Abstract
We aimed to evaluate prospectively the efficacy of positron emission tomography (PET)/computed tomography (CT) plus brain magnetic resonance imaging (MRI) for detecting extrathoracic metastases in lung adenocarcinoma. Metastatic evaluations were feasible for 442 consecutive patients (M:F=238:204; mean age, 54 yr) with a lung adenocarcinoma who underwent PET/CT (CT, without IV contrast medium injection) plus contrast-enhanced brain MRI. The presence of metastases in the brain was evaluated by assessing brain MRI or PET/CT, and in other organs by PET/CT. Diagnostic efficacies for metastasis detection with PET/CT plus brain MRI and with PET/CT only were calculated on a per-patient basis and compared from each other. Of 442 patients, 88 (20%, including 50 [11.3%] with brain metastasis) had metastasis. Regarding sensitivity of overall extrathoracic metastasis detection, a significant difference was found between PET/CT and PET/CT plus brain MRI (68% vs. 84%; P=0.03). As for brain metastasis detection sensitivity, brain MRI was significantly higher than PET/CT (88% vs. 24%; P<0.001). By adding MRI to PET/CT, brain metastases were detected in additional 32 (7% of 442 patients) patients. In lung adenocarcinoma patients, significant increase in sensitivity can be achieved for detecting extrathoracic metastases by adding dedicated brain MRI to PET/CT and thus enhancing brain metastasis detection.
Keywords: PET/CT Scan, Lung Neoplasms, Neoplasm Metastasis, Brain, Magnetic Resonance Imaging, Neoplasm Staging
INTRODUCTION
The incidence of distant metastases in non-small cell lung cancer (NSCLC) is reported in 40% or more of patients with the disease at the time of diagnosis (1, 2). In spite of the high percentage of extra-thoracic metastases, there has been no consensus recommendation for extra-thoracic metastasis evaluation using various imaging modalities. International medical associations have different opinions about the use of imaging (3, 4). One society suggests full scanning for metastatic disease, even if patients lack symptoms and signs of metastatic disease (3). Another society advocates no preoperative imaging of the brain or skeleton in NSCLC patients without symptoms or signs of a distant metastasis (4).
The use of integrated fluorine 18fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) is more efficient to assess the presence of a metastasis of both the mediastinal and extra-thoracic regions than the use of standard methods (5, 6). However, FDG PET or PET/CT has some limitation for brain metastasis evaluation, because it is difficult to differentiate FDG-avid metastases from the normal surrounding hyper-metabolic parenchyma in brain (7). Metastatic lesion to the brain may present in up to 18% of patients with NSCLC (8, 9). Particularly in adenocarcinoma of the lung, the brain metastasis rate has been reported to be 43% (58 of 136 patients) in one study (10). Considering the high percentage of the brain metastasis rate in lung adenocarcinoma patients and the limited efficacy of PET or PET/CT for detecting brain metastasis, PET or PET/CT plus an additional brain imaging modality (magnetic resonance imaging [MRI] or CT) may be needed for staging of lung adenocarcinoma. The purpose of our study was to evaluate prospectively the efficacy of PET/CT plus 3T brain MRI for detecting extrathoracic metastases in lung adenocarcinoma.
MATERIALS AND METHODS
The Institutional Review Board approved this prospective study and informed consent was obtained from all patients (2008-07-019).
Patients
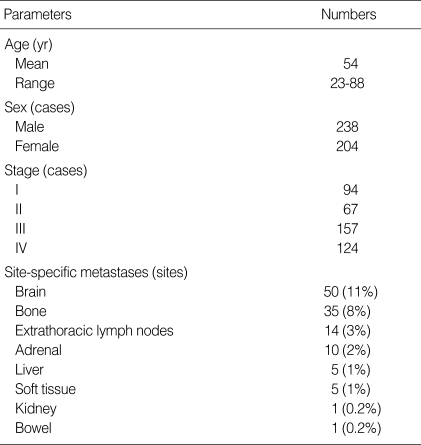
From November 2003 to December 2006, 520 consecutive patients with histopathologically (cytology or biopsy) proven adenocarcinoma of the lung (except for bronchioloalveolar cell carcinoma which mediastinal nodal or extra-thoracic metastasis is unusual) were enrolled. All patients were referred for cancer staging of lung adenocarcinoma. Of these 520 patients, 78 patients were excluded for the following reasons: 58 patients were unable to undergo brain MRI for the initial staging, and 20 patients were lost to follow-up. Ultimately, 442 patients (M:F=238:204; mean age, 54 yr) were included in this study, in whom both FDG PET/CT and dedicated brain MRI were performed for the initial staging and metastatic evaluations of M staging were feasible with pathological results or follow-up imaging studies.
Imaging and interpretation
The presence of metastases in the brain was evaluated by using brain MRI (Achieva, Philips Medical Systems, Best, The Netherlands) and PET/CT (Discovery LS; GE Medical Systems, Milwaukee, WI, U.S.A.), and the presence of metastases in other organs was determined by the use of PET/CT. PET/CT and MRI were performed within a one-week time interval (average time interval, 3.1 days; range, 0-7 days). Detailed imaging methods of integrated PET/CT have been described in previous reports (5, 11). Briefly, glucose level in peripheral blood was ≤150 mg/dL in all patients. The patients received an intravenous injection of 370 MBq (10 mCi) of FDG and then rested for over 45 min before scanning. PET/CT device consisted of a PET scanner (Advance NXi; GE Healthcare) and an eight-slice CT scanner (Light-Speed Plus; GE Healthcare). Immediately after taking nonenhanced CT, emission PET was performed in the identical transverse field of view. Co-registered images were displayed by Xeleris software (GE Healthcare). It took approximately 40 min to complete a PET/CT study. All brain MRI studies were performed by using a 3-Tesla scanner with a standard head coil. Brain MR images were obtained in the axial, sagittal, and coronal planes by using three sequences including a T2-weighted axial turbo spin-echo pulse sequence (repetition time 3,000 ms, echo time 80 ms) with fat suppression, a fluid-attenuation inversion-recovery (FLAIR) spin-echo sequence (repetition time 11,000 ms, echo time 125 ms, inversion time 2,800 ms) and a non-contrast enhanced and a contrast-enhanced T1-weighted spin-echo sequence (repetition time 500 ms, echo time 10 ms). The contrast-enhanced sequence was obtained after bolus injection of a dose of 0.2 mM/kg paramagnetic contrast agent (Magnevist, Schering, Berlin, Germany).
A chest radiologist with 18 yr of experience in CT interpretation and a nuclear medicine physician with four years of PET/CT interpretation jointly evaluated the integrated PET/CT images. Both clinicians were unaware of the brain MRI findings or the clinical and pathological evaluation results. An abnormal focal FDG uptake that accompanied a corresponding anatomic alteration was considered as the indicaton of a metastasis (5, 11). The maximum standardized uptake value (SUVmax) of the focal metastatic lesion was measured. All lymph nodes with abnormal FDG uptake (greater than mediastinal blood pool uptake) in the extrathoracic regions were considered as metastatic, unless they showed high attenuation over 70 HU or benign calcification (central nodular, laminated, popcorn-like and diffuse) at unenhanced CT (12). Two chest radiologists with three years of experience in whole-body MRI analysis, who were unaware of the clinical, PET/CT findings or histologic diagnoses, interpreted the brain MR images and decisions on findings were made by consensus. When the two chest radiologists had different opinions on the presence of brain metastasis, they sought for the third opinion from neuro-imaging radiologists and by a majority the presence or absence of brain metastasis was determined.
The probability of the presence of metastases on a per-patient basis was then evaluated by the use of a five-point visual scoring system: 0, definitely absent; 1, probably absent; 2, possibly present; 3, probably present; 4, definitely present. Before interpreting the images, the reviewers were informed that the categorization of confidence levels of 2 or higher belonged to a category of positive diagnosis for a metastasis. The largest diameter of the detected metastatic nodules on brain MR images and CT component images of PET/CT was measured and recorded.
Reference standard for metastases
The final decisions on the presence of extra-thoracic metastases in each patient were reached based on the results of dedicated standard imaging, a pathologic examination, or a follow-up examination.
Metastatic lesions were confirmed at 121 organ sites from 88 (20%) of the 442 patients. Sixteen lesions of metastases were pathologically proven. Ninety-four metastatic lesions were confirmed at the time of initial staging by using the use of specific organ-dedicated imaging studies, i.e., dedicated MRI at the time of staging for 58 lesions, dedicated CT for 21 lesions, and bone scan for 15 lesions. The remaining 11 lesions were detected by follow-up dedicated imaging studies in six months or more, and the lesions were regarded as metastatic (growth from a microscopic metastatic focus) because most of the primary cancers in these patients were resected surgically after the initial staging work-up.
The absence of a metastasis at 18 sites (from 18 patients) which were suspected as metastatic at the initial clinical-imaging studies was proven by following methods: negative biopsy results for metastasis (n=5); surgical excision (n=1) of a suspected adrenal lesion; organ-specific multiphase CT (n=2) for adrenal lesions; negative bone scan results (n=2); and negative results for brain metastasis (n=2) by the use of dedicated conventional MRI and three times of cerebrospinal fluid tapping. In the remaining six patients, the findings of clinico-laboratory follow-up studies served as reference standards for determining the absence of a metastasis. The mean clinical follow-up time for these six patients was 36 months (range, 30-41 months).
Statistical analysis
The diagnostic efficacy of PET/CT plus brain MRI was calculated on a per-patient basis. Receiver operating characteristic (ROC) curve analysis was used to compare diagnostic capabilities and it was compared by using a Z test, as described by Hanley and McNeil (12). Sensitivity, specificity, and accuracy of the two methods were statistically compared by use of the McNemar's test. Statistical differences in the size of PET-detected and PET-missed metastatic lesions were compared by use of the Mann-Whitney U test. A P value <0.05 was considered as a significant difference.
RESULTS
Patient demographics
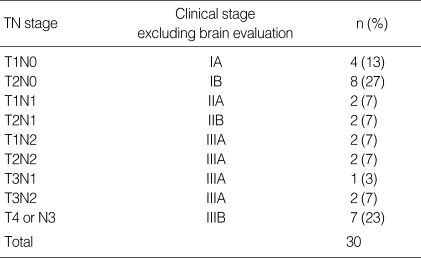
Details of the patient characteristics are summarized in Table 1. Extrathoracic metastatic lesions were confirmed at 121 organ sites for 88 (20%) of the 442 patients. Of the 50 patients with a brain metastasis, 30 (60%) had one organ (brain) metastasis and 35 (70%) patients had a single lesion metastasis. Sixteen (53%) of 30 patients with single organ metastasis to brain had early stage of cancer (stage I or II excluding brain evaluation) (Table 2). Seven (14%) patients had neurologic symptoms or signs (headache, dizziness, vomiting or a neurological deficit) at the time of initial presentation.
Table 1.
Characteristics of subjected patients
Table 2.
Tumor and nodal stage in patients with single organ metastasis to brain
Efficacy of MRI and PET/CT for metastasis detection
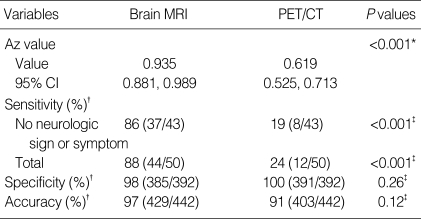
The diagnostic efficacies of the two modalities are summarized in Tables 3, 4. By adding contrast enhanced MRI to PET/CT, brain metastases were detected in additional 32 (7.2% of 442 patients; 44 in MRI plus PET/CT vs. 12 in PET/CT only) patients, in whom the treatment modality should have been changed. For the assessment of brain metastases on a per-patient basis, brain MRI showed a significantly greater area under the ROC curve (Az) than PET/CT (0.985 vs. 0.619; P<0.001) did. In terms of sensitivity for the detection of brain metastasis, a significant difference was found between brain MRI and PET/CT (88% vs. 24%; P<0.001). For the neurologically asymptomatic cases, the sensitivity of brain MRI was significantly higher than that of PET/CT (86% vs. 19%; P<0.001). Sizes (measured on enhanced T1-weighted images of brain MRI) of PET-detected brain metastases were significantly larger (mean, 27 mm; range, 10-47 mm) than those of PET-missed lesions (mean, 6 mm; range, 1-28 mm) (P=0.002).
Table 3.
ROC analysis and comparison of capability of detection of brain metastases on a per-patient basis
*P values were calculated by using the Z test; †Data in parentheses are the values used to calculate percentages; ‡P values were calculated by using McNemar's test.
Az, area under the ROC curve; CI, confidence interval.
Table 4.
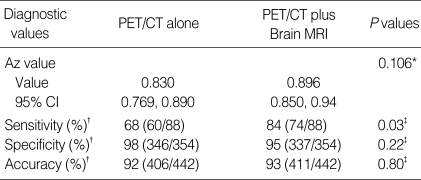
ROC analysis and sensitivity and specificity in detection of all extrathoracic metastases
*P values were calculated by using Z test; †Data in parentheses are the values used to calculate the percentage; ‡P values were calculated by using McNemar's test.
Az, area under the ROC curve; CI, confidence interval.
ROC analysis determined that PET/CT plus brain MRI was superior to PET/CT for the detection of overall extrathoracic metastases, although statistical significance was not reached (0.896 vs. 0.830; P=0.106). In terms of sensitivity for the detection of overall extrathoracic metastases, a significant difference was found between PET/CT and PET/CT plus brain MRI (68% vs. 84%; P=0.03).
False-negative and false-positive
A listing of false-positive and false-negative cases for brain metastases of lung adenocarcinoma patients identified by means of the two methods is shown in Table 5. Representative cases are shown in Figs. 1, 2. PET/CT could not depict 22 metastatic foci of <5 mm in diameter. At brain MRI, all false negative lesions were noted during the follow-up period and were not identified at the initial imaging study, retrospectively.
Table 5.
Causes of false-positive and false-negative lesions as determined by brain MRI and PET/CT
Numbers are per patient.
*Maximal size of the brain metastatic lesions.
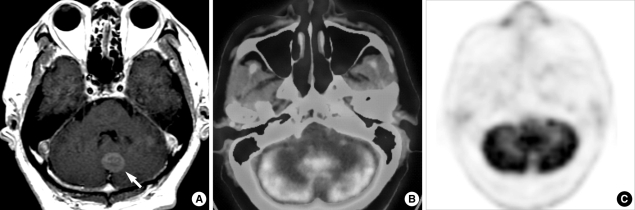
Fig. 1.
A 42-yr-old woman with lung adenocarcinoma and brain metastases. Contrast-enhanced T1-weighted brain MR image (A) clearly demonstrates the presence of cerebellar metastasis (arrow). PET/CT and PET images (B, C) show decreased FDG-uptake.
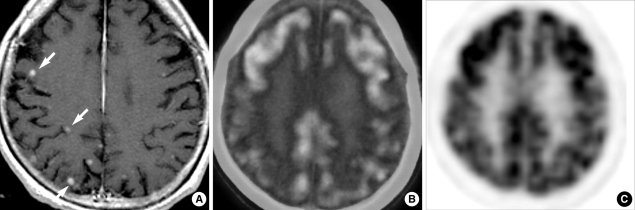
Fig. 2.
A 27-yr-old man with lung adenocarcinoma and multiple brain metastases. Contrast-enhanced T1-weighted brain MR image (A) shows the presence of multiple small metastases less than 5 mm in diameter (arrows). However, axial images (B, C) of PET/CT and PET images do not indicate the abnormal FDG-uptaking lesion.
DISCUSSION
Preoperative evaluation with diagnostic imaging for brain metastases in NSCLC patients remains controversial issue (14, 15). One meta-analysis in patients with no neurological abnormality on a clinical examination demonstrated the negative predictive value in this setting as 95% (16). In contrast, a recent report by Jena et al. (17) stated that an asymptomatic brain metastasis occurs in 17% (29 of 175; 158 [90%] of 175 patients had stage IV cancer) of lung cancer patients with almost equal number of symptomatic (33 of 62 [53%] patients with a brain metastasis) and asymptomatic (29 of 62 [47%] patients) metastases. In the current study (relatively even distribution of patient cancer stages, 124 [28%] of 442 patients had stage IV cancers), 50 (11%) of the 442 lung adenocarcinoma patients had a brain metastasis, and of these 50 patients, 30 (60%) patients had one organ (brain) metastasis only and 35 (70%) patients had a single lesion metastasis. Only seven (14%) patients complained of any neurological symptoms and signs.
The brain is often the only site of distant metastatic disease and approximately one-third of patients will have limited, potentially resectable lung tumors without distant metastases other than a brain metastasis (18). In the most recent ACCP guidelines (19), the use of routine staging of the central nervous system (CNS) system is considered in level 2C for stage III malignancies. However, 53% of the patients with single metastasis to brain in our study had stage I or II lung adenocarcinoma. The prognosis in patients with a brain metastasis who are untreated is extremely poor (about one month survival after diagnosis), whereas patients with NSCLC who were treated with local radiation therapy survived for about eight months (20, 21). Therefore, early and accurate diagnosis of a brain metastasis is crucial to improve quality of life and the poor survival rates of lung cancer patients.
For the assessment of brain metastases on a per-patient basis, our results showed that the Az value of brain MRI is significantly larger than that of PET/CT. In addition, the sensitivity of brain MRI is significantly higher than that of PET/CT. This result is in accord with findings of recent studies by Ohno et al. (21) and Yi et al. (22), which has found higher sensitivity of MRI than FDG PET or PET/CT for the detection of brain metastasis is determined. The disparity in sensitivity between brain MRI and PET/CT can be caused by the extremely high level of physiological tissue accumulation of FDG in the cerebral cortex as depicted at PET, which hinders the identification of metastatic disease in the brain, and by the insufficient spatial resolution of PET for the detection of a metastasis less than 5 mm.
On a per-patient basis, all false negative lesions on brain MRI were also assessed as false negative on PET, and there was no lesion which was detected on PET. The lesions were regarded as metastatic (growth from a microscopic metastatic focus) because all lesions were detected by follow-up studies in six months or more. Considering these results, torso PET plus brain MRI would provide enough information about brain metastasis and appears to be efficient for M staging in NSCLC patients as much as brain and body PET plus brain MRI.
In our study, by adding contrast enhanced MRI to PET/CT, brain metastases were detected in additional 32 (7% of 442) patients, in whom treatment modality should have been changed. Therefore, imaging surveillance of the brain for metastases is worthwhile in the context that neurologically asymptomatic metastases can be detected early to provide appropriate therapy, especially in patients with lung adenocarcinoma where asymptomatic metastases are more frequent than in other NSCLC (23-25).
Our study has several limitations. First and most importantly, because we did not administer IV contrast medium for CT image acquisition at PET/CT, CT component images might have played a role as transmission data only image coregistration and have provided little morphologic information for metastasis detection. This might have contributed to lowering sensitivity for detecting brain or other organ (e.g., hepatic, renal, and splenic) metastasis at PET/CT. Therefore, our comparison is more of PET alone versus PET plus MRI brain, rather than PET/CT alone versus PET/CT plus MRI brain. Obtaining contrast-enhanced CT component images at PET/CT would provide more precise anatomic information for abnormal lesions by enhancing attenuation differences between lesions and surrounding normal structures especially in such cases as brain, hepatic, renal or splenic metastasis. However, controversies on having enhanced CT component of PET/CT are still ongoing, because concerns over contrast medium injection in terms of technical, economic, work-flow, and radiation-safety issues has been raised. Second, we were unable to make a pathologic diagnosis of an extrathoracic metastasis at a given site in every patient. This may suggest that the sensitivity and specificity values in the current study might be biased. Third, brain MRI is not routinely included as a standard initial staging workup in clinical practice at other institutions and we did not calculate the cost-effectiveness of the addition of brain MRI to PET/CT. A further study needs to be performed as to the cost-effectiveness on our protocol for the addition of brain MRI to PET or PET/CT.
In conclusion, with the addition of dedicated brain MRI to PET/CT and thus with enhanced brain metastasis detection, a significant increase in diagnostic sensitivity can be achieved for detecting extrathoracic metastases in patients with lung adenocarcinoma. Thus, adding dedicated brain MRI to PET/CT appears to be efficient for M staging in patients who have a histopathologically confirmed lung adenocarcinoma.
References
- 1.Boring CC, Squires TS, Tong T. Cancer Statistics, 1992. CA Cancer J Clin. 1992;42:19–38. doi: 10.3322/canjclin.42.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 3.The Canadian Lung Oncology Group. Investigating extrathoracic metastatic disease in patients with apparently operable lung cancer. Ann Thorac Surg. 2001;71:425–433. [PubMed] [Google Scholar]
- 4.The American Thoracic Society and the European Respiratory Society. Pretreatment evaluation of non-small-cell lung cancer. The American Thoracic Society and the European Respiratory Society Consensus Report. Am J Respir Crit Care Med. 1997;156:320–332. doi: 10.1164/ajrccm.156.1.ats156.1. [DOI] [PubMed] [Google Scholar]
- 5.Shim SS, Lee KS, Kim BT, Chung MJ, Lee EJ, Han J, Choi JY, Kwon OJ, Shim YM, Kim S. Non-small cell lung cancer: prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]
- 6.De Wever W, Vankan Y, Stroobants S, Verschakelen J. Detection of extrapulmonary lesions with integrated PET/CT in the staging of lung cancer. Eur Respir J. 2007;29:995–1002. doi: 10.1183/09031936.00119106. [DOI] [PubMed] [Google Scholar]
- 7.Marom EM, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, Coleman RE, Herndon JE, Patz EF., Jr Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803–809. doi: 10.1148/radiology.212.3.r99se21803. [DOI] [PubMed] [Google Scholar]
- 8.Mintz BJ, Tuhrim S, Alexander S, Yang WC, Shanzer S. Intracranial metastases in the initial staging of bronchogenic carcinoma. Chest. 2004;86:850–853. doi: 10.1378/chest.86.6.850. [DOI] [PubMed] [Google Scholar]
- 9.Newman SJ, Hansen HH. Proceedings: frequency, diagnosis, and treatment of brain metastases in 247 consecutive patients with bronchogenic carcinoma. Cancer. 1974;33:492–496. doi: 10.1002/1097-0142(197402)33:2<492::aid-cncr2820330225>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 10.Mujoomdar A, Austin JH, Malhotra R, Powell CA, Pearson GD, Shiau MC, Raftopoulos H. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242:882–888. doi: 10.1148/radiol.2423051707. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Lee KS, Kim BT, Choi JY, Kim H, Kwon OJ, Shim YM, Yi CA, Kim HY, Chung MJ. Mediastinal nodal staging of nonsmall cell lung cancer using integrated 18F-FDG PET/CT in a tuberculosis-endemic country: diagnostic efficacy in 674 patients. Cancer. 2007;109:1068–1077. doi: 10.1002/cncr.22518. [DOI] [PubMed] [Google Scholar]
- 12.Lee EJ, Choi JY, Lee KS, Chung HW, Lee SJ, Cho YS, Choi Y, Choe YS, Lee KH, Kwon OJ, Shim YM, Kim BT. Improving diagnostic accuracy for malignant nodes and N staging in non-small cell lung cancer using CT-corrected FDG-PET. Korean J Nucl Med. 2005;39:231–238. [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method comparing the areas under receiver operator characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 14.Hillers TK, Sauve MD, Guyatt GH. Analysis of published studies on the detection of extrathoracic metastases in patients presumed to have operable non-small cell lung cancer. Thorax. 1994;49:14–19. doi: 10.1136/thx.49.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silvestri GA, Littenberg B, Colice GL. The clinical evaluation for detecting metastatic lung cancer: a meta-analysis. Am J Respir Crit Care Med. 1995;152:225–230. doi: 10.1164/ajrccm.152.1.7599828. [DOI] [PubMed] [Google Scholar]
- 16.Toloza EM, Harpole L, McCrory DC. Noninvasive staging of nonsmall cell lung cancer: a review of the current evidence. Chest. 2003;123:137S–146S. doi: 10.1378/chest.123.1_suppl.137s. [DOI] [PubMed] [Google Scholar]
- 17.Jena A, Taneja S, Talwar V, Sharma JB. Magnetic resonance (MR) patterns of brain metastasis in lung cancer patients: correlation of imaging findings with symptom. J Thorac Oncol. 2008;3:140–144. doi: 10.1097/JTO.0b013e318161d775. [DOI] [PubMed] [Google Scholar]
- 18.Yokoi K, Kamiya N, Matsuguma H, Machida S, Hirose T, Mori K, Tominaga K. Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest. 1999;115:714–719. doi: 10.1378/chest.115.3.714. [DOI] [PubMed] [Google Scholar]
- 19.Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E, Detterbeck F American College of Chest Physicians. Noninvasive staging of non-small cell lung cancer: ACCP evidencedbased clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):S178–S201. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol. 1988;6:1474–1480. doi: 10.1200/JCO.1988.6.9.1474. [DOI] [PubMed] [Google Scholar]
- 21.Zabel A, Milker-Zabel S, Thilmann C, Zuna I, Rhein B, Wannenmacher M, Debus J. Treatment of brain metastasis in patients with non-small cell lung cancer (NSCLC) by stereotactic linac-based radiosurgery: prognostic factors. Lung Cancer. 2002;37:87–94. doi: 10.1016/s0169-5002(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 22.Ohno Y, Koyama H, Nogami M, Takenaka D, Yoshikawa T, Yoshimura M, Kotani Y, Nishimura Y, Higashino T, Sugimura K. Whole-body MR imaging vs. FDG-PET: comparison of accuracy of M-stage diagnosis for lung cancer patients. J Magn Reson Imaging. 2007;26:498–509. doi: 10.1002/jmri.21031. [DOI] [PubMed] [Google Scholar]
- 23.Yi CA, Shin KM, Lee KS, Kim BT, Kim H, Kwon OJ, Choi JY, Chung MJ. Non-small cell lung cancer staging: efficacy comparison of integrated PET/CT versus 3.0-T whole-body MR imaging. Radiology. 2008;248:632–642. doi: 10.1148/radiol.2482071822. [DOI] [PubMed] [Google Scholar]
- 24.Cox JD, Yesner R. Adenocarcinoma of the lung: recent results from the veterans administration lung group. Am Rev Respir Dis. 1979;120:1025–1029. doi: 10.1164/arrd.1979.120.5.1025. [DOI] [PubMed] [Google Scholar]
- 25.Cox JD, Scott CB, Byhardt RW, Emami B, Russell AH, Fu KK, Parliament MB, Komaki R, Gaspar LE. Addition of chemotherapy to radiation therapy alters failure patterns by cell type within non-small cell carcinoma of lung (NSCCL): analysis of radiation therapy oncology group (RTOG) trials. Int J Radiat Oncol Biol Phys. 1999;43:505–509. doi: 10.1016/s0360-3016(98)00429-5. [DOI] [PubMed] [Google Scholar]
- 26.Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM. Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol. 2001;19:1344–1349. doi: 10.1200/JCO.2001.19.5.1344. [DOI] [PubMed] [Google Scholar]
- 27.Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: double-dose delayed CT vs contrast enhanced MR imaging. AJNR Am J Neuroradiol. 1991;12:293–300. [PMC free article] [PubMed] [Google Scholar]