Abstract
Early gastric cancer (EGC) is a "curable" disease with a high cure rate made possible through proper surgical treatment; nonetheless, some patients sustain a disease recurrence after curative resection. The aim of this study was to identify the clinicopathological characteristics of recurrent EGC and determine predictable immunohistochemical markers for recurrence. We investigated the clinicopathological features of 1,786 EGC cases, and using tissue microarray, the expression of c-erbB-2, EGFR, MLH1, MSH2, p53, and AQP1 was examined in group with recurrence and control group without recerrence. In the clinical analysis, 32 of 1,786 (1.79%) patients showed recurrence, with a 2.04% five-year cumulative recurrence rate. Age, submucosal invasion, and lymph node metastasis significantly correlated with tumor recurrence (P=0.044, 0.019, and <0.001, respectively). Multivariate analysis showed lymph node status and old age (≥57 yr) as independent risk factors of recurrence. In a case-control study, immunopositivity for c-erbB-2 was significantly associated with disease recurrence (P=0.024). There is the probability that EGC patients with old age (≥57 yr), lymph node metastasis, submucosal invasion, and c-erbB-2 immunopositivity will experience recurrence; therefore, it is critical that patients with these risk factors be followed-up closely and considered candidates for adjuvant treatment.
Keywords: Early Gastric Cancer; Recurrence; Lymph Node Metastasis; Immunohistochemistry; Genes, erbB-2
INTRODUCTION
The number of early gastric cancer (EGC) diagnoses has increased in recent decades, due to developments in diagnostic procedures. In Korea, the proportion of EGC patients is increasing; according to a recent report of the Korea Gastric Cancer Association, it increased from 28.6% in 1995 to 47.4% in 2004 (1).
In general, EGC is characterized by a malignant tumor that is considered "curable", given that the outcome of surgical treatment is excellent, even in patients with node-positive disease (2). However, some cases of EGC recur after radical surgery, in the form of lymphatic spread, blood-borne metastasis, or peritoneal dissemination. Although several studies have been conducted to identify the clinicopathological features of such exceptional cases (3), the predictive histopathological and immunohistochemical (IHC) parameters for recurrence of EGC are not well known.
In the current study, the clinicopathological characteristics and ancillary IHC markers of recurrent EGC were investigated, in comparison to non-recurrent cancer.
MATERIALS AND METHODS
Case selection
A total of 1,788 patients who had undergone curative gastrectomy for primary EGC at the Asan Medical Center in Seoul between 1991 and 2001 were retrospectively analyzed. All patients had undergone a gastrectomy with sufficient lymph node dissection, in accordance with the General Rules for the Gastric Cancer Study of the Japanese Research Society for Gastric Cancer (4).
The medical records and database of the Division of Gastric Surgery, Department of Surgery, Asan Medical Center were used, as were telephone interviews for gathering follow-up information. The recurrence of disease was confirmed by radiological studies, endoscopic examination with biopsy, surgery, and the cytological diagnosis of body fluids. Cases with multiple primary cancers-such as synchronous and metachronous tumors-were excluded. Thirty-four cases with recurrence were found, pathologically reexamined and recorded according to the Guide of the Gastrointestinal Pathology Study Group of the Korean Society of Pathologists (5). Via pathological reviews, two cases with proper muscle involvement of tumor cells were found and excluded from this study.
The patterns of recurrence were classified into four types: locoregional recurrence of perigastric lymph nodes and anastomosis site; distant recurrence to the liver, lung, brain, and distant lymph nodes; peritoneal recurrence, including Krukenberg's tumor and peritoneal carcinomatosis with malignant ascites; and mixed-type recurrence (6).
Selection of control group without recurrence
For IHC stains, 32 control cases were selected from 1,754 cases of non-recurrent EGC, via an age and gender-matched random sampling method. Patient data are summarized in Table 1.
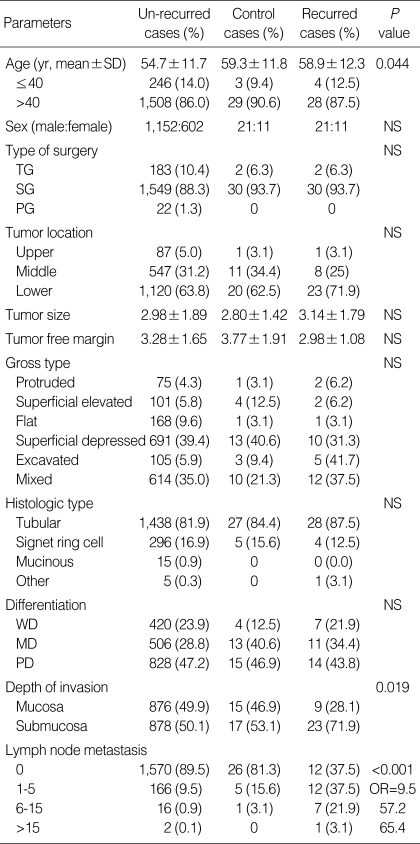
Table 1.
Clinicopathological parameters in relation to the study of recurrent early gastric cancer
SD, standard deviation; TG, total gastrectomy; SG, subtotal gastrectomy; PG, proximal gastrectomy; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; NS, not significant; OR, odds ratio.
Tissue microarray design and immunohistochemical stains
A tissue microarray (TMA) was constructed from the paraffin-embedded blocks of 64 recurrent and non-recurrent control cases; for this purpose, a tissue arrayer device (Beecher Instruments Inc., Sun Prairie, WI, U.S.A.) was used as previously described (7). Stomach cancers were histologically reviewed and representative tumor areas were marked in the corresponding paraffin blocks. Three selected cylinders (1.5 mm in the largest dimension) from three different tumor areas were included for each case. TMA blocks were each sectioned at a thickness of 4 µm; the sections were mounted on precoated glass slides and deparaffinized. IHC assays were performed using a Ventana NX automated immunohistochemistry system (Ventana Medical Systems, Tucson, AZ, U.S.A.) with monoclonal/polyclonal primary antibodies to c-erbB-2 (A0485, rabbit polyclonal, Dako, Glostrup, Denmark; dilution 1:500), EGFR (NOVO-L-EGFR, rabbit monoclonal, Novocastra, Newcastle upon Tyne, U.K.; dilution 1:50), MLH1 (13291A, mouse monoclonal, PharMingen, San Diego, CA, U.S.A.; dilution 1:50), MSH2 (65051A, mouse monoclonal, PharMingen; dilution 1:500), p53 (M7001, mouse monoclonal, Dako; dilution 1:3,000), and AQP1 (AQP11-4, rabbit polyclonal, Alpha Diagnostic International Inc., San Antonio, TX, U.S.A.; dilution 1:50).
Immunohistochemical evaluation
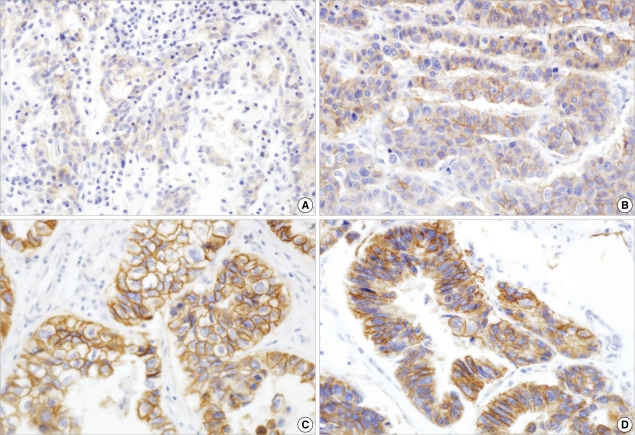
According to the scoring system recently proposed by Hofmann et al. (8), the results of IHC staining for c-erbB-2 were scored as follows: 0, no reactivity or membranous reactivity in <10% of tumor cells; 1+, faintly/barely perceptible membranous reactivity in ≥10% of tumor cells; 2+, weak to moderate membranous complete or basolateral membranous reactivity in ≥10% of tumor cells; 3+, moderate to strong complete or basolateral membranous stain in ≥10% of tumor cells (Fig. 1). Only a membranous stain-either complete or incomplete-was considered meaningful, not a cytoplasmic stain. The score of IHC stain for c-erbB-2 was classified as negative (score 0 and 1+), equivocal (score 2+), and positive (score 3+).
Fig. 1.
c-erbB2 immunohistochemical analysis. (A) 1+, faint (×100); (B) 2+, weak to moderate complete membranous (×200); (C) 3+, strong and complete membranous (×400); and (D) 3+, strong basalateral membranous reactivities (×400).
For EGFR, reactivity was scored as 0 when there was no membranous reactivity within the tumor; the positive samples were classified further into 1+, 2+, and 3+, based on intensity of reactivity. The highest intensity of reactivity of all tissue cores from the same tumor was used as the final IHC result for that tumor. Scores of 2+ and 3+ were considered suggestive of overexpression (9). A positive stain for MLH1/MSH2 was defined as an unequivocal nuclear stain in the neoplastic cells, and for AQP1, as membranous and/or cytoplasmic stain. Cases with nuclear staining in >10% of tumor cells were defined as p53-positive reactions.
Statistical analysis
Univariate analyses-including a t-test, a Pearson's chi-square test, and a logistic regression test-were used to compare data between the groups with and without recurrent cancer. In addition, a multivariate analysis was performed using the Cox proportional hazards model. The five-year cumulative recurrence rate of survival data was measured using the Kaplan-Meier method. All calculations were performed using the Statistical Package for the Social Sciences (SPSS) software program, version 14.0 (SPSS Inc., Chicago, IL, U.S.A.), and results were considered statistically significant when the P value was <0.05.
RESULTS
Recurrence rate and mortality
Of the 1,786 patients surgically treated for EGC, 32 patients (1.79%) had sustained a disease recurrence and 1,754 were disease-free. The five-year cumulative recurrence rate was 2.04%. Thirty-one of the 32 recurrent patients (93.9%) died. The sole surviving patient had undergone a primary operation in 1998 and a secondary resection in 2001 for lymphoepithelial carcinoma. The mean interval between operation and recurrence was 20 months, while the mean survival period after recurrence was 9.6 months.
Recurrence pattern
Of the 32 recurrent cases, nine (28.1%) had a locoregional recurrence, one (3.1%) had a peritoneal dissemination, 12 (37.5%) had distant metastasis and 10 (31.3%) had a mixed-type recurrence. The nine cases of locoregional recurrence included four cases with perigastric lymph node involvement and five with carcinoma in an anastomotic site, with no simultaneous cancer and sufficient tumor-free margins at the time of primary operation. Tumor recurrence in an anastomotic site occurred at a mean interval of 19 months. At initial diagnosis, one case had a mucosal tumor with perigastric lymph node metastasis and four had submucosal tumors with no lymph node metastasis. Four patients underwent reoperation, and 28 cases were managed with chemotherapy and conservative treatment.
Clinicopathological parameters predicting recurrence
The mean age of the patients with disease recurrence was 58.9±12.3 yr at the time of operation-somewhat older than the non-recurrent group (54.7±11.7). Age correlated with the incidence of recurrence (P=0.044) (Table 1).
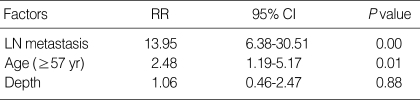
In the cases with recurrence, lymph node metastasis was positive in 20 cases (58.8%) and, in the group without recurrence, positive in 184 cases (10.5%). Recurrence was significantly frequent in cases with lymph node metastasis (P<0.001). Among the node-positive patients with recurrence, there were 12 patients with <6 positive node (N1), seven with 7-15 (N2), and one with >16 (N3). Logistic regression analysis indicated that the N-stage at the time of surgery was a strong predictor of recurrence (P<0.001; odds ratio [OR]=8 [N1], 49 [N2] 56 [N3]). In the recurrent group, there were 23 cases with submucosal invasion (71.9%), whereas there were 878 cases in the non-recurrent group (50.1%; P=0.019). According to multivariate analysis, old age (≥57 yr) and lymph node metastasis were independent risk factors of recurrence (Table 2).
Table 2.
Multivariate analysis of clinicopathological factors associated with early gastric cancer recurrence
RR, relative risk; CI, confidence interval.
Other clinicopathological features-including gender, tumor size, tumor location, histological type, gross type, differentiation, and distance from safety margin-did not correlate with recurrence.
Immunohistochemical markers predicting recurrence
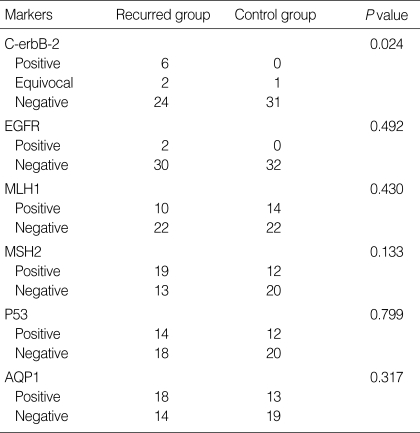
Positive staining for c-erbB-2 (score 3+) was found in six (18.8%) recurrent cases, but not in any non-recurrent control cases (P=0.024; Table 3). Three cases showed moderately to strongly basolateral membranous staining, and the other three cases sustained complete membranous staining. One case with tumor heterogeneity displayed 3+ tumor cells representing ≥10% of the tumor area. All c-erbB-2-positive cases were of the tubular and intestinal type, and one case showed poor differentiation. Regarding tumors with equivocal positivity (score 2+), there were two cases in the recurrent group and one in the non-recurrent control group. The mean survival period of immunopositive cases for c-erbB-2 was 21 months and that of the remaining c-erbB-2-negative and equivocally positive recurrent cases was 32.6 months.
Table 3.
Expression of immunohistochemical markers in group with recurrence and control group without recurrence
Only two (6.3%) recurrent cases showed membranous immunoreactivity for EGFR; they were of a tubular type and moderately differentiated. However, no non-recurrent case showed immunopositivity for EGFR (P=0.492). The mean survival period for EGRF-positive cases was 38.6 months. Nuclear immunoreactivity for MSH2 was found in 19 (59.4%) recurrent cases and 12 (37.5%) control cases (P=0.133). Regarding the expression of MLH1, AQP1, and p53, there was no statistically significant association between the recurrent and non-recurrent control cases (P=0.430, 0.317, and 0.799, respectively).
DISCUSSION
The recurrence of EGC is very rare in general, and the recurrence rate of EGC treated by curative surgery reportedly varies, from 1.4 to 6.4% (10-12). Recently, however, a study by Wu et al. (13) showed that post-gastrectomy recurrence was observed in 12.24% of the cases studied. This variation is due to what is considered recurrent carcinoma arising in the remnant gastric stump; some researchers take up the position that carcinoma in the residual stomach should be excluded from any recurrence analysis, because it is difficult to distinguish second primary carcinomas in the gastric stump from synchronous tumors that had been initially overlooked (12) or a metachronous tumor. In the current study, we included five cases found in the anastomotic site, by definition of multiple primary cancers (14). In none of the five cases were there any simultaneous cancers, and all five had a sufficient tumorfree margin at the time of primary operation. Furthermore, the initial tumor and secondary tumor showed the same morphological features.
With regards to EGC recurrence patterns, most researchers have reported that a distance recurrence via a hematogenous spread was the most common recurrence pattern. Folli et al. (15) reported that 14 out of 22 patients with recurrent EGC showed hematogenous recurrence, while Lee et al. (6) reported that distant recurrence was the most common pattern of recurrence (42.9%). The current study showed a similar result, with distance recurrence occurring in 37.5% of the cases, making it the most common recurrence pattern. In submucosal EGC, hematogenous recurrence could be explained by the mechanism of lymphatic and vascular invasions during the submucosal invasion of cancer cells (16). However, a satisfactory explanation for hematogenous recurrence has not yet been suggested vis-à-vis mucosal cancer.
The risk factors related to EGC recurrence have been discussed in several articles (6). Ichiyoshi et al. (10) reported that the elevated type, positive lymph node, and invasion of lymphatic and venous vessels were significantly associated with a high risk of recurrence. Shiozawa et al. (11) considered some other factors, such as submucosal invasion, a gross appearance of IIa+IIc type, and a tumor size >40 mm. Using multivariate analysis, Lee et al. (6) proposed that lymph node metastasis was the only significant prognostic factor, and that N-stage correlates with the five-year survival rate. Other studies (17, 18) repeatedly have proposed that lymph node metastasis is one of the most important diagnostic factors of EGC. As a rule, there is at least a thread of connection between lymphatic involvement at the time of operation and hematogenous and distant metastasis. A report of Wu et al. (13) stated that lymph node metastasis was an independent risk factor for short-term recurrence; however, in their series, 86% (26/30) of the recurrent patients had no lymph node metastasis at the time of operation, even though hematogenous and lymphatic metastasis was the most common recurrence pattern (33%). They proposed micrometastasis, as a means of reconciling these findings. Therefore, we assert that a pathologically careful examination of lymph node status is necessary with a surgically resected stomach. In any case, the citation of submucosal invasion and age as prognostic factors in EGC recurrence remains controversial (6, 12, 13, 19).
With respect to advanced gastric carcinoma, many studies have been performed to find predictive factor for recurrence. However, ancillary IHC markers have not been investigated in cases of EGC with recurrence, in comparison to non-recurrent cancer.
For IHC staining, we randomly selected from among the non-recurrent cases an age and gender-matched control group. The clinicopathological features of the control group represented most of the characteristics of the non-recurrent cases; however, despite the use of the random selection method, the proportion of cases with lymph node metastasis in the control group (18.7%) was higher than in the non-recurrent group (10.5%), and there were differences between the two groups in terms of such objects as tumor size and distance from safety margin (Table 1).
We chose to examine some IHC markers related to poor prognosis of gastric cancer. The EGF receptor/ligand system seems to be involved in the regulation of gastric mucosal proliferation and the progression of gastric carcinomas. In univariate and multivariate analyses, EGFR levels were found to be an independent indicator of poor prognosis (20, 21). Previous reports (22, 23) suggested that a high overexpression rate of the growth factor receptor oncogene c-erbB-2 was significantly related with metastasis and an advanced stage of stomach cancer, as well as poor tumor differentiation. We found the overexpression of c-erbB-2 could be considered a predictor of poor outcome in EGC as well as in advanced gastric cancer; however, we also found that the rates of c-erbB-2 immunoreactivity in tumors with well, moderate, and poor differentiation were 33% (2/6), 50% (3/6), and 17% (1/6), respectively. A high correlation between cerbB2 expression and intestinal type has been reported in many previous studies and confirmed in more recent studies (24, 25).
Unlike in breast carcinoma, the concordance between protein expression and gene amplification of c-erbB-2 in gastric cancer has been considered controversial (24, 25). In addition, the evaluation of c-erbB-2 immunohistochemisty with respect to gastric cancer tend to be more ambiguous than with breast cancer, due to tumor heterogeneity and higher frequency of glandular formations in the former. Therefore, the importance of an objective and specialized c-erbB-2 scoring system for gastric cancer becomes increasingly obvious. We used the proposed and modified HercepTest™ score for gastric cancer, as discussed in an international consensus meeting by Hofmann et al. (8) According to the validation study, identical scoring should be applied to samples with complete membranous reactivity, as well as those where reactivity was restricted to the basolateral membrane if such reactivity was noted in ≥10% of cells. Samples with cohesive 3+ tumor cells were considered positive, irrespective of size (8).
Gastric cancers have one of the highest prevalences of microsatellite instability (MSI) with up to 33% of cases (26). Wu et al. (27) demonstrated that a subset of sporadic gastric cancer with high-frequency MSI (MSI-H) showed a clinicopathological profile (i.e., higher frequency of antral location, intestinal subtype, and H. pylori seropositivity, but lower incidence of lymph-node metastasis) distinct from those with low-frequency MSI (MSI-L) or microsatellite stability (MSS). To more fully understand the implications of the more frequent immunoreactivities vis-à-vis MSH2 in our results, additional MSI-related studies are required.
The tumor suppressor gene p53 encodes for a nuclear protein that plays a key role in tumor progression by regulating DNA repair, cell division, and apoptosis. p53 expression was found to be higher in poorly differentiated gastric carcinomas than in well-differentiated ones (28). According to Pan et al. (29), tumors in the metastatic EGC group had higher p53 expression than those from the non-metastatic group.
Aquaporins (AQP) are integral membrane proteins having more than 10 isoforms in mammalian cells that serve as channels in the transfer of water and, in some cases, small solutes across the membrane. AQP1 found in the blood vessels, kidney proximal tubules, eye, and ear was considered to play a role in tumor angioinvasion and progression (30). However, in the current study, there were no differences between the recurrent and non-recurrent cases, with respect to staining patterns and intensities of AQP1.
In conclusion, the recurrence of EGC is very rare in general. However, it is well known that the management of recurrent tumors is difficult, owing to the presence of extra-gastrointestinal lesions and combined types of recurrence. The presence of lymph node metastasis, old age, submucosal invasion, and c-erbB-2 immunopositivities imply higher possibility of recurrence; therefore, post-gastrectomy patients possessing these traits should be closely followed and considered candidates for adjuvant treatment.
References
- 1.The Information Committee of the Korean Gastric Cancer Association. 2004 nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007;7:47–54. [Google Scholar]
- 2.Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F. Complete ten-year postgastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14–16. doi: 10.1016/0002-9610(89)90305-x. [DOI] [PubMed] [Google Scholar]
- 3.Matsusaka T, Kodama Y, Soejima K, Miyazaki M, Yoshimura K, Sugimachi K, Inokuchi K. Recurrence in early gastric cancer: a pathologic evaluation. Cancer. 1980;46:168–172. doi: 10.1002/1097-0142(19800701)46:1<168::aid-cncr2820460128>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Research Society for Gastric Cancer. Japanese classification of gastric carcinoma. 1st ed. Tokyo: Kanehara Shuppan; 1995. [Google Scholar]
- 5.Kim WH, Park CK, Kim YB, Kim YW, Kim HG, Bae HI, Song KS, Chang HK, Chang HJ, Chae YS. A standardized pathology report for gastric cancer. Korean J Pathol. 2005;39:106–113. [Google Scholar]
- 6.Lee HJ, Kim YH, Kim WH, Lee KU, Choe KJ, Kim JP, Yang HK. Clinicopathological analysis for recurrence of early gastric cancer. Jpn J Clin Oncol. 2003;33:209–214. doi: 10.1093/jjco/hyg042. [DOI] [PubMed] [Google Scholar]
- 7.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, Ochiai A, Ruschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimura M, Tsuda H, Morita D, Ichikura T, Ogata S, Aida S, Yoshizumi Y, Maehara T, Mochizuki H, Matsubara O. A proposal for diagnostically meaningful criteria to classify increased epidermal growth factor receptor and c-erbB-2 gene copy numbers in gastric carcinoma, based on correlation of fluorescence in situ hybridization and immunohistochemical measurements. Virchows Arch. 2004;445:255–262. doi: 10.1007/s00428-004-1048-7. [DOI] [PubMed] [Google Scholar]
- 10.Ichiyoshi Y, Toda T, Minamisono Y, Nagasaki S, Yakeishi Y, Sugimachi K. Recurrence in early gastric cancer. Surgery. 1990;107:489–495. [PubMed] [Google Scholar]
- 11.Shiozawa N, Kodama M, Chida T, Arakawa A, Tur G, Koyama K. Recurrent death among early gastric cancer patients: 20-years' experience. Hepatogastroenterology. 1994;41:244–247. [PubMed] [Google Scholar]
- 12.Sano T, Sasako M, Kinoshita T, Maruyama K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer. 1993;72:3174–3178. doi: 10.1002/1097-0142(19931201)72:11<3174::aid-cncr2820721107>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Wu B, Wu D, Wang M, Wang G. Recurrence in patients following curative resection of early gastric carcinoma. J Surg Oncol. 2008;98:411–414. doi: 10.1002/jso.21133. [DOI] [PubMed] [Google Scholar]
- 14.Moertel CG. Multiple primary malignant neoplasms: Historical perspectives. Cancer. 1977;40:1786–1792. doi: 10.1002/1097-0142(197710)40:4+<1786::aid-cncr2820400803>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Folli S, Dente M, Dell'Amore D, Gaudio M, Nanni O, Saragoni L, Vio A. Early gastric cancer: prognostic factors in 223 patients. Br J Surg. 1995;82:952–956. doi: 10.1002/bjs.1800820732. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi Y. Blood vessel invasion in gastric carcinoma. Surgery. 1990;107:140–148. [PubMed] [Google Scholar]
- 17.Lawrence M, Shiu MH. Early gastric cancer. Twenty-eight-year experience. Ann Surg. 1991;213:327–334. doi: 10.1097/00000658-199104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, Di Leo A, Gaudio M, Nanni O, Carli A, Cordiano C, Dell'Amore D, Vio A. Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Iitalian Research Group for Gastric Cancer (IRGGC) Jpn J Clin Oncol. 2001;31:495–499. doi: 10.1093/jjco/hye107. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Saku M, Kishihara F, Maehara Y. Effective follow-up for recurrence or a second primary cancer in patients with early gastric cancer. Br J Surg. 2005;92:235–239. doi: 10.1002/bjs.4758. [DOI] [PubMed] [Google Scholar]
- 20.Kim MA, Lee HS, Lee HE, Jeon YK, Yang HK, Kim WH. EGFR in gastric carcinomas: prognostic significance of protein overexpression and high gene copy number. Histopathology. 2008;52:738–746. doi: 10.1111/j.1365-2559.2008.03021.x. [DOI] [PubMed] [Google Scholar]
- 21.Garcia I, Vizoso F, Martin A, Sanz L, Abdel-Lah O, Raigoso P, Garcia-Muniz JL. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol. 2003;10:234–241. doi: 10.1245/aso.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- 23.Ougolkov A, Yamashita K, Bilim V, Takahashi Y, Mai M, Minamoto T. Abnormal expression of E-cadherin, beta-catenin, and c-erbB-2 in advanced gastric cancer: its association with liver metastasis. Int J Colorectal Dis. 2003;18:160–166. doi: 10.1007/s00384-002-0427-2. [DOI] [PubMed] [Google Scholar]
- 24.Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 25.Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, Son BH, Cho EY, Chae SW, Kim EJ, Sohn JH, Ryu SH, Sepulveda AR. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371–1379. doi: 10.1007/s10620-005-9057-1. [DOI] [PubMed] [Google Scholar]
- 26.Rhyu MG, Park WS, Meltzer SJ. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994;9:29–32. [PubMed] [Google Scholar]
- 27.Wu MS, Lee CW, Shun CT, Wang HP, Lee WJ, Chang MC, Sheu JC, Lin JT. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000;27:403–411. [PubMed] [Google Scholar]
- 28.Feng CW, Wang LD, Jiao LH, Liu B, Zheng S, Xie XJ. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: Correlation with clinical features. BMC Cancer. 2002;2:8. doi: 10.1186/1471-2407-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan W, Ishii H, Ebihara Y, Gobe G. Prognostic use of growth characteristics of early gastric cancer and expression patterns of apoptotic, cell proliferation, and cell adhesion proteins. J Surg Oncol. 2003;82:104–110. doi: 10.1002/jso.10204. [DOI] [PubMed] [Google Scholar]
- 30.Takata K, Matsuzaki T, Tajika Y. Aquaporins: water channel proteins of the cell membrane. Prog Histochem Cytochem. 2004;39:1–83. doi: 10.1016/j.proghi.2004.03.001. [DOI] [PubMed] [Google Scholar]