body irradiation; GvHD, graft versus host disease; SDS, standard deviation score.
Obesity and body mass index
Four patients (3.6%) met the criteria for obesity during the follow-up period, and nineteen (17.1%) had abnormal lipid profiles (Table 2). The BMI of the female patients who attained their final height was 21.0±3.3 kg/m2 (range 15.5-28.7 kg/m2). The BMI of the male patients who attained their final height was 20.1±3.1 kg/m2 (range 15.3-24.7 kg/m2).
DISCUSSION
The results of this study show that a quarter of the patients who underwent HSCT during childhood or adolescence developed growth impairment. The risk of attaining a final height lower than -2.0 SD was around 20%. The clinical determinants related to a decrease of the final height were conditioning regimen with TBI and younger (prepubertal) age at HSCT.
Many factors have been suggested to contribute to growth failure after HSCT (12-14). TBI used for the preparation of HSCT can impair linear growth by various mechanisms, such as GH deficiency and skeletal dysplasia (15). Some investigators have reported that disproportionate growth of the spine or long bones account for height loss after TBI (16, 17). Younger age at HSCT is a well known risk factor for a decrease in adult height (14, 18, 19). An increase in the incidence of GH deficiency after HSCT has been reported in several studies (14, 20), but transiency of the GH deficient state has also been reported (19, 21). In our study, the incidence of GH deficiency among patients with growth impairment was about 20%. The effect of GH replacement on the final height of patients who underwent HSCT during childhood has not been determined.
Among children and adolescents who were treated without HSCT, radiation exposure to the head and neck is a known risk factor for subsequent hypothyroidism. Following radiation doses above 15 cGy, the incidence of primary hypothyroidism was reported to be 40-90% in patients with Hodgkin's disease, non-Hodgkin's lymphoma or head/neck malignancies (22, 23). Meanwhile, according to a recent study, chemotherapy, either alone or in combination with radiation, was not associated with an increase in the incidence of hypothyroidism (23).
In our analysis, the incidence of thyroid dysfunction after HSCT was 11.7%. Most of the cases with thyroid dysfunction had subclinical hypothyroidism, while central hypothyroidism was rare. Hypothyroidism is a well known common complication of TBI conditioning regimens. The overall incidence of primary hypothyroidism after fractionated TBI has been reported to be 10 to 28% (19, 20, 24). However, reports of thyroid dysfunction in patients conditioned with a chemotherapy-only based regimen (25) have indicated that irradiation of the thyroid gland is not the only risk factor. It was reported that abnormalities of thyroid function including severe primary hypothyroidism occurred after HSCT in 10.8% of children with immunodeficiency who received chemotherapy conditioning (with busulfan/cyclophosphamide) without TBI (26). In our study, five (12.7%) out of 48 patients treated with a conditioning regimen without TBI developed hypothyroidism. Therefore, children and adolescents who undergo HSCT should be considered to be at high risk for thyroid dysfunction whether or not they receive TBI.
In a large multi-center study of long-term thyroid outcome after HSCT, Berger et al. (9) showed that the incidence of thyroid dysfunction was increased with prolongation of the follow-up period. The maximum interval between HSCT and the diagnosis of thyroid dysfunction was reported to be up to 11 yr (9) and was 6.2 yr in our study. We did not find evidence of an increased risk for thyroid neoplasms or autoimmune thyroid disease following HSCT.
Gonadal dysfunction after HSCT was observed at a very high rate in both females (65.5%) and males (64.3%). The use of busulfan/cyclophosphamide in the conditioning regimen was found to be a significant risk factor for gonadal dysfunction in the female patients. The increased incidence of gonadal dysfunction in the female patients with AML might reflect the impact of busulfan/cyclophosphamide on ovaries, because most of the AML patients were conditioned using busulfan/cyclophosphamide in our protocol. Our findings did not show an association between TBI or patient age and gonadal dysfunction in females. These results might have been due to a confounding influence of chemotherapy including busulfan/cyclophosphamide or by the small number of the subjects. It has been reported that ovarian failure developed in all patients older than 10 yr at the time they are treated with TBI (27, 28). By contrast, ovarian failure, following treatment with busulfan/cyclophosphamide, has been observed in patients treated before and after the onset of puberty (29).
Previous studies have shown that Leydig cell function generally is preserved in males treated with TBI-based conditioning regimens (12, 27). By contrast, germ cell dysfunction has been reported to be present in most males treated with TBI as indicated by elevated FSH levels (30). In our study, damage to Leydig cell function after HSCT indicated by abnormally elevated LH levels was observed in 17.9% of the males. Meanwhile, abnormally elevated FSH levels suggesting germ cell dysfunction was observed in 57.1% of the males. The impact of HSCT on germ cell function could vary depending on the follow-up duration and the age at the time of treatment, and requires more specific evaluation methods for confirmation.
Interestingly, loss of final height compared to midparental height was observed in females with normal gonadal function and in hypogonadic males, but not in hypogonadic females and in males with normal gonadal function. This observation suggests that sex hormone deficiency has a different impact on the final height in males and females. Further study is needed to determine whether delayed initiation of sex steroid replacement in hypogonadic females or early initiation of treatment in hypogonadic males result in a better height outcome.
There are some limitations to this study, in addition to the variety of the initial diagnoses included, that needs to be considered when interpreting the results. Chemotherapeutic agents or radiation therapy performed before HSCT has considerable influence on long-term endocrine functions. In this study, the chemotherapy protocols and doses of radiation therapy used before HSCT yielded too many combinations that we were unable to isolate the impact of HSCT from the influence of pre-HSCT treatment on endocrine functions. To overcome this limitation, further analysis of patients with homogenous diagnosis and common protocols needs to be done.
In conclusion, the results of this study show that after HSCT during childhood and adolescence a significant number of patients develop growth impairment, a reduced final height and/or subclinical hypothyroidism. Gonadal dysfunction was observed in approximately two thirds of the patients who underwent HSCT. The risk of endocrine complications was most commonly associated with the conditioning regimen including TBI and chemotherapy including busulfan/cyclophosphamide. However, factors such as gender and the age at the time of treatment also had some impact on the complications observed. Multiple endocrine functions should be monitored regularly after HSCT until the patients reach adult age.
References
- 1.Thomas ED. History of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–339. doi: 10.1111/j.1365-2141.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldman JM, Schmitz N, Niethammer D, Gratwohl A. Allogeneic and autologous transplantation for haematological disease, solid tumors and immune disorders: current practice in Europe in 1998. Accreditation Sub-Committee of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:1–7. doi: 10.1038/sj.bmt.1701089. [DOI] [PubMed] [Google Scholar]
- 3.Hows JM, Passweg JR, Tichelli A, Locasciulli A, Szydlo R, Bacigalupo A, Jacobson N, Ljungman P, Cornish J, Nunn A, Bradley B, Socié G IMUST Study Participating Centers; Late Effects Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006;38:799–805. doi: 10.1038/sj.bmt.1705531. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EI, Dopfer RE, Klingebiel T, Scheel-Walter H, Ranke MB, Niethammer D. Longitudinal gonadal function after bone marrow transplantation for acute lymphoblastic leukemia during childhood. Pediatr Transplant. 1999;3:38–44. doi: 10.1034/j.1399-3046.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 5.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, Kim YT, Lee CG. 2007 Korean National Growth Charts: review of developmental process and outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 6.Tanner JM, Whitehouse RH. Clinical longitudinal standards for Ht, weight, Ht velocity, and stages of puberty. Arch Dis Child. 1976;51:170–179. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greulich WW, Pyle SI. Radiograhic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford: Stanford University Press; 1959. [Google Scholar]
- 8.Shalitin S, Phillip M, Stein J, Goshen Y, Carmi D, Yaniv I. Endocrine dysfunction and parameters of the metabolic syndrome after bone marrow transplantation during childhood and adolescence. Bone Marrow Transplant. 2006;37:1109–1117. doi: 10.1038/sj.bmt.1705374. [DOI] [PubMed] [Google Scholar]
- 9.Berger C, Le-Gallo B, Donadieu J, Richard O, Devergie A, Galambrun C, Bordigoni P, Vilmer E, Plouvier E, Perel Y, Michel G, Stephan JL. Late thyroid toxicity in 13 long-term survivors of allogeneic bone marrow transplantation for acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;35:991–995. doi: 10.1038/sj.bmt.1704945. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Insititute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Mayer EI, Dopfer RE, Klingebiel T, Scheel-Walter H, Ranke MB, Niethammer D. Longitudinal gonadal function after bone marrow transplantation for acute lymphoblastic leukemia during childhood. Pediatr Transplant. 1999;3:38–44. doi: 10.1034/j.1399-3046.1999.00006.x. [DOI] [PubMed] [Google Scholar]
- 12.Chemaitilly W, Sklar CA. Endocrine complications of hematopoietic stem cell transplantation. Endocrinol Metab Clin North Am. 2007;36:983–998. doi: 10.1016/j.ecl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A, Rovelli A, Bakker B, Uderzo C, van Lint MT, Esperou H, Gaiero A, Leiper AD, Dopfer R, Cahn JY, Merlo F, Kolb HJ, Socié G. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: a study by the Working Party for Late Effects-EBMT. Blood. 1999;93:4109–4115. [PubMed] [Google Scholar]
- 14.Clement-De Boers A, Oostdijk W, Van Weel-Sipman MH, Van den Broeck J, Wit JM, Vossen JM. Final height and hormonal function after bone marrow transplantation in children. J Pediatr. 1996;129:544–550. doi: 10.1016/s0022-3476(96)70119-1. [DOI] [PubMed] [Google Scholar]
- 15.Brennan BM, Shalet MS. Endocrine late effects after bone marrow transplantation. Br J Haematol. 2002;118:58–66. doi: 10.1046/j.1365-2141.2002.03527.x. [DOI] [PubMed] [Google Scholar]
- 16.Chemaitilly W, Boulad F, Heller G, Kernan NA, Small TN, O'Reilly RJ, Sklar CA. Final height in pediatric patients after hyperfractionated total body irradiation and stem cell transplantation. Bone Marrow Transplant. 2007;40:29–35. doi: 10.1038/sj.bmt.1705694. [DOI] [PubMed] [Google Scholar]
- 17.Bakker B, Oostdijk W, Geskus RB, Stokvis-Brantsma WH, Vossen JM, Wit JM. Patterns of growth and body proportion after total body irradiation and hematopoietic stem cell transplantation during childhood. Pediatr Res. 2006;59:259–264. doi: 10.1203/01.pdr.0000199550.71887.ba. [DOI] [PubMed] [Google Scholar]
- 18.Sanders JE, Guthrie KA, Hoffmeister PA, Woolfrey AE, Carpenter PA, Appelbaum FR. Final adult height of patients who received hematopoietic cell transplantation in childhood. Blood. 2005;105:1348–1354. doi: 10.1182/blood-2004-07-2528. [DOI] [PubMed] [Google Scholar]
- 19.Holm K, Nysom K, Rasmussen MH, Hertz H, Jacobsen N, Skakkebaek NE, Krabbe S, Müsller J. Growth, growth hormone and final height after BMT. Possible recovery of irradiation-induced growth hormone insufficiency. Bone Marrow Transplant. 1996;18:163–170. [PubMed] [Google Scholar]
- 20.Ogilvy-Stuart AL, Clark DJ, Wallace WH, Gibson BE, Stevens RF, Shalet SM, Donaldson MD. Endocrine deficit after fractionated total body irradiation. Arch Dis Child. 1992;67:1107–1110. doi: 10.1136/adc.67.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couto-Silva AC, Trivin C, Esperou H, Michon J, Fischer A, Brauner R. Changes in height, weight and plasma leptin after bone marrow transplantation. Bone Marrow Transplant. 2000;26:1205–1210. doi: 10.1038/sj.bmt.1702718. [DOI] [PubMed] [Google Scholar]
- 22.Glatstein E, McHardy-Young S, Brast N, Eltringham JR, Kriss JP. Alterations in serum thyrotropin (TSH) and thyroid function following radiotherapy in patients with malignant lymphoma. J Clin Endocrinol Metab. 1971;32:833–841. doi: 10.1210/jcem-32-6-833. [DOI] [PubMed] [Google Scholar]
- 23.Sklar C, Whitton J, Mertens A, Stovall M, Green D, Marina N, Greffe B, Wolden S, Robison L. Abnormalities of the thyroid in survivors of Hodgkin's disease: data from the childhood cancer survivor study. J Clin Endocrinol Metab. 2000;85:3227–3232. doi: 10.1210/jcem.85.9.6808. [DOI] [PubMed] [Google Scholar]
- 24.Boulad F, Bromley M, Black P, Heller G, Sarafoglou K, Gillio A, Papadopoulos E, Sklar C. Thyroid dysfunction following bone marrow transplantation using hyperfrationated radiation. Bone Marrow Transplant. 1995;15:71–76. [PubMed] [Google Scholar]
- 25.Toubert ME, Socié G, Gluckman E, Aractingi S, Espérou H, Devergie A, Ribaud P, Parquet N, Schlageter MH, Beressi JP, Rain JD, Vexiau P. Short and long-term follow-up of thyroid dysfunction after allogeneic bone marrow transplantation without the use of preoperative total body irradiation. Br J Haematol. 1997;98:453–457. doi: 10.1046/j.1365-2141.1997.2433060.x. [DOI] [PubMed] [Google Scholar]
- 26.Slatter MA, Gennery AR, Cheetham TD, Bhattacharya A, Crooks BN, Flood TJ, Cant AJ, Abinun M. Thyroid dysfunction after bone marrow transplantation for primary immunodeficiency without the use of total body irradiation in conditioning. Bone Marrow Transplant. 2004;33:949–953. doi: 10.1038/sj.bmt.1704456. [DOI] [PubMed] [Google Scholar]
- 27.Sarafoglou K, Boulad F, Gillio A, Sklar C. Gonadal dysfunction after bone marrow transplantation for acute leukemia during childhood. J Pediatr. 1997;130:210–216. doi: 10.1016/s0022-3476(97)70345-7. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto M, Shinohara O, Ishiguro H, Shimizu T, Hattori K, Ichikawa M, Yabe H, Kubota C, Yabe M, Kato S. Ovarian function after bone marrow transplantation before menarche. Arch Dis Child. 1999;80:452–454. doi: 10.1136/adc.80.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel G, Socié G, Gebhard F, Bernaudin F, Thuret I, Vannier JP, Demeocq F, Leverger G, Pico JL, Rubie H, Mechinaud F, Reiffers J, Gratecos N, Troussard X, Jouet JP, Simonin G, Gluckman E, Maraninchi D. Late effects of allogeneic bone marrow transplantation for children with acute myeloblastic leukemia in first complete remission: the impact of conditioning regimen without total-body irradiation-a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 1997;15:2238–2246. doi: 10.1200/JCO.1997.15.6.2238. [DOI] [PubMed] [Google Scholar]
- 30.Sanders JE, Hawley J, Levy W, Gooley T, Buckner CD, Deeg HJ, Doney K, Storb R, Sullivan K, Witherspoon R, Appelbaum FR. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. [PubMed] [Google Scholar]