Abstract
The cholinergic theory of aging states that dysfunction of cholinergic neurons arising from the basal forebrain and terminating in the cortex and hippocampus may be involved in the cognitive decline that occurs during aging and Alzheimer’s disease. Despite years of research, pharmacological interventions to treat or forestall the development of Alzheimer’s disease have primarily focused on enhancing cholinergic transmission, either through increasing acetylcholine (ACh) synthesis or inhibition of the acetylcholinesterase enzyme responsible for ACh hydrolysis. However, recent studies have indicated that dietary supplementation can impact the cholinergic system, particularly during aging. The purpose of the present review is to examine the relevant research suggesting that cholinergic functioning may be maintained during aging via consuming a diet containing polyunsaturated fatty acids (PUFAs). The data reviewed herein indicate that, at least in animal studies, inclusion of PUFAs in the diet can improve cholinergic transmission in the brain, possibly leading to improvements in cognitive functioning.
Keywords: Acetylcholine, Aging, Cholinergic, Essential fatty acids
Introduction
For many years, it has been known that dysfunction of cholinergic neurons arising from the basal forebrain and terminating in the cortex and hippocampus are involved in the cognitive decline that occurs during aging as well as during the development of Alzheimer’s disease. This “cholinergic theory of aging” has received support from numerous studies demonstrating that the disruption of cholinergic function via anticholinergic drugs or basal forebrain lesions in young animals produces cognitive deficits commonly seen in aged animals [1]. In addition, aged animals exhibit cholinergic dysfunction evidenced as a reduced choline uptake in the hippocampus, striatum, and cortex [2]. Studies on postmortem human tissue have also shown that basal forebrain cholinergic cells undergo atrophy, loss of choline acetyltransferase activity and a slight decline in cell number [3].
Unfortunately, despite years of research into the biology of amyloid beta and tau, the actual interventions to treat or forestall the development of Alzheimer’s disease have primarily focused on enhancing cholinergic transmission, either through increasing acetylcholine (ACh) synthesis or inhibition of the acetylcholinesterase (AChE) enzyme responsible for ACh hydrolysis [4]. AChE inhibitors have, thus, far proven most efficacious in improving cognitive performance in the Alzheimer’s disease patients and include both synthetic AChE inhibitors (tacrine and donepezil) and natural compounds [5, 6]. In fact, one of the most widely used treatments for AD is the drug galantamine, originally isolated from the Galanthus worownii plant [7]. Galantamine exerts dual effects on the cholinergic system: inhibition of AChE and stimulation of postsynaptic nicotinic receptors [8]. Based on the efficacy of galantamine, screening studies have led to the identification and isolation of natural AChE inhibitors from herbs [9], plants [10], seeds and fruits [11]. In addition, recent studies have indicated that dietary polyunsaturated fatty acids (PUFAs) may be able to modulate cholinergic activity, either through enhancement of ACh release or inhibition of ACh hydrolysis (Table 1). If this is the case, and if treatments to increase cholinergic function remain at the forefront of the fight against Alzheimer’s disease, it might be possible to obtain these cholinergic enhancements via dietary means. The purpose of the present review is to examine the relevant research suggesting that cholinergic functioning may be maintained via a diet containing PUFAs.
Table 1.
Effects of dietary polyunsaturated fatty acids on cholinergic parameters
| Model | PUFA | Duration | Effect | References |
|---|---|---|---|---|
| SHRSP rats, 6 weeks old | DHA 1% or 5% (w/w) of diet | 14 weeks | Increased ACh in hippocampus and cortex | [41] |
| Wistar rat, 18 months | 75 mg DHA/ 100 g diet | 3 months | Increased basal and evoked ACh in hippocampus | [22] |
| Wistar rat, weanling | 2 or 3 g DHA/kg diet | 2–3 months | Higher maximal ACh release, lower basal release in hippocampus No effect on AChE |
[23] |
| CFY rats, weanling | Coconut, mustard, groundnut, safflower oil 20% (w/w) of diet | 16 weeks | Safflower and mustard oil lowered AChE activity in synaptosomes of cerebrum, cerebellum, brainstem | [33] |
| Wistar rats, 10 weeks old | 300 mg DHA/ kg body weight | 12 weeks | No change in cortical synaptic plasma membrane AChE activity | [34] |
| Sprague–Dawley rats, young adult | Safflower oil 38% kcal of diet; fish oil 36% kcal of diet | 8 weeks | Safflower oil decreased M2/M4 receptor expression in striatum and hippocampus | [26] |
Dietary PUFAs
Polyunsaturated fatty acids are structurally characterized as simple lipids containing two or more double bonds. The two major classes of PUFAs are the n-3 and the n-6 fatty acids, which share metabolic enzymes, but can have vastly different functions. Linoleic acid (C18:2 n-6, LA) and alpha-linolenic acid (C18:3 n-3, ALA) are the main essential fatty acids, so-called “essential” because they cannot be synthesized in vivo and must be obtained from the diet. LA can be metabolized to arachidonic acid (C20:4 n-6, AA) and docosapentaenoic acid (C22:5 n-6, DPA) while ALA is converted to eicosapentaenoic acid (C20:5 n-3, EPA) and docosahexaenoic acid (C22:6 n-3, DHA). The predominant sources of n-3 PUFAs are vegetable oils and fish: ALA and LA are found in green leafy vegetables, oils, and nuts while DHA and EPA are found in fish [12]. Brain availability of dietary PUFAs depends on metabolism of ALA and LA to DHA and AA by the liver, followed by uptake from circulation. Oral administration of ALA results in brain accumulation of EPA and DHA, but not ALA itself. Conversely, administration of LA results in a short-lived enrichment of LA in brain tissue along with a long-term accumulation of AA [13]. Dietary DHA has been reported to result in DHA enrichment of neuronal plasma membranes [14], although no association between dietary DHA and brain DHA has also been reported in aged animals [15].
PUFAs and the aging brain
The coordination and execution of cognitive processes depends on the appropriate detection and propagation of signals from both the environment and surrounding cells in the brain. The responsivity of each cell depends on the composition of the cell membrane, through which all signals must pass. Neuronal membranes are especially rich in fatty acids, which participate in structural maintenance, generation of second messengers and signaling molecules, and modulation of enzyme activity [16]. The aged brain exhibits lower levels of PUFA in neuronal membranes as compared to young animals, which could account for functional neuronal declines in the aged brain [17]. Less free and phospholipid-bound fatty acids are found in the aged brain, particularly in the cortex and hippocampus [18]. These alterations contribute to a decrease in membrane fluidity, which is particularly evident in the hippocampus, cortex, cerebellum and striatum of aged rats [19]. The age-related decline in neuronal membrane fatty acid composition also contributes to alterations in neuronal morphology and reduced synaptic plasticity [20]. Deficits in membrane fatty acid composition could, therefore, affect a number of neuronal functions, including the ability of cells to respond to environmental stimuli as well as the ability of cells to successfully propagate and transmit signals.
ACh release
As integral constituents of neuronal plasma membranes, PUFAs might be expected to affect the formation and exocytosis of synaptic vesicles containing neurotransmitter. Indeed, Lesa et al. have demonstrated that successful cholinergic transmission in C. elegans depends on the presence of long-chain PUFAs. The deletion of the enzyme responsible for the conversion of ALA to DHA led to a depletion of synaptic vesicles and loss of cholinergic tone at neuromuscular junctions. Addition of DHA to the growth media was able to rescue this phenotype in C. elegans [21]. In terms of diet, DHA has also been shown to impact cholinergic transmission in animal models, although these effects appear to be sensitive to the dietary source of DHA. In aged animals, supplementation with DHA in the form of egg phospholipids or pig brain was able to restore the age-related decline in membrane DHA composition. Animals were maintained on a balanced diet containing ALA and LA from birth to 18 months, during which time spontaneous ACh release steadily declined in the hippocampus. While the egg phospholipid diet restored both the DHA and AA content of neuronal membranes and ACh release in the hippocampus, the pig brain diet had no effect on membrane composition, but was able to restore ACh release, albeit to a lesser extent than the egg phospholipid diet [22]. In a similar study, rats were fed with diets supplemented with increasing levels of DHA derived from either egg phospholipids or tuna oil. In animals fed with >2 g DHA/kg diet, basal release of ACh was actually lower in the hippocampus although evoked release of ACh was higher in animals supplemented with 2 or 3 g DHA/kg diet [23]. The beneficial effects of PUFAs on neuronal function have traditionally been assumed to be mediated via fatty acid enrichment of neuronal membranes and alterations in membrane biophysical properties [19]. However, one study demonstrated an increase in ACh release despite no changes in membrane PUFA content [22] indicating that the effects of fatty acids on cholinergic transmission may, therefore, not be mediated exclusively by alterations in membrane composition.
In addition to affecting ACh release, PUFAs have also been shown to impact ACh receptors, both nicotinic and muscarinic. In Xenopus oocytes transfected with nicotinic ACh receptors, addition of either ALA or LA resulted in a biphasic effect of ACh receptor currents: short-term depression followed by long-term enhancement of cholinergic transmission [24]. The long-term enhancement was mediated through phosphokinase C activation and phosphorylation of the receptor. PUFAs were also shown to modulate the muscarinic receptor, which has been implicated in the establishment and maintenance of long-term potentiation in the hippocampus, a cellular mechanism of synaptic plasticity underlying memory formation [25]. In animals fed with an n-6 supplemented diet for 8 weeks, M2/M4 receptor expression was reduced in the striatum and hippocampus, M1/M4 receptor binding was not affected by n-6 PUFA administration, and n-3 PUFA supplementation did not affect the expression of either receptor subtype [26]. The decrease in M2/M4 receptors could reflect an adaptation secondary to the enhancement of ACh release to the hippocampus: more neurotransmitter leads to fewer receptors. In fact, one study by Almeida et al. [27] demonstrated a link between muscarinic receptor stimulation and ACh release in the hippocampus. In hippocampal slice cultures, muscarinic stimulation led to AA release, which in turn enhanced ACh release from hippocampal nerve terminals. Since AA is formed from LA in the diet, it is possible that dietary LA could provide a pool of AA to act as a retrograde messenger to increase ACh release and strengthen synaptic connectivity in the aging hippocampus.
AChE inhibition
Dietary fats have been shown to modulate membrane-bound enzymes on erythrocytes as early as 1973 [28], when it was noted that AChE activity was correlated with changes in membrane biophysical properties. Subsequent studies demonstrated that various oils administered through the diet could affect erythrocyte AChE activity, with the highest level of AChE inhibition seen in animals consuming mustard oil, containing primarily monounsaturated fatty acids (MUFAs) as well as the PUFAs ALA and LA [29]. ALA and LA were further implicated as AChE inhibitors when erythrocyte enzyme activity was investigated in rats fed diets of varying ratios of ALA and LA. It was found that a diet containing a ratio of LA:ALA of 8:1 induced more potent inhibition of erythrocyte AChE than a diet of LA:ALA of 40:1 [30].
This data on erythrocyte AChE activity, along with the expansion of the cholinergic hypothesis of aging, led to the investigation of the potential for dietary fatty acids to function as AChE inhibitors in the basal forebrain–hippocampal system in the brain. An initial study investigated the effects of dietary soybean oil, sunflower oil, and soybean phosphatidylcholine on synaptosomal AChE activity in young rats [31]. Animals consuming sunflower oil, which contains primarily LA, exhibited an increase in the activity of AChE in isolated synaptosomes as compared to synaptosomes isolated from animals consuming a soybean oil diet containing both LA and ALA [31]. A subsequent study found no differences in AChE activity in the brains of animals fed either ALA-rich perilla oil or safflower oil, which is high in LA [32]. In contrast, Srinivasarao et al. [33] demonstrated that safflower oil supplementation at 20% of the diet resulted in lower AChE activity in synaptosomal membranes of cortex, cerebellum, and brainstem of young male rats. The aforementioned study compared the synaptosomal AChE activity in animals fed either safflower oil (high in LA), peanut oil (high in MUFAs but containing approximately 40% LA and 2% ALA), mustard oil (primarily composed of MUFAs, but containing ALA and LA at approximately equal amounts), and coconut oil, which is high in saturated fatty acids, but does contain a small amount of LA. In terms of AChE activity, coconut and peanut oil fed groups exhibited significantly higher AChE activity than either safflower or mustard oil fed animals [33]. These results primarily implicate ALA as a dietary source of AChE inhibition. In addition, this study demonstrates that the effects of dietary oils on AChE activity may be complex and difficult to predict: whereas an oil with primarily MUFAs and 38% LA increased AChE activity, safflower oil containing a majority of fats as PUFAs and approximately 79% LA lowered AChE activity in synaptosomes.
Although LA and ALA have been shown to modulate AChE activity in the brain, DHA has thus far not proven as effective as an AChE inhibitor. When adult male rats were given DHA for 12 weeks, the DHA content of cell membranes was increased with a concomitant increase in the fluidity of the membrane, but AChE activity in the cortex was unchanged by DHA supplementation [34]. These differences in the effects of PUFAs on AChE activity may be due to the extent of incorporation of specific PUFAs into the cell membrane. DHA supplementation could increase disorganization of the membrane lipid core [35], thereby leaving the fluidity of the cell surface untouched and the activity of cell surface-attached AChE unaffected. It is also possible that AChE modulation is a function exclusive of ALA or LA and not associated with DHA.
Dietary fatty acids and age-related cognitive decline
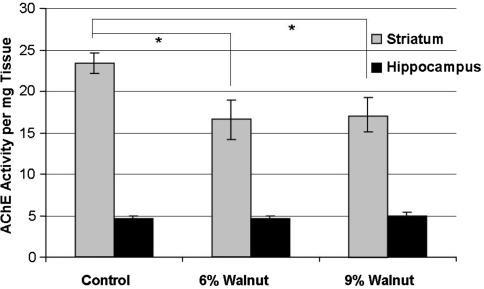
Although several studies have demonstrated that DHA supplementation can restore membrane content and enhance ACh release [22, 23], few studies have reported a beneficial effect of DHA, EPA or fish oil on cognitive behavior in aged animals [36, 37]. DHA supplementation was found to be ineffective at improving overall Morris water maze performance when given at 9.4 g/kg diet to aged mice throughout life [36], 11.2% of dietary fatty acids to aged rats for 4 weeks [38], or 3.5 g/kg diet to aged APP/PS1 mice [39]. When diets supplemented with DHA-rich fish oil were provided to APP/PS1 mice, the mice did spend significantly more time in the correct quadrant of the maze during the probe trial, but the amount of time spent in the correct quadrant was not better than chance [39]. In other behavioral tasks and at higher dietary concentrations, DHA has been shown to improve maze performance in aged animals. 9-month-old mice consuming a diet supplemented with 20% DHA in the form of Chlorella vulgaris for 8 weeks made fewer total and fewer working memory errors in the 8-arm radial arm maze [40]. In stroke-prone spontaneously hypertensive rats, dietary DHA administration from 6 to 20 months was able to normalize hippocampal ACh levels and improve performance on passive avoidance learning [41]. Similarly, in a footshock-motivated T-maze, 10-month-old senescence-accelerated SAMP8 mice fed a high DHA diet (14% DHA) for 8 weeks exhibited improved acquisition and retention of the learning task when compared with animals maintained on a low DHA (0.3%) diet [42]. In other studies, DHA supplementation was only found to improve cognitive performance in aged animals only when compared with diets deficient in key nutrients [43] or diets enriched in saturated fats [44, 45]. Several studies have investigated the effects of perilla oil (approximately 13% LA, 60% ALA) and safflower oil (approximately 75% LA, 0.1% ALA) on learning in aged animals; these studies have generally demonstrated improved brightness discrimination learning in 19-month-old rats fed throughout life [46], 7-month-old senescence-accelerated SAMP8 mice fed from birth [47], and 15-month-old senescence-resistant SAMR1 mice fed from birth [48]. Other than the previously mentioned oil supplementation studies, few reports exist on the effects of ALA and LA on cognitive function in aged animals. Our most recent work with dietary PUFAs demonstrated that supplementation of aged animals with 6% walnut extract in the diet improved performance on cognitive and motor tasks [49]. Walnuts contain approximately 9 g of ALA and 38 g of LA per 100 g of walnuts, the richest whole food source of ALA [50]. For animal studies, this translates to 5.4 g of ALA and 22.9 g of LA per kg of the 6% diet and 8.2 g of ALA and 34.28 g of LA per kg of 9% diet. As one potential explanation for the beneficial effects of walnuts on cognition, we subsequently found that walnut consumption was associated with significantly lower AChE activity in one brain region of aged animals (Fig. 1). These results are in concert with the previously mentioned studies of fatty acid supplementation and reveal a compelling benefit of walnut consumption which is undergoing further investigation.
Fig. 1.
Walnut supplementation significantly decreases acetylcholinesterase activity in the aged striatum, but not in the hippocampus. AChE activity was determined using Ellman’s colorimetric method (data presented as mean ± SEM; control n = 5, 6% n = 7, 9% n = 6; *P < 0.05)
Summary and conclusions
In conclusion, a number of studies have demonstrated that natural substances and dietary components can impact the cholinergic system of the brain. Of these, PUFAs from the diet may represent one source of cholinergic modulators. Although this area of research is in its infancy, studies have revealed that the consumption of essential fatty acids can affect ACh release, cholinergic receptor expression, and activity and ACh hydrolysis. Coupled with numerous other experiments showing that berry fruits such as blueberries, blackberries, and strawberries can reverse motor and cognitive deficits in aging, findings revealing that PUFAs can affect the cholinergic system suggest once more that diet can be a powerful modifier of the course of behavioral decline and perhaps increase the health span in aging.
Footnotes
This article belongs to a special issue on Nutrients and Brain Health.
Contributor Information
Lauren Meredith Willis, Email: lauren.meredith.willis@gmail.com.
James A. Joseph, Phone: +1-617-5563178, FAX: +1-617-5563222, Email: james.joseph@ars.usda.gov
References
- 1.Schliebs R (2005) Basal forebrain cholinergic dysfunction in Alzheimer’s disease—interrelationship with beta-amyloid, inflammation and neurotrophin signaling. Neurochem Res 30:895–908 [DOI] [PubMed]
- 2.Sherman KA, Friedman E (1990) Pre- and post-synaptic cholinergic dysfunction in aged rodent brain regions: new findings and an interpretative review. Int J Dev Neurosci 8:689–708 [DOI] [PubMed]
- 3.Schliebs R, Arendt T (2006) The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J Neural Transm 113:1625–1644 [DOI] [PubMed]
- 4.Viegas C Jr, Bolzani Vda S, Barreiro EJ, Fraga CA (2005) New anti-Alzheimer drugs from biodiversity: the role of the natural acetylcholinesterase inhibitors. Mini Rev Med Chem 5:915–926 [DOI] [PubMed]
- 5.Orhan G, Orhan I, Subutay-Oztekin N, Ak F, Sener B (2009) Contemporary anticholinesterase pharmaceuticals of natural origin and their synthetic analogues for the treatment of Alzheimer’s disease. Recent Patents CNS Drug Discov 4:43–51 [DOI] [PubMed]
- 6.Loizzo MR, Tundis R, Menichini F (2008) Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem 15:1209–1228 [DOI] [PubMed]
- 7.Lopez S, Bastida J, Viladomat F, Codina C (2002) Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci 71:2521–2529 [DOI] [PubMed]
- 8.Marco L, do Carmo Carreiras M (2006) Galanthamine, a natural product for the treatment of Alzheimer’s disease. Recent Patents CNS Drug Discov 1:105–111 [DOI] [PubMed]
- 9.Akhondzadeh S, Abbasi SH (2006) Herbal medicine in the treatment of Alzheimer’s disease. Am J Alzheimers Dis Other Dementias 21:113–118 [DOI] [PMC free article] [PubMed]
- 10.Mukherjee PK, Kumar V, Mal M, Houghton PJ (2007) Acetylcholinesterase inhibitors from plants. Phytomedicine 14:289–300 [DOI] [PubMed]
- 11.Nakajima A, Yamakuni T, Haraguchi M, Omae N, Song SY, Kato C, Nakagawasai O, Tadano T, Yokosuka A, Mimaki Y, Sashida Y, Ohizumi Y (2007) Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J Pharmacol Sci 105:122–126 [DOI] [PubMed]
- 12.Benatti P, Peluso G, Nicolai R, Calvani M (2004) Polyunsaturated fatty acids: biochemical, nutritional and epigenetic properties. J Am Coll Nutr 23:281–302 [DOI] [PubMed]
- 13.Lin YH, Salem N Jr (2007) Whole body distribution of deuterated linoleic and {alpha}-linolenic acids and their metabolites in the rat. J Lipid Res 48:2709–2724 [DOI] [PubMed]
- 14.Martin DS, Spencer P, Horrobin DF, Lynch MA (2002) Long-term potentiation in aged rats is restored when the age-related decrease in polyunsaturated fatty acid concentration is reversed. Prostaglandins Leukot Essent Fatty Acids 67:121–130 [DOI] [PubMed]
- 15.Little SJ, Lynch MA, Manku M, Nicolaou A (2007) Docosahexaenoic acid-induced changes in phospholipids in cortex of young and aged rats: a lipidomic analysis. Prostaglandins Leukot Essent Fatty Acids 77:155–162 [DOI] [PubMed]
- 16.Youdim KA, Martin A, Joseph JA (2000) Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci 18:383–399 [DOI] [PubMed]
- 17.Ulmann L, Mimouni V, Roux S, Porsolt R, Poisson JP (2001) Brain and hippocampus fatty acid composition in phospholipid classes of aged-relative cognitive deficit rats. Prostaglandins Leukot Essent Fatty Acids 64:189–195 [DOI] [PubMed]
- 18.Lopez GH, Ilincheta de Boschero MG, Castagnet PI, Giusto NM (1995) Age-associated changes in the content and fatty acid composition of brain glycerophospholipids. Comp Biochem Physiol 112B:331–343 [DOI] [PubMed]
- 19.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI (2002) The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging 23:843–853 [DOI] [PubMed]
- 20.Mora F, Segovia G, del Arco A (2007) Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev 55:78–88 [DOI] [PubMed]
- 21.Lesa GM, Palfreyman M, Hall DH, Clandinin MT, Rudolph C, Jorgensen EM, Schiavo G (2003) Long chain polyunsaturated fatty acids are required for efficient neurotransmission in C. elegans. J Cell Sci 116:4965–4975 [DOI] [PubMed]
- 22.Favreliere S, Perault MC, Huguet F, De Javel D, Bertrand N, Piriou A, Durand G (2003) DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol Aging 24:233–243 [DOI] [PubMed]
- 23.Aid S, Vancassel S, Linard A, Lavialle M, Guesnet P (2005) Dietary docosahexaenoic acid [22: 6(n-3)] as a phospholipid or a triglyceride enhances the potassium chloride-evoked release of acetylcholine in rat hippocampus. J Nutr 135:1008–1013 [DOI] [PubMed]
- 24.Nishizaki T, Ikeuchi Y, Matsuoka T, Sumikawa K (1997) Short-term depression and long-term enhancement of ACh-gated channel currents induced by linoleic and linolenic acid. Brain Res 751:253–258 [DOI] [PubMed]
- 25.Jerusalinsky D, Kornisiuk E, Izquierdo I (1997) Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochem Res 22:507–515 [DOI] [PubMed]
- 26.du Bois TM, Bell W, Deng C, Huang XF (2005) A high n-6 polyunsaturated fatty acid diet reduces muscarinic M2/M4 receptor binding in the rat brain. J Chem Neuroanat 29:282–288 [DOI] [PubMed]
- 27.Almeida T, Cunha RA, Ribeiro JA (1999) Facilitation by arachidonic acid of acetylcholine release from the rat hippocampus. Brain Res 826:104–111 [DOI] [PubMed]
- 28.Bloj B, Morero RD, Farias RN, Trucco RE (1973) Membrane lipid fatty acids and regulation of membrane-bound enzymes. Allosteric behaviour of erythrocyte Mg 2+-ATPase (Na++ K+)-ATPase and acetylcholinesterase from rats fed different fat-supplemented diets. Biochim Biophys Acta 311:67–79 [DOI] [PubMed]
- 29.Vajreswari A, Narayanareddy K (1992) Effect of dietary fats on erythrocyte membrane lipid composition and membrane-bound enzyme activities. Metabolism 41:352–358 [DOI] [PubMed]
- 30.Vajreswari A, Rupalatha M, Rao PS (2002) Effect of altered dietary n-6-to-n-3 fatty acid ratio on erythrocyte lipid composition and membrane-bound enzymes. J Nutr Sci Vitaminol 48:365–370 [DOI] [PubMed]
- 31.Foot M, Cruz TF, Clandinin MT (1983) Effect of dietary lipid on synaptosomal acetylcholinesterase activity. Biochem J 211:507–509 [DOI] [PMC free article] [PubMed]
- 32.Tsutsumi T, Yamauchi E, Suzuki E, Watanabe S, Kobayashi T, Okuyama H (1995) Effect of a high alpha-linolenate and high linoleate diet on membrane associated enzyme activities in rat brain-modulation of Na+, K+ ATPase activity at suboptimal concentrations of ATP. Biol Pharm Bull 18:664–670 [DOI] [PubMed]
- 33.Srinivasarao P, Narayanareddy K, Vajreswari A, Rupalatha M, Prakash PS, Rao P (1997) Influence of dietary fat on the activities of subcellular membrane-bound enzymes from different regions of the brain. Neuochem Int 31:789–794 [DOI] [PubMed]
- 34.Shahdat H, Hashimoto M, Shimada T, Shido O (2004) Synaptic plasma membrane-bound acetylcholinesterase activity is not affected by docosahexaenoic acid-induced decrease in membrane order. Life Sci 74:3009–3024 [DOI] [PubMed]
- 35.Mitchell DC, Litman BJ (1998) Effect of cholesterol on molecular order and dynamics in highly polyunsaturated phospholipid bilayers. Biophys J 75:896–908 [DOI] [PMC free article] [PubMed]
- 36.Carrie I, Guesnet P, Bourre JM, Frances H (2000) Diets containing long-chain n-3 polyunsaturated fatty acids affect behaviour differently during development than ageing in mice. Br J Nutr 83:439–447 [PubMed]
- 37.Fedorova I, Salem N Jr (2006) Omega-3 fatty acids and rodent behavior. Prostaglandins Leukot Essent Fatty Acids 75:271–289 [DOI] [PubMed]
- 38.Barcelo-Coblijn G, Hogyes E, Kitajka K, Puskas LG, Zvara A, Hackler L Jr, Nyakas C, Penke Z, Farkas T (2003) Modification by docosahexaenoic acid of age-induced alterations in gene expression and molecular composition of rat brain phospholipids. Proc Natl Acad Sci USA 100:11321–11326 [DOI] [PMC free article] [PubMed]
- 39.Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Kiliaan AJ (2009) DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis 33:482–498 [DOI] [PubMed]
- 40.Sugimoto Y, Taga C, Nishiga M, Fujiwara M, Konishi F, Tanaka K, Kamei C (2002) Effect of docosahexaenoic acid-fortified Chlorella vulgaris strain CK22 on the radial maze performance in aged mice. Biol Pharm Bull 25:1090–1092 [DOI] [PubMed]
- 41.Minami M, Kimura S, Endo T, Hamaue N, Hirafuji M, Togashi H, Matsumoto M, Yoshioka M, Saito H, Watanabe S, Kobayashi T, Okuyama H (1997) Dietary docosahexaenoic acid increases cerebral acetylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacol Biochem Behav 58:1123–1129 [DOI] [PubMed]
- 42.Petursdottir AL, Farr SA, Morley JE, Banks WA, Skuladottir GV (2008) Effect of dietary n-3 polyunsaturated fatty acids on brain lipid fatty acid composition, learning ability, and memory of senescence-accelerated mouse. J Gerontol A Biol Sci Med Sci 63:1153–1160 [DOI] [PubMed]
- 43.Suchy J, Chan A, Shea TB (2009) Dietary supplementation with a combination of alpha-lipoic acid, acetyl-l-carnitine, glycerophosphocholine, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr Res 29:70–74 [DOI] [PubMed]
- 44.Shirai N, Suzuki H (2004) Effect of dietary docosahexaenoic acid and catechins on maze behavior in mice. Ann Nutr Metab 48:51–58 [DOI] [PubMed]
- 45.Lim SY, Suzuki H (2000) Intakes of dietary docosahexaenoic acid ethyl ester and egg phosphatidylcholine improve maze-learning ability in young and old mice. J Nutr 130:1629–1632 [DOI] [PubMed]
- 46.Yamamoto N, Okaniwa Y, Mori S, Nomura M, Okuyama H (1991) Effects of a high-linoleate and a high-alpha-linolenate diet on the learning ability of aged rats: evidence against an autoxidation-related lipid peroxide theory of aging. J Gerontol 46:B17–B22 [DOI] [PubMed]
- 47.Umezawa M, Ohta A, Tojo H, Yagi H, Hosokawa M, Takeda T (1995) Dietary alpha-linolenate/linoleate balance influences learning and memory in the senescence-accelerated mouse (SAM). Brain Res 669:225–233 [DOI] [PubMed]
- 48.Umezawa M, Kogishi K, Tojo H, Yoshimura S, Seriu N, Ohta A, Takeda T, Hosokawa M (1999) High-linoleate and high-alpha-linolenate diets affect learning ability and natural behavior in SAMR1 mice. J Nutr 129:431–437 [DOI] [PubMed]
- 49.Willis LM, Shukitt-Hale B, Cheng V, Joseph JA (2009) Dose-dependent effects of walnuts on motor and cognitive function in aged rats. Br J Nutr 101:1140–1144 [DOI] [PubMed]
- 50.Ros E, Mataix J (2006) Fatty acid composition of nuts—implications for cardiovascular health. Br J Nutr 96(Suppl 2):S29–S35 [DOI] [PubMed]