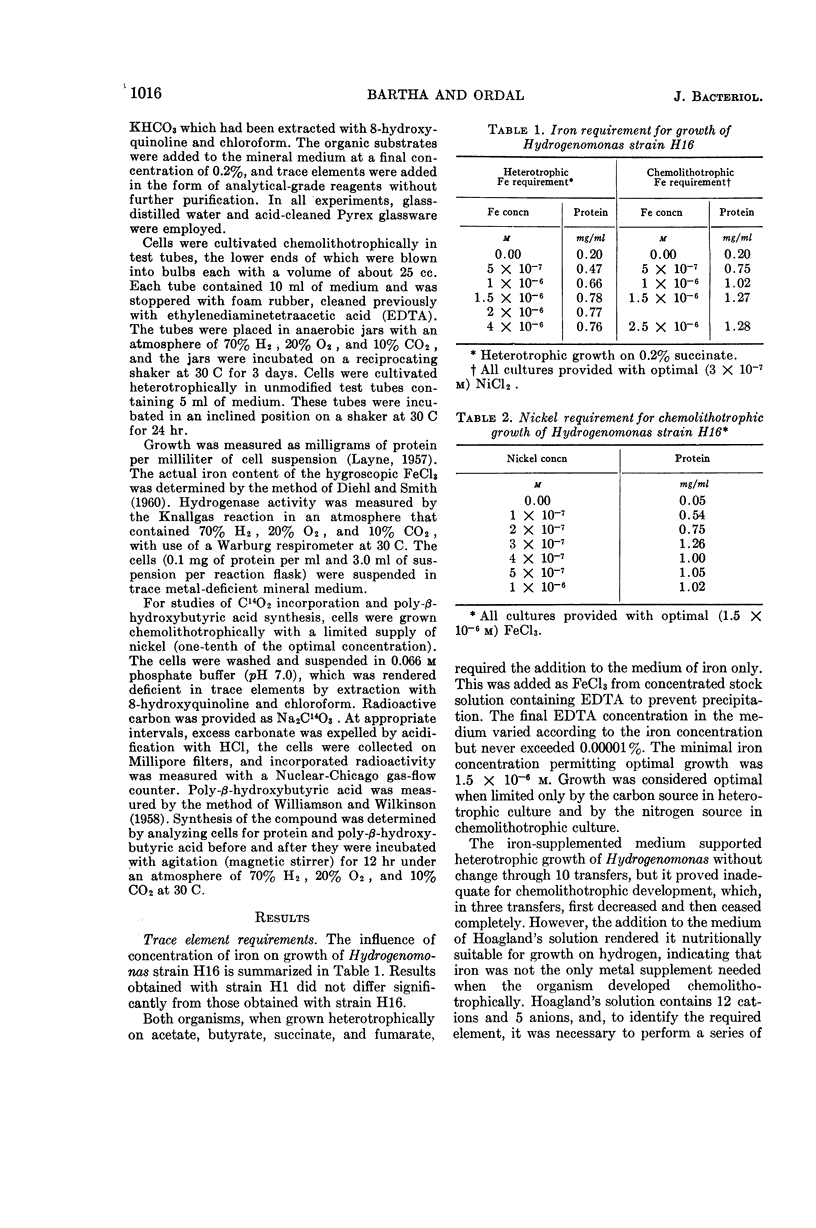
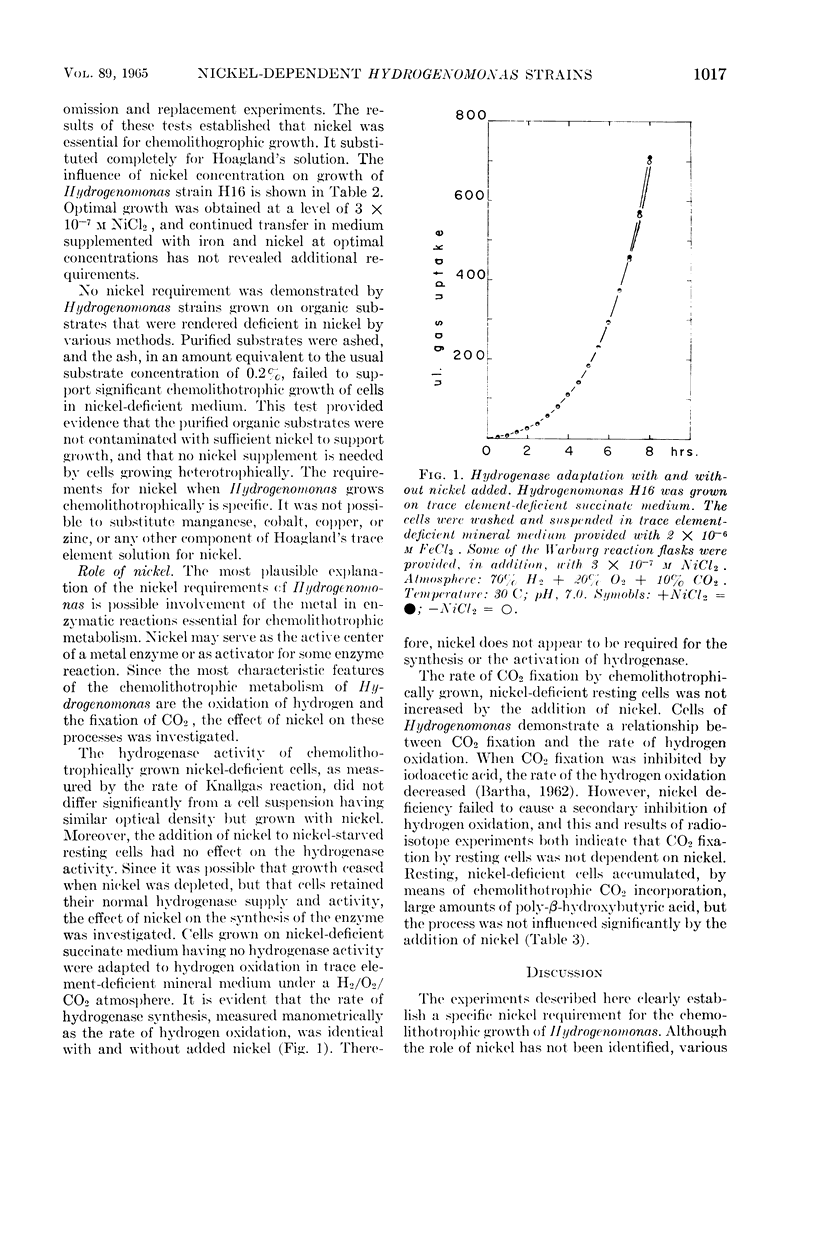
Abstract
Bartha, R. (University of Washington, Seattle), and E. J. Ordal. Nickel-dependent chemolithotrophic growth of two Hydrogenomonas strains. J. Bacteriol. 89:1015–1019. 1965.—The trace element requirements for growth of facultative chemolithotrophic Hydrogenomonas strains H1 and H16 were investigated under both autotrophic and heterotrophic conditions. The organisms were grown in a mineral medium, rendered deficient in trace elements by extraction with 8-hydroxyquinoline and chloroform, and, in some cases, by coprecipitation with copper. The organic substrates, succinate and fumarate, used for heterotrophic growth were treated in a similar fashion. Acetate and butyrate were purified by redistillation. It was found that iron alone was required for heterotrophic growth (optimal concentration, 1.5 × 10−6m Fe+++), but cells grown chemolithotrophically on molecular hydrogen required the addition of nickel. The yield of protein was proportional to the nickel added, reaching a maximum at 3 × 10−7m Ni++. Manganese, cobalt, copper, and zinc, alone or in combination, failed to substitute for nickel in the experiments with Hydrogenomonas. Although nickel is required specifically for the chemolithotrophic growth of Hydrogenomonas, nickel deficiency did not affect: (i) the synthesis or activation of hydrogenase, (ii) the Knallgas reaction, (iii) the assimilation of CO2 by resting cells, or the synthesis of the storage material poly-β-hydroxybutyric acid. It is suggested that nickel participates in some reaction involved in CO2 fixation by growing cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTHA R. [Physiological studies on the chemolithotropic metabolism of recently isolated Hydrogenomonas strains]. Arch Mikrobiol. 1962;41:313–350. [PubMed] [Google Scholar]
- HIRSCH P. CO2-FIXIERUNG DURCH KNALLGASBAKTERIEN. II. CHROMATOGRAPHISCHER NACHWEIS DER FRUEHZEITIGEN FIXIERUNGSPRODUKTE. Arch Mikrobiol. 1963 Jul 18;46:53–78. [PubMed] [Google Scholar]
- HIRSCH P., GEORGIEV G., SCHLEGEL H. G. CO2-FIXIERUNG DURCH KNALLGASBAKTERIEN. III. AUTOTROPHE UND ORGANOTROPHE CO2-FIXIERUNG. Arch Mikrobiol. 1963 Jul 18;46:79–95. [PubMed] [Google Scholar]
- HIRSCH P., SCHLEGEL H. G. CO2-FIXIERUNG DURCH KNALLGASBAKTERIEN. I. EINBAU UND FRAKTIONIERUNG. Arch Mikrobiol. 1963 Jul 18;46:44–52. [PubMed] [Google Scholar]
- PAIGEN K. The properties of particulate phosphoprotein phosphatase. J Biol Chem. 1958 Aug;233(2):388–394. [PubMed] [Google Scholar]
- TURNER D. H., TURNER J. F. Uridine diphosphoglucose pyrophosphorylase of pea seeds. Biochem J. 1958 Jul;69(3):448–452. doi: 10.1042/bj0690448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., WILKINSON J. F. The isolation and estimation of the poly-beta-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol. 1958 Aug;19(1):198–209. doi: 10.1099/00221287-19-1-198. [DOI] [PubMed] [Google Scholar]
- WOLD F., BALLOU C. E. Studies on the enzyme enolase. I. Equilibrium studies. J Biol Chem. 1957 Jul;227(1):301–312. [PubMed] [Google Scholar]


