Abstract
Rigidity or hypertonicity is a cardinal symptom of Parkinson's disease (PD). We hypothesized that hypertonicity of the body axis affects functional performance of tasks involving balance, walking and turning. The magnitude of axial postural tone in the neck, trunk and hip segments of 15 subjects with PD (both ON and OFF levodopa) and 15 control subjects was quantified during unsupported standing in an axial twisting device in our laboratory as resistance to torsional rotation. Subjects also performed six functional tests (walking in a figure of eight [Figure of Eight], Timed Up & Go, Berg Balance Scale, supine rolling task [rollover], Functional Reach, and standing 360-deg turn-in-place) in the ON and OFF state. Results showed that PD subjects had increased tone throughout the axis compared to control subjects (p=0.008) and that this increase was most prominent in the neck. In PD subjects, axial tone was related to functional performance, but most strongly for tone at the neck and accounted for an especially large portion of the variability in the performance of the Figure of Eight test (rOFF=0.68 and rON=0.74, p<0.05) and the Rollover test (rOFF=0.67and rON=0.55, p<0.05). Our results suggest that neck tone plays a significant role in functional mobility and that abnormally high postural tone may be an important contributor to balance and mobility disorders in individuals with PD.
Keywords: Parkinson's disease, postural tone, neck tone, functional performance, balance
Introduction
Rigidity (or muscle hypertonicity), defined as increased resistance to passive movement, is a cardinal sign of Parkinson's disease (PD) (Foster 1892). Previous studies suggest that rigidity may underlie many of the common motor disabilities associated with PD (Nutt et al., 1992; Shenkman et al, 2001). Research on rigidity in subjects with PD has concentrated mainly on the effects of hypertonicity in the distal limb musculature, even though rigidity in axial and proximal muscles may be an important contributor to limited functional mobility (Nagumo et al., 1993; Nagumo et al., 1996; Nutt et al., 1992; Schenkman et al., 2001; Steiger et al., 1996).
A number of studies (Horak et al., 1996; Lakke 1985; Nagumo et al., 1993; Schenkman et al., 2000; Steiger et al., 1996) have attempted to relate axial rigidity to functional performance. However, these studies did not use direct objective measures of axial tone because of the complexity of quantifying tone in the axial segments. For example, some studies have quantified axial trunk tone in the supine position (Nagumo et al., 1993; Nagumo et al., 1996) by passively moving the legs and hips. The shortcoming of this technique is that tone is assessed in a resting position, when the body is relaxed such that postural tone is reduced. The clinical measure of rigidity in the UPDRS (Unified Parkinsons Disease Rating Scale, Fahn et al., 1987) is also limited by the subjective estimation of tone in the extremities and the neck with a rating scale when the patient is sitting.
In the present study, we used a device recently developed in our laboratory to objectively quantify axial tone in standing PD subjects when the nervous system is actively controlling balance (Gurfinkel et al., 2006; Wright et al., 2007). This device, called “Twister,” quantifies axial tone by measuring resistance to passively applied torsional rotation at the neck, trunk and/or hips without constraining anterior-posterior, lateral, or vertical body position (Gurfinkel et al., 2006). Body position is constrained only in the torsional direction. Using the Twister device, we showed that axial tone in healthy adults is not static, but rather involves flexible, active shortening and lengthening reactions (Gurfinkel et al., 2006). Unlike stretch reflexes, in which muscles are activated during muscle lengthening and silenced during muscle shortening, shortening reactions are associated with increased activity during muscle shortening and decreased activity during muscle lengthening (see Sherrington 1909). In another study, we showed that subjects with PD have elevated tone in the trunk and hips compared to age-matched control subjects (Wright et al., 2007); however, this study did not investigate axial tone at the neck.
Axial hypertonicity may contribute to motor disability in PD (Schenkman et al., 2000; Wright et al., 2007). For example, hypertonicity is thought to contribute to the absence of body rotation during sleep (Nutt et al., 1992; Stack et al., 2006a) and may also contribute to abnormal intersegmental coordination during walking and turning (Crenna et al., 2007; Mesure et al., 1999; Vaugoyeau et al., 2006). While these observations suggest a causal relationship between axial tone and functional performance in PD, no study has directly measured axial tone and its relation to functional performance.
The axial trunk tone might affect the performance during walking and turning because it may hinder normal dissociated rotation of the head and trunk (Stack et al., 2006b). Axial hip tone may also affect gait speed and turning because it could impair the control of pelvis on hip rotation (Vaugoyeau et al., 2006). More importantly, increased neck tone may have a major impact on walking, turning and twisting since the head must be free to move to scan surrounding objects and to steer locomotion (Paquette et al., 2006). Therefore, we hypothesize that neck tone will be the locus of hypertonicity and most related to performance impairments in balance, walking and turning tasks in persons with PD. To test this hypothesis, we correlated axial postural tone in the neck, trunk and pelvis segments measured objectively using Twister with measures of balance and mobility in both subjects with PD and age-matched control subjects.
Methods
Subjects
Fifteen male subjects (63 ± 8 years) with a clinical diagnosis of “idiopathic” PD, treated with levodopa and 15 healthy male control subjects (64 ± 9 years old), matched for age, weight and height, participated in the study. The PD subjects had no history suggesting “atypical” PD symptoms, as defined by Hughes et al. (1992) or other existing neuromuscular disorders, including severely flexed posture. We included only PD subjects with Hoehn &Yahr scores of 2 or 3. Characteristics of the PD subjects are presented in Table 1. The control subjects had no recent or unresolved history of musculoskeletal, neuromuscular or motor disorders. Control and PD subjects were able to stand independently for at least 10 minutes. All subjects provided informed consent in accordance to the Oregon Health & Science University Internal Review Board regulations for human subjects' studies and the Helsinki Declaration.
Table 1.
PD subject characteristics
| UPDRS Motor Part III |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Rigidity |
PIGD | ADL | H&Y | Medication | ||||||||||||
| Total | Neck | ||||||||||||||||
| PD Subj |
Sex | Duratio n of PD (yrs) |
Age (yrs) |
Ht (cm) |
Wt (kg) |
Side | ON | OFF | ON | OFF | ON | OFF | ON | OFF | L-DOPA | ||
| 1 | M | 8 | 64 | 180 | 99 | R | 36 | 52.5 | 7 | 11 | 3 | 4 | 5 | 7.5 | 28 | 3 | 900 |
| 2 | M | 9 | 56 | 175 | 83 | L | 24 | 47 | 9 | 13 | 2 | 4 | 2 | 4 | 9 | 2.5 | 1450 |
| 3 | M | 12 | 81 | 175 | 77 | L | 40.5 | 46 | 12 | 16 | 3 | 4 | 4 | 4 | 13 | 3 | 800 |
| 4 | M | 3.5 | 46 | 180 | 81 | R | 31 | 44.5 | 4 | 8 | 1 | 2 | 1 | 2.5 | 14 | 3 | 333 |
| 5 | M | 10 | 53 | 178 | 68 | R | 17 | 39.5 | 3 | 8 | 2 | 3 | 1.5 | 5 | 6 | 3 | 900 |
| 6 | M | 4 | 66 | 185 | 82 | R | 30 | 37 | 4 | 7 | 1 | 1 | 4 | 4 | 14 | 2.5 | 850 |
| 7 | M | 5 | 60 | 168 | 73 | L | 26.5 | 34.5 | 10 | 12 | 2 | 3 | 2.5 | 4.5 | 8 | 2.5 | 650 |
| 8 | M | 1.5 | 70 | 170 | 94 | L | 29 | 32 | 6 | 7 | 3 | 3 | 4.5 | 5 | 3 | 2 | 125 |
| 9 | M | 10 | 66 | 180 | 86 | R | 23.5 | 31 | 5.5 | 8.5 | 2 | 3 | 2 | 3.5 | 14 | 2 | 700 |
| 10 | M | 3 | 71 | 179 | 94 | S | 16.5 | 23.5 | 1 | 2.5 | 1 | 1 | 3.5 | 5 | 9 | 2 | 450 |
| 11 | M | 9 | 65 | 183 | 75 | R | 20.5 | 30 | 3 | 5 | 0 | 1 | 2 | 5 | 18 | 2 | 683 |
| 12 | M | 2 | 56 | 180 | 80 | R | 16 | 27 | 2 | 3 | 0 | 1 | 2 | 2 | 11 | 2.5 | 133 |
| 13 | M | 9 | 66 | 175 | 88 | L | 19.5 | 30.5 | 1 | 1 | 1 | 1 | 2.5 | 3.5 | 7 | 2 | 875 |
| 14 | M | 12 | 64 | 165 | 68 | R | 14.5 | 22 | 4 | 5 | 2 | 2.5 | 1.5 | 3 | 10 | 2 | 1200 |
| 15 | M | 1.5 | 65 | 168 | 70 | L | 14 | 18 | 0 | 2 | 0 | 1 | 1 | 1 | 6 | 2 | 100 |
| Mean | - | 6.5 | 63 | 176 | 81 | - | 24 | 34.5 | 5 | 7 | 1.5 | 2 | 2.5 | 4 | 11 | 2.5 | |
| Mean | Cntrls | - | 64 | 174 | 79 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - |
Duration of PD- years since the PD diagnosis, Side – affected side, L- left, R - right, S-symmetrical, Total rigidity - UPDRS, item 22 Neck rigidity - UPDRS, neck part of item 22, PIGD - Postural Instability and Gait Difficulty (UPDRS item 27-30 UPDRS), ADL-Activities of Daily Living, L-DOPA - daily dose in mg/day., H & Y= Hoehn & Yahr
Protocol
PD subjects were tested OFF-medication (OFF) the morning after abstaining from levodopa overnight (washout period =12 h). The experiment began with the measurement of axial tone at the neck, trunk and hips. Subjects were then instrumented with reflective markers on the skin over bony prominences to measure accurately their active range of motion at the neck, trunk and hips while standing using motion analysis. Six functional performance tests were then performed by each subject: 1) The Figure of Eight test; 2) Supine Rolling task on a therapy mat; 3) the Timed-Up-and-Go (TUG) test; 4) the Berg Balance Scale ([BBS]; 5) the Functional Reach test; 6) Standing, 360 deg turn-in-place. The motor part of the UPDRS (Fahn et al., 1987) was then performed by each subject.
The OFF testing session ended with PD subjects taking their usual morning dose of medication, followed by a rest period of one hour. During that time, the subjects rated themselves on activities of daily living (ADL) as part of the UPDRS (Fahn et al., 1987) to quantify activity impairments. After the rest period and once the subjects reported that they felt “ON”, the protocol was repeated (i.e., range of motion, functional performance tests, UPDRS and axial tone measurement) with PD subjects ON-medication (ON). Control subjects only did the first part of the protocol (i.e., same as for PD OFF).
Measurement of axial tone
Axial tone was quantified in standing subjects using Twister, which has been shown to be a repeatable and reliable measure of axial postural tone in healthy individuals (Gurfinkel et al., 2006) and PD subjects (Wright et al., 2007). Briefly, subjects stood blindfolded on a horizontal platform that slowly (1 deg/s) rotated left and right (±10 deg) with certain axial body segments attached either to an earth-fixed frame or to the rotating surface in order to isolate the rotation to specific axial segments (i.e., hips, trunk or neck). Body segments were fixed to the earth-fixed frame or rotating surface with a helmet, shoulder harness, or pelvis harness. A torque sensor (Futek TFF400, Irvine, CA), located between the earth-fixed frame and the axial segment under study, was used to measure that segment's axial resistance to passive rotation. A double-hinged attachment of the torque sensor restricted torsion but allowed motion in all other directions so subjects actively maintained stance balance. The following configurations were used to quantify tone at different body segments: 1) neck tone: head attached to the earth-fixed frame such that platform motion caused the legs, pelvis and trunk to rotate torsionally as a unit relative to head, 2) trunk tone: trunk attached to the earth-fixed frame and pelvis attached to the rotating platform such that platform motion caused the legs and pelvis to rotate as one unit, and 3) hip tone: pelvis attached to the earth-fixed frame such that platform motion caused the legs to rotate relative to the fixed pelvis.
During the measurement of axial tone in each subject, the platform rotated under the feet for 4 complete cycles ±10 deg from the neutral position (i.e., facing straight ahead) at 1 deg/s (Fig. 1A, left panel bottom trace). A potentiometer signaled the angular position of the platform. The rate of rotation was constant to minimize effects of inertia, except during directional changes where the rotation followed a parabolic trajectory, reducing acceleration to <12 deg/s2. The resistive torque at the hip, trunk and neck was digitized and recorded at 100 samples/s using Spike2 software (Cambridge Electronic Devices, Cambridge, UK) and analyzed offline using Matlab software (Mathworks, Natick, MA).
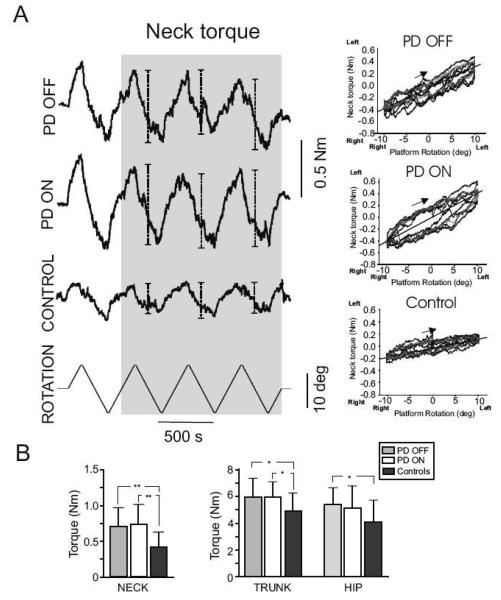
Fig. 1.
Panel A shows the raw data of the neck resistance when the head was fixed and the body was twisted in one representative PD subject both OFF and ON medication and one control subject. The peak torque measured for each cycle of left and right rotation used for the analysis are indicated by bars in the shaded area. Bottom trace illustrates the signal from the rotating plate, ±10°, 1°/s. The torque-angle relation is shown in the same subjects. A flatter slope of the torque- angle relationship indicates relatively little resistance to axial rotation. Panel B shows the mean and SD of the neck, trunk and hip resistance to torsional rotation in all groups. ** denotes p<0.01and *denotes p<0.05.
The first cycle of rotation was excluded from the analysis of the torsional resistance since that cycle might be affected by the subject's initial postural set or resting short-range stiffness. This difference between the first and subsequent cycles of rotation was confirmed by a 3-way analysis of variance (ANOVA) on Group (PD vs. Controls) X Cycles (1st vs. subsequent) X Segment (head, trunk, pelvis) with repeated measures on the last 2 factors. An interaction effect was found between the first and subsequent cycles in the hips segment (F[1,42]=407.5, p>0.001), while no interaction was found between Group and Cycles (F[2,42]=1.4, p=0.255). The resistive torque in the neck, trunk and hips was quantified as the mean peak-to-peak torque amplitude for cycles 2-4 (bars in the shaded area of Fig. 1A, left panel). We also calculated the cross-covariance between resistive torque and torsional position at the neck, trunk and hips. The cross-covariance is the mean-removed cross-correlation sequence, and it quantifies the similarity between two waveforms as a function of a time-lag applied to one of the waveforms. Cycles 2-4 were also used for this calculation.
Measurement of active range of axial movement
Active range of motion was measured at the neck, trunk and pelvis in PD subjects and control subjects while standing. The subjects were positioned with their feet together and arms crossed. Maximum horizontal voluntary range of motion was measured with an 8-camera video system (Hi-Res, Motion Analysis System, Santa Rosa, CA). The cameras tracked the 3-dimensional positions of 14 reflective markers affixed to the head, trunk, pelvis and feet. An experimenter instructed the subjects to first look straight ahead (center, 0° position) then to twist their body in the horizontal plane as far as possible to the left or right while keeping their feet in the same position. The subjects were then instructed to come back to center position and then to rotate in the opposite direction, repeating this process once (i.e., right-left-right-left or left-right-left-right). Every position, including the center position, was held for 3 to 5 seconds.
For range of motion, segment angular rotation in the horizontal plane was obtained by computing the angular displacement of each segmental vector (i.e., head, trunk and pelvis) in each of the 2 axes defining the horizontal plane. The reference position (0° position) for all three segments corresponds to the center, straight ahead position, normalized to the beginning of the trial when subjects were facing forward. For interval-detection purposes, 2 Hz low-pass filter (4th order, dual-pass Butterworth filter) was used. A criterion of null angular velocity (0±3 deg/s) over at least 30 frames was used to determine the intervals when the subjects had reached their maximum right or left position or center. The maximum active range of motion value selected represents the position of each segment averaged over the interval when subjects maintained their maximum position. The outcome measures retained were total range of motion (maximum left + maximum right) of the neck (i.e., head movement minus trunk movement), trunk (i.e., trunk movement minus pelvis movement) and pelvis.
Functional performance assessments
Functional performance tests commonly used by therapists in clinical settings were performed to assess functional mobility in PD subjects both ON and OFF medication and in control subjects. The following 6 functional performance measures were assessed in random order across subjects:
1) The Figure of Eight test timed subjects walking in a figure-8 trajectory (Jarnlo and Nordell, 2003). The figure-8 trajectory was marked with 4 cm-wide tape on the floor, each loop having an internal diameter of 163 cm. The time to walk two complete cycles was measured with a hand-held stopwatch. The subject started in the center of the figure-8 and the onset time was based on the first detectable movement of the subject following a “Go!” command from the experimenter. Steps outside or inside the line were quantified as described in Jarnlo and Nordell (2003). The task was performed twice, but the first test was considered a training trial and not used for analysis.
2) The rollover task began with subjects in supine position on a therapy mat. The subjects were instructed to execute a 360 deg-rolling movement to the left as fast as possible after a “Go!” command (stopwatch started). When the evaluator determined that the maneuver had reached a full 360 deg, the subject immediately received a “Return” command and the subject rolled 360 deg to the right as fast as possible to the supine position. A stopwatch was used to time the duration of the task. The task was performed twice, and the second trial duration was used for analysis.
3) In the Timed Up and Go test (TUG; Morris et al., 2001; Podsiadlo et al., 1991) subjects began from a seated position, rose from the chair, walked 3 m straight ahead, turned 180 deg, returned to the chair, and sat down. The entire sequence was timed with a stopwatch and all subjects performed the TUG three times. The first trial was considered a training trial and the mean of the second and third trials was used for analysis. In addition, the 180 degree-turning sequence during the TUG was videotaped and post processed according to procedures described by Dite and Temple (2002).
4) All 14 items of the Berg Balance Scale (BBS) were assessed as described by Berg et al. (1992).
5) In the Functional Reach test (Duncan et al., 1992), subjects stood beside a horizontally oriented measuring tape on the wall at their shoulder level. The subjects were oriented with shoulders perpendicular to the measuring tape with both arms stretched out in a 90° shoulder flexion. The subjects were then instructed to “reach forward as far as you can without losing balance or taking a step” and the number of centimeters they reached from the initial position was measured.
6) A 360 deg turn-in-place task was timed using the motion analysis system with reflective markers on the subjects' feet. The subjects were instructed to turn in their preferred direction 360 deg when they were ready to do so (self-initiated). Turn duration was computed from the first movement of the toe or heel off the floor until the last vertical contact of the foot (toe or heel) on the floor after a full 360 deg turn.
Statistical analysis
Statistical analysis for both axial tone and range of motion measures consisted of a two-way analysis of variance (ANOVA) on Group (PD OFF meds vs. controls) X axial segments (neck, trunk and hip) with repeated measures on the last factor to compare PD subjects to control subjects. To test for the effect of medication in the PD group (ON vs. OFF) X the axial segments (neck, trunk and hip), an ANOVA with repeated measurement on both factors was used. Planned comparisons were used to analyze significant main effects.
T-tests were used to analyze differences in the functional performance tests between the PD and control groups (independent) and between PD ON and OFF (dependent). In the specific cases of the BBS, UPDRS and oversteps in the Figure of Eight test, differences were analyzed with Mann-Whitney U-test statistics (independent) and Wilcoxon Matched Pairs test (dependent).
The Pearson product moment correlation was used to investigate the relationship between tone and range of motion as well as the relationship between tone and functional performance tests. In the specific cases of the BBS and UPDRS, the Spearman Rank Order Correlation was used. The significance level was set at p<0.05. Subject 9 was excluded from the correlation analysis between tone and the functional performance tests due to prominent dystonia in the left foot that influenced his performance in the clinical tests. In addition, subject 8 did not perform the TUG in the OFF medication state due to fatigue. One of the control subject' Achilles tendon was painful during gait but had no symptoms when standing quietly. Thus this subject did not perform the Figure of Eight and the TUG and only his tone and range of motion were retained for analysis.
Results
Magnitude of axial tone
Figure 1A (left panel) shows example traces of neck resistive torque in response to torsional rotation of the neck from a representative control subject and a subject with PD (ON and OFF levodopa). A main effect of group was observed (Fig. 1B), in which PD subjects showed significantly higher (43%) axial torque amplitude compared to the control group (PD OFF vs. controls: neck 0.7 ± 0.3 Nm vs. 0.4 ± 0.2 Nm; trunk: 6.0 ± 1.4 Nm vs. 4.9 ± 1.4 Nm; hip 5.4 ± 1.3 Nm vs. 4.1 ± 1.7 Nm; F(1, 28)=8.2, p=0.008). Most of the difference between the PD and control groups was due to the neck segment, in which tone was 75% higher in the PD group. As expected, a main effect of segment was found, with neck<hip<trunk tone, F(2, 56)=214.2, p>0.001. Figure 1A, right panel shows the torque changes plotted against changes in platform rotation. A linear relationship between torque and platform rotation was observed in both PD subjects and normal controls. The increased axial tone in PD subjects is also reflected by their steeper torque/rotation slopes. Thus, the difference in torque amplitude for each subject was dependent on the subject's stiffness (i.e., slope) and independent of the degree of rotation, consistent across the ±10 deg tested in this study.
Medication (PD OFF vs. PD ON) had no significant effect on the resistive torque in the neck, trunk or hip segment, F(1, 14)=0.6, p=0.460 (Fig. 1B).
Flexibility of axial tone
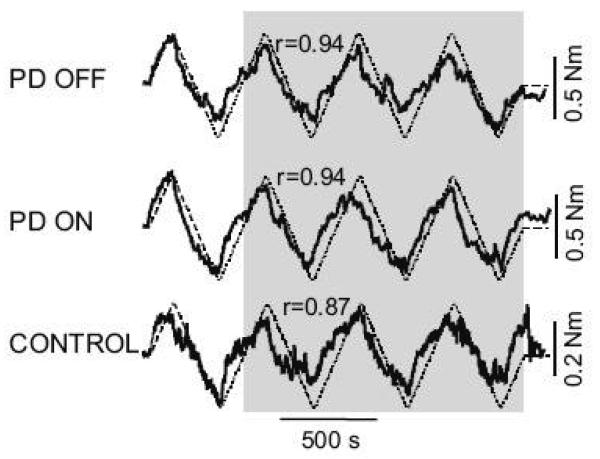
The PD OFF group's axial resistance was more dependent on the torsional rotation (platform position), that is, these subjects were less “flexible” than the control subjects (Fig. 2). A cross-covariance analysis revealed a higher correlation in the neck between axial torque and platform position in the PD OFF group compared to the control group (PD OFF: r=0.94 vs. controls: r=0.85, p=0.005) and in the hip segment (PD OFF: r=0.98 vs. controls r=0.94, p=0.04). In contrast, the resistance of the trunk segment was highly dependent on the torsional rotation in both groups (PD OFF: r=0.99 vs. controls: r=0.98, p=0.102). There were no significant differences in correlation coefficients between PD ON and OFF at all 3 axial levels (p>0.05). Also, the cross-covariation analysis revealed that, during head rotation, neck torque led neck position by 3.9 s in the control subjects and only by 2.9 s in the PD subjects OFF (p=0.002), while no significant group differences in phase were observed in the hip or trunk segments of control or PD OFF subjects (p>0.05).
Fig. 2.
Illustrates the cross-covariance between the torque signal and the rotation signal in one PD subject ON and OFF their medication and one control subject.
Active range of motion
PD OFF subjects and control subjects had similar active axial range of rotation (PD OFF: 90° ± 13°; controls: 95° ± 14°, p=0.36). No significant change in range of axial rotation was observed when PD subjects went ON their medication (PD ON: 91° ± 16°, p=0.842). Subjects were generally symmetric in their left and right trunk rotation amplitude despite the fact that PD subjects were asymmetric in their limb PD signs as recorded on the UPDRS. There was no significant relation between axial tone and total range of motion in any segment (neck range-of-motion vs. neck resistance; rOFF=0.06, rON= −0.37, rControls=−0.15: trunk range-of-motion vs. trunk resistance rOFF=0.27, rON= 0.24, rControls=−0.13 and pelvis range-of-motion vs. hip resistance rOFF=0.03, rON= −0.19, rControls=−0.30).
Functional performance tests
As seen in Table 2, all functional performance tests showed better scores in the control group than in the PD OFF group. Only the Figure of Eight, TUG, and BBS tests improved significantly with levodopa. The Figure of Eight test improved by 8.4 seconds in the ON, compared to the OFF state. The oversteps/errors counted during the Figure of Eight test were also significantly larger in the PD OFF than the control group (Median 1 [range 0-8] vs. Median 0 [range 0-4], respectively; p=0.012) but not between the PD OFF and ON groups (p=0.575). With levodopa, the TUG improved by one second and the BBS decreased by one unit.
Table 2.
Mean and SD of the performance on the clinical tests and comparison between controls vs. PD OFF and PD OFF vs. PD ON.
| Controls | PD OFF meds | PD ON meds | Control vs. PD OFF |
PD OFF vs. PD ON |
||||
|---|---|---|---|---|---|---|---|---|
| Clinical tests | Mean | SD | Mean | SD | Mean | SD | P-value | P-value |
| Figure of Eight (s) | 18.8 | (3.2) | 34.4 | (10.2) | 26.0 | (7.5) | 0.000 | 0.001 |
| Rollover (s) | 4.0 | (0.6) | 6.2 | (2.0) | 5.9 | (2.3) | 0.000 | 0.408 |
| Timed Up & Go (s) | 6.9 | (0.7) | 9.4 | (1.7) | 8.4 | (1.5) | 0.000 | 0.004 |
| BBS (14 items, score 0-4) | 55 | (1.2) | 53 | (2.3) | 54 | (2.1) | 0.001 | 0.032 |
| Functional Reach (cm) | 34 | (6.5) | 29.5 | (4.5) | 29.5 | (5.3) | 0.029 | 0.547 |
| 360 deg turn (s) | 3.8 | (0.7) | 5.3 | (1.6) | 5.1 | (1.9) | 0.007 | 0.654 |
| 180 deg turn (s) | 1.0 | (0.3) | 1.6 | (0.4) | 1.3 | (0.3) | 0.000 | 0.018 |
italic=significantly different from zero at p<0.05.
Axial tone affects functional performance in PD
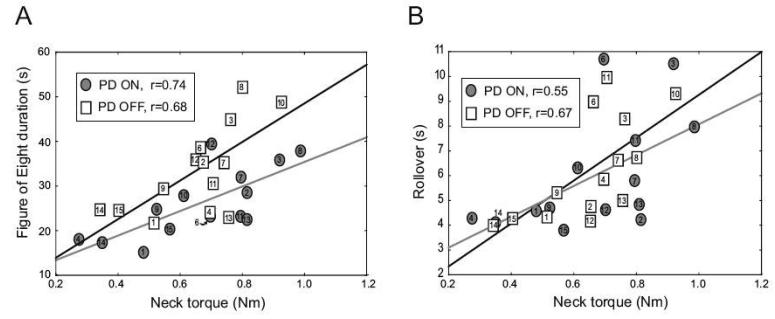
The functional performance of the PD subjects was correlated with their axial tone, especially by their neck tone (Table 3). Neck tone accounted for a large portion of the variability in the performance of the Figure of Eight test, both OFF (r=0.68, r2=0.46, p<0.05) and ON (r=0.74, r2=0.55, p<0.05) medication (Fig. 3A). However, the change in the Figure of Eight time OFF and ON medication did not correlate with the change in the subjects' neck torque (r=0.23, p>0.05), or with the change in UPDRS (r=0.02, p>0.05). Indicating a strong relation between performance on the Figure of Eight test and the neck tone measured with Twister, although the improvement in performing the Figure of Eight was not due to a change in tone, or for that matter UPDRS.
Table 3.
Correlation between neck, trunk and hip torque and functional performance tests in controls and PD ON and OFF.
| Controls | PD OFF | PD ON | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinical tests | Neck | Trunk | Hip | Neck | Trunk | Hip | Neck | Trunk | Hip |
| Figure of Eight (s) | 0.32 | 0.30 | 0.16 | 0.68 | 0.26 | −0.24 | 0.74 | 0.63 | 0.25 |
| Rollover (s) | 0.30 | 0.47 | 0.29 | 0.67 | 0.26 | 0.01 | 0.55 | 0.39 | 0.10 |
| Timed Up & Go (s) | 0.17 | 0.36 | −0.02 | 0.63 | 0.27 | 0.11 | 0.48 | 0.36 | 0.21 |
| BBS (14 items, score 0-4) | 0.21 | −0.19 | −0.10 | −0.37 | −0.33 | 0.26 | −0.74 | −0.67 | −0.32 |
| Functional Reach (cm) | −0.09 | −0.37 | 0.24 | −0.50 | −0.34 | 0.13 | −0.72 | −0.61 | −0.30 |
| 360 deg turn (s) | 0.41 | 0.29 | 0.44 | 0.20 | 0.13 | −0.35 | −0.01 | 0.15 | −0.04 |
| 180 deg turn during TUG (s) |
0.12 | −0.19 | −0.05 | −0.12 | −0.46 | −0.57 | 0.31 | 0.17 | 0.06 |
italic=significantly different from zero at p<0.05.
Fig. 3.
Relation between the peak to peak neck torque and the Figure of Eight (A) and the rollover (B) in both PD OFF (boxes) and ON (circles). Regression lines are shown for the PD subjects OFF (solid black) and ON (solid grey) medication.
The Supine Rolling task (rollover) was also influenced by the subjects' neck tone, both OFF and ON medication (r=0.67, r2=0.45, p<0.05 and r=0.55, r2=0.30, p<0.05, see Fig. 3B). When the PD subjects were ON their medication, the performance of the Functional Reach and BBS was also significantly influenced by their neck and trunk tone. When the PD subjects were OFF medication, the TUG correlated with their neck tone (see Table 3). In contrast to the subjects with PD, the neck, trunk or hip tone did not correlate with the functional performance tests in the control group (Table 3).
Relation between postural tone and the UPDRS motor part
The total UPDRS motor score decreased significantly from the OFF to the ON state (p<0.001) from 34 ± 10 (OFF) to 24 ± 8 (ON). However, no significant relationship (r=0.09-0.47, p>0.05) was found between postural tone in the three axial segments (i.e., measured with Twister) and the total score of the UPDRS (motor part), the rigidity (item 22), the neck rigidity (part of item 22), or the postural alignment (item 28). The only significant relation was found between our measure of axial tone and PIGD (postural instability and gait difficulty, items 27-30). The PIGD correlated significantly with neck tone (r=0.71) in PD subjects OFF and also with the trunk tone in PD subjects, both OFF and ON (r=0.64 and r=0.78, respectively).
Relation between functional performance and the UPDRS motor part
In contrast to the quantitative measure of axial tone from Twister, the neck rigidity measure of the UPDRS motor was not significantly correlated with the functional performance tests (r= −0.34-0.28, p>0.05). The only functional performance task that significantly correlated with the total UPDRS score was the rollover, when the PD subjects were ON medication (r=0.62, p<0.05).
Discussion
In this study, we demonstrate that axial tone, particularly at the neck, is abnormally high in subjects with PD. The increase in neck tone was more strongly related to functional performance than were the increases in trunk and hip axial tone, suggesting that neck muscle tone plays a critical role in the control of balance, mobility, and coordination.
Causes of increased postural neck tone in PD
Consistent with our earlier studies in subjects with PD and elderly controls, trunk tone was greater than hip tone (Gurfinkel et al., 2006; Wright et al., 2007) and tone was smallest in the neck (Gurfinkel et al., 2006). This is the first study to quantify axial tone at the neck in PD subjects and we find it to be 75% higher, compared to 22% higher at the trunk and 32% higher at the hips, compared to age-matched control subjects. We do not think that the increased tone, especially the increased neck tone, was due to a stooped postural alignment of our subjects with PD. Our subjects did not have severely stooped posture and the flexed postural alignment (Item 28 of the UPDRS) was not significantly correlated with our subjects' axial tone. In addition, the observed increase in resistance to axial rotation around the vertical axis was not due to limitations in their passive or active range-of-motion.
Alternatively, high axial tone in PD could arise from a generalized increase in flexor muscle tone (Zhang et al., 1999), or from compensatory hyperactivity in the extensor muscles to maintain antigravity posture during increased flexor activity. Even in healthy subjects, the center of mass of the head is in front of the neck, so neck extensor muscles must remain active to prevent the head from falling forward. Disturbances in cervical muscle tone have been described for many years in patients with PD. James Parkinson first reported that “the chin was almost immovably bent down upon the sternum” (Parkinson, 1817). Purdue Martin (1967) noted a loss of “fixation activity” in cervical extensor muscles in patients with advanced PD. This dropped head sign (also called anterocollis) could result from imbalanced muscle rigidity between anterior and posterior neck muscles (Yoshiyama et al., 1999; Kashihara et al., 2006; van de Warrenburg et al., 2007). In fact, we previously found larger increases in flexor, than extensor, EMG activity during stance in subjects with PD (Burleigh et al, 1995). Even when PD subjects show a flexed head posture, they can voluntarily hold their heads upright, indicating a disturbance in involuntary tonic muscle activity and not in voluntary muscle activity (Martin, 1967; Kashihara et al., 2006).
The physiological basis for rigidity is not well understood. Studies have suggested that rigidity is associated with increased tonic activity of motoneurons, lack of reciprocal inhibition of spinal circuits, reduced phasic stretch reflexes or abnormal tonic stretch reflexes, myofacial and histological changes in muscle fibers, abnormal firing patterns of motoneurons, abnormal feedback from secondary afferents of muscle spindles, larger evoked potentials from supraspinal supersensitivity, hyperexcitable motoneurons, and deficits in cortical inhibition onto motoneuron pools (Granit, 1970; Meyer et al., 1977; Mori, 1987; Kandel et al., 2000). In our study, higher peak resistance to rotation and faster change in resistance (Fig. 1) were both associated with higher axial tone in PD. In addition to the higher amplitude of axial tone, the cross-covariance between tone and rotation showed an “inflexibility” of the tone, that is, axial tone was more driven by the platform rotation with the muscles acting more as a passive, rubber band in the subjects with PD than in the control subjects (Fig. 2). This dependence of tone on muscle length may suggest more peripheral, than central, control of tone in the subjects with PD. Consistent with less central control over postural tone in PD, Hiraoka et al. (2005) found that the central inhibition of the H-reflex during gait initiation was inversely related to the severity of PD, indicating an abnormality of descending central commands in more severely affected patients. This abnormality in H-reflex inhibition has also been seen in PD subjects during standing (Hayashi et al., 1997). Consistent with an inflexibility of postural tone, previous studies showed that, unlike control subjects, PD subjects with rigidity do not flexibly inhibit their leg muscle responses to postural perturbations when the initial conditions of support change (Horak et al., 1992; Chong et al., 2000).
Higher tone in the neck, than the trunk and hips, in subjects with PD may be related to the special descending and ascending innervations of neck motoneurons. The innervation of cervical muscles is especially complex (Peterson, 2004). Neck muscles receive more afferent control than trunk and hip muscles and afferents from neck muscles project more widely to the central nervous system (Peterson, 2004). Neck motoneurons receive input from reticulospinal neurons, vestibular nuclei, cerebellum (via fastigial nucleus), the motor cortex, as well as from the frontal and occipital cortex, through primary motor cortex (Peterson, 2004). There are also medial vestibulospinal neurons that project only to neck motoneurons (Perlmuter et al.,1998). In addition, cervical muscles contain a higher density of muscle spindles (ratio of mean spindle content to the mean weight of muscle in grams) than any other axial muscles (Cooper et al., 1963; Richmond et al., 1979; Kulkarni et al., 2001). The high connectivity of neck motoneurons may be responsible for a greater vulnerability of neck tone to degenerative processes that disturb muscle tone regulation with PD (Samantaray et al., 2008). For example, PD is known to be associated with abnormal proprioceptive processing (Khudados et al., 1999; O'Suilleabhain et al., 2001), abnormal reciprocal inhibition of muscle activation (Dimitrova et al., 2004a), and abnormal synchronisation of muscle activation (Dimitrova et al., 2004b).
Neck tone also had a stronger correlation with functional performance measures than either trunk or hip tone, suggesting that neck tone may be even more important than trunk and hip tone for functional mobility. Laboratory studies have shown that neck muscles are particularly important for control of posture. Activation of proprioceptors in cervical muscles with vibration causes a number of powerful perceptual and motor effects. For example, activation of muscle spindles with vibration of neck muscles in standing causes larger postural disturbances than vibration of other muscles of the trunk and legs (Gregoric et al., 1978). In addition, changing posture of the head greatly alters the direction of postural sway due to both leg muscle vibration and galvanic vestibular stimulation, consistent with an effect of head posture on postural reference frames (Lund et al., 1983; Gurfinkel et al., 1995). Damage to cervical, but not other axial, muscles can result in gait ataxia and cervicogenic dizziness in both humans and animals, supporting their special role in spatial orientation (Brandt, 1996). Cervical muscles also appear to receive higher descending levels of tonic central readiness as shown by their lower thresholds to startle-reactions and to otolith stimulation and by their sudden relaxation with sleep (Brown et al., 1991; Halmagyi et al., 1983).
Contribution of axial tone to functional performance
The unique ability of Twister to measure axial tone while subjects actively controlled posture and stance equilibrium (Gurfinkel et al., 2006) may be why we were able to observe a significant relationship between axial tone and clinical measures of balance and mobility, even though the more subjective rigidity item 22 of the UPDRS did not correlate with functional performance. However, we did see a relation between the PIGD scores (Items 27-30) of the UPDRS and both neck and trunk tone, consistent with an influence of axial postural tone on functional posture and gait.
The strongest relationship between neck tone and the functional performance measures was with the Figure of Eight test, which had been previously used with healthy elderly subjects (Jarnlo and Nordell, 2003; Shkuratova et al., 2004), subjects with Alzheimer's disease (Pettersson et al., 2002), and subjects with arthritis (Noren et al., 2001). We found no previous studies using the Figure of Eight test on subjects with PD, despite the clinical observation that individuals with PD often have difficulty turning (Bloem et al., 2001). The Figure of Eight test requires continuous turning during gait with an emphasis on accuracy (avoid oversteps), speed (timed task), and switching of motor patterns during the cross-over from the clockwise to the counter-clockwise loop. Hicheur et al. (2005) studied body kinematics during Figure of Eight walking in 10 healthy subjects, revealing anticipatory head turns related to both the geometrical form of the path and to the transfer of body mass from one foot to the other during stepping. Given the nature of PD mobility problems with en bloc turns, adapting locomotor patterns and quick switching of motor patterns (Crenna et al., 2007; Huxham et al., 2008), the Figure of Eight test seems to be a sensitive functional performance measure of mobility for individuals with PD.
The rollover and the TUG, which require twisting and turning of the head, also had a strong correlations with neck tone (Table 3). The rollover requires inter-segmental coordination of the head and trunk to initiate the movement as well as to stop it and reverse direction. The TUG involves coordination of the head and trunk during postural transitions of the sit-to-stand and the stand-to-sit components, as well as counter-rotation of the head, shoulder and pelvis during gait and turns, especially when looking at the chair prior to sitting down at the end of the sequence. Since the duration of the 180-degree turn of the TUG was not correlated with axial tone, it is unlikely that the turn, alone, was responsible for the correlation between the TUG with neck tone. However, straight walking also involves coordinated counter-rotation of the head, trunk, and pelvis with precise multisegmental timing of axial segments (Murray et al., 1966, Stokes et al., 1989), which may be reduced by increased axial tone.
There are many examples of everyday motor tasks that require inter-segmental coordination of axial segments to maintain balance and mobility. Stability and mobility in functional motor activities depend on the precise regulation of axial phasic and tonic activity that is carried out automatically, without conscious awareness (Gurfinkel et al., 2006). In PD, posture and gait movements involving axial counter-rotation are often problematic. During walking and turning, individuals with PD tend to rotate the head and trunk simultaneously, whereas healthy subjects lead turns with prior head rotation (Crenna et al., 2007; Mesure et al., 1999, Stack et al., 2006b; Vaugoyeau et al., 2006). Effective postural responses to recover equilibrium also require precise spatial-temporal coordination of the axis (Horak et al., 1986). For example, control of sagittal stability requires coordinated counter-rotation of the upper and lower body segments and lateral postural stability requires coordination of lateral trunk flexion with trunk rotation; coordinations that are disrupted by PD (Henry et al., 1998; Dimitrova et al., 2004b; Horak et al., 2005; Maurer et al., 2006).
The role of levodopa on axial tone and functional performance
Levodopa did not alter axial tone in our study, consistent with previous studies (Burleigh et al., 1995; Pavese et al., 2006; Weinrich et al., 1988) that found this drug to be ineffective for axial hypertonia. Our recent study on axial tone in PD subjects (Wright et al., 2007) also showed that hypertonicity in the trunk and hips is insensitive to levodopa. This agrees with our previous findings that high amplitude of muscle activity during stance is significantly reduced in the distal, but not proximal, muscles with levodopa (Burleigh et al., 1995; however, cf. Bejjani et al., 2000). Fatigue is a common problem in PD (Friedman et al., 2007) and might have influenced these non-significant differences between ON and OFF state since our experimental protocol was twice as long for the PD subjects compared to controls. On the other hand, we provided pauses and, as mentioned earlier, our result are consistent with previous studies (Burleigh et al., 1995; Pavese et al., 2006; Weinrich et al., 1988; Wright et al., 2007), although in some cases subjects were collected in OFF and ON state on different days (Pavese et al., 2006). Moreover, although axial tone was similar OFF and ON medication the performance in some clinical tests improved. Our results support the theory that axial musculature is controlled by nondopaminergic central structures and pathways that are separate from the levodopa-sensitive pathways for distal muscles (Bloem, 1992).
Although scores on the BBS and the TUG improved with medication in our study, these improvements were too small to be clinically significant. Steffen and Seney (2008) showed that a minimally detectable change in BBS to identify a significant difference in score from measurement error is >5 of the total 56 points in PD subjects. However, none of the PD subjects in the present study improved by >5 points in the BBS with medication. However, we observed a ceiling effect of the Berg in both PD subjects and controls, similar to the study by Steffen and Seney (2008). Although significant, the 1 s improvement in the TUG we observed cannot be distinguished from measurement error. In the study by Steffens and Seney (2008) the minimal detectable difference for the TUG was 11 s in the PD group.
In the present study, only the Figure of Eight test had a clinically meaningful improvement (8.4 s) with levodopa. This improvement with levodopa in the Figure of Eight, without improvement in axial tone, suggests that the Figure of Eight test is also constrained by mobility components, such as bradykinesia and limb rigidity, which are improved by levodopa. A similar explanation may account for the correlation between axial tone and the BBS and the Functional Reach test in PD subjects ON, but not OFF. In the ON state, the primary constraint to perform these tasks may be axial tone, whereas in the OFF state other symptoms of the disease, such as gait deficits and bradykinesia may be more limiting to functional performance.
Limitations in measuring axial tone and functional performance
Our measure of neck tone did not correlate with clinical neck rigidity as measured with the UPDRS. Whereas our measure of tone with Twister showed no significant difference in neck tone ON and OFF levodopa, the UPDRS measures of rigidity were reduced (rigidity: Item 22 and neck rigidity: part of Item 22; Table 1). There are many possible reasons for poor correlations between our measure of tone and the clinical measure of rigidity, including:
Objectivity of tonic measurements—the experimenter assessing UPDRS was not blinded to the medication status of subjects (OFF or ON) and the measure of rigidity with UPDRS is based on subjective feeling of rigidity by the rater.
Sitting vs. standing posture—The subjects were sitting down when assessed tone with the UPDRS, whereas in our measure of axial resistance, the subjects were standing and actively maintaining their balance.
Planes of movement assessed in neck rigidity—Twister quantified rigidity in torsion only, whereas the UPDRS score is obtained by summing the resistance in torsion, flexion/extension, and lateral flexion.
Duration of rigidity test—Twister sampled axial resistance over 160 s at each segment while the UPDRS measure of tone was tested over only a few seconds.
It is unknown exactly how axial tone affects mobility in individuals with PD. While we were able to demonstrate correlations between axial tone and functional performance, there remain many questions about what specific aspects of motor control these clinical tests actually assess. Future studies employing both kinematic and EMG measures of functional performance are needed to study how multi-segmental coordination is affected by axial rigidity in PD. Future studies also need to explore how active axial tone measured with Twister can be influenced by fatigue, postural alignment and attention. Although we have shown good reliability in young subjects (Gurfinkel et al., 2006), the test-retest reliability in PD subjects has yet to be investigated.
Conclusions
This study suggests that abnormally high postural tone, especially in the neck, may contribute significantly to balance and gait disorders in individuals with PD. Many falls in patients with PD associated with sudden changes in postural orientation, such as turning, may be due to inflexible control of axial postural tone (Bloem et al., 2001). A better understanding of the relationship between axial postural tone and motor disability is needed to develop more effective therapeutic interventions for PD.
Acknowledgements
The authors thank Triana Nagel-Nelson for help with subject recruitment and data collection. This study was supported by the NIH R37-AG006457, NIH R01-DC004082, Swedish Brain Foundation, the Center for Health Care Sciences at Karolinska Institutet and Karolinska University Hospital, Sweden.
List of abbreviations
- ADL
Activities of daily living
- ANOVA
Analysis of variance
- BBS
Berg Balance Scale
- PD
Parkinson's Disease
- PIGD
Postural instability and gait difficulty, items 27-30 in UPDRS
- TUG
Timed Up & Go test
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bejjani BP, Gervais D, Arnulf I, Papadopoulos S, Demeret S, Bonnet AM, Cornu P, Damier P, Agid Y. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68:595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K, Wood-Dauphinn,e S, Williams JI, Gayton D. Measuring balance in the elderly: Preliminary development of an instrument. Physiother Can. 1989;41:304–311. [Google Scholar]
- Bloem BR. Postural instability in Parkinson's disease. Clin Neurol Neurosurg. 1992;94(Suppl):S41–45. doi: 10.1016/0303-8467(92)90018-x. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Brandt T. Cervical vertigo--reality or fiction? Audiol Neurootol. 1996;1:187–196. doi: 10.1159/000259201. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114(Pt 4):1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Burleigh A, Horak F, Nutt J, Frank J. Levodopa reduces muscle tone and lower extremity tremor in Parkinson's disease. Can J Neurol Sci. 1995;22:280–285. doi: 10.1017/s0317167100039470. [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson's disease impairs the ability to change set quickly. Journal of the neurological sciences. 2000;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM. Muscle Spindles in Man; Their Morphology in the Lumbricals and the Deep Muscles of the Neck. Brain. 1963;86:563–586. doi: 10.1093/brain/86.3.563. [DOI] [PubMed] [Google Scholar]
- Crenna P, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R, Ferrarin M. The association between impaired turning and normal straight walking in Parkinson's disease. Gait & Posture. 2007;26:172–178. doi: 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Horak FB, Nutt JG. Postural muscle respnses to multidirectional translations in patients with Parkinson's disease. J Neurophysiol. 2004a;91:489–501. doi: 10.1152/jn.00094.2003. [DOI] [PubMed] [Google Scholar]
- Dimitrova D, Nutt J, Horak FB. Abnormal force patterns for multidirectional postural responses in patients with Parkinson's disease. Exp Brain Res. 2004b;156:183–195. doi: 10.1007/s00221-003-1770-4. [DOI] [PubMed] [Google Scholar]
- Dite W, Temple VA. Development of a clinical measure of turning for older adults. Am J Phys Med Rehabil. 2002;81:857–866. doi: 10.1097/00002060-200211000-00010. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Studenski S, Chandler J, Prescott B. Functional reach: predictive validity in a sample of elderly male veterans. J. Gerontol. 1992;47:M93–M98. doi: 10.1093/geronj/47.3.m93. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson's disease rating scale. In: Fahn S, Marsden D, Calne D, editors. Recent Developments in Parkinson Diseases. Macmillan; London: 1987. pp. 153–163. [Google Scholar]
- Foster MA. The Central Nervous System. Macmillan; London: 1892. Text Book of Physiology, part III. [Google Scholar]
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS, Marsh L, Nail L, Shulman L, Taylor CB. Fatigue in Parkinson's disease: a review. Mov Disord. 2007;22:297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- Granit R. The Basis of Motor Contol. Academic Press; London and New York: 1970. [Google Scholar]
- Gregoric M, Takeya T, Baron JB, Bessineton JC. Influence of vibration of neck muscles on balance control in man. Agressologie. 1978;19:37–38. [PubMed] [Google Scholar]
- Gurfinkel V, Cacciatore TW, Cordo P, Horak F, Nutt J, Skoss R. Postural muscle tone in the body axis of healthy humans. J Neurophysiol. 2006;96:2678–2687. doi: 10.1152/jn.00406.2006. [DOI] [PubMed] [Google Scholar]
- Gurfinkel VS, Ivanenko YP, Levik YS. The influence of head rotation on human upright posture during balanced bilateral vibration. Neuroreport. 1995;7:137–140. [PubMed] [Google Scholar]
- Halmagyi GM, Gresty MA. Eye blink reflexes to sudden free falls: a clinical test of otolith function. Journal of neurology, neurosurgery, and psychiatry. 1983;46:844–847. doi: 10.1136/jnnp.46.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R, Tokuda T, Tako K, Yanagisawa N. Impaired modulation of tonic muscle activities and H-reflexes in the soleus muscle during standing in patients with Parkinson's disease. J Neurol Sci. 1997;153:61–67. doi: 10.1016/s0022-510x(97)00175-5. [DOI] [PubMed] [Google Scholar]
- Henry S, Fung J, Horak FB. Control of stance during lateral and anterior/ posterior surface translations. IEEE Trans Rehabil Eng. 1998;6:32–42. doi: 10.1109/86.662618. [DOI] [PubMed] [Google Scholar]
- Hicheur H, Vieilledent S, Berthoz A. Head motion in humans alternating between straight and curved walking path: combination of stabilizing and anticipatory orienting mechanisms. Neurosci Lett. 2005;383:87–92. doi: 10.1016/j.neulet.2005.03.046. [DOI] [PubMed] [Google Scholar]
- Hiraoka K, Matsuo Y, Abe K. Soleus H-reflex inhibition during gait initiation in Parkinson's disease. Mov Disord. 2005;20:858–864. doi: 10.1002/mds.20448. [DOI] [PubMed] [Google Scholar]
- Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson's disease. Experimental neurology. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Horak FB, Frank JS, Nutt J. Effects of dopamine on postural control in Parkinsonian subjects: scaling, set and tone. J Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. Journal of neurophysiology. 1986;55:1369–1381. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. Journal of the neurological sciences. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxham F, Baker R, Morris ME, Iansek R. Footstep adjustments used to turn during walking in Parkinson's disease. Mov Disord. 2008;23:817–823. doi: 10.1002/mds.21932. [DOI] [PubMed] [Google Scholar]
- Jarnlo GB, Nordell E. Reliability of the modified figure of eight-a balance performance test for elderly women. Physiother Theory Practice. 2003;19:35–43. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM, editors. Principles of neural science. 4th Edition McGraw-Hill; 2000. [Google Scholar]
- Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson's disease. Mov Disord. 2006;21:1213–1216. doi: 10.1002/mds.20948. [DOI] [PubMed] [Google Scholar]
- Khudados E, Cody FW, O'Boyle DJ. Proprioceptive regulation of voluntary ankle movements, demonstrated using muscle vibration, is impaired by Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:504–510. doi: 10.1136/jnnp.67.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni V, Chandy MJ, Babu KS. Quantitative study of muscle spindles in suboccipital muscles of human fetuses. Neuro India, 2001;44:355–359. [PubMed] [Google Scholar]
- Lakke JPWF. Axial apraxia in Parkinson's disease. J Neurol Sci. 1985;69:37–46. doi: 10.1016/0022-510x(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Experimental brain research. Experimentelle Hirnforschung. 2006;171:231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- Martin JP. The Basal Ganglia and Posture. Pitman Medical Publishing Co; London: 1967. [Google Scholar]
- Mesure S, Azulay JP, Pouget J, Amblard B. Strategies of segmental stabilization during gait in Parkinson's disease. Exp Brain Res. 1999;129:573–581. doi: 10.1007/s002210050927. [DOI] [PubMed] [Google Scholar]
- Meyer DL, Bullock TH. The hypothesis of sense-organ-dependent tonus mechanisms: history of a concept. Ann N Y Acad Sci. 1977;290:3–17. doi: 10.1111/j.1749-6632.1977.tb39712.x. [DOI] [PubMed] [Google Scholar]
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987;28:161–195. doi: 10.1016/0301-0082(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. 2001;81:810–818. doi: 10.1093/ptj/81.2.810. [DOI] [PubMed] [Google Scholar]
- Murray MP, Kory RC, Clarkson BH, Sepic SB. Comparison of free and fast speed walking patterns of normal men. Am J Phys Med. 1966;45:8–23. [PubMed] [Google Scholar]
- Nagumo K, Hirayama K. A study on truncal rigidity in parkinsonism--evaluation of diagnostic test and electrophysiological study. Rinsho Shinkeigaku. 1993;33:27–35. [PubMed] [Google Scholar]
- Nagumo K, Hirayama K. Axial (neck and trunk) rigidity in Parkinson's disease, striatonigral degeneration and progressive supranuclear palsy. Rinsho Shinkeigaku. 1996;36:1129–1135. [PubMed] [Google Scholar]
- Noren AM, Bogren U, Bolin J, Stenström C. Balance assessment in patients with peripheral arthritis: applicability and reliability of some clinical assessments. Physiother Res Int. 2001;6:193–204. doi: 10.1002/pri.228. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Hammerstad JP, Gancher ST. Parkinson's Disease. Mosby Year Book; St. Louis, MO: 1992. [Google Scholar]
- O'Suilleabhain P, Bullard J, Dewey R. Proprioception in Parkinson's disease is acutely depressed by dopamine medications. J Neurol, Neurosur, Psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette C, Paquet N, Fung J. Aging affects coordination of rapid head motions with trunk and pelvis movements during standing and walking. Gait & Posture. 2006;24:62–69. doi: 10.1016/j.gaitpost.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Parkinson J. An essey on the shaking palsy. Whittingham and Rowland; London: 1817. [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurol. 2006;67:1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Perlmutter SI, Iwamoto Y, Baker JF, Peterson BW. Interdependence of spatial properties and projection patterns of medial vestibulospinal tract neurons in the cat. Journal of neurophysiology. 1998;79:270–284. doi: 10.1152/jn.1998.79.1.270. [DOI] [PubMed] [Google Scholar]
- Peterson BW. Current approaches and future directions to understanding control of head movement. Progress in Brain Research. 2004;143:369–381. doi: 10.1016/s0079-6123(03)43035-5. [DOI] [PubMed] [Google Scholar]
- Pettersson AF, Engardt M, Wahlund LO. Activity level and balance in subjects with mild Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;13:213–216. doi: 10.1159/000057699. [DOI] [PubMed] [Google Scholar]
- Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. JAGS. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Abrahams VC. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol. 1979;42:604–617. doi: 10.1152/jn.1979.42.2.604. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Butler JT, Ray SK, Banik NL. Extranigral neurodegeneration in Parkinson's disease. Ann N Y Acad Sci. 2008;1139:331–336. doi: 10.1196/annals.1432.002. [DOI] [PubMed] [Google Scholar]
- Schenkman M, Clark K, Xie T, Kuchibhatla M, Shinberg M, Ray L. Spinal movement and performance of a standing reach task: participants with and withour Parkinson's disease. Phys Ther. 2001;81:1400–1141. doi: 10.1093/ptj/81.8.1400. [DOI] [PubMed] [Google Scholar]
- Schenkman M, Morey M, Kuchibhatla M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson's disease. J Gerontol A-Biol. 2000;55A:M441–M445. doi: 10.1093/gerona/55.8.m441. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. A mammalian spinal preparation. J Physiol. 1909;38:375–383. doi: 10.1113/jphysiol.1909.sp001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkuratova N, Morris ME, Huxham F. Effects of age on balance control during walking. Arch Phys Med Rehabil. 2004;85:582–588. doi: 10.1016/j.apmr.2003.06.021. [DOI] [PubMed] [Google Scholar]
- Stack EL, Ashburn AM. Impaired bed mobility and disordered sleep in Parkinson's disease. Mov Disord. 2006a;21:1340–1342. doi: 10.1002/mds.20944. [DOI] [PubMed] [Google Scholar]
- Stack EL, Ashburn AM, Jupp KE. Strategies used by people with Parkinson's disease who report difficulty turning. Parkinsonism Relat Dis. 2006b;12:87–92. doi: 10.1016/j.parkreldis.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified parkinson disease rating scale in people with parkinsonism. Phys Ther. 2008;88:733–746. doi: 10.2522/ptj.20070214. [DOI] [PubMed] [Google Scholar]
- Steiger MJ, Thompson PD, Marsden CD. Disordered axial movement in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1996;61:645–648. doi: 10.1136/jnnp.61.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes VP, Andersson C, Forssberg H. Rotational and translational movement features of the pelvis and thorax during adult human locomotion. J Biomec. 1989;22:43–50. doi: 10.1016/0021-9290(89)90183-8. [DOI] [PubMed] [Google Scholar]
- van de Warrenburg BP, Cordivari C, Ryan AM, Phadke R, Holton JL, Bhatia KP, Hanna MG, Quinn NP. The phenomenon of disproportionate antecollis in Parkinson's disease and multiple system atrophy. Mov Disord. 2007;22:2325–2331. doi: 10.1002/mds.21634. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Viallet F, Aurenty R, Assaiante C, Mesure S, Massion J. Axial rotation in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2006;77:815–821. doi: 10.1136/jnnp.2004.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Koch K, Garcia F, Angel RW. Axial versus distal motor impairment in Parkinson's disease. Neurol. 1988;38:540–545. doi: 10.1212/wnl.38.4.540. [DOI] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol. 2007;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Takama J, Hattori T. The dropped head sign in parkinsonism. Journal of the neurological sciences. 1999;167:22–25. doi: 10.1016/s0022-510x(99)00129-x. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wei G, Yan Z, Ding M, Li C, Ding H, Xu S. Quantitative assessment of Parkinson's disease deficits. Chin Med J (Engl) 1999;112:812–815. [PubMed] [Google Scholar]