Scientific abstract
Although widespread alterations in cortical structure have been documented in individuals with autism, the functional implications of these alterations remain to be determined. Here, we adopted a novel inter-subject (inter-SC) and intra-subject (intra-SC) correlation technique to quantify the reliability of the spatio-temporal responses of functional MR activity across the entire cortex in adults with autism during free-viewing of a popular audio-visual movie. Whereas these complex stimuli evoke highly reliable shared response time courses in typical individuals, cortical activity was more variable across individuals with autism (low inter-SC). Interestingly, when we measured the responses within an autistic individual across repeated presentations of the movie, we observed a unique, idiosyncratic response time course that was reliably replicated within each individual (high intra-SC). Encouragingly, after filtering out the idiosyncratic responses from each individual time course, we were able to uncover a more typical response profile, which resembles the shared responses seen in the typical subjects. These findings indicate that, under conditions approximating real-life situations, the neural activity of individuals with autism is characterized by individualistic responses that, although reliable within an autistic individual, are both highly variable across autistic individuals and different from the responses observed within the typical subjects. These idiosyncratic responses may underlie the atypical behaviors observed in autism. At the same time, we are encouraged by the presence of the more typical activation pattern lurking beneath these idiosyncratic fluctuations. Taken together, these findings may pave the way to future research aimed at characterizing the idiosyncratic response profiles, which, in turn, might contribute to a better understanding of the heterogeneity of the autism spectrum and its diagnosis.
Introduction
Autism is a neurodevelopmental disorder in which affected individuals exhibit atypical behaviors in social interaction and communication, and have restricted or stereotyped patterns of behaviors (Baron-Cohen & Belmonte, 2005; Behrmann, Thomas, & Humphreys, 2006; Frith & Happe, 2005). Consistent with the fact that multiple cognitive and affective behaviors are implicated in autism (Williams, Goldstein, & Minshew, 2006), neuroanatomical studies have reported widespread changes in cerebral grey and white matter in, among other regions, the amygdala, hippocampus, caudate nucleus and cerebellum (Amaral, Schumann, & Nordahl, 2008; Bachevalier & Loveland, 2006; Belmonte, et al., 2004; Herbert, 2005; Herbert, et al., 2004; McAlonan, et al., 2005). Additionally, recent neuroimaging studies have revealed disruptions in the structural and functional connectivity within and between cortical regions in individuals with autism during the execution of predefined tasks as well as during resting state conditions (Barnea-Goraly, et al., 2004; Hrdlicka, 2008; Kennedy, Redcay, & Courchesne, 2006; Kleinhans, et al., 2008; Vandenbroucke, Scholte, Engeland, Lamme, & Kemner, 2008). What remains unknown is how these neural disruptions manifest under real-life circumstances, and to what extent they have functional consequences for the individual's behavioral and cognitive profile.
To characterize the cortical response under conditions approximating real-life circumstances, we used fMRI to map the whole-brain activation profile in adults with autism during free-viewing of an engaging movie and we compared this profile to that evinced by typical participants. The carefully orchestrated audio-visual movie sequence is well-suited for driving reliable activation simultaneously in multiple brain areas (Hanson, Gagliardi, & Hanson, 2008; Hasson, Furman, Clark, Dudai, & Davachi, 2008; Hasson, Nir, Levy, Fuhrmann, & Malach, 2004; Hasson, Yang, Vallines, Heeger, & Rubin, 2008a; Jaaskelainen, et al., 2008; Wilson, Molnar-Szakacs, & Iacoboni, 2008). The data were analyzed by comparing the evoked fMRI response time courses across different subjects (inter-subject correlation, henceforth inter-SC; (Hasson, et al., 2004), and by comparing response time courses elicited by repeated presentations of the same stimulus within the same individual (intra-subject correlation, henceforth intra-SC; (Golland, et al., 2007). Computing the inter-SC within the typical individuals (typical-typical), on a voxel-by-voxel basis, quantifies the reliability of the response time courses in each brain area in the typical group. Using this analysis, we demonstrated that, across typical observers, approximately 30%–65% of the cerebrum evinces similar shared response time courses under free viewing of complex naturalistic stimuli (Hasson, Furman, et al., 2008; Hasson, et al., 2004; Hasson, Yang, Vallines, Heeger, & Rubin, 2008b). Moreover, these reliable responses, although widespread, are nonetheless selective (i.e., the response time courses differ from one brain area to another; (Hasson, Malach, & Heeger, submitted).
Here, we compare the cortical responses of individuals with autism against this typical response benchmark. Computing the inter-SC between the typical and the autism groups (typical-autism) provides a measure of similarity in the functional response in each brain area across the two groups. Low inter-SC between the typical-autism groups in conjunction with high inter-SC within the typical group would indicate that the response time course in a given brain area is markedly different in individuals with autism. Moreover, high inter-SC within the autism group (autism-autism) in conjunction with low inter-SC between the typical-autism groups can identify reliable response time courses, which are unique to the autism group and are not observed in the typical subjects. Finally, computing the intra-SC within each autistic individual across repeated presentations can reveal the unique set of response time courses, which, although possibly variable across the group, are nonetheless reliable within an individual. Critically, this type of functional analysis does not make any a priori assumptions about affected regions. Rather, the within- and between-groups inter-SC and within-individual intra-SC analyses permit an unbiased exploration of the entire cortex in the quest for differences in the patterns of brain responses between members of the two groups.
Methods
Subjects
Twelve adults with autism and eight typical subjects were scanned at the Brain Imaging Research Center in Pittsburgh. All subjects watched a 10-minute excerpt of a complex audio-visual movie sequence (see description below in the main movie experiment section); and an object localizer was used to define particular regions of interest (see description below in the object localizer experiment section). Eight of the subjects (4 from each group) watched the movie twice. To identify and localize a set of functional regions of interest (ROI) independently, an additional eight typical subjects were scanned at NYU's Center for Brain Imaging. All subjects had normal or corrected-to-normal vision and provided written informed consent. All protocols and procedures were approved by the University Committee on Activities Involving Human Subjects at the University of Pittsburgh, Carnegie Mellon University, and New York University. Data from three autism participants were removed from all analysis due to excessive head movements during the main movie watching experiment.
All participants with autism had Wechsler Full Scale and Verbal IQ scores of 80 or above (Autism mean VIQ=102, mean PIQ=105) and their diagnosis was confirmed on the Autism Diagnostic Revised Interview (Lord, Rutter, & Le Couteur, 1994), the Autism Diagnostic Observation Schedule (social mean=8; communication mean=4.8; (Lord, Cook, Leventhal, & Amaral, 2000) and by expert clinical diagnosis. Exclusion criteria for autism included associated disorders such as fragile-X syndrome or tuberous sclerosis, evidence of birth asphyxia, head injury, or seizure disorder, based on neurological history and examination and chromosomal analysis. The typical participants were community volunteers matched to the autism individuals in age, gender, and handedness as closely as possible. Although the IQ data were not available for all typical participants, no obvious link has been found between IQ and performance on non-speeded visual perceptual tasks in either typical (Deary, McCrimmon, & Bradshaw, 1997) or autism (Behrmann, et al., 2006) individuals, nor is there a statistically reliable IQ-fMRI relationship in autism (Kennedy & Courchesne, 2008).
MRI setup
Subjects were scanned in a 3T Siemens Allegra scanner equipped with a standard head coil (same scanner at Pittsburgh and NYU). Blood oxygenation level dependent (BOLD) contrast was acquired using gradient-echo echo-planner imaging (EPI) sequence (TE=35 msec, flip angle=90°, FOV=210×210mm2, matrix size 64×64). A TR of 3000ms was used for the main experiments. The scanned volume included 35 axial slices of 3mm thickness with no gap. High-resolution anatomical scans (T1-weighted 3D MPRAGE) were acquired for cortical segmentation, reconstruction, and volume-based statistical analysis (TE=3.49, flip angle=80°, FOV=256×256mm2, matrix size=256×256, slice thickness=1mm, number of slices=160–192, sagittal orientation).
Main movie experiment
We compared the patterns of brain activation of typical and autistic individuals across multiple cortical regions under maximally naturalistic conditions. To drive activity simultaneously in as many brain areas as possible, the participants viewed a 10-minute uninterrupted excerpt from the classic Western feature film, “The Good, the Bad and the Ugly,” directed by Sergio Leone (Hasson, et al., 2004); for a similar approach, see (Hanson, et al., 2008; Jaaskelainen, et al., 2008; Wilson, et al., 2008). The experiment started with a 30-second blank followed by 9 seconds of patterned stimuli, which were excluded from the analysis. To ensure that participants comprehended the movie sequence, following the movie, we administered a set of comprehension questions outside of the scanner. The questions were designed to determine whether the participants were able to track events or characters from the excerpt, for example, “Where does the movie take place?” (Wild West) and “What do the main characters look like?”, as well as questions about the main events that occurred such as “What happened prior to the main character being captured?”. All participants from both groups provided accurate answers to all questions.
Data analysis
Data analysis was performed using BrainVoyager QX (Brain Innovation, Maastricht, Netherlands) and complementary in-house software written in Matlab. For each subject, the cortical surface was reconstructed and flattened (see Figure 1). Preprocessing of functional scans included filtering out of low frequencies (e.g., slow drift), up to five cycles per experiment, and 3D-motion correction. The 3D algorithm adjusts for small head movements by rigid body transformations of all slices to the first reference volume (see note above regarding exclusion of three individuals with autism who showed excessive motion).
Figure 1. Inter-subject correlation.
The average inter-SC across all pair-wise comparisons (A) within the typical group and (B) within the autism group, (C) between the autism-typical groups. Correlation maps are shown on inflated (top) and unfolded (bottom) left and right hemispheres. Posterior areas (P) are toward the middle of each panel, while anterior areas (A) are facing the sides. The estimated face-, object-, and building-related borders (red, blue, and green rings, respectively) are superimposed on the cortical map. Black dotted lines denote estimated borders of retinotopic visual areas V1, V2, V3, VP, V3A, V4/V8 obtained from a representative subject.
Mapping inter-subject correlation
To measure the reliability of the response time courses between corresponding regions across subjects (inter-subject correlation, inter-SC) and within a subject (intra-subject correlation, intra-SC), we first transformed all brains into the Talairach coordinate system (Talairach & Tournoux, 1988), and applied a Gaussian filter of 8mm full width at half maximum value (FWHM) to the data. To remove pre-processing artifacts, we excluded from the analysis the first and last 10 time points of the experiment. The inter-SC and intra-SC were computed between the entire response time courses in each pair of subjects/or within a subject across repeated presentations of the movie on a voxel-by-voxel basis. In a separate analysis, we computed the inter-SC and intra-SC for the average time courses within each predefined ROI. We computed the inter-SC and the intra-SC separately within the typical group (typical-typical) and within the autistic group (autism-autism). We also computed the inter-SC between the groups (typical-autism). We then calculated the average correlation coefficient (r) per voxel or per ROI, after applying the Fisher transformation to the individual coefficients. To ensure that these mean correlation values were not biased by outliers, we performed a second order t-test analysis on the pair-wise values within each comparison to confirm that the mean was significantly different from zero.
To correct for the multiple comparisons in the inter-SC and intra-SC analyses, we estimated the arbitrary correlation values that might arise in such a complex data set across the entire volume (all voxels), when taking two unrelated time courses. This was achieved by flipping the time course of each subject and calculating the correlations between the forward and flipped time courses (using same procedure as outlined above). Because the forward and reversed time courses are not aligned, the correlation between them should be low and, as expected, this was the case. The highest value exhibited by any voxel in this analysis served as a conservative statistical criterion for thresholding the inter-SC and intra-SC maps (r>0.14).
Object localizer
To assess further the reliability of responses within each group, we analyzed the time courses in several regions of interest (ROIs). Regions of interests (ROIs) were defined using independent fMRI measurements of cortical activity evoked by select object categories. This experiment was composed of 32 epochs (15 seconds long) of movie clips, divided into four categories: shots of faces under various natural situations (e.g., walking in the street), navigation of the camera through a building area, navigation of the camera through open fields, and movie clips of miscellaneous images from various object categories (machines, cars); (for additional information, see (Hasson, et al., 2004). This task has been used successfully to evaluate cortical activity in autism (Humphreys, Hasson, Avidan, Minshew, & Behrmann, 2008).
Auditory localizer
A five-minute soundtrack taken from an audio book (“Alice's Adventures in Wonderland”) was played to the subjects. The soundtrack was muted every 10 seconds for 6 seconds to create block-like alterations with 17 non-continuous 10-second segments of the story soundtrack followed by 6 seconds of silence.
Selection of regions of interest (ROI)
Consistent with other studies (Hadjikhani, et al., 2004; Humphreys, et al., 2008; Pierce, Muller, Ambroses, Allen, & Courchesne, 2001), the cortical activation pattern for different object categories (faces, buildings, and common objects) in the autism individuals was highly irregular, relative to the typical pattern (Hasson, Harel, Levy, & Malach, 2003), with hyperactivation for the objects and hypoactivation for the face stimuli (also see (Humphreys, et al., 2008; Schultz, et al., 2000). Given this irregularity, identifying a functionally defined face- and object-related ROI for each individual with autism could not be done reliably at a corrected threshold. Although we were able to identify consistent ROIs in all typical subjects, the use of these ROIs would bias the analysis toward the typical group. We therefore used the average ROIs, obtained from a separate and independent group of 8 typical subjects, and applied their coordinates to derive ROIs in each of the current participants. This ensures that the activation profile measured from voxels within each ROI is not biased towards the typical group and ensures that the strong inter-SC within the typical group is not a function of the ROI selection per se. To confirm further that the reliance on an independent typical group for selecting these ROIs did not bias the responses in favor of the typical subjects, we also defined the same ROIs based on the responses obtained in our autistic group for the same stimuli. Given the inherent variability in the autism group, we used an uncorrected fixed effect analysis for this procedure. This procedure biased the responses toward the autism group, but, importantly, resulted in similar results, thereby confirming the outcome of the previous analysis (see Supplementary Figure 1).
Eye movement analysis
To ensure that any group differences did not result from differential viewing of the movie and to confirm that participants were indeed watching the movie at all times, we monitored their eye movement trajectories in the scanner, using an infrared video camera equipped with a custom-built MRI telephoto lens (Applied Sciences Laboratories, Model 504LRO; http://www.a-s-l.com). The camera was focused on the right eye through the same mirror with which participants viewed the visual display. For 8 runs (4 from each group), we also measured the precise eye gaze pattern during the fMRI sessions, using the same camera and sampling x, y eye position at 60Hz. Nine points on the screen were used to calibrate the coordinates of the eye tracker at the beginning and end of each run. Eye traces were median-filtered, normalized, and converted to video frame coordinates. A cross correlation index was calculated independently for x and y.
Power spectrum
As a means of ensuring that the differences in BOLD response between the autism and typical individuals were not simply due to a decrease in the overall response amplitude in the autism individuals, we measured the power spectral density in each subject. Tests comparing power spectrum were conducted on time courses sampled from the average time course of all voxels correlated to the ventral occipital temporal lobe (VOT). Power spectrum was defined as the base-10 logarithm of the squared absolute value of the Fast Fourier Transform components. Significant differences in the power spectrum were defined by a paired t-test (p<0.05) separately for each frequency range. This analysis was used to demonstrate that observed differences in correlation values across groups were not a result of a differential decrease in the response amplitudes in any frequency band.
Results
Increased variability in response time courses to natural stimuli within the autism group
To characterize the shared response time courses within the typical group, we used functional magnetic resonance imaging (fMRI) to measure cortical activity while observers viewed complex, naturalistic stimuli (a segment from an engaging commercial film). After normalizing all brains to the Talairach coordinate system, we calculated the inter-SC across the entire movie sequence within the typical group on a voxel-by-voxel basis (see Methods). This was done separately for every voxel. High inter-SC within the typical group indicates that response time courses in a particular brain region are similar, hence shared, across subjects. Low inter-SC reliability is interpreted as a different temporal evolution of neuronal events across the two groups.
Despite the seemingly uncontrolled (free viewing) task and complex nature of the stimuli, and, consistent with our previous findings, the movie evoked highly reliable brain activity in many brain areas within the typical subjects (Figure 1A; typical subjects, n=8 subjects, see Supplementary Figure 2A for the inter-SC maps of each typical individual). Compared to the typical group (Figure 1A), the response time courses were highly variable within the autistic group (Figure 1B; autistic subjects, n=9 subjects; see Supplementary Figure 2B for the inter-SC maps of each autistic individual) with some inter-SC in early visual and auditory areas (Fig. 1B). Importantly, there were no regions that exhibited shared responses (high inter-SC) within the autistic individuals alone but not within the typical individuals. Finally, as in the autism-autism comparison, the analysis of the inter-SC between the autistic (n=9) and typical subjects (n=8) also reveals robust and widespread disruption of the inter-subject correlation of the BOLD signal (Fig. 1C) with, again, somewhat better correlation in primary sensory regions (auditory and visual cortices) and with systematic decrement as one proceeds to higher-order areas (see also Figure 2D).
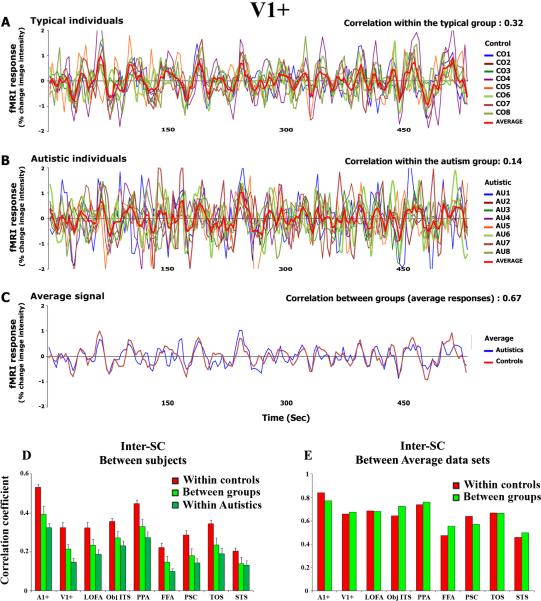
Figure 2. Signal fluctuations within regions of interest.
Visual cortex (V1+) response time courses for (A) each typical subject and (B) each participant with autism. (C) The average signal for the typical (red line) and autism (blue line) group. (D) The mean inter-SC values for the within-typical group (typical-typical, red bars), within-autism group (autism-autism, light green bars), and between the two groups (autism-typical, green bars) for selected ROIs. ROI abbreviations: A1+, primary and secondary auditory cortices; V1+, primary and secondary visual cortices; LOFA, lateral occipital cortex responsive to pictures of faces; Obj-ITS, object-related area in the inferior temporal sulcus; PPA, parahippocampal place area; FFA, fusiform face area; PCS, posterior central sulcus responsive to pictures of objects; TOS, transverse occipital sulcus responsive to pictures of places; STS-Face, area in superior temporal sulcus responsive to pictures of faces. (E) The inter-SC between the average autism-typical time courses (green bars) and the typical-typical time courses (red bars) in each ROI (same abbreviations as in D). Note the extent of variability in signal fluctuation in the autism individuals relative to the typical subjects. Moreover, note that by averaging the time courses within a group the responses become highly correlated across groups.
To assess further the reliability of responses within each group, we analyzed the time courses in several regions of interest (ROIs), functionally defined based on the activation patterns of 8 subjects whose data were not included in subsequent analyses (see Methods). Figure 2A shows the activation profile sampled from the vicinity of the calcarine sulcus, which include the primary visual cortex and nearby early visual areas (termed area V1+ in the paper) for each individual in each group, plotted across the entire movie sequence. This area was chosen as an illustrative example of the findings; similar results were obtained in the other independently defined ROIs (see Figure 2D). As is evident, the response time courses in area V1+ were reliable across all typical subjects (inter-SC: 0.32±0.01), whereas the time courses from the same area in the individuals with autism, although highly fluctuating (rather than remaining unchanged, see also spectral analysis below), show much greater variability across subjects (inter-SC: 0.13±0.02; see Figure 2B).
The reduction in the reliability of the response time courses in individuals with autism held across large regions of posterior cortex. Figure 2D presents the average inter-SC values for the within- and between-group comparisons across the preselected ROIs. The high correlation values within the group of typical subjects (typical-typical: red) replicate our previous findings (Hasson, et al., 2004) and, additionally, validate the use of the externally defined unbiased ROIs. In all ROIs (including early and higher-order regions), the autism-autism analysis (dark green) and the autism-typical analysis (light green) showed a substantially reduced correlation of about 40–50%, relative to the typical subjects' inter-SC values. Although the overall inter-SC values are higher in primary sensory cortices, the reduction in the reliability of responses was evident in all regions. Also, as mentioned above, no region exhibited a high correlation within the autistic individuals but not within the typical individuals. To ensure further that our procedure for selecting the ROIs did not bias the responses in favor of the typical subjects, we also defined the same set of ROIs based on the autism group (see Methods). Although, such a selection naturally biases the responses toward the autism group, similar results were obtained for this analysis as for the independent-ROI definition analysis (see Supplementary Figure 1). In sum, the reduction in inter-SC observed within the individuals with autism appears to be driven by signal fluctuations (Figure 2B), which deviate from the typical response time courses observed in typical subjects (Figure 2A), and diminish the reliability of responses across individuals with autism (Figure 2D).
The variable responses in autistic individuals are partially attributable to idiosyncratic responses
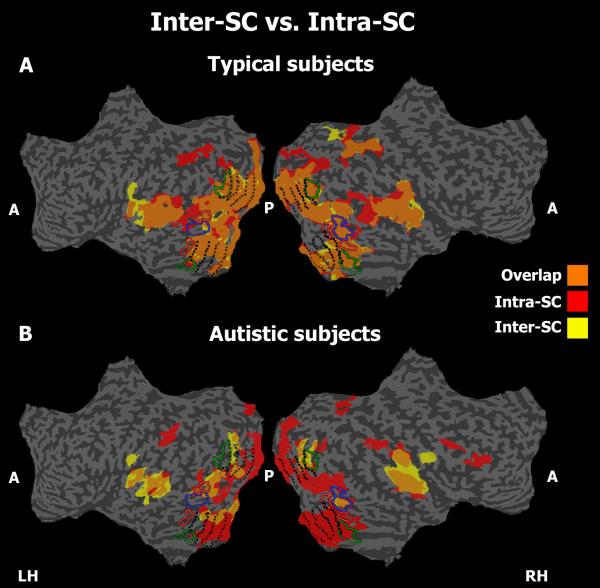
To evaluate whether the variable fluctuations in the autism group result from a consistent but unique viewing approach employed by each individual with autism, we calculated the intra (within)-SC and inter (between)-SC across repeated presentations of the movie on a voxel-by-voxel basis in eight individuals, four from each group (see Figure 3). High intra-SC implies that the responses to the movie stimuli are reliable within an individual, and high inter-SC adds information regarding the similarity of responses across all members of the group. In the typical subjects, we observed a high degree of overlap between the intra-SC and inter-SC maps (Figure 3A; orange), indicating that most response time courses are similar both within and between all individuals. In contrast, in the autism individuals, we detected a unique idiosyncratic signal that was apparently stable and reliable within a given individual across repeated presentations (high intra-SC; red), but was not similar across autistic individuals (low inter-SC; notice the absence of orange in the display). Thus, the increased intra-SC suggests that at least some of the variability in the autism group might be attributed to the idiosyncratic response time courses, which are uniquely and differentially characteristic of each individual.
Figure 3. Inter (between)- and intra (within)-subject correlation for both typical and autism groups.
The average intra-SC and inter-SC across all pair-wise comparisons within the (A) typical group and (B) autism group. Similar abbreviations as in Figure 1. The intra-SC was computed first within each subject across repeated presentations of the movie and then averaged separately for the subjects in the typical (n=4) and autism group (n=4). For comparison, we plot the inter-SC computed for the same subjects (similar to the procedure above, see Figure 1). Note that in the typical subjects the signal was reliable within and across subjects (overlap between the inter-SC and intra-SC; orange color), in the autism group, we detected reliable responses within each autistic individual (high intra-SC; red color) even in areas that fail to show reliable responses across group members (low inter-SC).
The idiosyncratic signal fluctuations do not abolish the processing of the movie and the overall activation level is not altered in autism
One possible explanation for the reduction in inter-SC in autism may be that the individuals with autism were distracted and did not consistently attend to the movie. However, the eye movement patterns (acquired via a built-in video camera in the scanner, see Method) assured us that each individual was indeed looking at the movie. Moreover, finding high intra-SC within each autistic individual (Figure 3B) implies that the variability in response time courses across the autistic individuals (Figures 1B, C; 2B, D) is not attributable to some erratic process, but, rather, emerges from replicable processes within an individual. The reduction in the inter-SC is also not attributable to decreased activation in the autism group; analyses of the variance and spectral content of the time courses did not reveal any significant differences between the two groups at any frequency (see Methods and time courses in Figure 2B).
The idiosyncratic signal fluctuations mask the underlying stimulus-evoked activity
Despite the variable signal fluctuations, a post-scan questionnaire revealed that all autistic individuals achieved at least a reasonable understanding of the movie plot. This finding prompted us to search for a more typical response time course embedded within the variable signal fluctuations, which might mediate the apparent comprehension of the movie plot. When the cortical responses are averaged across the individuals of the autism group (rather than considered individual-by-individual), the average response sampled from V1+ begins to be better correlated with the average response of the typical subjects (Figure 2C). Thus, the average time course of the autism group (red curve) and that of the typical group (blue curve) yields a correlation of 0.67, noticeably higher than the average inter-SC between the pairwise typical subjects (correlation of 0.32). A similar increment in the between-groups correlations values was apparent in each ROI (Figure 2E, green bars). To assess the impact of averaging the data on the typical group, we calculated the correlation between the average signals obtained from splitting the typical group into two subgroups (n=4 in each group), averaging the signal within each ROI, and then computed the correlation between the two average time courses (Figure 2E, red bars). As can be seen, aggregating the signal across all autistic individuals averaged out some of the uncorrelated and idiosyncratic fluctuations, and revealed a more typical response time course in all cortical regions that is similar to the signal seen in the typical subjects (Figure 2C, E).
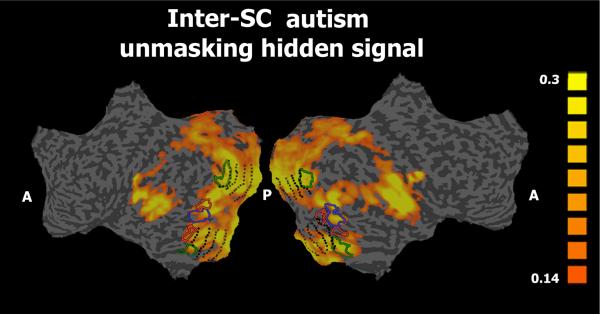
Finally, to unmask the typical activation patterns within each autistic individual on a voxel-by-voxel basis, we used the average spatio-temporal activation profile of all typical subjects as a predictor for the activity pattern in each autistic brain. Correlating the autistic individuals' response time courses with the average typical response time courses enabled us to recover more typical response time courses in each individual with autism (Figure 4 presents the average mean inter-SC map across all autistic individuals, Supplementary Figure 2C presents the inter-SC maps for each autistic individual). This analysis revealed typical response time courses in each autistic individual across all regions that exhibited high inter-SC in typical subjects (compare with Figure 1A). Thus, beneath the variable and uncorrelated internal signal fluctuations (as seen in Figures 2B and 2D), we uncovered more standard responses in many brain regions in the autism individuals (Figures 2E, 4 and Supplementary Figure 2C).
Figure 4. Unmasking the hidden signal in the autism group.
The mean inter-SC between each individual with autism and the average spatio-temporal response time courses of the typical subjects. Similar abbreviations as in Figure 1. Note that by correlating the response time courses of the participants with autism with the average typical response time courses, we succeeded in recovering a more typical signal from the autism individuals.
Ruling out alternative explanations for fluctuations in autism
The idiosyncratic cortical fluctuations found within each autistic individual (high intra-SC, Figure 3B) can not be attributable to idiosyncratic repeatable head movement artifact within each individual (recall that three individuals with head motion were already excluded from the analysis). To detect and correct for head motion in the current individuals, we applied a correction algorithm and calculated the magnitude of the head movements as the square root sum of all x, y, z rotations and translations in space (mm). No observable head motion patterns were found between the derivative of the head motion across repeated presentations of the movie within autistic individuals (correlations 0.035±0.02).
It is also not the case that the low inter-SC in the autism group emerges from the autism individuals' failure to watch the display. The eye movement patterns (acquired via a built-in video camera in the scanner) assured us that each individual was looking at the movie (see above). It is the case, however, that unusual eye movement patterns are not uncommon in autism (Klin, Jones, Schultz, Volkmar, & Cohen, 2002b; Pelphrey, Morris, & McCarthy, 2005; Ristic, et al., 2005) and may lead to reduced activation in, for example, the Fusiform Face Area (FFA; (Dalton, et al., 2005; Morris, Pelphrey, & McCarthy, 2007). To monitor individuals' eye movement trajectories, we recorded the eye movements of four typical and four autism subjects. As evident in Figure 5, eye movement patterns were similar across the two presentations of the movie for the typical (x-axis r=0.28±0.02; y-axis r=0.27±0.03) but not for the autistic individuals (x-axis r=0.09±0.09; y-axis r=0.11±0.02), replicating the reports of irregular eye movement trajectories in individuals with autism (Cody, Pelphrey, & Piven, 2002). The differences across the groups in eye movements, however, do not offer a complete explanation of the current findings, as we see typical mean response time courses (after removing the idiosyncratic responses) within autistic individuals (see Figures 2C, 4) even in the absence of reliable eye movement trajectories across repeated presentations. Thus, the variability in eye movements within the autism group is not sufficient for abolishing the typical responses within each autistic individual. It will be interesting to explore whether the eye movements within an individual with autism are reliable across repeated presentations, hence contributing to the increase in intra-SC observed in the autistic individuals.
Figure 5. Intrinsic fluctuations cannot be attributed to differences in eye movements.
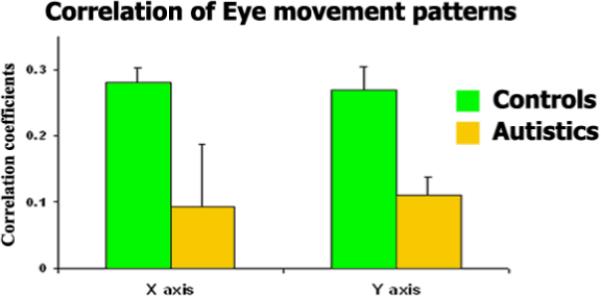
Correlation of the eye movement pattern (computed separately for the x-axis and y-axis) within the typical and autism groups during the movie watching. The decreased correlation in the eye movement patterns within the individuals with autism points to an irregular eye movement pattern across repeated presentations of the movie.
Discussion
Autism is a profound disorder of brain development and, despite the rigorous scientific attention paid to it, many fundamental questions remain unanswered. Classically, the study of autism has focused on specific aspects such as the atypicalities in social cognition, theory of mind, joint attention, language, and emotional regulation, and the alterations in those brain regions that underlie these behaviors (Behrmann, et al., 2006; Dakin & Frith, 2005; Klin, Jones, Schultz, Volkmar, & Cohen, 2002a; Luna, et al., 2002; Peterson, Wellman, & Liu, 2005). Here, using naturalistic, rich sensory stimulation, and an assumption-free inter-SC and intra-SC analysis, we explore the whole-brain activation profile in high-functioning adults with autism, and demonstrate that the functional response time courses in autism during natural viewing are markedly atypical across many brain regions (Figure 1B). In particular, the expected cortical response profile is masked by idiosyncratic alterations in the response time courses in areas ranging from primary sensory cortices all the way through to high-level association areas (Figure 3B).
Specifically, we document three major findings. First, relative to the well-defined and predictable patterns of activity in typical subjects, the cortical activation patterns in individuals with autism were highly disrupted in multiple cortical areas, including both primary sensory areas and higher-order areas (Figures 1B–C and 2D). This suggests that autism is associated with a broad neuronal dysfunction, affecting multiple, disparate cortical areas. Second, within each individual with autism, retested under identical circumstances, the intra-SC was higher than the inter-SC (Figure 3B). This suggests that at least part of the variability observed in each autistic individual might emerge from a consistent but idiosyncratic activation pattern in response to the sensory input (Rogers, Hepburn, Stackhouse, & Wehner, 2003). Finally, given that we were able to uncover typical response time courses in each autistic individual (Figure 4 and Supplementary Figure 2), the idiosyncratic fluctuations appear to interfere with, but not entirely abolish, the neuronal processing of the movie. Indeed, all of the individuals with autism achieved at least some understanding of the movie's plot, as revealed by the post-scan questionnaire, indicating that the fluctuations observed in each autistic individual did not eliminate their ability to comprehend the external input. At the same time, the breakdown in the inter-SC observed within the individuals with autism (Figure 1B–C) attests to the presence of idiosyncratic signal fluctuations even while the individuals are actively watching the movie.
These idiosyncratic response time courses are not attributable to irregular head movements, or to the irregular eye movement patterns observed in individuals with autism across repeated presentations of the movie, or to group differences in signal amplitude. Finally, the finding that each individual with autism was able to follow the movie's plot, together with the fact that we were able to uncover a typical signal in the autism group and the test-retest replicability, argue against the likelihood that the idiosyncratic response time courses are simply induced by a lack of attention to the movie.
What is the source of the idiosyncratic response time courses observed in each autistic individual? First, the responses might be related to a set of individualized strategies employed by each autism subject in response to the rich sensory input. These strategies can vary from individual to individual, but still be highly reliable within an autistic individual. Thus, it could be that the increase in reliable responses within each autistic individual (increased intra-SC within the autism group; Figure 3B) uncovers the singularity of the neuronal responses in each autistic individual. These individual profiles may serve as the neural correlate for the extensive heterogeneity in behavioral symptoms (social behavior, communication abilities, and restricted, repetitive or stereotyped patterns of behavior) expressed, not just across the autism spectrum, but even within individuals falling at the same point along the continuum. Note that, in contrast to the substantial inter-individual variability observed in individuals who are diagnosed with autism that is co-morbid with attention deficit disorder (Geurts, et al., 2008), we see relatively stable, repeatable patterns within, although not between, individuals. Further studies are needed in order to identify the ramifications of these individual profiles and to assess whether it is indeed the case that the behavioral heterogeneity is mediated by the differing individual neural patterns. It is, of course, also crucial to understand how the observed variability across autistic individuals gives rise to the well-known triad of symptoms common in autism.
Another possibility, albeit not mutually exclusive, is that these idiosyncratic responses are related to more generalized physiological and/or structural alternations in the autistic's brain. For example, the idiosyncratic activity in autism may result from disproportionately higher levels of excitation and/or lower levels of inhibition in the individuals (Polleux & Lauder, 2004; Rubenstein & Merzenich, 2003), resulting in hyperexcitable cortex. This would be consistent with reports of high levels of noisy spike activity on EEG/MEG (Daoust, Limoges, Bolduc, Mottron, & Godbout, 2004; Hurley, Lewine, Jones, Orrison, & Taber, 2000) and increased seizure incidence in autism (Hughes & Melyn, 2005; Tuchman, 2000). However, it is important to note that fMRI provides only an indirect measure of the neuronal responses (Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001; Mukamel, et al., 2005), and thus we cannot directly assess this hypothesis with the current data set. Moreover, the idiosyncratic response time courses may be due to an excess of white matter tracts in autism, relative to matched controls (Courchesne, et al., 2001; Freitag, et al., 2009; Hendry, et al., 2005; Herbert, 2005; Herbert, et al., 2004). Regardless of the source of the variability across individuals with autism, whether it be physiological or structural, our results indicate that these irregularities are expressed in a lawful manner within each individual under real-life viewing conditions. In other words, the idiosyncratic responses are reliably induced (i.e., time locked) to the external stimuli with each autistic individual, and cannot be attributed to an internally induced erratic and unstable set of responses.
Finally, our findings of idiosyncratic response time courses may be linked with the claim that individuals with autism experience sensory overload (for example, (Crane, Goddard, & Pring, 2009). These results are also consistent with the recent “intense world syndrome” model of autism (Markram, Rinaldi, & Markram, 2007), in which the excessive neuronal processing in circumscribed circuits in individuals with autism leads to hyper-reactivity, which interferes with the processing of the incoming information, which, in turn, leads to social and environmental withdrawal. On this account, in response to this excessive neuronal activation, the neural system is thought to “lock down” the individual to a small repertoire of idiosyncratic behaviors, which are repeated with high frequency. In our data, we observed strong idiosyncratic fluctuations (see Figure 2B) and these may be associated (either as cause or effect) with the hypersensitivity and hyperfunctionality observed in autistic individuals.
Conclusion
In sum, we have exploited a novel inter-correlation approach, which has enabled us to document both typical and atypical aspects of the cortical activation patterns in high functioning adults with autism. Our study exposes the extensive presence of idiosyncratic response time courses in the individuals with autism during the processing of complex, dynamic external stimuli. Any full theoretical account of autism will need to be able to explain both the common aspects of the response as well as the apparent idiosyncratic, repeatable within-subject neural profile. We note that this analytic approach has much potential to elucidate the neural dynamics that are activated in naturalistic conditions in individuals with autism, as reflected by its ability to distinguish clearly between individuals with autism and their typical counterparts. Taken together, these findings may pave the way to future research focusing on identifying and characterizing the underline sources of such idiosyncratic fluctuations, which may also help developing diagnostic tools for autism in at-risk populations.
Supplementary Material
Acknowledgements
Special thanks to Rafael Malach for his valuable input to this project. We thank David Heeger, Nava Rubin, Ifat Levy, Kate Humphreys, Cibu Thomas, and Yoram Bonneh for fruitful discussions and comments on the manuscript. Funding was provided by an International Human Frontier Science Program Organization long-term fellowship (U. H.) and NICHD/NIDCD PO1/U19 (M. B. and N. M.), which is part of the NICHD/NIDCD Collaborative Programs for Excellence in Autism. We thank Grace Lee Leonard, Lauren Lorenzi, and Stacy Cho for assistance in data collection and the individuals at the Collaborative Program for Excellence in Autism research at the University of Pittsburgh for their help in recruiting, scheduling, and testing subjects.
Footnotes
The authors declare no competing financial interests.
References
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008 doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends Cogn Sci. 2006;10(6):258–264. doi: 10.1016/j.tics.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Jr., Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy(1) Mol Psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Cody H, Pelphrey K, Piven J. Structural and functional magnetic resonance imaging of autism. Int J Dev Neurosci. 2002;20(3–5):421–438. doi: 10.1016/s0736-5748(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Sensory processing in adults with autism spectrum disorders. Autism. 2009;13(3):215–228. doi: 10.1177/1362361309103794. [DOI] [PubMed] [Google Scholar]
- Dakin S, Frith U. Vagaries of visual perception in autism. Neuron. 2005;48(3):497–507. doi: 10.1016/j.neuron.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust AM, Limoges E, Bolduc C, Mottron L, Godbout R. EEG spectral analysis of wakefulness and REM sleep in high functioning autistic spectrum disorders. Clin Neurophysiol. 2004;115(6):1368–1373. doi: 10.1016/j.clinph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Deary IJ, McCrimmon RJ, Bradshaw J. Visual information processing and intelligence. Intelligence. 1997;24(3):461–479. [Google Scholar]
- Freitag CM, Luders E, Hulst HE, Narr KL, Thompson PM, Toga AW, et al. Total Brain Volume and Corpus Callosum Size in Medication-Naive Adolescents and Young Adults with Autism Spectrum Disorder. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Happe F. Autism spectrum disorder. Curr Biol. 2005;15(19):R786–790. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RP, Verte S, Oosterlaan J, Roeyers H, van Kammen SM, et al. Intra-individual variability in ADHD, autism spectrum disorders and Tourette's syndrome. Neuropsychologia. 2008;46(13):3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, et al. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17(4):766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Chabris CF, Joseph RM, Clark J, McGrath L, Aharon I, et al. Early visual cortex organization in autism: an fMRI study. Neuroreport. 2004;15(2):267–270. doi: 10.1097/00001756-200402090-00011. [DOI] [PubMed] [Google Scholar]
- Hanson SJ, Gagliardi AD, Hanson C. Solving the brain synchrony eigenvalue problem: conservation of temporal dynamics (fMRI) over subjects doing the same task. J Comput Neurosci. 2008 doi: 10.1007/s10827-008-0129-z. [DOI] [PubMed] [Google Scholar]
- Hasson U, Furman O, Clark D, Dudai Y, Davachi L. Enhanced intersubject correlations during movie viewing correlate with successful episodic encoding. Neuron. 2008;57(3):452–462. doi: 10.1016/j.neuron.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37(6):1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Hasson U, Malach R, Heeger D. Reliability of cortical activity during natural stimulation. Trends Cogn Sci. doi: 10.1016/j.tics.2009.10.011. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A hierarchy of temporal receptive windows in human cortex. J Neurosci. 2008a;28(10):2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Yang E, Vallines I, Heeger DJ, Rubin N. A Hierarchy of Temporal Receptive Windows in Human Cortex. J Neurosci. 2008b;28(10):2539–2550. doi: 10.1523/JNEUROSCI.5487-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry J, Devito T, Gelman N, Densmore M, Rajakumar N, Pavlosky W, et al. White matter abnormalities in autism detected through transverse relaxation time imaging. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005;11(5):417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55(4):530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Hrdlicka M. Structural neuroimaging in autism. Review. Neuro Endocrinol Lett. 2008;29(3):281–286. [PubMed] [Google Scholar]
- Hughes JR, Melyn M. EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin EEG Neurosci. 2005;36(1):15–20. doi: 10.1177/155005940503600105. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Functional mapping of category-related object areas in high-functioning adults with autism. Autism Research. 2008;1 doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RA, Lewine JD, Jones GM, Orrison WW, Jr., Taber KH. Application of magnetoencephalography to the study of autism. J Neuropsychiatry Clin Neurosci. 2000;12(1):1–3. doi: 10.1176/jnp.12.1.1. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen PI, Koskentalo K, Balk HM, Autti T, Kauramaki J, Pomren C, et al. Inter-Subject Synchronization of Prefrontal Cortex Hemodynamic Activity During Natural Viewing. The Open Neuroimaging Journal. 2008;2(1):14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci U S A. 2006;103(21):8275–8280. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, et al. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(Pt 4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Defining and quantifying the social phenotype in autism. American Journal of Psychiatry. 2002a;159:895–908. doi: 10.1176/appi.ajp.159.6.895. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002b;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28(2):355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luna B, Minshew NJ, Garver KE, Lazar NA, Thulborn KR, Eddy WF, et al. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59(6):834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, Markram K. The intense world syndrome – an alternative hypothesis for autism. Frontiers in Neuroscience. 2007;1(1):77–96. doi: 10.3389/neuro.01.1.1.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128(Pt 2):268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Controlled scanpath variation alters fusiform face activation. Soc Cogn Affect Neurosci. 2007;2(1):31–38. doi: 10.1093/scan/nsl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309(5736):951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128(Pt 5):1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Peterson CC, Wellman HM, Liu D. Steps in theory-of-mind development for children with deafness or autism. Child Dev. 2005;76(2):502–517. doi: 10.1111/j.1467-8624.2005.00859.x. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambroses J, Allen G, Courchesne E. Face processing occurs outside the fusiform `face area' in autism: evidence from fMRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Brain Res Cogn Brain Res. 2005;24(3):715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tuchman R. Treatment of seizure disorders and EEG abnormalities in children with autism spectrum disorders. J Autism Dev Disord. 2000;30(5):485–489. doi: 10.1023/a:1005572128200. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MW, Scholte HS, Engeland HV, Lamme VA, Kemner C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain. 2008 doi: 10.1093/brain/awm321. [DOI] [PubMed] [Google Scholar]
- Williams DL, Goldstein G, Minshew NJ. Neuropsychologic functioning in children with autism: further evidence for disordered complex information-processing. Child Neuropsychol. 2006;12(4–5):279–298. doi: 10.1080/09297040600681190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Molnar-Szakacs I, Iacoboni M. Beyond superior temporal cortex: intersubject correlations in narrative speech comprehension. Cereb Cortex. 2008;18(1):230–242. doi: 10.1093/cercor/bhm049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.