Abstract
Retinoblastoma protein (RB) plays crucial roles in cell cycle control and cellular differentiation. Specifically, RB impairs the G1 to S phase transition by acting as a repressor of the E2F family of transcriptional activators while also contributing towards terminal differentiation by modulating the activity of tissue-specific transcription factors. To examine the role of RB in Sertoli cells, the androgen-dependant somatic support cell of the testis, we created a Sertoli cell-specific conditional knockout of Rb. Initially, loss of RB has no gross effect on Sertoli cell function because the mice are fertile with normal testis weights at 6 wk of age. However, by 10–14 wk of age, mutant mice demonstrate severe Sertoli cell dysfunction and infertility. We show that mutant mature Sertoli cells continue cycling with defective regulation of multiple E2F1- and androgen-regulated genes and concurrent activation of apoptotic and p53-regulated genes. The most striking defects in mature Sertoli cell function are increased permeability of the blood-testis barrier, impaired tissue remodeling, and defective germ cell-Sertoli cell interactions. Our results demonstrate that RB is essential for proper terminal differentiation of Sertoli cells.
Conditional knockout of Rb in murine Sertoli cells results in irregular terminal differentiation, misregulation of known and novel RB-regulated genes, and infertility.
The Sertoli cell is the somatic support cell of mammalian spermatogenesis. This mesenchymal epithelial cell operates uniquely and diversely to support the germ cells of the seminiferous epithelium. In the mouse, at embryonic d 10.5–12, Sertoli cells differentiate from their female counterpart, the granulosa cells, and begin expressing SOX9 and anti-Müllerian hormone (AMH) (1). Immature Sertoli cells continue to be proliferative until postnatal d 12–17, at which point they permanently exit the cell cycle (2). During this time, AMH is down-regulated just as p27 (CDKN1B), a cyclin-dependent kinase inhibitor (CDKI) and a marker of Sertoli cell maturation and mitotic quiescence, is up-regulated (1,3). Sertoli cells also begin to produce secreted proteins important for germ cell development and establish the tight junctions of the blood-testis barrier (1). Thus, in Sertoli cells as in many other cell types, terminal differentiation is closely related to cell cycle exit (4).
The retinoblastoma protein (RB) pathway represents one of the most well-studied mechanisms of cell cycle regulation. In a simplified version of this model, G1 to S phase progression is mediated through the hyperphosphorylation of RB by cyclin/CDK complexes, preventing the binding of RB to E2F transcriptional activators (i.e. E2F1, E2F2, and E2F3a) (5). Loss of RB binding derepresses E2F proteins and allows the transcription of genes important for cell cycle progression (5). When cyclin/CDK complex activity is blocked by inhibitors like p27, RB remains hypophosphorylated and able to bind to E2Fs (5). Whereas hypophosphorylated RB is clearly a marker of cell cycle exit, its role in replicative senescence appears to be highly tissue specific (6).
The RB pathway has also been implicated in the terminal differentiation of a variety of cell types, which has led to the hypothesis that RB is a master regulator coordinating cell cycle exit with differentiation (4). Conditional knockout studies of Rb in skeletal myoblasts (7), osteoblasts (8), and preadipocytes (9) reveal that RB is essential for the proper differentiation of these progenitor cell types. It has been proposed that RB binding to tissue-specific and tissue-nonspecific transcription factors is necessary for proper differentiation of these and other cell types (6,10). Studies in retinal interneurons also suggest that the inhibition of E2Fs, specifically E2F3a, by RB may be critical for their terminal differentiation and that this function is not related to cell cycle exit (11).
Due to the well-defined link between Sertoli cell maturity and mitotic quiescence, Sertoli cells represent a unique and interesting cell type to study the involvement of RB in the coordination of cell cycle exit and terminal differentiation. In mice, knockout of p27 with or without concomitant knockout of p21 (Cdkn1a), another CDKI, results in an overall increase in Sertoli cell number; however, the proliferative lifespan of Sertoli cells remains unchanged (12), suggesting that loss of CDKIs increased the rate of proliferation but does not abrogate replicative senescence. Other rodent models implicate the RB-E2F pathway in Sertoli cell cycle exit even more strongly. For example, transgenic mice expressing simian virus 40 (SV40) large T-antigen in Sertoli cells develop testicular tumors (13), and cellular division resumes in postmitotic rat Sertoli cell cultures when inhibitor of DNA binding 1 (ID1) or ID2 is overexpressed (14). Both SV40 T-antigen and the ID proteins are known to bind and inactivate RB. When replicative senescence is already overcome, as in the case of inhibin α (Inha) knockout mice that universally undergo Sertoli cell oncogenesis (15), perturbations of upstream regulators of RB can worsen (16) or lessen (17) tumor progression. Also, RB is a known transcriptional coregulator of androgen receptor (AR) (18,19), a steroid hormone receptor that is indispensable for proper mature Sertoli cell function (20,21,22). Stage-specific staining of RB in Sertoli cells is closely related to that of AR as both are maximally expressed in stage VII–VIII (23), and changes are seen in this staining when androgens are suppressed (24). All of these observations, not to mention the importance of RB in the differentiation of granulosa cells (25), suggest that RB may be important for the coordination of cell cycle exit and terminal differentiation of Sertoli cells.
In this report, we explore the functions of RB in Sertoli cells by creating and studying a Sertoli cell-specific RB conditional knockout mouse model. Our findings show that mutant Sertoli cells are capable of supporting the first wave of spermatogenesis, but the function of these Sertoli cells rapidly declines, leading to infertility. Our results suggest that RB is dispensable for initial maturation of Sertoli cells but is essential for their sustained terminal differentiation, which includes maintenance of maturing germ cells and cell cycle quiescence.
Results
Generation of Sertoli cell-specific Rb mutant mice
To investigate the roles of RB in Sertoli cells specifically, we employed the Cre-loxP system. We used this conditional knockout system for two reasons. First, the homozygous null mutation of Rb uniformly causes lethality around embryonic d 14.5 (26,27,28). Second, although replacing RB in the placenta can rescue these pups allowing them to be viable until the perinatal period (29), RB is also expressed in spermatogonia (23), and we did not want to experience any secondary effects. To create a Sertoli cell-specific conditional knockout, we employed a transgenic mouse expressing Cre recombinase driven by the AMH promoter (Amh-Cre), which has been shown to have high activity as early as embryonic d 14.5 (20). To obtain the most complete deletion possible, we intercrossed Rbflox/flox mice (30,31) with Rbwt/− Amh-Cre+ mice (26) so that Cre-mediated recombination would occur on a heterozygous background. Throughout this study, we refer to two different groups of mice generated by this cross: control (Rbflox/−) and Rb cKO (Rbflox/− Amh-cre+).
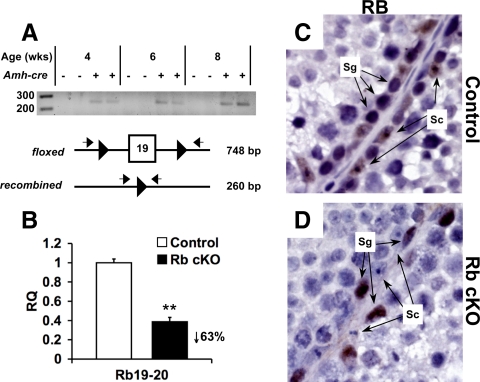
The deletion of Rb occurred specifically in Sertoli cells and was very efficient. First, we showed that recombination occurred in whole-testis DNA (Fig. 1A) by performing PCR. If excision of exon 19 had occurred, a 260-bp product would be amplified (30,31). DNA from Rb cKO testes at 4, 6, and 8 wk of age all contained this 260-bp product, whereas the age-matched control testis DNA was negative. Quantitative RT-PCR against the exon 19 to 20 junction of Rb showed a 63% reduction in RNA levels of Sertoli cells isolated from Rb cKO testes vs. the control Sertoli cells (Fig. 1B). In addition, immunohistochemical staining of 6-wk-old testes showed that loss of RB staining in Rb cKO was specific to Sertoli cells and did not affect its expression in spermatogonia (Fig. 1, panel D vs. panel C).
Figure 1.
Deletion of RB in mutant testes occurs specifically in Sertoli cells. A, PCR amplification of DNA extracted from whole testis of Rbflox/− mice of 4, 6, and 8 wk of age. Primers flanking the loxP sites amplify the recombined Rb conditional allele producing a 260-bp product. B, Quantitative RT-PCR of RNA extracted from isolated Sertoli cells of 6-wk-old animals. Primers were designed flanking the exon 19–20 boundary to show recombination of the Rb conditional allele (**, P < 0.01; control, n = 6; Rb cKO, n = 5). Immunohistochemical staining of RB in 6-wk-old control (C) vs. Rb cKO (D) testis in which Rb staining is ablated in Sertoli cells (Sc) but persistent in spermatogonia (Sg). RQ, Relative quantification.
Infertility in Rb cKO males is progressive
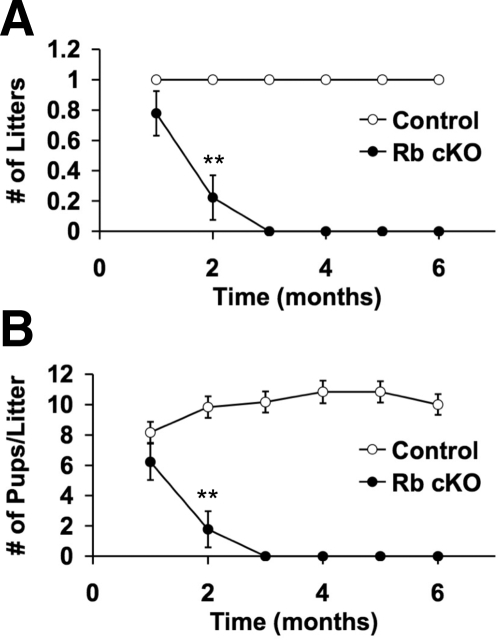
Because mice reach sexual maturity by postnatal d 42, we set up 6-wk-old control and Rb cKO males with age-matched wild-type females to observe their fertility (Fig. 2). During the first month of breeding, there were no significant differences between control and Rb cKO mice in either the number of mice that produced litters [Fig. 2A, control = 1.0 ± 0 (mean litters per mouse ± se); Rb cKO = 0.78 ± 0.15; P > 0.05] or the average size of their litters [Fig. 2B, control = 8.2 ± 0.7 (mean pups per litter per mouse ± se); Rb cKO = 6.2 ± 1.2; P > 0.05]. However, in the second month of breeding, there was a significant reduction in both parameters in Rb cKO mice as compared with control [Fig. 2, control = 1.0 ± 0 (mean litters per mouse ± se); Rb cKO = 0.22 ± 0.15; P < 0.01; control = 9.8 ± 0.7 (mean pups per litter per mouse ± se); Rb cKO = 1.8 ± 1.2; P < 0.01], and in the third month onward, Rb cKO mice were uniformly infertile and produced no pups (Fig. 2). At the end of the fertility tests, when the mice were 7.5 months old, there were no mortalities or cachectic mice in either the Rb cKO or the control groups. Testes harvested from this age group showed no signs of cancer; however, the Rb cKO testes were extremely small and dysgenic [control = 102.2 ± 8.1 (mean testis weight in milligrams ± se); Rb cKO = 9.3 ± 0.5; 92% reduction in testis weight].
Figure 2.
Sertoli cell-specific Rb ablation causes progressive infertility. The fertility of control mice (n = 6) was compared with Rb cKO mice (n = 9) over 6 months. Comparisons of litter size per month per mouse (A) and pups per litter per mouse (B) show the progressive nature of the infertility. **, P < 0.01.
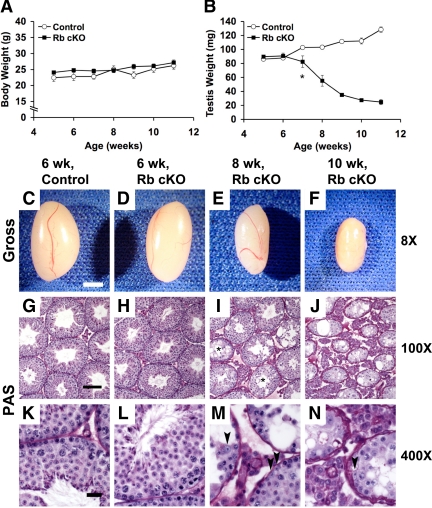
To investigate the onset and progression of the Rb cKO phenotype, we collected testes from mice between 5 and 11 wk of age. There were no significant differences in body weights between Rb cKO and control males between 5 and 11 wk of age (Fig. 3A). Likewise, at 5 and 6 wk of age, when the Rb cKO mice were fertile, there were no significant differences in testis weights between the two groups (Fig. 3B). However, beginning at 7 wk of age, after the completion of the first wave of spermatogenesis, there was a significant decrease in Rb cKO testis weights [Fig. 3B, control = 102.8 ± 2.2 (mean testis weight in milligrams ± se); Rb cKO = 82.5 ± 2.2; P < 0.05], which dramatically declined to 19% of the control weights by 11 wk of age (Fig. 3B). Histologically, the 6-wk-old control (Fig. 3, G and K) and mutant (Fig. 3, H and L) testes were indistinguishable: both had a full complement of germ cells and normal tubular architecture. At 8 wk of age, signs of Sertoli cell dysfunction began to emerge (Fig. 3, I and M). Many seminiferous tubules had lost all elongating spermatids and spermatozoa (Fig. 3I, asterisks), the most mature forms of germ cells contained in the seminiferous epithelium. Tubular width is normally supported by Sertoli cell fluid secretion (32), so the decreasing widths of seminiferous tubules seen at this age were another sign of dysfunction (Fig. 3I). Vacuolization, another hallmark of unhealthy Sertoli cells (32), could also be observed in many tubules (Fig. 3, I and M). All of these signs of Sertoli cell failure were even more severe in the 10-wk-old testis and, in addition, even more immature types of germ cells (round spermatids and many spermatocytes) were also lost (Fig. 3, J and N).
Figure 3.
Progressive testicular atrophy and seminiferous tubule breakdown occurs in Rb cKO mice. Comparisons of body weights (A) and testis weights (B) from 5–11 wk of age show that although body weights do not vary significantly between groups, Rb cKO testis weights significantly decrease at 7 wk of age (P < 0.05) and progressively decrease to less than 19% of the control weights by 11 wk of age (n = 6–10 per data point). Gross morphology (C–F) and periodic Schiff (PAS) staining (G–N) of control (C, G, and K) compared with Rb cKO (D–F, H–J, and L–N) testes. A full complement of germ cells can be observed in control (G and K) and Rb cKO (H and L) testes at 6 wk of age, but representative sections from 8 (I and M) and 10-wk-old (J and N) testes show progressive loss of maturing germ cells in addition to reduction of tubular and luminal widths and increased vacuolization. Asterisks (I) indicate tubules in the 8-wk-old Rb cKO testis that had lost elongating spermatids and spermatozoa. Progressive germ cell loss is presumably not completely caused by loss of Sertoli cells because their presence can be identified in these dysfunctional tubules (arrowheads, M and N). C–F, 2 mm; G–J, 100 μm; K–N, 20 μm.
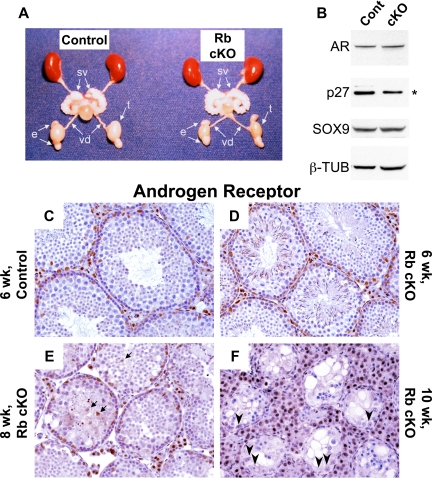
To determine whether the deletion of Rb in Sertoli cells resulted in any hormone-related changes, we performed hormone detection assays on the serum from 6- and 8-wk-old mice and found that there were no significant differences in LH or testosterone in either age group (Table 1). These results were further supported by the normal size of androgen-dependent organs, such as the epididymis and seminal vesicles, in 8-wk-old Rb cKO mice (Fig. 4A). On the other hand, FSH levels were significantly increased in 8-wk-old mutant mice as compared with the control mice (Table 1). Because FSH levels may be related to Sertoli cell number and function (33), we decided to use AR immunohistochemistry to stain Sertoli cells and measure their maturity. At 6 wk of age, AR staining in Rb cKO mice (Fig. 4D) was comparable to the control (Fig. 4C) correlating to the results of Western blot analysis (Fig. 4B). By 8 wk of age, Sertoli cell sloughing could be observed as evidenced by AR-positive cells in the lumen of the seminiferous tubules (Fig. 4E, arrows). By 10 wk of age, although we identified a number of Sertoli cells that remained along the basement membrane, these Sertoli cells displayed profound variability in the intensity of AR staining (Fig. 4F, arrowheads). All of these results indicated that Sertoli cells in the Rb cKO mice had defects in sustained differentiation after the first wave of spermatogenesis.
Table 1.
Serum hormone levels for control and Rb cKO males
| Age (wk) | Genotype | FSH (ng/ml) | LH (ng/ml)a | T (ng/dl)a |
|---|---|---|---|---|
| 6 | Rbflox/− | 34.0 ± 3.5b (8) | 0.26 ± 0.08 (7) | 65.0 ± 13.6 (8) |
| 6 | Rbflox/−; Amh-cre+ | 39.9 ± 4.2b (12) | 0.46 ± 0.11 (11) | 93.3 ± 24.8 (11) |
| 8 | Rbflox/− | 31.1 ± 2.5b (10) | 0.55 ± 0.20 (10) | 88.1 ± 16.6 (10) |
| 8 | Rbflox/−; Amh-cre+ | 51.8 ± 5.9b (10) | 0.99 ± 0.27 (9) | 226.1 ± 88.4 (9) |
Values are means ± se. Values in parentheses are the number of samples used per group.
, Not significant (P < 0.05 by one-way ANOVA).
Differing superscript letters represent statistically different groups as determined Tukey-Kramer honestly significant difference test (P < 0.05).
Figure 4.
Rb cKO Sertoli cells have abnormal expression of markers of maturity. A, Although the testes of 8-wk old Rb cKO mice are smaller than those of the control, the other androgen-dependant organs of the Rb cKO urogenital system appear unchanged (sv, seminal vesicle; vd, vas deferens; e, epididymis; t, testis). B, Western blot analysis of the Sertoli cell markers AR and SOX9 in the control (lane 1) and Rb cKO (lane 2) 6-wk-old whole testis lysates show comparable expression whereas p27 is decreased significantly in Rb cKO [*, P < 0.05, comparing band densitometry between groups (n = 3,3) normalized to β-tubulin (β-TUB)]. C–F, Changes in Sertoli cell function are seen by comparing AR immunohistochemistry in 8- (E) and 10-wk-old (F) Rb cKO testes to 6-wk-old control (C) and Rb cKO (D) testes. Arrows (E) indicate sloughed Sertoli cells present in the tubular lumen of 8-wk-old Rb cKO mice. Arrowheads (F) indicate misregulated expression of AR in 10-wk-old Rb cKO Sertoli cells showing variable intensities of staining. Cont, Control.
Gene expression changes of Rb cKO Sertoli cells indicate improper differentiation
To better assess the mechanism leading to the Rb cKO phenotype, we obtained purified Sertoli cell samples. To do this, we crossed our mice with Sox9-GFP knockin mice, which, in the testis, express green fluorescent protein (GFP) exclusively in Sertoli cells (34). This allowed us to utilize fluorescence-activated cell sorting to enrich for Sertoli cells without culturing (supplemental Fig. S1, A–D, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Also, we decided to use Sertoli cells that had been isolated from 6-wk-old mice, because this was the age at which control and mutant mice were histologically similar and Rb cKO Sertoli cells showed no signs of failure. RNA isolated from Sertoli cells of control (n = 3) and Rb cKO (n = 3) mice were used for microarray analysis (GEO accession no. GSE 17904).
By our analysis (see Materials and Methods), we discovered that deletion of Rb resulted in the down-regulation of 137 genes and the up-regulation of 372 genes (supplemental Fig. S1E and supplemental Table S1). Using the online bioinformatics tool DAVID (35,36), we were able to categorize our results into a number of functional pathways (supplemental Table S2). We observed an up-regulation of many genes involved in cell cycle control, DNA synthesis, and apoptosis and the misregulation of genes that may be important for proper Sertoli cell function such as cell adhesion, tight junction formation, and tissue remodeling (i.e. proteases and their inhibitors). Because Sertoli cell maturity is linked to mitotic quiescence, these results suggest an essential role for RB in the regulation of genes important for the maintenance of the terminally differentiated state of adult Sertoli cells. These points will be described in greater detail in the following sections.
Irregular cell cycle progression seen in Rb cKO Sertoli cells may be mediated by E2Fs
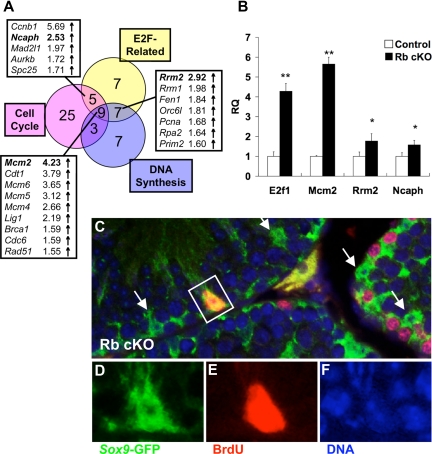
As mentioned above, our microarray analysis revealed the up-regulation of a number of genes involved in cell cycle control and DNA synthesis in Rb cKO Sertoli cells. We hypothesized that these gene changes may be related to E2F activity because RB is a known repressor of E2F transcription factors. To investigate this possibility, we compared our gene changes with those seen in a study investigating the effect of overexpression of E2F1 or E2F3 in mouse embryonic fibroblasts (37); we will refer to this group of genes herein as “E2F-related.” Consistent with our hypothesis, we discovered that many of the genes that were up-regulated by E2F1 or E2F3 in those studies were also up-regulated in Rb cKO Sertoli cells (Fig. 5A and supplemental Table S2). In addition, there is a significant overlap between the genes that are E2F related and those involved in cell cycle control (Fig. 5A, 14 of 42; P < 0.01) and DNA synthesis (Fig. 5A, 16 of 26; P < 0.01). Although E2F3 and E2F4 are the only E2Fs that are normally expressed in adult Sertoli cells (38), we saw a significant up-regulation of E2f1 in Rb cKO Sertoli cells (Fig. 5B) that may have been the result of derepressed E2F3 transcriptional activity (39).
Figure 5.
RB deletion causes impairment of Sertoli cell cycle quiescence. A, Venn diagram of microarray changes in E2F-related, cell cycle-regulatory, and DNA synthesis genes shows the overlap between these pathways. B, Quantitative RT-PCR of select (bold) genes from panel A, and E2f1 confirms the up-regulation of these genes in Rb cKO Sertoli cells (*, P < 0.05; **, P < 0.01; control, n = 6; Rb cKO, n = 5). RQ, Relative quantification. C–F, Immunofluorescent analysis of BrdU (red) and Sox9-GFP (green) indicates that occasional 6-wk-old Rb cKO Sertoli cells show persistent DNA synthesis (S phase). Arrows (C) indicate Sertoli cells that were not BrdU positive.
We used quantitative RT-PCR to determine the relative levels of Mcm2, Rrm2, and Ncaph in Rb cKO Sertoli cells (n = 5) vs. control (n = 6) because these were all putative E2F-target genes that were also involved in DNA synthesis/cell cycle control. We found that these genes were up-regulated in Rb cKO Sertoli cells by both microarray as well as quantitative RT-PCR (Fig. 5B). Minichromosome maintenance deficient 2 (Mcm2) is an indispensable component of the MCM2–7 complex that is loaded onto chromatin during G1 in preparation for S phase (40). Inappropriate expression of MCM2 protein has also been implicated as an early premalignant change (40). Transcription of ribonucleotide reductase M2 (Rrm2) is maximal during S phase (41), and small interfering RNA inhibition of this gene led to decreased proliferation of a variety of cell lines in vitro as well as in vivo (42). Transcription of non-SMC condensin I complex, subunit H (Ncaph) is thought to occur exclusively in proliferating cells with highest expression in the G2 phase of the cell cycle (43). Normally, adult Sertoli cells are postmitotic; therefore, the up-regulation of genes expressed in proliferating cells suggested that Rb cKO Sertoli cells might have been aberrantly cycling. To examine this, we injected 6-wk-old mice with 5-bromo-2-deoxyuridine (BrdU) and stained their testes for BrdU, the cellular incorporation of which is indicative of DNA synthesis/S phase. We never observed BrdU-positive Sertoli cells in control mice, but there were occasional Sertoli cells that labeled with BrdU in Rb cKO mice (Fig. 5, C–F). Performing similar studies in 4-wk-old and 8-wk-old mice revealed that Rb cKO Sertoli cells at both ages were also occasionally BrdU-positive (supplemental Fig. S2), suggesting that Rb cKO Sertoli cells do not completely stop proliferating at any time point in the period that is normally postmitotic.
Increased death of Rb cKO Sertoli cells may be due to the p53 apoptotic pathway
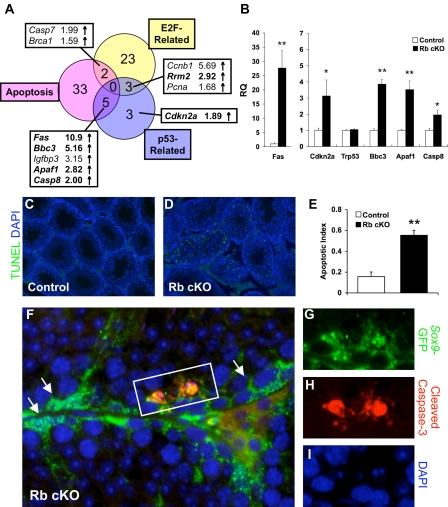
Although we saw a profound up-regulation of cell cycle-related genes in Rb cKO Sertoli cells, the number of cycling Sertoli cells was quite low, and these mice never developed testicular cancer. Due to these findings and our observations of Sertoli cell sloughing (Fig. 4E), we hypothesized that Sertoli cell cycling triggered apoptotic signals that subsequently eliminated these abnormal cells from the testis. Rb cKO microarray pathway analysis showed the up-regulation of 40 genes related to apoptosis (Fig. 6A and supplemental Table S2), thus supporting our hypothesis. Interestingly, pathway analysis also revealed the up-regulation of genes related to the p53 pathway, and the correlation between apoptotic genes and p53-related genes was significant (Fig. 6A, 5 of 40; P < 0.01). When we verified select genes by quantitative RT-PCR, we noticed that although the mRNA level of Trp53, the gene encoding p53, was unchanged, the genes encoding its upstream activator, Cdkn2a (ARF), and its downstream targets, Fas (44), Apaf1 (45), and Bbc3 (46), were up-regulated, suggesting that p53 may have been activated posttranslationally in Rb cKO Sertoli cells (Fig. 6B). Despite the fact that a number of these p53-related genes were not contained in our “E2F-related” gene list, p53 activation may have still been E2F1 dependant because Cdkn2a is a direct transcriptional target of E2F1 (47).
Figure 6.
RB deletion in Sertoli cells causes activation of apoptotic pathways. A, Venn diagram of microarray changes in E2F-related, p53-related, and apoptotic genes shows the overlap between these pathways. B, Quantitative RT-PCR of select (bold) genes from panel A, and Trp53 confirms the up-regulation of these genes in Rb cKO Sertoli cells (*, P < 0.05; **, P < 0.01; control, n = 6; Rb cKO, n = 5). RQ, Relative quantification. C–E, TUNEL (green) staining in 6-wk-old testes is increased in Rb cKO (D) as compared with control (C) as determined by apoptotic index (E) (**, P < 0.01; control, n = 3; Rb cKO, n = 4). F–I, Immunofluorescent analysis of cleaved caspase-3 (red) and Sox9-GFP (green) reveals the activation of caspase-mediated apoptosis in 6-wk-old Rb cKO Sertoli cells. Arrows (I) indicate Sertoli cells that did not stain for cleaved caspase-3. DAPI, 4′,6-diamidino-2-phenylindole.
The genes that we verified by quantitative RT-PCR (Fig. 6B) represented two main apoptosis-initiating pathways, the death receptor pathway and the mitochondrial apoptogenic factor release pathway. In the death receptor pathway, the binding of Fas ligand by Fas death receptor (FAS) induces receptor trimerization and recruitment and cleavage/activation of procaspase-8 (CASP8) (48). In the mitochondrial apoptogenic factor release pathway, BCL2 binding component 3 (BBC3/PUMA) indirectly promotes permeabilization of the mitochondrial membrane leading to the release of cytochrome c and apoptotic peptidase-activating factor 1 (APAF1), which form a holoenzyme complex with procaspase-9 (48,49). These two pathways converge because procaspase-3 is a substrate of both active caspase-8 and caspase-9 (48), and the cleavage of caspase-3 is integral to its activation, which induces DNA fragmentation, a hallmark of apoptosis (48). To determine the functional significance of the up-regulation of these pathways, we examined apoptotic DNA fragmentation in situ by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis. Our results showed a significant increase in the apoptotic index of 6-wk-old Rb cKO testes as compared with controls, but the TUNEL-positive cells appeared to be a mixture of both Sertoli and germ cells (Fig. 6, C–E). To determine whether the apoptotic pathways that we saw up-regulated in our microarray were activated in Sertoli cells specifically, we performed cleaved caspase-3/GFP costaining and found that there were indeed multiple Rb cKO Sertoli cells that stained positively for this key effector of apoptosis (Fig. 6, F–I). This once again suggested that tumor surveillance of the p53 pathway was responsible for preventing a cancer phenotype in Rb cKO testes.
Relationship of Sertoli cell maturational defect to AR transcriptional activity
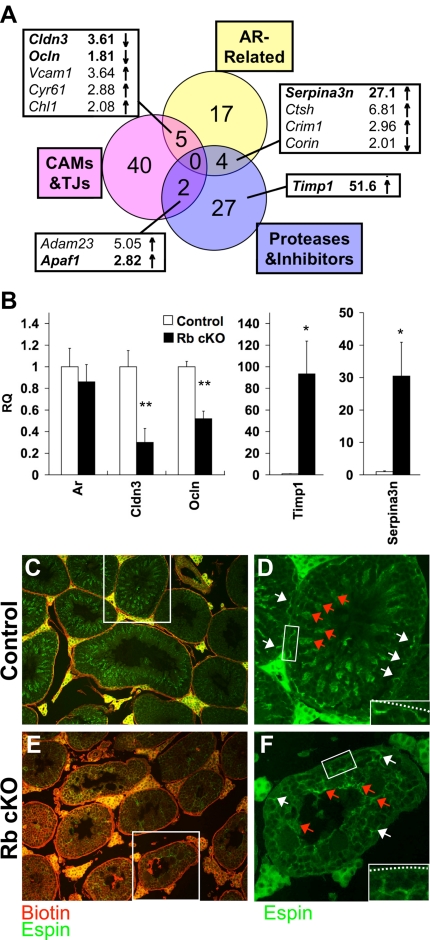
Although increased apoptosis could partially explain the Rb cKO phenotype, it seemed an insufficient explanation for the widespread loss of maturing germ cells because many tubules still contained Sertoli cells (Fig. 3, M and N, and Fig. 4, E and F). Because RB is a known coregulator of AR transcriptional activity (18,19), we hypothesized that defects in Sertoli cell function were due, in part, to misregulation of androgen-responsive genes by RB. Because we had ensured that AR mRNA (Fig. 7B) and protein (Fig. 4B) levels were comparable between 6-wk-old control and Rb cKO mice, we were able to test our hypothesis by comparing our microarray data to Sertoli cell gene lists from the literature that contained putative androgen-regulated genes (50,51,52,53), which we will refer to herein as “AR-related.” Through this comparison, we were able to identify 26 AR-related genes in our study, six of which were down-regulated and 20 of which were up-regulated (Fig. 7A and supplemental Table S2). Of those genes, five were related to cell adhesion molecules and tight junctions, and four were related to proteases and their inhibitors (supplemental Table S2). The overlap between these pathways and AR-related genes was significant (Fig. 7A, nine of 74; P < 0.01), and the alteration of these pathways was also noteworthy because it showed that the histological changes seen in Rb cKO testes may be due to defects in tissue remodeling important for the maintenance of the dynamic interface of cell-cell junctions between Sertoli cells and other cells of the seminiferous tubule (51,54).
Figure 7.
Infertility in Rb cKO mice may be caused by abnormal expression of genes important to tight junction formation and tissue remodeling. A, Venn diagram of changes in AR-related, cell adhesion molecules (CAMs) and tight junctions (TJs), and proteases and their inhibitors shows the overlap between these pathways. B, Quantitative RT-PCR of select (bold) genes from panel A and Ar confirms the misregulation of these genes in Rb cKO Sertoli cells (*, P < 0.05; **, P < 0.01; control, n = 6; Rb cKO, n = 5). RQ, Relative quantification. C and E, A biotin tracer (red) was injected into 6-wk-old control (C) and Rb cKO (E) testes. Presence of biotin in the adluminal space of the Rb cKO mice indicates that these mice have a defect in blood-testis barrier function. C–F, Espin (green) marks the basal ectoplasmic specializations (white arrows) of the blood-testis barrier. The normal curvilinear staining of the basal ectoplasmic specializations in the control (D) has been lost in Rb cKO (F). The insets show the proximity between the blood-testis barriers and the basement membranes (dashed line). Espin also marks the apical ectoplasmic specializations (red arrows) that are important for formation of the sperm head, and this staining is neither organized nor polarized in Rb cKO (F) as they are in the control (D).
One such cell-cell junction is the blood-testis barrier, a specialized occluding junction formed between neighboring Sertoli cells that must cyclically undergo remodeling to allow the passage of maturing germ cells from the basal to the adluminal compartment (54). Two tight junction components of the blood-testis barrier, claudin 3 (Cldn3) and occludin (Ocln), were down-regulated in Rb cKO Sertoli cells (Fig. 7B). Claudin 3 had previously been reported to be transiently associated with newly formed tight junctions at the time that germ cells move from the basal to the adluminal compartment, and its down-regulation was thought to be the main cause of increased permeability of the seminiferous tubules seen in Sertoli cell-specific AR knockout mice (55). To test whether this conclusion was also true in our mouse model, we investigated the permeability of the Rb cKO blood-testis boundary by injecting a biotin tracer into the testicular interstitium. In 4-wk-old mice, biotin remained in the basal compartment of the seminiferous tubule in both the control and Rb cKO mice (supplemental Fig. S3, red), suggesting that the blood-testis barrier was normally established in both genotypes. In 6-wk-old mice, biotin remained in the basal compartment of the seminiferous tubule, close to the basement membrane in the control mice (Fig. 7C, red), but biotin staining pervaded the tubule in the Rb cKO mice (Fig. 7E, red). Loss of integrity of the Rb cKO blood-testis barrier suggested that RB was initially dispensable for the formation of the blood-testis barrier but might be indispensable for its remodeling as maturing germ cells crossed from the basal into the adluminal compartment and that this function might be directly related to the down-regulation of tight junction genes.
Defects in Sertoli cell junction remodeling are both AR related and unrelated
Dynamic assembly of tight and adherens junctions of the seminiferous tubules are thought to be regulated by the balance between proteases and protease inhibitors (54), and protease inhibitors such as tissue inhibitor of metalloproteinase 1 (Timp1) and serine (or cysteine) peptidase inhibitor, clade A, member 3N (Serpina3n) were highly up-regulated in Rb cKO Sertoli cells (Fig. 7B). Although Timp1 is induced by a myriad of factors in the testis (56), one in vitro model suggests that when the collagen network of the basement membrane is perturbed by matrix metalloproteinases, Timp1 expression is induced by negative feedback to limit protease activity (57). Our microarray analysis showed that a variety of extracellular matrix components, such as isoforms of type IV collagen, were up-regulated in Rb cKO Sertoli cells (supplemental Table S2), which also supported the theory that the collagen network was perturbed. Because alterations in the basement membrane are thought to affect blood-testis barrier stability (57), we wanted to visualize the blood-testis barrier of Rb cKO mice. Ectoplasmic specializations are modified adherens junctions that are present between Sertoli cells (basal) and between germ cells and Sertoli cells (apical) (54). Basal ectoplasmic specializations colocalize with the blood-testis barrier (54); therefore, we visualized these structures by staining for espin, a marker of ectoplasmic specializations (58). The blood-testis barrier appeared very disorganized in 6-wk-old Rb cKO testes (Fig. 7F, green, white arrows), and any contact between the barrier and the basement membrane appeared to be lost (Fig. 7F, inset). Because direct contact between tight junctions and basement membrane components is related to junction stability (57), the increased permeability of the blood-testis barrier may be secondary to disruption of the collagen network in addition to altered tight junction expression levels.
In contrast to matrix metalloproteinase inhibitors, serine protease inhibitors are thought to affect adherens junction dynamics between germ cells and Sertoli cells (54). The AR-related serine protease inhibitor Serpina3n was up-regulated in Rb cKO Sertoli cells (Fig. 7B); therefore, to determine the significance of this gene change, we investigated the localization of apical ectoplasmic specializations. Apical ectoplasmic specializations are thought to be important for spermiogenesis, the process of forming the sperm head, and for the prevention of premature release of elongated spermatids from seminiferous tubules (54). Proper function and localization of apical ectoplasmic specializations may be dependant on constant remodeling because the shape and location of the head of elongating spermatids are constantly changing (54). Our studies showed that, similar to the basal ectoplasmic specializations, apical ectoplasmic specializations were also highly disorganized in Rb cKO mice (Fig. 7F, green, red arrows). These observations supported the notion that remodeling of germ cell-Sertoli cell junctions was also impaired and contributed to the most severe defect of Sertoli cell function in Rb cKO mice, the loss of maturing germ cells.
Discussion
Much work has been dedicated to the link between cessation of replication and terminal differentiation. This developmental work is invaluable to determining how cells can overcome replicative senescence and cause cancer. In our study of a Sertoli cell-specific knockout of Rb, we found that although RB was dispensable for initial maturation of Sertoli cells, it was essential for the full maturity of Sertoli cells. Initially, maturation of Rb cKO Sertoli cells was normal as evidenced by testis weights, germ cell maturation, and fertility that were comparable to control. After 6 wk of age, however, decreasing testis weights, loss of maturing germ cells, and infertility indicated a progressive decline in Sertoli cell function. When Sertoli cells were isolated and profiled for changes in gene expression, we observed an up-regulation of genes important for cell cycle progression and a misregulation of genes important for tubular remodeling of cell-cell junctions. These dysfunctional Sertoli cells were progressively eliminated from Rb cKO testes, and our data suggest that this process was mediated by p53-dependant apoptosis.
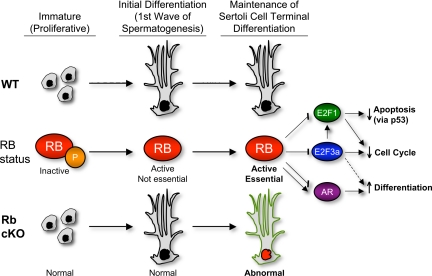
In our model of cell cycle control, RB is indispensable to maintenance of Sertoli cell terminal differentiation through a variety of interactions with transcription factors (Fig. 8). RB’s inhibition of E2F3a may be essential to preventing the up-regulation of E2f1, the expression of which, in turn, could be causing persistent cell cycling. This hypothesis is supported by observations in a variety of other tissues in which concomitant knockout of E2f1 and Rb has rescued abnormal cell cycle reentry (8,11,59). Recent findings revealed that Sertoli cells exit terminal mitosis and reenter the cell cycle when isolated and cultured in vitro (60), and other studies of Sertoli cell-specific knockouts of connexin 43 (Gja1), a gap junction protein important for cell-cell communication, also showed persistent Sertoli cell proliferation beyond the normal proliferative period (61). These results, taken together, indicate that the maintenance of adult Sertoli cells in a nonproliferative state in vivo may depend upon: 1) a fine balance between cell cycle inducers and inhibitors and/or 2) undisturbed Sertoli cell communication with other cells of the seminiferous tubule. Because abnormal Sertoli cell cycling is observed in both our knockout as well as the connexin 43 knockout model and because both knockouts also have impaired support of germ cell maturation, it will be important to ascertain whether deletion of E2f1 from Rb cKO Sertoli cells could prevent persistent Sertoli cell cycling and/or germ cell loss. The result of this double-knockout model may shed light on the possible role of cell cycle proteins in the maintenance of the cycling of the seminiferous tubule.
Figure 8.
A summary of the postulated role of RB in Sertoli cells. In wild-type (WT) Sertoli cells, inhibition of E2F3a by RB prevents expression of E2F1. In Rb cKO Sertoli cells, the aberrant activation of E2F3a and, thus, E2F1 leads to derepression of cell cycle and apoptotic genes. RB may also modulate the expression of AR-regulated genes in WT Sertoli cells; therefore, the lack of RB in Rb cKO Sertoli cells may directly lead to their misregulation. Repression of E2F3a by RB may be responsible for AR-independent gene changes involved in adult Sertoli cell function (i.e. Timp1 down-regulation).
Inappropriate expression of E2F1 may also cause the activation of the p53-dependent apoptotic pathway. We have previously discussed the activation of the p53 pathway by CDKN2A/ARF. Additional gene changes that we discovered in our microarray analysis, such as the up-regulation of Cdt1 (chromatin licensing and DNA replication factor 1) and Cdc6 (cell division cycle 6 homolog), have also been linked to p53 stabilization, which is necessary for its activation because p53 normally has a very short half-life (62). Thus, although replicative senescence had been overcome, activation of p53-dependant apoptosis may be responsible for the prevention of tumor formation. In a variety of other Rb conditional knockout models of cancer, including ovarian cancer (63), small cell lung cancer (64), and medulloblastoma (65), concomitant p53 inactivation has been reported to be indispensable to precipitate oncogenesis. In addition, the p53 pathway is particularly intriguing to investigate especially when considering that a phenotype of progressive Sertoli cell loss, similar to that seen in our model, is observed in the knockout model of Bcl2l2/Bclw, a member of the BCL2 family of antiapoptotic proteins (66,67). In Rb cKO mice, Bbc3/PUMA up-regulation by p53 may be responsible for inhibiting the BCLW protein that would indirectly lead to activation of the proapoptotic protein, BAX, and the induction of apoptosis (68,69). In the Sertoli cell-specific transgenic SV40 large T antigen model in which Sertoli cell proliferation leads to cancer (13), transformation could rely not only on RB inactivation but also the inactivation of p53, another known function of T antigen (70). In the future, it will be important to investigate whether a double knockout of Rb and Trp53 could lead to Sertoli cell oncogenesis.
Alternatively, loss of Sertoli cell expression of RB may only cause impairment of permanent cell cycle quiescence rather than the activation of cell cycle progression. Perhaps hyperplasia or cancer in our Rb cKO model cannot be achieved without a concomitant mitogenic signal (e.g. activins, FSH). It will be interesting to examine a double knockout of Inha and Rb to determine whether the combination of loss of Rb with increased activin signaling can precipitate early tumorigenesis, because these mice show the earliest signs of cancer at 4 wk of age (71). Conversely, although serum FSH, another known Sertoli cell mitogen, is increased in 8-wk-old Rb cKO mice, these cells do not exhibit tumorigenesis. It will be important to examine the activation of FSH signaling pathways in Trp53/Rb double knockouts to determine whether the mitogenic signaling of FSH is p53-dependent.
In addition to investigating the cell cycle and apoptotic defects, it will also be important to determine the ultimate cause of impaired terminal differentiation in our model. Although many of the gene changes relating to differentiation may be caused by misregulation of AR, a number of gene changes, such as the induction of Timp1, have not been reported to be regulated by AR. Still, our evidence suggested that RB may interact specifically with AR in Sertoli cells, meaning these cells may be regulated postmitotically like skeletal muscle in which RB is essential to modulate the tissue-specific transcription factors that control differentiation (72). Alternatively, is it the balance of cell cycle inducers (E2Fs) and cell cycle inhibitors (RB, p27) that perform an essential role in regulating Sertoli cells, specifically regarding their stage-specific functions? Future work should determine whether a double knockout of RB and E2F3a would rescue the Rb cKO functional phenotype without rescuing the cell cycle phenotype as was seen in the elegant studies performed in retinal interneurons (11). If so, this would suggest that proper terminal differentiation of Sertoli cells is dependant more on the balance of the expression of cell cycle proteins rather than the actual maintenance of cell cycle quiescence. This hypothesis is also supported by the extensive amount of literature linking AR to cell cycle proteins both in Sertoli cells (23,24) and prostate cancer cells (73,74). However, it is also possible that activation of p53 alone is responsible for the progressive infertility, if the germ cell loss seen in the Bcl2l2 knockout is intrinsic to Sertoli cell function and not germ cell function as has been hypothesized (75).
In closing, our Rb cKO model that demonstrates Sertoli cell dysfunction has allowed us to arrive at two general conclusions. First, with regard to human infertility, understanding the function of Sertoli cells will lead to better in vitro methods of germ cell maturation that will not only help infertile couples conceive but will also provide a framework for better studies of human causes of male infertility. In addition, the contribution of Sertoli cell dysfunction to human infertility is unknown and has not been very carefully studied. Further understanding the function of Sertoli cells in mouse models will be of great benefit to the understanding of human infertility. Second, our studies attempt to dissect the essential role of RB in differentiation of Sertoli cells. Although we achieved the conclusions in this study by making careful comparisons to existing microarrays and mouse models, we propose that further delineation of these pathways using compound genetic models, as described above, will result in a better understanding of RB’s role in Sertoli cell function which, by comparative studies, will lead to a more universal understanding of how RB controls differentiation and cell cycle regulation in other tissue types as well.
Materials and Methods
Mouse lines and genotyping
Generation of mice containing a null (26) or floxed (65) mutation in the Rb gene and transgenic mice carrying a Cre recombinase driven by the AMH promoter (Amh-Cre) (20) have been described previously. Generation of Sox9-GFP knock-in mice will be described in detail elsewhere (Akiyama, H., manuscript in preparation). Rbwt/− Amh-cre+ Sox9-GFP+ mice were crossed to Rbflox/flox mice to generate control and experimental animals. Tail DNA was used for PCR genotyping and was performed for all alleles according to the manufacturer’s protocol (New England Biolabs, Ipswich, MA). Primers for the Rb-null allele, adapted from Ref. 26, are as follows: RX3, 5′-GCATCTGCATCTTTATCGCAG-3′; RI3.1: 5′-CACCTTAGGCCGGGCAGTG-3′; PGK, 5′-GAAGAACGAGATCAGCAGCC-3′) and produced wild-type (724-bp) and mutant (400-bp) products. Primers for Rb-floxed allele, described in Ref. 30, are as follows: Rb212, 5′-GAAAGGAAAGTCAGGGACATTGGG-3′; Rb18, 5′-GGCGTGTGCCATCAATG-3′) yielding a 748-bp product for the Rb-floxed allele and a 260-bp product for the recombined allele. PCR for Amh-Cre: McreAMH, 5′-AGCTCAGGCCTCTGCAGTTA-3′; McreGene, 5′-AATCGCGAACATCTTCAGGT-3′) produced a 443-bp product. PCR for Sox9-GFP: GfpF, 5′-CAAGATCCGCCACAACATCG-3′; GfpR, 5′-CCAGCAGGACCATGTGATCG-3′ produced a 170-bp product. All primers designed by the laboratory were made using Primer3 online software (76).
Animal care and treatment
Mice were housed with unlimited access to food and water and exposure to 12-h light, 12-h dark cycles in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Baylor College of Medicine Institutional Animal Care and Use Committee. For BrdU incorporation studies, mice were injected with 125–155 mg BrdU/kg body weight (77). For serum collection, mice were anesthetized, and blood was collected by cardiac puncture. Microtainer tubes (BD Biosciences, Franklin Lakes, NJ) were used for serum isolation and sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (http://www.healthsystem.virginia.edu/internet/crr) for detection of FSH, LH, and testosterone. Immediately after cardiac puncture or 2 h after BrdU injection, the mice were euthanized, and the desired tissues were harvested. In general, one testis was fixed for histological analysis and the other was frozen for DNA (−20 C) or protein (−80 C) analyses, or both testes were collected for Sertoli cell isolation.
Sertoli cell isolation, RNA extraction, microarray, and quantitative PCR
After removal of the tunica albuginea, testes were dissociated by sequential collagenase and trypsin treatment (78). Sox9-GFP+ Sertoli cells were isolated from this single-cell preparation in the Baylor College of Medicine Cytometry and Cell Sorting Core using a FACSAriaII cell sorter (BD Biosciences). Representative graphs showing gating of channels are displayed in supplemental Fig. S1. Collected cells were centrifuged, and RNA was extracted using the RNeasy Micro Kit (QIAGEN, Valencia, CA). Microarray was performed by the Children’s Health and Nutrition Research Center Core of Baylor College of Medicine utilizing the MouseWG-6 v2.0 chip (Illumina, San Diego, CA). Results were analyzed using GeneSpring GX Version 9.0.0 (Agilent Technologies, Palo Alto, CA) by filtering for flags and then applying an unpaired t test (P < 0.05) on genes that had expression above the 20th percentile and were more than 1.5-fold different from control. For quantitative PCR applications, Sertoli cell RNA isolated from control (n = 6) and Rb cKO (n = 5) mice was converted to cDNA using SuperScriptIII (Invitrogen, Carlsbad, CA). Microarray data were deposited into National Center for Biotechnology Information’s Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo; accession no. GSE17904). Sybr Green primers were designed using PrimerExpress (Applied Biosystems, Foster City, CA) and are listed in Table 2 or were previously reported (25,79). The remaining genes were assayed with Taqman probes (Applied Biosystems): Rrm2 (Mm00485881_g1), Ncaph (Mm00522764_m1), Bbc3 (Mm00519268_m1), Serpina3n (Mm00776439_m1). Quantitative PCRs were performed as described elsewhere (25) using Gapdh as an endogenous control for relative quantification.
Table 2.
Quantitative PCR primer sequences
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Apaf1 | AGCACAACTCCGGTGCAAA | TGTGCCTCAGGGTTTTCTCTCT |
| Ar | GGAATTCCTGTGCATGAAAGC | GTAGTTCATTCGAAGTTCATCAAAGAA |
| Casp8 | GAACCTGGTATATTCAGTCACTTTGC | CCAGTCAGGATGCTAAGAATGTCA |
| Cdkn2a | AGACCGACGGGCATAGCTT | TAGCTCTGCTCTTGGGATTGG |
| Cldn3 | GCTGGCATCTCCCCTTCTC | TTTGTCCATTCGGCTTGGA |
| Fas | CCGCCCGCTGTTTTCC | TTAACTGTGAGCCAGCAAGCA |
| Mcm2 | CACCGATGAGGACGTGAAGA | GGTGCAATGCTGGCAAAGAT |
| Ocln | CTGGATGACTACAGAGAGGAGAGTGA | TTCATCAGCAGCAGCCATGT |
| Timp1 | CCACCCACAGACAGCCTTCT | GGTATAAGGTGGTCTCGTTGATTTC |
Western blot analysis
Western blot analysis was performed as described in Ref. 80. Before storage at −80 C, testis samples were homogenized in RIPA buffer [50 mm Tris, 150 mm NaCl, 0.1% sodium dodecyl sulfate, 1.0% Nonidet P-40, 0.5% sodium deoxycholate (pH 8.0), and complete EDTA-free protease inhibitor cocktail tablets (Roche, Indianapolis, IN)]. After determination of protein concentration, 100 μg of each sample was separated using 4–12% Bis-Tris gels (Invitrogen). Proteins were transferred onto a nitrocellulose membrane (Whatman, Dassel, Germany) as instructed by the manufacturer. Membranes were probed at 4 C overnight with primary antibodies diluted in 5% milk/PBSt (PBS with 0.05% Tween-20) followed by the appropriate peroxidase-conjugated secondary antibody (1:10,000 dilution; Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature. Primary antibodies were diluted as follows: AR (sc-816; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1:250; p27 (sc-528, Santa Cruz), 1:500; Sox9 (sc-20095, Santa Cruz), 1:500; and β-tubulin (T4026, Sigma), 1:3000. Detection was performed using SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical Co., Rockford, IL) and Kodak BioMax XAR film (Sigma, St. Louis, MO). Band densitometry was determined using ImageJ software (National Institutes of Health, Bethesda, MD).
Histology, immunohistochemistry, and immunofluorescence
Tissues were fixed in Bouin’s fixative or 10% neutral buffered formalin before paraffin embedding. Tissue embedding, sectioning, and staining for periodic acid Schiff and hematoxylin were performed by the Histology Core of the Department of Pathology of Baylor College of Medicine. Immunohistochemistry and immunofluorescence were performed on tissues fixed in formalin as described elsewhere (79, 81) with minor modifications. Briefly, 5-μm sections were cleared and rehydrated. Antigen retrieval was performed by boiling the samples in 10 mm citrate buffer (pH 6.0). Samples were blocked (3% BSA/10% serum in PBS) for 1 h at room temperature and then incubated with primary antibodies overnight at 4 C. This was followed by incubation in the appropriate biotinylated secondary antibody (Vector Laboratories, Inc., Burlingame, CA; 1:200) or fluorescent secondary antibody (Molecular Probes, Inc., Eugene, OR; 1:500). Primary antibodies were diluted as follows: AR (sc-816, Santa Cruz), 1:200; RB (G3–245, BD Biosciences), 1:100; BrdU (Bu20a, DAKO Corp., Carpinteria, CA), 1:100; GFP (A11122, Invitrogen), 1:50; and GFP (CLONTECH Laboratories, Inc., Palo Alto, CA), 1:50. Vectastain ABC kit (Vector Laboratories) followed by diaminobenzidine incubation was used to visualize biotinylated secondary antibodies for immunohistochemistry which were counterstained with hematoxylin. Fluorescent samples were mounted with Vectashield containing 4′,6-diamidino-2-phenylindole (Vector).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
TUNEL analysis was performed on 6-wk-old testis sections as described previously (25) utilizing the ApopTag Plus fluorescein in situ apoptosis detection kit (Chemicon, Temecula, CA) as per the manufacturer’s guidelines. Apoptotic index was determined by counting the fraction of tubules that contained one or more apoptotic cells and was assessed in at least 10 high-powered fields (×100) for at least three biological samples per genotype.
Biotin tracer assay
This assay was performed as described previously (55) with minor modifications. Animals (6 wk of age) were anesthetized, and after their testes were exposed, a small opening was made in the tunica albuginea. Twenty-five microliters of 10 mg/ml EZ-Link Sulfo-NHS-LC-Biotin (Thermo Scientific, Rockland, IL) that had been freshly prepared in PBS containing 1 mm CaCl2 was injected into the interstitium. The contralateral testis was injected with calcium chloride solution alone and served as a negative control. After 30 min had elapsed, the animals were euthanized, and the testes were harvested and fixed in 10% neutral buffered formalin. Testis sections were probed sequentially with streptavidin-Texas Red (1:400 in PBS; Thermo Scientific) to detect biotin and then anti-espin antibody (1:400, BD Biosciences) followed by Alexa Fluor 488 donkey anti-mouse secondary antibody (Molecular Probes).
Statistical analysis
Statistical analysis used JMP 7.0.1 software (SAS institute). Statistical significance was determined by one-tailed t test assuming unequal variance for two-sample comparison and by one-way ANOVA followed by Tukey honestly significant difference test for multiple sample comparisons. Χ2 test was used to determine the significance of association between groups in the Venn diagrams of the microarray (i.e. if the overlap between groups was at an expected frequency or at a higher frequency than would be predicted if there was no association).
Supplementary Material
Acknowledgments
We thank Dr. Tyler Jacks and Dr. Anton Berns for generously depositing the Rb-null and Rb-floxed mice, respectively, in the National Cancer Institute’s Mouse Repository of the Mouse Models of Human Cancer Consortium. We also thank Dr. Richard Behringer for providing us with access to the Sox9-GFP mice and Dr. Yi-Nan Lin and Dr. Jing Meng for assistance with experimental protocols.
This work was supported by National Institutes of Health Grant CA60651 (to M.M.M.) and the Edward J. and Josephine G. Hudson Scholar Fund (to R.L.N.). R.L.N. is also supported by the Baylor College of Medicine Medical Scientist Training Program. The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development/National Institutes of Health (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934. These funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: AMH, anti-Müllerian hormone; AR, androgen receptor; BrdU, 5-bromo-2-deoxyuridine; CDK, cyclin-dependent kinase; CDKI, CDK inhibitor; GFP, green fluorescent protein; ID, inhibitor of DNA binding; RB, retinoblastoma; SV40, simian virus 40; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
References
- Skinner MK, Griswold MD 2005 Sertoli cell biology. Boston: Elsevier Academic Press [Google Scholar]
- Vergouwen RP, Jacobs SG, Huiskamp R, Davids JA, de Rooij DG 1991 Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil 93:233–243 [DOI] [PubMed] [Google Scholar]
- Walker WH 2003 Molecular mechanisms controlling Sertoli cell proliferation and differentiation. Endocrinology 144:3719–3721 [DOI] [PubMed] [Google Scholar]
- Lipinski MM, Jacks T 1999 The retinoblastoma gene family in differentiation and development. Oncogene 18:7873–7882 [DOI] [PubMed] [Google Scholar]
- Nguyen DX, McCance DJ 2005 Role of the retinoblastoma tumor suppressor protein in cellular differentiation. J Cell Biochem 94:870–879 [DOI] [PubMed] [Google Scholar]
- Goodrich DW 2006 The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene 25:5233–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh MS, Parker MH, Scimè A, Parks R, Rudnicki MA 2004 Rb is required for progression through myogenic differentiation but not maintenance of terminal differentiation. J Cell Biol 166:865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SD, Yuan TL, Miller ES, Lee EY, Caron A, Lees JA 2008 The retinoblastoma protein tumor suppressor is important for appropriate osteoblast differentiation and bone development. Mol Cancer Res 6:1440–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimè A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA 2005 Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1α. Cell Metab 2:283–295 [DOI] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ 2001 Retinoblastoma protein partners. Adv Cancer Res 82:1–54 [DOI] [PubMed] [Google Scholar]
- Chen D, Opavsky R, Pacal M, Tanimoto N, Wenzel P, Seeliger MW, Leone G, Bremner R 2007 Rb-Mediated neuronal differentiation through cell-cycle-independent regulation of E2f3a. PLoS Biol 5:e179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsberger DR, Buchold GM, Leal MC, Kiesewetter SE, O'Brien DA, Hess RA, Françca LR, Kiyokawa H, Cooke PS 2005 Cell-cycle inhibitors p27Kip1 and p21Cip1 regulate murine Sertoli cell proliferation. Biol Reprod 72:1429–1436 [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Behringer RR, Cate RL, Harwood KA, Idzerda RL, Brinster RL, Palmiter RD 1992 Directed expression of an oncogene to Sertoli cells in transgenic mice using mullerian inhibiting substance regulatory sequences. Mol Endocrinol 6:1403–1411 [DOI] [PubMed] [Google Scholar]
- Chaudhary J, Sadler-Riggleman I, Ague JM, Skinner MK 2005 The helix-loop-helix inhibitor of differentiation proteins induce post-mitotic terminally differentiated Sertoli Cells to re-enter the cell cycle and proliferate. Biol Reprod 72:1205–1217 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG, Hsueh AJW, Bradley A 1992 α-Inhibin is a tumor-suppressor gene with gonadal specificity in mice. Nature 360:313–319 [DOI] [PubMed] [Google Scholar]
- Cipriano SC, Chen L, Burns KH, Koff A, Matzuk MM 2001 Inhibin and p27 interact to regulate gonadal tumorigenesis. Mol Endocrinol 15:985–996 [DOI] [PubMed] [Google Scholar]
- Burns KH, Agno JE, Sicinski P, Matzuk MM 2003 Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin α knockout mice. Mol Endocrinol 17:2053–2069 [DOI] [PubMed] [Google Scholar]
- Lu J, Danielsen M 1998 Differential regulation of androgen and glucocorticoid receptors by retinoblastoma protein. J Biol Chem 273:31528–31533 [DOI] [PubMed] [Google Scholar]
- Yeh S, Miyamoto H, Nishimura K, Kang H, Ludlow J, Hsiao P, Wang C, Su C, Chang C 1998 Retinoblastoma, a tumor suppressor, is a coactivator for the androgen receptor in human prostate cancer DU145 cells. Biochem Biophys Res Commun 248:361–367 [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE 2004 Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467 [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Kero J, Suominen J, Toppari J 2001 Differential expression and regulation of the retinoblastoma family of proteins during testicular development and spermatogenesis: roles in the control of germ cell proliferation, differentiation and apoptosis. Oncogene 20:1343–1356 [DOI] [PubMed] [Google Scholar]
- Tan KA, Turner KJ, Saunders PT, Verhoeven G, De Gendt K, Atanassova N, Sharpe RM 2005 Androgen regulation of stage-dependent cyclin D2 expression in Sertoli cells suggests a role in modulating androgen action on spermatogenesis. Biol Reprod 72:1151–1160 [DOI] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Chen R, Matzuk MM 2008 Conditional deletion of the retinoblastoma (Rb) gene in ovarian granulosa cells leads to premature ovarian failure. Mol Endocrinol 22:2141–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA 1992 Effects of an Rb mutation in the mouse. Nature 359:295–300 [DOI] [PubMed] [Google Scholar]
- Clarke AR, Maandag ER, van Roon M, van der Lugt NM, van der Valk M, Hooper ML, Berns A, te Riele H 1992 Requirement for a functional Rb-1 gene in murine development. Nature 359:328–330 [DOI] [PubMed] [Google Scholar]
- Lee EY, Chang CY, Hu N, Wang YC, Lai CC, Herrup K, Lee WH, Bradley A 1992 Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359:288–294 [DOI] [PubMed] [Google Scholar]
- Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, Yang Y, Opavska J, Wilson P, Thompson JC, Ostrowski MC, Rosol TJ, Woollett LA, Weinstein M, Cross JC, Robinson ML, Leone G 2003 Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421:942–947 [DOI] [PubMed] [Google Scholar]
- Vooijs M, van der Valk M, te Riele H, Berns A 1998 Flp-mediated tissue-specific inactivation of the retinoblastoma tumor suppressor gene in the mouse. Oncogene 17:1–12 [DOI] [PubMed] [Google Scholar]
- Vooijs M, te Riele H, van der Valk M, Berns A 2002 Tumor formation in mice with somatic inactivation of the retinoblastoma gene in interphotoreceptor retinol binding protein-expressing cells. Oncogene 21:4635–4645 [DOI] [PubMed] [Google Scholar]
- Russell LD 1990 Histological and histopathological evaluation of the testis, 1st ed. Clearwater, FL: Cache River Press [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS 2003 Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125:769–784 [DOI] [PubMed] [Google Scholar]
- Nel-Themaat L, Vadakkan TJ, Wang Y, Dickinson ME, Akiyama H, Behringer RR 2009 Morphometric analysis of testis cord formation in Sox9-EGFP mice. Dev Dyn 238:1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003 DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3 [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA 2009 Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- Ishida S, Huang E, Zuzan H, Spang R, Leone G, West M, Nevins JR 2001 Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol Cell Biol 21:4684–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Darwish KS, Parvinen M, Toppari J 2006 Differential expression of members of the E2F family of transcription factors in rodent testes. Reprod Biol Endocrinol 4:63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao KM, McMahon SL, Farnham PJ 1994 Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Genes Dev 8:1526–1537 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Dutta A 2005 Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabes A, Thelander L 2000 Controlled protein degradation regulates ribonucleotide reductase activity in proliferating mammalian cells during the normal cell cycle and in response to DNA damage and replication blocks. J Biol Chem 275:17747–17753 [DOI] [PubMed] [Google Scholar]
- Heidel JD, Liu JY, Yen Y, Zhou B, Heale BS, Rossi JJ, Bartlett DW, Davis ME 2007 Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin Cancer Res 13:2207–2215 [DOI] [PubMed] [Google Scholar]
- Cabello OA, Eliseeva E, He WG, Youssoufian H, Plon SE, Brinkley BR, Belmont JW 2001 Cell cycle-dependent expression and nucleolar localization of hCAP-H. Mol Biol Cell 12:3527–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH 1998 p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 188:2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni MC, Hickman ES, Lazzerini Denchi E, Caprara G, Colli E, Cecconi F, Müller H, Helin K 2001 Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol 3:552–558 [DOI] [PubMed] [Google Scholar]
- Wang P, Yu J, Zhang L 2007 The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci USA 104:4054–4059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanian A, Iaquinta PJ, Verona R, Lees JA 2004 Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev 18:1413–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr I, Debatin KM 2001 Cellular stress response and apoptosis in cancer therapy. Blood 98:2603–2614 [DOI] [PubMed] [Google Scholar]
- Callus BA, Moujallad DM, Silke J, Gerl R, Jabbour AM, Ekert PG, Vaux DL 2008 Triggering of apoptosis by Puma is determined by the threshold set by prosurvival Bcl-2 family proteins. J Mol Biol 384:313–323 [DOI] [PubMed] [Google Scholar]
- Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, Braun RE 2007 Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol 21:895–907 [DOI] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, Verhoeven G 2006 The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol 20:321–334 [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant'Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C 2006 Androgen receptor in sertoli cell is essential for germ cell nursery and junctional complex formation in mouse testes. Endocrinology 147:5624–5633 [DOI] [PubMed] [Google Scholar]
- Sadate-Ngatchou PI, Pouchnik DJ, Griswold MD 2004 Identification of testosterone-regulated genes in testes of hypogonadal mice using oligonucleotide microarray. Mol Endocrinol 18:422–433 [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY 2004 Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25:747–806 [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE 2005 Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA 102:16696–16700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønning LM, Wang JE, Ree AH, Haugen TB, Taskén K, Taskén KA 2000 Regulation of tissue inhibitor of metalloproteinases-1 in rat Sertoli cells: induction by germ cell residual bodies, interleukin-1α, and second messengers. Biol Reprod 62:1040–1046 [DOI] [PubMed] [Google Scholar]
- Siu MK, Lee WM, Cheng CY 2003 The interplay of collagen IV, tumor necrosis factor-α, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology 144:371–387 [DOI] [PubMed] [Google Scholar]
- Bartles JR, Wierda A, Zheng L 1996 Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci 109:1229–1239 [DOI] [PubMed] [Google Scholar]
- Chau BN, Wang JY 2003 Coordinated regulation of life and death by RB. Nat Rev Cancer 3:130–138 [DOI] [PubMed] [Google Scholar]
- Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, de Rooij DG 2009 Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod 80:1084–1091 [DOI] [PubMed] [Google Scholar]
- Sridharan S, Brehm R, Bergmann M, Cooke PS 2007 Role of connexin 43 in Sertoli cells of testis. Ann NY Acad Sci 1120:131–143 [DOI] [PubMed] [Google Scholar]
- Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, Wagle N, Hwang DS, Dutta A 2003 A p53-dependent checkpoint pathway prevents rereplication. Mol Cell 11:997–1008 [DOI] [PubMed] [Google Scholar]
- Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY 2003 Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res 63:3459–3463 [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A 2003 Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4:181–189 [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A 2000 Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev 14:994–1004 [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, MacGregor GR 1998 Testicular degeneration in Bclw-deficient mice. Nat Genet 18:251–256 [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Köntgen F, Adams JM, Cory S 1998 Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 95:12424–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Amy SP, Mahar PL, Lindsten T, Knudson CM, Thompson CB, Korsmeyer SJ, MacGregor GR 2001 BCLW mediates survival of postmitotic Sertoli cells by regulating BAX activity. Dev Biol 239:295–308 [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC 2005 Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17:393–403 [DOI] [PubMed] [Google Scholar]
- Levine AJ 2009 The common mechanisms of transformation by the small DNA tumor viruses: the inactivation of tumor suppressor gene products: p53. Virology 384:285–293 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Shou W, Coerver KA, Lau AL, Behringer RR, Finegold MJ 1996 Transgenic models to study the roles of inhibins and activins in reproduction, oncogenesis, and development. Recent Prog Horm Res 51:123–157 [PubMed] [Google Scholar]
- De Falco G, Comes F, Simone C 2006 pRb: master of differentiation. Coupling irreversible cell cycle withdrawal with induction of muscle-specific transcription. Oncogene 25:5244–5249 [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C 2002 Androgen receptor (AR) coregulators: an overview. Endocr Rev 23:175–200 [DOI] [PubMed] [Google Scholar]
- Martinez ED, Danielsen M 2002 Loss of androgen receptor transcriptional activity at the G(1)/S transition. J Biol Chem 277:29719–29729 [DOI] [PubMed] [Google Scholar]
- Russell LD, Warren J, Debeljuk L, Richardson LL, Mahar PL, Waymire KG, Amy SP, Ross AJ, MacGregor GR 2001 Spermatogenesis in Bclw-deficient mice. Biol Reprod 65:318–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H 2000 Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- Yan W, Kero J, Huhtaniemi I, Toppari J 2000 Stem cell factor functions as a survival factor for mature Leydig cells and a growth factor for precursor Leydig cells after ethylene dimethane sulfonate treatment: implication of a role of the stem cell factor/c-Kit system in Leydig cell development. Dev Biol 227:169–182 [DOI] [PubMed] [Google Scholar]
- Zhao M, Rohozinski J, Sharma M, Ju J, Braun RE, Bishop CE, Meistrich ML 2007 Utp14b: a unique retrogene within a gene that has acquired multiple promoters and a specific function in spermatogenesis. Dev Biol 304:848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Vieyra C, Chen R, Matzuk MM 2007 Effects of granulosa cell-specific deletion of Rb in Inha-α null female mice. Endocrinology 148:3837–3849 [DOI] [PubMed] [Google Scholar]
- Lin YN, Roy A, Yan W, Burns KH, Matzuk MM 2007 Loss of zona pellucida binding proteins in the acrosomal matrix disrupts acrosome biogenesis and sperm morphogenesis. Mol Cell Biol 27:6794–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, Braun RE, Rajkovic A, Matzuk MM 2006 TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci USA 103:4982–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.