Abstract
The transcriptional repressor Bcl6 is a male-specific rat liver gene product and one of 24 early GH-response genes encoding DNA-binding proteins. Presently, the sex specificity of Bcl6 was shown to emerge at puberty, when hepatic Bcl6 mRNA was induced in males and repressed in females by the female plasma GH profile. Hepatic Bcl6 mRNA was increased to near-normal male levels in hypophysectomized females and was extinguished in intact males given a continuous GH infusion (female-like GH pattern). Bcl6 was also repressed in adult male somatostatin-deficient mice, where plasma GH profiles are female like. Hepatic Bcl6 RNA was rapidly down-regulated by GH pulse treatment, both in hypophysectomized male rats and in primary rat hepatocytes. Bcl6 was substantially induced in female mice deficient in hepatic signal transducer and activator of transcription (STAT)5a/STAT5b, suggesting that these STAT transcriptional mediators of GH signaling repress Bcl6. Indeed, STAT5 was bound to Bcl6 STAT5-binding region-B, previously associated with Bcl6 repression, in both male and female liver chromatin. STAT5 also bound to Bcl6 region-A in male chromatin but only during a plasma GH pulse. Analysis of primary transcripts (heterogenous nuclear RNA) across the Bcl6 gene revealed a novel mechanism of GH-dependent sex specificity, with two apparent blocks in Bcl6 transcription elongation seen in female liver and in continuous GH-treated male liver, one early in intron 4 and one in exon 5, which together reduced transcription beyond exon 5 more than 300-fold. Finally, Bcl6 was bound to a subset of STAT5-binding sites in male liver chromatin, including a Socs2 STAT5-binding site where Bcl6 binding increased substantially between plasma GH pulses, i.e. when STAT5 binding was low. Bcl6 and STAT5 binding are thus inversely coordinated by the endogenous pulses of pituitary GH release, suggesting this male-specific transcriptional repressor modulates hepatic GH signaling to select STAT5 target genes.
Female-specific plasma GH profile blocks transcriptional elongation of male-specific rat hepatic Bcl6. Male plasma GH pulses inversely coordinate binding of Bcl6 and STAT5 to Socs2.
The liver is a sexually dimorphic organ, with sex differences reported for the expression of more than 1000 genes in both rats and mice (1,2,3). These sex differences are dictated by the distinct temporal profiles of pituitary GH release, which impart distinct temporal patterns of GH stimulation of male and female hepatocytes, as seen in rats, mice and humans (4,5,6). In adult male rats, liver cells are stimulated by GH pulses of high amplitude every 3–4 h, followed by periods of no detectable circulating GH (pulsatile GH profile), whereas in adult females plasma GH stimulation is nearly continuous. Liver gene expression is feminized in adult males given a continuous GH infusion via an osmotic minipump, which elevates the basal, interpulse GH level and renders the overall GH profile more female like (7,8,9). GH binding to its cell surface receptor activates multiple signaling pathways, including signaling via STAT5b (signal transducer and activator of transcription 5b) (10,11). STAT5b and the closely related STAT5a (>90% identical) are transcription factors that are activated in direct response to each plasma GH pulse in adult male rat liver (12,13,14). STAT5 activity is much lower in female than in male rat liver, reflecting partial desensitization of GH signaling to STAT5 by the female plasma GH profile (15,16,17). Ablation of STAT5b in mice leads to down-regulation of approximately 90% of male-specific genes in males with a loss of liver sexual dimorphism (1,18), suggesting that STAT5b might directly activate male-specific gene transcription. Whereas this may be the case for the male-specific Mup genes (19), which are rapidly activated after a single a plasma GH pulse (2), other male-specific genes are likely to be regulated by STAT5b indirectly, via a transcriptional cascade (20). Further complexity is indicated by the fact that GH/STAT5 activation of genes such as Igf1 and Socs2 (21,22,23) is associated with direct binding to high-affinity STAT5 sites in both male and female liver without major sex differences (19). Moreover, STAT5 can trans-activate genes showing female-specific expression, most notably Cyp2c12, A1bg, and Hnf6/Onecut1 (24,25,26). These latter findings suggest that liver STAT5 binding and/or its transcriptional activity can be modulated in a sex-dependent manner.
STAT5b is proposed to be a primary, upstream regulator of a large transcriptional network that targets sex-specific genes in the liver (20). In an effort to elucidate this network, we carried out microarray analysis leading to the discovery of early sex-specific genes that respond to GH rapidly and that may represent direct targets of GH-activated STAT5b (2). One of the sex-specific early GH response genes identified was Bcl6, which encodes a sequence-specific zinc-finger transcriptional repressor (27,28). Bcl6 protein is highly expressed in germinal center B cells (29), and its mRNA is found in many tissues, including liver (30). Bcl6 is frequently deregulated in B cell lymphoma and may contribute to B cell transformation (31,32,33). Bcl6 represses genes involved in B cell activation and lymphocyte differentiation, inflammation, and cell cycle regulation (34). The DNA recognition motif of Bcl6 (27,28) resembles the STAT5 consensus site TTCN3GAA (35), raising the possibility that Bcl6 and STAT proteins may regulate overlapping sets of genes. STAT5 target genes, such as β-casein (36) and MIP1α (37), are repressed by Bcl6 (34,38). Bcl6 can bind to a variety of STAT sites in vitro and can repress transcription from promoters regulated by STAT3, STAT5, and STAT6 (39,40,41,42,43). STAT5 also regulates the expression of Bcl6 in hematopoietic cell lines (44) and human memory B cells (45). Given this interplay between Bcl6 and STAT5, and our finding that Bcl6 is an early liver GH-response gene that shows male specificity by microarray analysis (2), we presently investigate the sex-specific regulation of Bcl6 and the potential for Bcl6 to regulate GH-responsive genes in the liver. Our findings reveal a novel GH-regulatory mechanism, whereby GH confers sex-specific expression through sex-specific effects on Bcl6 transcription elongation. Furthermore, we demonstrate that Bcl6 binds to a subset of STAT5-binding sites in liver chromatin and that Bcl6 and STAT5 both bind to the STAT5-binding region of Socs2 in male liver in a manner that is inversely coordinated by natural plasma GH pulses. Bcl6 may thus contribute to GH-regulated liver gene expression by moderating GH-stimulated expression of a subset of STAT5-dependent genes in male liver.
Results
Male-specific expression of Bcl6 RNA and its responsiveness to hypophysectomy and GH treatment
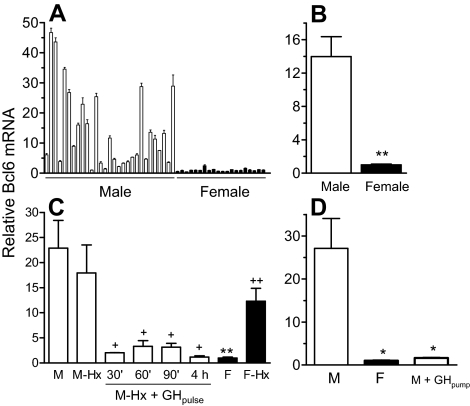
GH regulates many early response genes in rat liver, including 24 that encode DNA-binding proteins (2). One of these genes, Bcl6, codes for a transcriptional repressor that showed higher signal intensity in male compared with female liver in a microarray study (2). This finding was verified by quantitative PCR (qPCR) analysis of total liver RNA prepared from a large set of individual adult male and female rats. Large variation in Bcl6 mRNA levels was observed in a set of 29 individual male livers (Fig. 1A), where the mean level was 14-fold higher than in females (Fig. 1B). Hypophysectomy had little effect on the expression of Bcl6 in male liver but stimulated a large increase in expression in females (Fig. 1C). Thus, Bcl6 can be classified as a class IIA male-specific gene (2), i.e. it is strongly up-regulated in female liver after hypophysectomy, indicating strong negative regulation by the female pituitary hormone profile. Treatment of hypophysectomized male rats with a single, physiological injection of GH decreased Bcl6 mRNA to intact female levels within 30 min, and this down-regulation persisted for at least 4 h (Fig. 1C). This rapid response indicates that Bcl6 is directly suppressed by GH and suggests that the up-regulation of Bcl6 seen in hypophysectomized female liver results from the loss of GH suppression. To further characterize the role of GH in the sex-specific expression of Bcl6, male rats were infused with GH continuously for 7 d, to mimic the adult female plasma GH pattern. Bcl6 mRNA was suppressed to untreated female levels (Fig. 1D), supporting the hypothesis that the male, but not the female, plasma GH profile is permissive for Bcl6 expression.
Figure 1.
Male-specificity and GH-responsiveness of Bcl6 in rat liver. Liver Bcl6 mRNA levels were determined by qPCR analysis in individual adult male and female rats that were untreated, hypophysectomized (Hx), or treated with GH as indicated below. The qPCR primer pair used in this assay targets Bcl6 exons 9 and 10. RNA levels were normalized to the 18S rRNA content of each liver and presented relative to the average RNA level in untreated females, which was set at 1. A and B, Bcl6 mRNA levels were determined in livers of individual untreated male (n = 29) and female (n = 20) rats and graphed as mean ± sd for each individual (A) or as mean ± sem for each group (B). C, Bcl6 mRNA levels were determined in individual livers for the following groups of rats: untreated males (M; n = 8); hypophysectomized males (M-Hx; n = 7); hypophysectomized males given a single injection of rat GH (M-Hx + GH pulse) and killed after 30 min (30′, n = 2), after 60 min (60′, n = 3), after 90 min (90′, n = 3), or after 4 h (4 h, n = 3); untreated females (F, n = 8) and hypophysectomized females (F-Hx, n = 8). Data are graphed as mean ± sem. D, Bcl6 mRNA was determined in the livers of individual untreated male (n = 6) and female rats (n = 6) and in the livers of individual male rats given a continuous infusion of GH via an osmotic minipump for 7 d (n = 5; two rats were infused with rat GH and three rats were infused with human GH, with no difference in response between the two GH treatments). Data are graphed as mean ± sem. Data were analyzed using Student’s t test with two-tail unequal variance due to high variability of mRNA expression levels in the untreated male and hypophysectomized female groups: * and **, P < 0.05 and P < 0.01, respectively, for untreated male vs. untreated female (B–D), or untreated male vs. GH-treated male (D); + and ++, P < 0.05 and P < 0.01, respectively, for untreated male vs. hypophysectomized male, or untreated female vs. hypophysectomized female, or hypophysectomized male vs. GH-treated hypophysectomized male (C). One-way ANOVA with Tukey post hoc test for panel C confirmed these results for untreated male vs. untreated female (P < 0.001) and for hypophysectomized male vs. GH-treated hypophysectomized males combined as a group (P < 0.05).
GH responsiveness of Bcl6 in primary rat hepatocyte culture
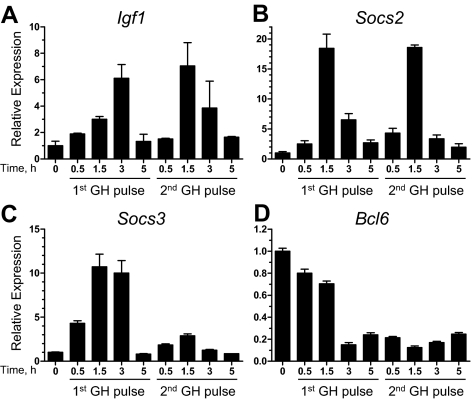
Next, we investigated whether the rapid GH suppression of Bcl6 seen in intact rats reflects the direct action of GH on the liver, as determined using primary cultures of rat hepatocytes. Cells cultured under conditions that induce partial hepatocyte redifferentiation (46) were treated with two pulses of GH, 30 min in duration and spaced 4 h apart. RNA was prepared from the cells at various time points and assayed for the expression of the GH-responsive genes Igf1, Socs2, and Socs3. All three hepatic genes are rapidly induced by GH (47,48) and in the case of Igf1 and Socs2 represent direct STAT5 targets (19,23,49). Igf1 and Socs2 were induced after each GH treatment (Fig. 2, A and B), although Socs3 was induced by the first GH pulse but showed a weak response to the second pulse (Fig. 2C). Presumably, the GH-activated signaling pathway that leads to induction of Socs3 did not fully reset in time for the second pulse (50,51). In contrast to the induction observed for Igf1 and the Socs genes, GH suppressed Bcl6 expression within 3 h of the first hormone pulse with no recovery seen at later time points (Fig. 2D). Moreover, strong suppression of the primary transcript of Bcl6 (assayed across the junction of exon 5/intron 5) was evident within 30 min of GH treatment (data not shown). GH also rapidly suppressed Bcl6 mRNA in the GH-responsive liver cell line CWSV-1 (52) (data not shown). Thus, GH acts directly on liver cells to suppress Bcl6.
Figure 2.
Effect of GH on the expression of three GH-responsive genes and Bcl6 in primary rat hepatocytes. Primary rat hepatocytes cultured for 6 d were stimulated once or twice with GH for 30 min, followed by change of the medium. The second GH treatment started 4 h after the start of the first treatment. The hepatocytes were lysed either before the first GH treatment (‘0′ time point) or at times indicated after the start of each GH treatment. Relative RNA levels of Igf1 (A), Socs2 (B), Socs3 (C), and Bcl6 (assayed at the exon 9/exon 10 junction) (D) were determined by qPCR. Values were normalized to the 18S rRNA content of each sample and expressed relative to the average RNA level in untreated hepatocytes (‘0′ time point), which was set to 1. Data shown are mean ± sd values for triplicate qPCR determinations.
Impact of somatostatin and Stat5a/Stat5b gene deficiency on Bcl6 expression in mouse liver
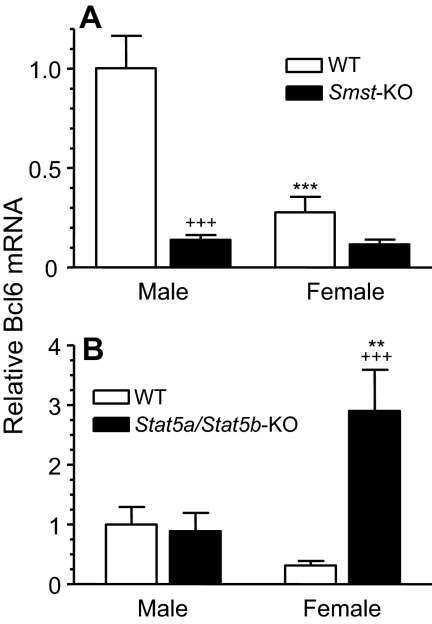
The impact of circulating GH profiles on Bcl6 expression was further investigated in mice deficient in somatostatin, a hypothalamic peptide hormone that inhibits GH secretion by the pituitary gland (53). Male somatostatin knockout mice (Smst−/−) display marked increases in baseline plasma GH levels, which feminizes liver gene expression (54). In wild-type mice, hepatic expression of Bcl6 was male specific (Fig. 3A), although the male-female ratio was lower than in rats (3- to 4-fold in mice vs. 14-fold in rats). Liver Bcl6 mRNA levels were down-regulated with somatostatin deficiency, by approximately 7-fold in males and by about 2.4-fold in females, with a loss of sex specificity (Fig. 3A). Thus, the near-continuous plasma GH profile of somatostatin-deficient mice (54) suppresses Bcl6 in both males and females, consistent with the suppressive response to continuous GH seen in rats (Fig. 1D). The decrease in Bcl6 mRNA seen in somatostatin-deficient female mice indicates that the endogenous plasma GH profile of wild-type female mice [which have well-defined GH-off times, in contrast to female rats (5)] is partially permissive for Bcl6 expression and that the more continuous GH profile of the somatostatin-deficient female mice overrides this permissive expression.
Figure 3.
Effect of somatostatin- and hepatocyte-specific STAT5a/STAT5b deficiency on mouse liver Bcl6 mRNA. RNA isolated from individual mouse livers was analyzed by qPCR for expression of Bcl6. Bcl6 mRNA levels were normalized to the 18S rRNA content of each sample and expressed relative to the average level in wild-type (WT) males, which was set to 1. Data shown are mean ± se for each group. A, Bcl6 mRNA was determined in livers of WT (Smst+/+) male (n = 11) and female (n = 8) mice and somatostatin-deficient (KO; Smst−/−) male (n = 13) and female (n = 14) mice. B, Bcl6 RNA was determined in livers of WT (Stat5a/Stat5b-flox) male and female mice and in Stat5a/Stat5b-deficient (KO) male and female mice (n = 8 per group). Data were analyzed using one-way ANOVA with Tukey post hoc test: ** and ***, P < 0.01 and P < 0.001, respectively, for male vs. corresponding female group; +++, P < 0.001, for WT vs. KO. Down-regulation of Bcl6 in Smst-KO female did not reach statistical significance. KO, Knockout.
Given the essential role of STAT5b for sex-specific gene expression in mouse liver (1), we investigated the impact of STAT5 deficiency on Bcl6 expression. Hepatocyte-specific loss of STAT5a/STAT5b (18,55) had no effect on Bcl6 expression in male liver (Fig. 3B), as was also the case when GH activation of STAT5 was abrogated by hypophysectomy (Fig. 1C). In contrast, hepatic Bcl6 expression was substantially up-regulated in STAT5-deficient females (∼10-fold increase) (Fig. 3B), indicating that STAT5 is required for GH suppression of Bcl6 in female liver.
Continuous GH-dependent, adult female-specific block in Bcl6 transcription elongation
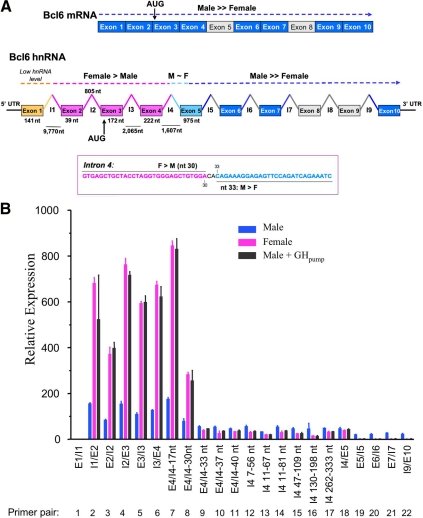
GH regulates several sex-specific rat CYP genes at the level of transcription initiation, as was shown in run-on transcription assays (56,57) and confirmed by analysis of their primary [heterogenous nuclear RNA (hnRNA)] transcripts (2,57). qPCR analysis of hepatic Bcl6 hnRNA revealed that transcripts containing sequences spanning the junction of exon 5 and intron 5 display the same male specificity as mature Bcl6 mRNA. To our surprise, however, Bcl6 hnRNA amplified across the junction of exon 3 and intron 3 was approximately 5-fold more abundant in female than in male liver (Table 1). These initial findings prompted us to quantify Bcl6 transcripts using a series of exon-exon and exon-intron junction primers spanning the length of the Bcl6 gene. In contrast to mature Bcl6 mRNA, which showed strong male specificity in both early and late exonic sequences (Table 1 and Fig. 4A, top), the male-female expression ratio of Bcl6 hnRNA increased dramatically as transcription proceeded through the gene. This is seen in Fig. 4B, where the quantified hnRNA levels are graphed on a relative basis after normalizing for qPCR primer efficiency. Thus, Bcl6 hnRNA sequences from the junction of intron1/exon 2 through the junction of exon 4/intron 4 were about 5-fold more abundant in adult females than in adult males. However, beginning early in intron 4, Bcl6 transcript levels in female liver were markedly decreased compared with the earlier hnRNA sequences, such that the male-female ratio increased from approximately 0.2 to 1.8 (Fig. 4B and Table 2). The transition of Bcl6 hnRNA from female-specific to male-specific sequences was mapped to nucleotides (nts) 30–33 of intron 4 (Fig. 4A, bottom; Fig. 4B, primer pairs 8–9) and is consistent with there being a significant block in transcription elongation in this region in female liver. No further change in steady state hnRNA levels was observed until exon 5, where there was a small (∼2-fold) decrease in transcript levels in males but a further 15-fold decline in females, increasing the male-female hnRNA expression ratio to approximately 12 (Table 2). This substantial male specificity was maintained through the junction of intron 9/exon 10 (Fig. 4B). Male rats given GH as a continuous infusion for 7 d showed the same pattern of liver Bcl6 hnRNA as untreated females (Fig. 4B). Thus, continuous GH treatment suppresses mature Bcl6 mRNA by inducing a block in transcript elongation early in intron 4 and a second block in exon 5. This results in a 325-fold overall accumulation in female liver of primary transcripts containing the intron 3/exon 4 junction and earlier sequences; a similar, 380-fold accumulation of early transcript sequences was seen in continuous GH-treated male liver (Table 2). Finally, a very low steady-state level was observed for Bcl6 hnRNA sequences that include the junction of exon 1/intron 1, but not for those that contain the junction of intron 1/exon 2 (Fig. 4B, primer pairs 1 vs. 2). This suggests hepatic Bcl6 RNA contains an uncharacterized exon between exons 1 and 2.
Table 1.
Relative levels of Bcl6 mRNA and hnRNA in rat liver
| Bcl6 | qPCR primer location | Relative RNA levels (%)
|
|
|---|---|---|---|
| Male | Female | ||
| mRNA | Exon 3/exon 4 | 100 ± 18 | 5.4 ± 0.9 |
| Exon 9/exon 10 | 100 ± 17 | 7.2 ± 0.7 | |
| hnRNA | Exon 3/intron 3 | 100 ± 14 | 531 ± 44 |
| Exon 5/intron 5 | 19 ± 5 | 2.4 ± 0.3 | |
Relative mRNA and hnRNA levels were determined by qPCR analysis of individual untreated adult male (n = 29) and female (n = 20) rat liver RNA samples (as in Fig. 1A) using primer pairs complementary to Bcl6 exon 3/exon 4 and exon 9/exon 10 sequences, for mRNA analysis, and Bcl6 exon 3/intron 3 and exon 5/intron 5 sequences for hnRNA. RNA levels were normalized to the 18S rRNA content of each liver. The levels of mRNA are presented relative to the average level of the corresponding mRNA in males, which was set to 100%. Bcl6 hnRNA levels are adjusted for the primer efficiency and calculated relative to the average male level of hnRNA containing exon 3/intron 3 junction, which was set to 100%. Data are mean values ± se. Bcl6 mRNA levels determined with the two sets of exonic primer pairs showed an excellent correlation (r2 = 0.98) across the full set of 49 livers.
Figure 4.
Sex-dependent, continuous GH-regulated block in Bcl6 transcription elongation. A, Bcl6 mRNA and hnRNA structure with points of regulation by continuous GH. Shown at the top in dark blue are exonic regions of Bcl6 mRNA that exhibit male specificity by qPCR analysis, using primers that amplify across adjacent exons (see supplemental Table S1). Shown in the middle are regions of Bcl6 hnRNA that show female specificity (exons and introns shown in pink), a region extending from nt 30–33 of intron 4 to exon 5 that shows moderate male specificity (light blue), and sequences downstream of exon 5, which show strong male specificity (dark blue). Exon 1-intron 1 transcripts are present at very low levels in both males and females (orange). Shown at the bottom is the sequence of the transcriptional block mapped to the vicinity of intron 4, nts 30–33, based on data shown in panel B and in Table 2. Segments of Bcl6 mRNA and hnRNA not tested are shown in gray (introns) and white (exons). B, Relative Bcl6 hnRNA levels in rat liver. Total liver RNA samples prepared from individual adult rats [untreated males (n = 8), untreated females (n = 8), and males treated with continuous GH via an osmotic pump (GHpump) for 7 d (n = 5, as in Fig. 1D)] and were pooled within each group. The samples were then assayed by qPCR for Bcl6 hnRNA using qPCR primers that span the indicated Bcl6 genomic regions (see supplemental Table S1). hnRNA levels were normalized to the 18S rRNA content of each liver, adjusted for relative primer efficiency determined using rat genomic DNA template, and presented relative to the average level in untreated males of RNA amplified within exon 1, which was set at 1. Data shown are mean ± sd values based on triplicate qPCRs. E, Exon; I, intron. X-axis labels such as E4/I4–30 indicate that the qPCR-amplified region begins in exon 4 and ends at nt 30 of intron 4; labels such as I4 11–67 nt indicate that the amplified region begins at nt 11 and ends at nt 67 of intron 4. AUG, Translation initiation site; F, female; M, male; UTR, untranslated region.
Table 2.
Quantification of rat hepatic Bcl6 mRNA and hnRNA levels across the Bcl6 gene
| Bcl6 region assayed | Male | Female | Male + GHcont | Male-female ratio | |
|---|---|---|---|---|---|
| hnRNA | Intron 1–exon 4 | 134 ± 34 | 654 ± 163 | 614 ± 150 | 0.21 ± 0.02 |
| Intron 4–exon 5 | 48 ± 7 | 29 ± 8 | 33 ± 10 | 1.8 ± 0.5 | |
| Exon 5–exon 10 | 23 ± 3 | 2.0 ± 0.7 | 1.6 ± 0.3 | 12 ± 4 | |
| mRNA | Exon 9–intron 9 | 27.1 ± 6.9 | 1.00 ± 0.15 | 1.67 ± 0.11 | 27.1 ± 6.9 |
Relative levels of liver Bcl6 hnRNA presented in Fig. 4B were averaged across three distinct regions of the Bcl6 gene: intron 1 through exon 4 (primer pairs numbered 2–7 in Fig. 4B), intron 4 through exon 5 (primer pairs 9–18), and exon 5 through exon 10 (primers pairs 19–22); data shown are mean ± sd values. Relative levels of Bcl6 mRNA (mean ± se.) are based on Fig. 1D. Based on these data, the transcriptional block in female liver and in continuous GH-treated (GHcont) male liver decreased steady-state levels of Bcl6 hnRNA sequences beyond exon 5 by 325-fold (in females; i.e. 654/2.0) or by 380-fold (in continuous GH-treated males; i.e. 614/1.6) when compared with hnRNA sequences before intron 4. The corresponding decrease in hnRNA transcript levels across the gene in male liver was only 5.8-fold (i.e. 134/23), which may reflect increased processing of the longer transcripts to mature mRNA.
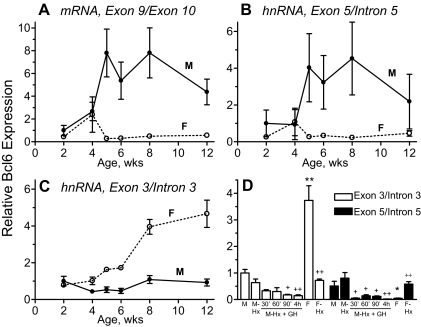
Next, we reasoned that if the block in Bcl6 transcription seen in female liver is induced by continuous GH exposure, it should onset at puberty (i.e. after 4 wk of age), when the sex differences in pituitary GH secretion are first established (58,59). At 2 wk of age, mature Bcl6 mRNA was expressed at a low level in both male and female liver (Fig. 5A). In females, Bcl6 expression was suppressed after 4 wk of age, whereas in males it increased to adult levels. This developmental pattern parallels that of the prototypical male-specific GH-regulated gene Cyp2c11 (e.g. Ref. 60). The developmental profile of Bcl6 exon 5/intron 5 hnRNA was very similar to that of the mature mRNA (Fig. 5B). In contrast, Bcl6 primary transcripts containing exon 3/intron 3 sequences (i.e. before the first transcription block), although present at a low level in males throughout postnatal development, were elevated in females after 4 wk of age (Fig. 5C). Thus, the sex-dependent expression of Bcl6 hnRNA and mRNA both begin at puberty and are regulated by the continuous GH-dependent transcription block mechanism discussed above.
Figure 5.
Developmental profiles and GH pulse responsiveness of Bcl6 mRNA and hnRNA. A–C, Total RNA samples prepared from livers of individual male (M) and female (F) rats ranging in age from 2–12 wk were assayed by qPCR for Bcl6 mRNA (A), hnRNA at the exon 5/intron 5 junction (B), and hnRNA at exon 3/intron 3 junction (C). RNA levels were normalized to the 18S rRNA content of each sample and expressed relative to the average level in 2-wk-old males, which was set to 1 in each case. The data shown are mean ± se based n = 3–5 individuals for each sex and age group, which accounts for the high variability seen in the males in A and B (cf. high variability in Fig. 1A). D, Rat liver RNA samples described in Fig. 1C were assayed by qPCR for Bcl6 hnRNA levels using qPCR primers specific for the exon 3/intron 3 and exon 5/intron 5 junctions. hnRNA levels were normalized to the 18S rRNA content of each liver, adjusted for primer efficiency, and presented relative to the average level in untreated males of hnRNA amplified at the junction of exon 3/intron 3, which was set at 1. Data are mean ± se. Data in D were analyzed using Student’s t test with two-tail unequal variance: * and **, P < 0.05 and P < 0.01, respectively, for untreated male vs. untreated female; + and ++, P < 0.05 and P < 0.01, respectively, for hypophysectomized male vs. GH-treated hypophysectomized male. One-way ANOVA with Tukey post hoc test analysis of data in D: for exon 3/intron 3 hnRNA, P < 0.001 for intact male vs. female, and P < 0.001 for untreated female vs. hypophysectomized female; for exon 5/intron 5 hnRNA, P < 0.001 for hypophysectomized male vs. GH-treated hypophysectomized males combined as a group, and P < 0.05 for untreated female vs. hypophysectomized female. Hx, Hypophysectomized.
Finally, we investigated whether the block in Bcl6 transcription elongation is released when the female GH profile is ablated by hypophysectomy. Hypophysectomy of female rats decreased the steady-state level of accumulated Bcl6 hnRNA sequences upstream of the block (assayed at the exon 3/intron 3 junction) whereas it increased the abundance of hnRNA sequences downstream of the block (i.e. across exon 5/intron 5) (Fig. 5D). Thus, the block in transcription is relieved when the female GH profile is ablated. Hypophysectomy of males had no significant effect on either early or late Bcl6 hnRNA sequences, indicating that the plasma GH pulses in males do not have a major affect on the rate of Bcl6 transcriptional elongation through the sequences that impart the block in female liver. Indeed, treatment of hypophysectomized male rats with a single GH injection not only led to rapid suppression of exon 5/intron 5 hnRNA, consistent with the response of mature Bcl6 mRNA (Fig. 1C), but also stimulated a decrease, and not an increase, in exon 3/intron 3 hnRNA (Fig. 5D). Thus, the rapid decline in mature Bcl6 RNA after GH pulse treatment proceeds by a mechanism that is distinct from the transcription elongation block mechanism that suppresses the formation of mature Bcl6 mRNA in intact females and in continuous GH-treated males.
Male specificity of Bcl6 protein and DNA-binding activity in rat liver nuclei
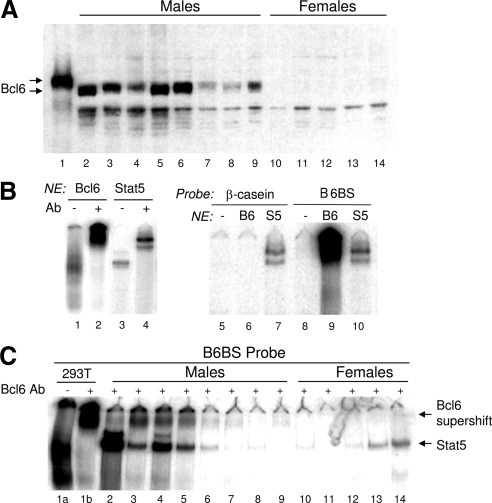
Next, we determined whether the male specificity of Bcl6 mRNA is associated with male-specific expression of a functional, DNA-binding Bcl6 protein in adult liver. Western blot analysis revealed Bcl6 protein present in male, but not female, liver nuclear extracts, albeit with individual variability (Fig. 6A). DNA-binding sequences bound by Bcl6 (28,61) resemble and overlap with the STAT5 consensus sequence, TTC-NNN-GAA (35). Accordingly, Bcl6 and STAT5 can both bind to a classic Bcl6 EMSA probe (Fig. 6B, lanes 1–4). However, Bcl6 does not bind to all STAT5 sequences, as exemplified by an EMSA probe from the β-casein promoter (Fig. 6B, lanes 5–7 vs. 8–10). The use of supershifting antibodies enabled us to visualize the relatively diffuse Bcl6-DNA complex in the form of a supershift complex the migration of which is distinct from that of an unshifted STAT5 complex (Fig. 6, B and C). Using this Bcl6 supershift assay, strong Bcl6-DNA binding was detected in three of eight male liver nuclear samples, and weak binding was seen in four other males; however, Bcl6 binding was not detected in any of the five female samples examined (Fig. 6C). A general correlation between Bcl6 and STAT5 DNA-binding activity was apparent in many but not all individual male livers (cf. Fig. 6C, lane 2, where STAT5 binding is high but Bcl6 binding is low).
Figure 6.
Male-specific rat liver Bcl6 protein and EMSA activity. A, Nuclear extracts (30 μg/lane) prepared from livers of individual adult male (lanes 2–9) and female rats (lanes 10–14) were analyzed by Western blotting using Bcl6 antibody sc-858. Lane 1, nuclear extract (3 μg/lane) prepared from HEK293T cells transfected with mouse Bcl6 cDNA served as a positive control. Note the apparent size difference between rat liver Bcl6 protein and the cDNA-encoded mouse Bcl6 protein. B, EMSA analysis of STAT5 and Bcl6 binding to the B6BS EMSA probe (lanes 1–4 and 8–10) and to the β-casein EMSA probe (lanes 5–7). Binding was assayed using nuclear extracts (NE) (4 μg/lane) prepared from HEK293T cells that were either transfected with mouse Bcl6 (lanes 1, 2, 6, 9), transfected with STAT5b and GH receptor and stimulated with GH for 30 min (lanes 3, 4, 7, 10), or untransfected (lanes 5, 8). Samples were supershifted with antibody to Bcl6 (lanes 2, 6, 9) or STAT5 (lanes 4, 7, 10). C, Nuclear extracts from individual rat livers (5 μg/lane) were analyzed by EMSA using the B6BS probe. Nuclear extract (5 μg/lane) from HEK293T cells transfected with Bcl6 (lanes 1a and 1b) was used as a positive control. All samples, except for the HEK293T cell sample in lane 1a, were incubated with Bcl6 antibody sc-368 (0.4 μg/sample) to separate the supershifted Bcl6-DNA complex from the STAT5-DNA complex (lower band). The band marked STAT5 was identified by supershift analysis, as in B (data not shown). Ab, Antibody; B6, Bcl6; S5, STAT5.
Binding of Bcl6 to STAT5 sites in liver chromatin
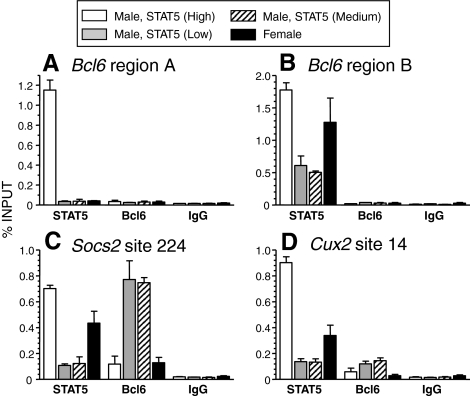
Next, we performed chromatin immunoprecipitation of rat liver tissue to determine whether Bcl6 can bind to STAT5 sites associated with GH-regulated genes in vivo. We also investigated the relationship between the binding of Bcl6 and STAT5 to these sites using liver chromatin prepared from female rats and from male rats killed at different points in the plasma GH pulse/trough cycle, where the occurrence of a plasma GH pulse directly correlates with the STAT5 activity content of the liver (12,13). STAT5 and Bcl6 binding was assayed at four STAT5-binding regions: regions A and B of the Bcl6 gene (44), a STAT5-binding site of Socs2 [(23); described as site 224 in Ref. 19], and a predicted STAT5-binding site upstream of the continuous GH-inducible, female-specific transcription factor Cux2 (60) (site 14; see Materials and Methods). High levels of STAT5 binding were observed at all four chromatin regions in livers of male rats with high STAT5 activity, i.e. rats killed during a plasma GH pulse (Fig. 7). STAT5 binding to Bcl6 region A was very low or undetectable in male livers with medium or low STAT5 activity, i.e. rats killed between plasma GH pulses (Fig. 7A). This finding evidences the dynamic, GH pulse-dependent nature of STAT5 binding in vivo and suggests that Bcl6 region A corresponds to a low-affinity STAT5 site (19). In contrast, substantial STAT5 binding to Bcl6 region B was retained in male livers with medium or low STAT5 activity and in female livers (Fig. 7B). No binding of Bcl6 was detected at Bcl6 region A or region B.
Figure 7.
Chromatin immunoprecipitation analysis of STAT5 and Bcl6 binding in female liver and in male livers that differ in STAT5 activity content. STAT5 binding to the indicated genomic regions of Bcl6 (A and B), Socs2 (C), and Cux2 (D) were analyzed by chromatin immunoprecipitation using sets of male and female chromatin samples, prepared from individual livers of untreated adult female rats (n = 5) or adult male rats with high, medium, and low liver STAT5 activity (n = 2/group). The chromatin immunoprecipitation enrichment at each STAT5-binding region was quantified relative to input DNA. Enrichment values obtain with normal rabbit IgG (controls) are shown at the right. Data shown are mean ± sem for each group of rats.
Substantial binding of STAT5, and Bcl6, to liver chromatin was observed at the Socs2 and Cux2 STAT5 sites examined (Fig. 7, C and D). STAT5 binding to these sites was highest in livers of males killed at a peak of hepatic STAT5 activity and in female livers. In contrast, Bcl6 binding was highest in male livers where STAT5 binding was low. The inverse pattern of STAT5 and Bcl6 binding was most striking at the Socs2 site. Thus, Bcl6 binding to this site in males was highest when hepatic STAT5 activity was low and vice versa. This finding is consistent with the binding of these two factors to the Socs2 site being mutually exclusive due to overlap between the STAT5- and Bcl6-binding sequences. In female liver, low-level Bcl6 binding was observed at the Socs2 site, and no binding was observed at the other three genomic regions, consistent with the level of Bcl6 protein being low in female liver. EMSA analysis verified the intrinsic ability of Bcl6 and STAT5 to bind to the STAT5 sites of Socs2 and Cux2 (Fig. 8).
Figure 8.
STAT5-binding regions of Socs2 and Cux2 bind Bcl6 and STAT5 in vitro. Binding of Bcl6 and STAT5 to EMSA probes comprised of sequences of STAT5-binding site 224 of Socs2 or the core of STAT5-binding site 14 of Cux2 were assayed using nuclear extracts (NE, 7.5 μg/lane) prepared from HEK293T cells transfected with plasmids coding for Bcl6 (lanes 1, 2, 5, 6) or with STAT5b and GH receptor and stimulated with GH for 30 min (lanes 3, 4, 7, 8). Samples were supershifted with antibody to Bcl6 (0.4 μg/lane) (sc-368, lanes 2 and 6) or STAT5 (sc-835, Santa Cruz; lanes 4 and 8). Ab, Antibody.
Discussion
The transcriptional repressor Bcl6 was originally identified in a microarray study as one of 24 DNA-binding proteins that responds rapidly to GH in hypophysectomized rat liver (2). Presently, we characterize the mechanisms that govern the sex specificity of hepatic Bcl6 expression. Bcl6 mRNA was male specific in both rat and mouse liver, with the male to female ratio being higher in rats (∼14-fold) than in mice (∼3- to 4-fold). An unusually high interindividual variability in hepatic Bcl6 mRNA levels was observed in adult males. Given our finding that hepatic Bcl6 mRNA was rapidly suppressed by a single, physiological pulse of GH, both in hypophysectomized male rat liver and in primary rat hepatocyte cultures, the high variability of Bcl6 mRNA seen in individual intact rats could reflect the suppressive effects of a recent plasma GH pulse. Indeed, hypophysectomy decreased the frequency of individual males with very low Bcl6 mRNA levels (data not shown), although the mean Bcl6 mRNA level in the population sampled was not increased compared with sham-operated controls. Given the 3- to 4-h time period between plasma GH pulses in adult male rats (62), this proposal would require that the suppressive effects of a plasma GH pulse be sufficiently short lived to allow for recovery and reexpression of Bcl6 mRNA by the time of the next GH pulse. However, in our studies of hypophsectomized male rats, no recovery of hepatic Bcl6 mRNA or Bcl6 hnRNA was seen 4 h after a single GH pulse. This could indicate that other pituitary-dependent hormones (e.g. thyroid hormone and/or glucocorticoids) are required for rapid reexpression of Bcl6.
Bcl6 was strongly up-regulated by hypophysectomy in females, in contrast to the modest effect of hypophysectomy in eliminating very low expression in individual males discussed above. Moreover, Bcl6 was strongly down-regulated by continuous GH infusion in males. These findings indicate that the female pattern of near-continuous plasma GH stimulation is strongly inhibitory to Bcl6 expression and is the major determinant of the very low level of hepatic Bcl6 expression that is uniformly seen in females. This conclusion is supported by the strong down-regulation of hepatic Bcl6 mRNA in somatostatin-deficient male mice (Fig. 3), where basal (interpulse) plasma GH levels are substantially increased and there is a near continuous presence of GH in plasma in both males and females owing to the absence of the inhibitory effects of somatostatin on pituitary GH release (54). Hepatic Bcl6 RNA was also strongly up-regulated (derepressed) in hepatocyte STAT5a/STAT5b-deficient female mice, indicating that STAT5a and/or STAT5b is required for the inhibitory effects of the female plasma GH profile on Bcl6 expression. Indeed, Bcl6 was not suppressed by continuous GH infusion in hepatocyte STAT5-deficient male mouse liver (data not shown), a finding reminiscent of our earlier observations with the class II male-specific mouse Cyp2d9 (18). STAT5 has been proposed to mediate the rapid suppressive effects of GH on certain liver RNAs in hypophysectomized rat liver (23) and might also mediate the rapid suppression of Bcl6 seen in the present study.
STAT5 regulates the Bcl6 gene in hematopoietic cells and in a subpopulation of germinal center cells, with the mode of regulation (up- or down-regulation) dependent on the cell type (44,45). Two species-conserved STAT5-binding regions have been identified, one in the first exon of Bcl6 (region B), which was associated with Bcl6 repression (44), and one in the first intron (region A). In a hematopoietic cell line where IL-2-activated STAT5 represses Bcl6, STAT5 occupies Bcl6 region B, but not region A, as shown by chromatin immunoprecipitation (44). We found that in untreated rat liver, STAT5 binds strongly to liver chromatin at Bcl6 regions A and B in males but only to region B in females, consistent with the proposal that the GH/STAT5-dependent repression discussed above involves direct binding of STAT5 to Bcl6. The mechanism(s) by which STAT5 represses target genes is largely unknown, with only moderate STAT5-dependent suppression of transcriptional activity seen using luciferase reporter constructs containing Bcl6 region B (44). Indirect mechanisms are also possible, as in the case of STAT5-dependent repression of Igfbp1 transcription, where GH-activated STAT5 impairs the actions of the Foxo1 transcription factor on the Igfbp1 promoter (63). A similar mechanism could apply to Bcl6, which is also activated by Foxo1 and related factors (39,64). Further work is required to elucidate the role of STAT5 in the repressive effects of GH on liver Bcl6 expression and to determine what contribution STAT5 binding to Bcl6 regions A and B might make to this process.
Bcl6 and STAT proteins exhibit distinct, but overlapping, consensus DNA-binding sequences, giving Bcl6 the potential to repress the expression of select STAT-regulated genes (40,41,44). Bcl6 and GH-activated STAT5b both bound to the Bcl6 binding site probe B6BS, whereas STAT5, but not Bcl6, bound strongly to a STAT5 site of the β-casein promoter (Fig. 6). In addition, we observed that Bcl6 binds strongly to the high affinity Socs2 STAT5-binding site 224 (19) in untreated male liver chromatin. Furthermore, we observed that the binding of Bcl6 and STAT5 to the Socs2 site was inversely coordinated by natural pulses of pituitary GH secretion. Thus, at the time of a plasma GH pulse (identified by high content of active STAT5 in the liver), STAT5 binding to the Socs2 site was high and Bcl6 binding was low, whereas during the GH interpulse interval, Bcl6 binding was high and STAT5 binding was low. This inverse coordination of the binding of Bcl6 and STAT5 to Socs2 suggests that Bcl6, a strong transcriptional repressor, moderates STAT5-dependent expression of Socs2, which itself is a negative feedback regulator of GH signaling to STAT5 (65). This proposed action of Bcl6 in male liver could be an important mechanism for maintaining SOCS2 at a similar level in male and female liver, thus counteracting the tendency for increased Socs2 expression in males due to the much higher level of STAT5 occupancy that is seen in males at three other Socs2 sites (19). Our finding of an inverse, plasma GH-dependent binding of STAT5 and Bcl6 to Socs2 in liver in vivo is consistent with a recent report that in cultured adipocytes, Bcl6 binding to the same genomic region of Socs2 decreases progressively with time whereas STAT5 binding increases after GH treatment (66). Further studies, including investigation of other genes that are dual targets for Bcl6 and STAT5, may clarify the role of Bcl6 in GH/STAT5-dependent gene regulation.
In contrast to the strong binding of Bcl6 to the STAT5-binding site of Socs2, weak binding of Bcl6 was found at a newly predicted STAT5-binding site upstream of the rat Cux2 gene, whereas no Bcl6 binding was observed at STAT5-binding regions A and B of the Bcl6 gene itself. Of note, all four rat genomic regions bound similar levels of STAT5 during a plasma GH pulse (Fig. 7), highlighting the fact that Bcl6 binds to a subset of STAT5 sites. Given the male specificity of hepatic Bcl6 expression, we considered the possibility that in male rat liver Bcl6 might bind to, and thereby repress, female-specific genes that are susceptible to GH/STAT5 activation, and thereby help enforce the sex specificity of liver gene expression. One such gene is Cyp2c12, which harbors a pair of upstream STAT5 sites that show GH responsiveness in a luciferase reporter construct after direct injection of a naked plasmid DNA reporter in rat liver (24). However, although Bcl6 and STAT5 could both bind to these Cyp2c12 STAT5 sites in vitro in EMSA assays, very weak STAT5 binding and no Bcl6 binding were seen in male and female rat liver chromatin (data not shown). In contrast, strong binding of STAT5, and to a lesser extent Bcl6, was presently shown to occur in rat liver chromatin at a STAT5 site upstream of Cux2, a highly female-specific transcription factor that is strongly induced by continuous GH treatment (60). STAT5 binding to this site was particularly strong in male liver at the time of a plasma GH pulse, raising the possibility that this site might contribute to the repression of Cux2 in male liver (60).
Finally, the present study revealed a novel mechanism for the sex-specific actions of GH on Bcl6 expression. Comparison of hepatic Bcl6 mRNA and hnRNA levels led us to the unexpected discovery of a partial block in Bcl6 transcriptional elongation in female liver, with hepatic Bcl6 hnRNA transcripts extending through exon 4 being approximately 5-fold enriched, both in females and in continuous GH-treated males, compared with untreated male liver; in contrast, mature Bcl6 mRNA was more than 10-fold more abundant in male liver. Two apparent transcriptional blocks (sites of transcriptional stalling or pausing) were identified, one early in intron 4 and another within exon 5; in combination, these reduced Bcl6 transcription elongation more than 300-fold. This phenomenon is distinct from the promoter-proximal pausing described for RNA polymerase II, which initiates transcription of many genes that are expressed at a low mRNA level, but stalls after transcribing only 20–50 nts of the gene (67). The block in Bcl6 transcription described here has another unique feature, namely, its responsiveness to the plasma GH profile, as evident from the inhibition of transcription elongation seen in livers of female rats and in male rats receiving continuous infusion of GH, but not in untreated males. To our knowledge, this represents the first example in which regulation of transcriptional elongation is used as a mechanism to impart sex specificity to gene expression. Analysis of the DNA sequences in the vicinity of the first transcriptional block (Fig. 4A) indicates the absence of a STAT5 consensus sequence, suggesting that the block in elongation is not due to direct binding of STAT5 to this transcriptional regulatory region. Further study will be required to determine the mechanism whereby the female plasma GH pattern imposes this transcriptional block and whether it contributes to GH regulation of other sex-specific genes in the liver.
Materials and Methods
Rat and mouse models
Livers were collected from individual adult male and female Fischer 344 rats, 9–13 wk of age. Intact male rats were continuously infused with recombinant rat or human GH for 7 d via an Alzet osmotic mini-pump (20–25 ng of GH/g body weight per h) (57) as specified in each experiment. Hypophysectomy was performed by the supplier (Taconic Farms, Inc., Hudson, NY) at 8 wk of age. Some hypophysectomized male rats were given a single ip injection of recombinant rat GH (60 ng/g body weight) and killed 30 min to 4 h later; no other pituitary-dependent hormone supplementation was provided (2,68). Livers from untreated Fischer 344 rats ranging in age from 2–12 wk (12) were used for the postnatal developmental expression studies. Livers collected from individual male and female hepatocyte-specific STAT5a/STAT5b knockout mice (8–12 wk; C57BL/6 X 129J X FVB/N) and corresponding floxed control mice were described previously (18). Livers from adult male and female somatostatin-deficient and control mice (9–12 wk) were those used previously (60) and were kindly provided by Drs. R. M. Luque and R. D. Kineman (University of Illinois at Chicago, Chicago, IL) (69).
Total RNA preparation and qPCR
Total RNA prepared from cultured cells or individual frozen livers using TRIzol reagent (Invitrogen, Carlsbad, CA) was treated with RQ1 ribonuclease-free deoxyribonuclease (Promega Corp., Madison, WI) for 1 h at 37 C followed by heating for 5 min at 75 C. Reverse transcription of 1 μg of RNA per sample was performed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Samples were diluted in 50 μg/ml yeast RNA (Applied Biosystems/Ambion, Austin, TX) for qPCR analysis performed using Power SYBR Green PCR Master Mix (Applied Biosystems) on an ABS 7900HT sequence detection system (Applied Biosystems). Primer sequences are listed in supplemental Table S1 (published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Primary rat hepatocyte culture
Primary rat hepatocytes were isolated from livers of adult male Fischer 344 rat as described (46) except that perfusion with Ca2+-free buffer (10 mm HEPES, pH 7.4; 142 mm NaCl; 6.7 mm KCl) was followed by perfusion with buffer containing 0.54 mg/ml type II collagenase and 0.7 mg/ml CaCl2 · 2 H2O. Hepatocytes were cultured in modified Chee’s medium (no. 88–5046EA, Life Technologies, Inc., Gaithersburg, MD) (70) containing 0.1 μm dexamethasone, 3.7 g/liter sodium bicarbonate, 10 mg/liter thymidine, 4 mm l-glutamine, 6.25 μg/ml transferrin, 6.25 μg/ml insulin, 6.25 ng/ml selenium, 10 ng/ml epidermal growth factor, and 1 ng/ml hepatocyte growth factor. Dimethylsulfoxide was added to the medium at a final concentration of 2% (vol/vol) beginning on culture d 4 and maintained for the duration of each experiment. On culture d 6, the cells were treated with recombinant rat GH added to the culture medium for 30 min (final concentration 300 ng/ml), followed by a second GH treatment beginning 4 h after the start of the first treatment. At the end of each 30-min GH treatment, the cells were washed once and fresh medium without GH was added. Primary rat hepatocytes were lysed in TRIzol reagent either before the start of the first GH treatment (‘0′ time point) or 0.5, 1.5, 3, or 5 h after the beginning of each GH treatment.
Preparation of nuclear extracts and Western blot analysis
Human embryonic kidney (HEK)293T cells were transfected with expression plasmids for mouse STAT5b and rat GH receptor (kindly provided by Dr. Nils Billestrup, Hagedon Research Institute, Gentofe, Denmark) and stimulated with recombinant rat GH (200–300 ng/ml) for 30 min. HEK293T cells were also transfected with expression plasmid for mouse Bcl6 (mBcl6/pCMV-SPORT6.1, catalog no. MMM1013-9201254; Open Biosystems, Huntsville, AL). Nuclear extracts from HEK293T cells were prepared using a NucBuster Protein Extraction Kit (Novagen, Madison WI). Nuclear extracts from individual livers of untreated adult male and female rats were prepared as described (57). For Western blotting, nuclear extracts prepared from HEK293T cells transfected with Bcl6 expression plasmid (3 μg/lane) or individual rat livers (30 μg/lane) were resolved on a 9% sodium dodecyl sulfate-polyacrylamide gel and subjected to Western blotting using anti-Bcl6 antibody (sc-858; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Bcl6 antibody was diluted 1:1250 in Tris-buffered saline buffer with 0.1% Tween 20 and 5% milk, and the membrane was incubated for 2 h at room temperature. Horseradish peroxidase-conjugated secondary antibody (donkey antirabbit IgG; GE Healthcare, Piscataway, NJ) was used at a dilution 1:20,000. The proteins were detected with a SuperSignal West Femto Maximum Substrate (Pierce Chemical Co./Thermo Fisher Scientific, Rockford, IL).
EMSA
EMSA was performed as described elsewhere (15) with modifications. Nuclear extracts prepared from HEK293T cells or individual rat livers (4–5 μg) were mixed with EMSA buffer (1.6 mm HEPES, pH 7.9; 8 mm KCl; 1.6% glycerol; 0.67 mm MgCl2; 0.016 mm EDTA; 0.67 mm dithiothreitol final concentration), 0.13–0.2 μg/μl poly(deoxyinosinic-deoxycytidylic)acid and, where indicated, 0.4 μg of STAT5 antibody (sc-835, Santa Cruz Biotechnology) or 0.4 μg of Bcl6 antibody (sc-368, Santa Cruz) and incubated for 10 min at room temperature. Double-stranded DNA probe (0.25 nm final concentration) labeled on one end with 32P was added to the mixture to give a final volume of 15 μl, and the mixture was incubated for 20 min at room temperature. The samples were resolved on 5.5% nondenaturing polyacrylamide gels in 0.5× Tris-buffered EDTA buffer. Gels were exposed to phosphor screens (GE Healthcare), followed by analysis on a Typhoon Trio Variable Mode Imager (GE Healthcare). Oligonucleotides used in EMSA were as follows (the sequences of top strands are listed): B6BS probe (5′-GAAAATTCCTAGAAAGCATA-3′), containing a Bcl6-binding site (27); Socs2 probe (5′-GGCGGATTCCTGGAAAGTTCCTGGAAAGCGG-3′) and Cux2 probe (5′-GATCGCAGTTCTTGGAAGGCCT-3′), respectively, containing STAT5-binding site 224 of rat Socs2 (19) and the core binding site of predicted STAT5-binding region 14 of rat Cux2 (see below). β-Casein EMSA probe was that used earlier (15).
Chromatin immunoprecipitation assay
Immunoprecipitation with STAT5 antibody sc-836X or Bcl6 antibody sc-858X (Santa Cruz Biotechnology) was performed using chromatin samples prepared from frozen or fresh liver tissue from female rats and from male rats that differ in hepatic STAT5 activity. The STAT5 activity status of individual livers was determined by EMSA with the β-casein promoter probe as described elsewhere (19) and was designated high, medium, or low. High STAT5 activity male samples had relative activity values of 74% and 100%, medium activity male samples had values of 6% and 24%, and low activity male samples had values of 0.6% and 0.7%; female samples exhibited relative STAT5 activity values ranging from 3.5% to 22%. Chromatin samples were prepared and immunoprecipitation was performed as described (19). STAT5 sites associated with Socs2 [site 224 (19)] and Bcl6 (44) and a newly predicted STAT5 site of Cux2 (see below) were interrogated by qPCR using primers listed in supplemental Table S1. Results were calculated relative to input DNA. The Cux2 site (site 14) was predicted using the phylogenetic footprinting program described in Ref. 19 and was conserved in both rat [chromosome 12 (+): 35,751,791–35,751,837] and mouse [chromosome 5 (−): 122,469,474– 122,469,520].
Supplementary Material
Footnotes
This work was supported, in part, by National Institutes of Health Grant DK33765 (to D.J.W.).
Disclosure Summary: The authors have nothing to declare.
First Published Online October 1, 2009
Abbreviations: HEK, Human embryonic kidney; hnRNA, heterogenous nuclear RNA; nt, nucleotide; qPCR, quantitative PCR; STAT, signal transducer and activator of transcription.
References
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ 2006 Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20:1333–1351 [DOI] [PubMed] [Google Scholar]
- Wauthier V, Waxman DJ 2008 Sex-specific early growth hormone response genes in rat liver. Mol Endocrinol 22:1962–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ 2006 Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16:995–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson JO, Edén S, Isaksson O 1985 Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6:128–150 [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, Shapiro BH 1991 Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131:395–399 [DOI] [PubMed] [Google Scholar]
- Pincus SM, Gevers EF, Robinson IC, van den Berg G, Roelfsema F, Hartman ML, Veldhuis JD 1996 Females secrete growth hormone with more process irregularity than males in both humans and rats. Am J Physiol Endocrinol Metab 270:E107–E115 [DOI] [PubMed] [Google Scholar]
- Ahluwalia A, Clodfelter KH, Waxman DJ 2004 Sexual dimorphism of rat liver gene expression: regulatory role of growth hormone revealed by deoxyribonucleic acid microarray analysis. Mol Endocrinol 18:747–760 [DOI] [PubMed] [Google Scholar]
- Holloway MG, Laz EV, Waxman DJ 2006 Codependence of growth hormone-responsive, sexually dimorphic hepatic gene expression on signal transducer and activator of transcription 5b and hepatic nuclear factor 4α. Mol Endocrinol 20:647–660 [DOI] [PubMed] [Google Scholar]
- Pampori NA, Agrawal AK, Shapiro BH 2001 Infusion of gender-dependent plasma growth hormone profiles into intact rats: effects of subcutaneous, intraperitoneal, and intravenous routes of rat and human growth hormone on endogenous circulating growth hormone profiles and expression of sexually dimorphic hepatic cyp isoforms. Drug Metab Dispos 29:8–16 [PubMed] [Google Scholar]
- Hosui A, Hennighausen L 2008 Genomic dissection of the cytokine-controlled STAT5 signaling network in liver. Physiol Genomics 34:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, O'Connor C 2006 Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20:2613–2629 [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ 2000 Plasma growth hormone pulse activation of hepatic JAK-STAT5 signaling: developmental regulation and role in male-specific liver gene expression. Endocrinology 141:3245–3255 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Choi HK, Gurd W, Waxman DJ 2001 Temporal relationship between the sexually dimorphic spontaneous GH secretory profiles and hepatic STAT5 activity. Endocrinology 142:4599–4606 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Ram PA, Park SH, Choi HK 1995 Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem 270:13262–13270 [DOI] [PubMed] [Google Scholar]
- Choi HK, Waxman DJ 1999 Growth hormone, but not prolactin, maintains, low-level activation of STAT5a and STAT5b in female rat liver. Endocrinology 140:5126–5135 [DOI] [PubMed] [Google Scholar]
- Fernández L, Flores-Morales A, Lahuna O, Sliva D, Norstedt G, Haldosén LA, Mode A, Gustafsson JA 1998 Desensitization of the growth hormone-induced Janus kinase 2 (Jak 2)/signal transducer and activator of transcription 5 (Stat5)-signaling pathway requires protein synthesis and phospholipase C. Endocrinology 139:1815–1824 [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, Waxman DJ 1999 Down-regulation of liver JAK2-STAT5b signaling by the female plasma pattern of continuous growth hormone stimulation. Mol Endocrinol 13:213–227 [DOI] [PubMed] [Google Scholar]
- Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, Waxman DJ 2007 Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148:1977–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laz EV, Sugathan A, Waxman DJ 2009 Dynamic in vivo binding of STAT5 to growth hormone-regulated genes in intact rat liver. Sex-specific binding at low but not high affinity STAT5 sites. Mol Endocrinol 23:1242–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG 2009 Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DJ, Ono M, Woelfle J, Schlesinger-Massart M, Jiang H, Rotwein P 2006 Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J Biol Chem 281:3190–3197 [DOI] [PubMed] [Google Scholar]
- Eleswarapu S, Gu Z, Jiang H 2008 Growth hormone regulation of insulin-like growth factor-I gene expression may be mediated by multiple distal signal transducer and activator of transcription 5 binding sites. Endocrinology 149:2230–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal OM, Merino R, Rico-Bautista E, Fernandez-Perez L, Chia DJ, Woelfle J, Ono M, Lenhard B, Norstedt G, Rotwein P, Flores-Morales A 2007 In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol Endocrinol 21:293–311 [DOI] [PubMed] [Google Scholar]
- Endo M, Takahashi Y, Sasaki Y, Saito T, Kamataki T 2005 Novel gender-related regulation of CYP2C12 gene expression in rats. Mol Endocrinol 19:1181–1190 [DOI] [PubMed] [Google Scholar]
- Gardmo C, Mode A 2006 In vivo transfection of rat liver discloses binding sites conveying GH-dependent and female-specific gene expression. J Mol Endocrinol 37:433–441 [DOI] [PubMed] [Google Scholar]
- Lahuna O, Rastegar M, Maiter D, Thissen JP, Lemaigre FP, Rousseau GG 2000 Involvement of STAT5 (signal transducer and activator of transcription 5) and HNF-4 (hepatocyte nuclear factor 4) in the transcriptional control of the hnf6 gene by growth hormone. Mol Endocrinol 14:285–294 [DOI] [PubMed] [Google Scholar]
- Chang CC, Ye BH, Chaganti RS, Dalla-Favera R 1996 BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci USA 93:6947–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfert VL, Allman D, He Y, Staudt LM 1996 Transcriptional repression by the proto-oncogene BCL-6. Oncogene 12:2331–2342 [PubMed] [Google Scholar]
- Dent AL, Vasanwala FH, Toney LM 2002 Regulation of gene expression by the proto-oncogene BCL-6. Crit Rev Oncol Hematol 41:1–9 [DOI] [PubMed] [Google Scholar]
- Bajalica-Lagercrantz S, Piehl F, Farnebo F, Larsson C, Lagercrantz J 1998 Expression of the BCL6 gene in the pre- and postnatal mouse. Biochem Biophys Research Commun 247:357–360 [DOI] [PubMed] [Google Scholar]
- Baron BW, Nucifora G, McCabe N, Espinosa III R, Le Beau MM, McKeithan TW 1993 Identification of the gene associated with the recurring chromosomal translocations t(3;14)(q27;q32) and t(3;22)(q27;q11) in B-cell lymphomas. Proc Natl Acad Sci USA 90:5262–5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki T, Yano T, Clark HM, Bastard C, Kerckaert JP, Jaffe ES, Raffeld M 1995 Analysis of LAZ3 (BCL-6) status in B-cell non-Hodgkin’s lymphomas: results of rearrangement and gene expression studies and a mutational analysis of coding region sequences. Blood 85:2877–2884 [PubMed] [Google Scholar]
- Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Favera R 1993 Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262:747–750 [DOI] [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM 2000 BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13:199–212 [DOI] [PubMed] [Google Scholar]
- Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, Bucher P 2001 DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem 276:6675–6688 [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M, Happ B, Ball RK, Groner B 1992 Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding β-casein. Proc Natl Acad Sci USA 89:3130–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Walker SR, Alvarez JV, Frank DA 2004 Isolation of unique STAT5 targets by chromatin immunoprecipitation-based gene identification. J Biol Chem 279:54724–54730 [DOI] [PubMed] [Google Scholar]
- Logarajah S, Hunter P, Kraman M, Steele D, Lakhani S, Bobrow L, Venkitaraman A, Wagner S 2003 BCL-6 is expressed in breast cancer and prevents mammary epithelial differentiation. Oncogene 22:5572–5578 [DOI] [PubMed] [Google Scholar]
- Fernández de Mattos S, Essafi A, Soeiro I, Pietersen AM, Birkenkamp KU, Edwards CS, Martino A, Nelson BH, Francis JM, Jones MC, Brosens JJ, Coffer PJ, Lam EW 2004 FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol Cell Biol 24:10058–10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM 1997 Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276:589–592 [DOI] [PubMed] [Google Scholar]
- Harris MB, Chang CC, Berton MT, Danial NN, Zhang J, Kuehner D, Ye BH, Kvatyuk M, Pandolfi PP, Cattoretti G, Dalla-Favera R, Rothman PB 1999 Transcriptional repression of Stat6-dependent interleukin-4-induced genes by BCL-6: specific regulation of Iε transcription and immunoglobulin E switching. Mol Cell Biol 19:7264–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reljic R, Wagner SD, Peakman LJ, Fearon DT 2000 Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 192:1841–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TT, Dowbenko D, Jackson A, Toney L, Lewin DA, Dent AL, Lasky LA 2002 The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J Biol Chem 277:14255–14265 [DOI] [PubMed] [Google Scholar]
- Walker SR, Nelson EA, Frank DA 2007 STAT5 represses BCL6 expression by binding to a regulatory region frequently mutated in lymphomas. Oncogene 26:224–233 [DOI] [PubMed] [Google Scholar]
- Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, Wijnands E, Gimeno R, Vyth-Dreese FA, Blom B, Spits H 2005 STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol 6:303–313 [DOI] [PubMed] [Google Scholar]
- Su T, Waxman DJ 2004 Impact of dimethyl sulfoxide on expression of nuclear receptors and drug-inducible cytochromes P450 in primary rat hepatocytes. Arch Biochem Biophys 424:226–234 [DOI] [PubMed] [Google Scholar]
- Bichell DP, Kikuchi K, Rotwein P 1992 Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Mol Endocrinol 6:1899–1908 [DOI] [PubMed] [Google Scholar]
- Adams TE, Hansen JA, Starr R, Nicola NA, Hilton DJ, Billestrup N 1998 Growth hormone preferentially induces the rapid, transient expression of SOCS-3, a novel inhibitor of cytokine receptor signaling. J Biol Chem 273:1285–1287 [DOI] [PubMed] [Google Scholar]
- Woelfle J, Chia DJ, Rotwein P 2003 Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J Biol Chem 278:51261–51266 [DOI] [PubMed] [Google Scholar]
- Ram PA, Park SH, Choi HK, Waxman DJ 1996 Growth hormone activation of Stat 1, Stat 3, and Stat 5 in rat liver. Differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phosphorylation. J Biol Chem 271:5929–5940 [DOI] [PubMed] [Google Scholar]
- Ji S, Frank SJ, Messina JL 2002 Growth hormone-induced differential desensitization of STAT5, ERK, and Akt phosphorylation. J Biol Chem 277:28384–28393 [DOI] [PubMed] [Google Scholar]
- Gebert CA, Park SH, Waxman DJ 1999 Termination of growth hormone pulse-induced STAT5b signaling. Mol Endocrinol 13:38–56 [DOI] [PubMed] [Google Scholar]
- Guillermet-Guibert J, Lahlou H, Cordelier P, Bousquet C, Pyronnet S, Susini C 2005 Physiology of somatostatin receptors. J Endocrinol Invest 28(Suppl 11):5–9 [PubMed] [Google Scholar]
- Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M 2001 Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107:1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Hosui A, Sun R, Shen K, Gavrilova O, Chen W, Cam MC, Gao B, Robinson GW, Hennighausen L 2007 Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46:504–513 [DOI] [PubMed] [Google Scholar]
- Legraverend C, Mode A, Westin S, Ström A, Eguchi H, Zaphiropoulos PG, Gustafsson JA 1992 Transcriptional regulation of rat P-450 2C gene subfamily members by the sexually dimorphic pattern of growth hormone secretion. Mol Endocrinol 6:259–266 [DOI] [PubMed] [Google Scholar]
- Sundseth SS, Alberta JA, Waxman DJ 1992 Sex-specific, growth hormone-regulated transcription of the cytochrome P450 2C11 and 2C12 genes. J Biol Chem 267:3907–3914 [PubMed] [Google Scholar]
- Edén S 1979 Age- and sex-related differences in episodic growth hormone secretion in the rat. Endocrinology 105:555–560 [DOI] [PubMed] [Google Scholar]
- Gabriel SM, Roncancio JR, Ruiz NS 1992 Growth hormone pulsatility and the endocrine milieu during sexual maturation in male and female rats. Neuroendocrinology 56:619–625 [DOI] [PubMed] [Google Scholar]
- Laz EV, Holloway MG, Chen CS, Waxman DJ 2007 Characterization of three growth hormone-responsive transcription factors preferentially expressed in adult female liver. Endocrinology 148:3327–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata N, Miki T, Ohashi K, Suzuki K, Fukuda T, Hirosawa S, Aoki N 1994 Recognition DNA sequence of a novel putative transcription factor, BCL6. Biochem Biophys Res Commun 204:366–374 [DOI] [PubMed] [Google Scholar]
- Tannenbaum GS, Martin JB 1976 Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98:562–570 [DOI] [PubMed] [Google Scholar]
- Ono M, Chia DJ, Merino-Martinez R, Flores-Morales A, Unterman TG, Rotwein P 2007 Signal transducer and activator of transcription (Stat) 5b-mediated inhibition of insulin-like growth factor binding protein-1 gene transcription: a mechanism for repression of gene expression by growth hormone. Mol Endocrinol 21:1443–1457 [DOI] [PubMed] [Google Scholar]
- Shore AM, White PC, Hui RC, Essafi A, Lam EW, Rowe M, Brennan P 2006 Epstein-Barr virus represses the FoxO1 transcription factor through latent membrane protein 1 and latent membrane protein 2A. J Virol 80:11191–11199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Morales A, Greenhalgh CJ, Norstedt G, Rico-Bautista E 2006 Negative regulation of growth hormone receptor signaling. Mol Endocrinol 20:241–253 [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin G, Huo JS, Barney D, Wang Z, Livshiz T, States DJ, Qin ZS, Schwartz J 2009 Computational and functional analysis of growth hormone-regulated genes identifies the transcriptional repressor Bcl6 as a participant in GH-regulated transcription. Endocrinology 150:3645–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core LJ, Lis JT 2008 Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science 319:1791–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram PA, Waxman DJ 1999 SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274:35553–35561 [DOI] [PubMed] [Google Scholar]
- Luque RM, Gahete MD, Hochgeschwender U, Kineman RD 2006 Evidence that endogenous SST inhibits ACTH and ghrelin expression by independent pathways. Am J Physiol Endocrinol Metab 291:E395–E403 [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Morrissey JJ, Naik S, Jauregui HO 1990 Phenobarbital induction of cytochromes P-450. High-level long-term responsiveness of primary rat hepatocyte cultures to drug induction, and glucocorticoid dependence of the phenobarbital response. Biochem J 271:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.