Abstract
Several brittle culm (bc) mutants known in grasses are considered excellent materials to study the process of secondary cell wall formation. The brittle phenotype of the rice bc5 (brittle node) mutant appears exclusively in the developed nodes, which is distinct from other bc mutants (bc1, 2, 3, 4, 6 and 7) that show the brittle phenotype in culms and leaves. To address the defects of the rice bc5 mutant in node-specific cell wall formation, we analyzed tissue morphology and cell wall composition. The bc5 mutation was found to affect the cell wall deposition of node sclerenchyma tissues at 1 week after heading, the stage at which the cell wall sugar content is reduced, in the bc5 nodes, compared with wild-type nodes. Moreover, decreased accumulation of lignin and thickness of cell walls in the sclerenchyma tissues were also observed in the bc5 nodes. The amounts of cellulose and hemicellulose were reduced to 53 and 65% of those in the wild-type plants, respectively. Sugar composition and glycosidic linkage analyses of the hemicellulose showed that the accumulation of glucuronosyl arabinoxylan in bc5 nodes was perturbed by the mutation. The bc5 locus was narrowed to an approximately 3.1 Mb region of chromosome 2, where none of the other bc genes is located. The bc5 mutation appeared to reduce the expression levels of the OsCesA genes in the nodes after heading. The results indicate that the BC5 gene regulates the development of secondary cell walls of node sclerenchyma tissues.
Keywords: Cellulose • Glucuronosyl arabinoxylan • Lignin • Rice node • Sclerenchyma tissue • Secondary cell walls
Introduction
Plant cell walls constitute the skeletal structures of plant bodies, and determine the mechanical strength of the plant bodies. Cell wall polysaccharides, the major fraction of the cell walls, consisting mainly of pectin, hemicellulose and cellulose, are different between grasses and dicot plants, especially in terms of their non-cellulosic components: β-1,3:1,4-glucan is present exclusively in grasses, while dicot plants are rich in pectic substances and xyloglucan (Carpita 1996). Higher plants develop two different types of cell walls, i.e. primary and secondary cell walls. Secondary cell walls are formed inside primary cell walls after the cessation of cell growth, and develop particularly in sclerenchyma tissues and xylem elements (Reiter 2002). The developed secondary cell walls presumably provide plant bodies with mechanical strength (Carpita and Gibeaut 1993, Gibeaut and Carpita 1994). It is likely that the mechanism for the development of secondary cell walls in grasses is different from that in dicot plants.
In Arabidopsis, mutant screening related to the mechanical strength of inflorescence stems has identified several genes involved in the development of xylem elements and interfascicular fiber cells (Turner and Somerville 1997, Burk et al. 2001, Zhong et al. 2002, Zhong et al. 2004). The irregular xylem (irx) 1, irx3 and irx5 genes have defects in their cellulose synthase catalytic subunits (CesAs), and exhibit collapsed xylem caused by a lack of resistance to the negative pressure due to water transport (Taylor et al. 1999, Taylor et al. 2000, Taylor et al. 2003). IRX7/FRAGILE FIBER (FRA) 8, IRX8 and IRX9, which encode proteins belonging to glycosyltransferase (GT) families 47, 8 and 43, respectively (Bourne and Henrissat 2001), appear to be required for normal vessel morphology and the accumulation of xylan and cellulose in inflorescence stems (Zhong et al. 2005, Lee et al. 2007, Peña et al. 2007, Persson et al. 2007). Recently, transcript profiling of the genes expressed during xylem differentiation in Zinnia and Arabidopsis has led to the identification of a plant-specific family of transcription factors containing a NAC domain which regulate the formation of secondary cell walls. Arabidopsis vascular-related NAC domain (VND) 6 and VND7 are transcriptional switches for the differentiation of metaxylem and protoxylem vessels, respectively, which control morphogenetic events, including the formation of secondary cell walls in the tissues (Demura et al. 2002, Kubo et al. 2005). Other NAC genes, NAC SECONDARY WALL THICKENING PROMOTING FACTOR (NST) 1 and NST2, regulate secondary cell wall thickening in the anther endothelium essential for its normal dehiscence in Arabidopsis (Mitsuda et al. 2005). In addition, the NST3/SECONDARY WALL-ASSOCIATED NAC DOMAIN (SND) 1, MYB46 and INTERFASCICULAR FIBERLESS (IFL) 1 were reported to participate in transcriptional regulation in the formation of secondary cell walls (Zhong and Ye 1999, Zhong et al. 2006, Mitsuda et al. 2007, Zhong et al. 2007a). Although various factors involved in the biosynthesis of secondary cell walls in inflorescence stems, leaves and roots have been found in Arabidopsis, those in grasses remain to be identified. In particular, very few studies have investigated the mechanisms responsible for the formation of secondary cell walls in the nodes, which do not develop in dicots as in monocots.
Several bc mutants are classically known in grasses. The barley bc mutants, ohichi-hen, shiroseto-hen and kobinkatagi-hen, which have easily broken culms and leaves, show reduced levels of cellulose compared with the corresponding wild-type lines, whereas they have normal levels of lignin, pectin and hemicellulose (Kokubo et al. 1989, Kokubo et al. 1991). In rice, the disruption of CesA genes by the endogenous retrotransposon TOS17 causes a strong brittle culm phenotype and severe dwarfism due to deficient growth (Tanaka et al. 2003). The amounts of cellulose in these mutants are drastically reduced to 8.9–25.5% of those of wild-type plants, causing reduced secondary cell wall thickness. The rice classic mutant bc1 with a decreased level of cellulose has a defect in a COBRA-like protein that may function in the formation of secondary cell walls (Li et al. 2003). While rice bc1, 2, 3, 4, 6 and 7 show brittle phenotypes in culms and leaves (Nagao and Takahashi 1963, Takahashi et al. 1968, Iwata and Omura 1977, Librojo and Khush 1986, Singh et al. 1994, Yan et al. 2007), bc5 shows the brittle phenotype in nodes but not in culms or leaves. These observations suggest the intriguing possibility that the secondary cell wall formation in nodes is regulated independently from that in culms and leaves. In this study, we found that the bc5 mutant has reduced secondary cell wall thickness in the nodes, and the cell wall composition was specifically altered in the nodes. On the basis of these results, we have discussed the possible functions of the BC5 gene in the formation of secondary cell walls in nodes.
Results
General phenotype of the brittle culm 5 mutant
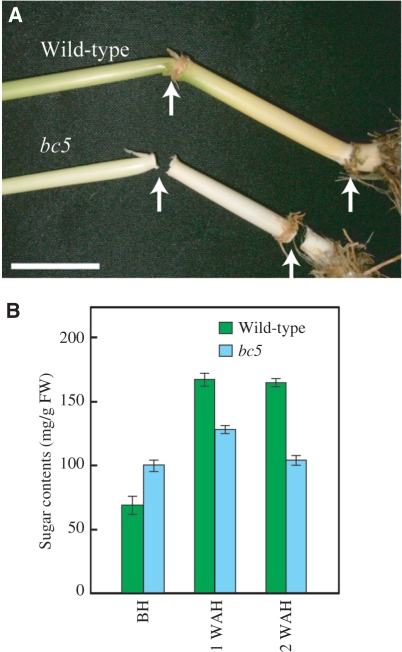
We first examined the brittle phenotype caused by bc5 mutation in various parts of rice plants. As the mutant has been known as ‘brittle nodes’, in spite of its registration as ‘brittle culm 5’, the brittle phenotype of the mutant is restricted to developed nodes, and is not observed in other parts of the plant, including culms and leaves (Fig. 1A). Other than the brittleness in the nodes, we could not find any phenotype different from that of wild-type plants. In most cases, the nodes of the bc5 mutant were broken at junctions to the upper culms. In addition, the brittle phenotype of the bc5 mutant was significant in matured nodes after heading (AH), but was rarely observed before heading (BH), indicating that the BC5 gene is involved in the formation of secondary cell walls in nodes.
Fig. 1.
‘Brittle node’ phenotype of the bc5 mutant. (A) Nodes of the bc5 mutant break easily by bending. Arrows indicate nodes of bc5 mutant and wild-type plants after hand-bending. The scale bar indicates 2 cm. (B) Total sugar content of cell walls in nodes BH, and at 1 and 2 WAH. Green and blue bars indicate the sugar content in wild-type and bc5 mutant plants, respectively. Vertical bars show standard errors (n = 3).
As alterations in the deposition of cell wall polysaccharides influence the mechanical strength of plant bodies, we measured the levels of cell wall polysaccharides in nodes at various developmental stages. In wild-type plants, the amounts of cell wall polysaccharides increased to 167 mg g−1 fresh weight at 1 week after heading (WAH), which was nearly twice as much as that BH. This observation indicates that polysaccharide deposition in secondary cell walls in the nodes occurs AH. On the other hand, the bc5 mutant did not show a significant increase in cell wall polysaccharide levels in the nodes at 2 WAH (Fig. 1B). These results suggest that the BC5 gene regulates the deposition of cell wall polysaccharides in nodes.
Morphology of sclerenchyma cells in the bc5 mutant
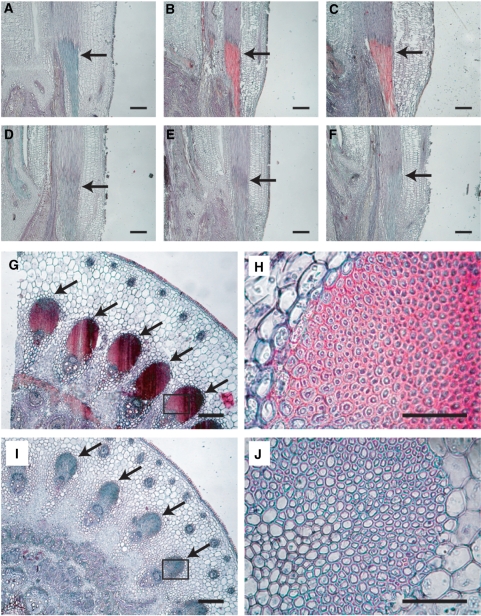
To determine the alterations in cell wall structure and thickness that are responsible for the brittle phenotype, we compared the cell wall morphology in bc5 nodes with that in wild-type nodes by microscopy. In rice nodes, peripheral vascular tissue cells and sclerenchyma cells under the epidermal layer develop thickened cell walls, and are presumed to provide mechanical strength to the plant body. In longitudinal sections, while the sclerenchyma tissues in wild-type nodes showed substantial staining with Safranin O and Fast Green FCF at 1 WAH (Fig. 2A–C), the tissues in the bc5 nodes were not stained even at 2 WAH (Fig. 2D–F). Although sclerenchyma tissues developed not only in nodes but also in culms, the brittle phenotype was not observed in the culms of the bc5 mutant. In addition, no significant difference was observed between the bc5 mutant and the wild-type plant, with regard to the sclerenchyma tissues of culms (Supplementary Fig. S1). This observation suggests that the formation of secondary cell walls in nodes is regulated independently from that in culms. In transverse sections, the bc5 mutant appeared to have a defect in cell wall thickening in the sclerenchyma tissues of the nodes (Fig. 2G–J). These results suggest that the bc5 mutation affects the formation of the secondary cell walls of nodes, particularly in the sclerenchyma tissues. The reduced thickness of the sclerenchyma cell walls is probably due to alterations in the deposition of cell wall components.
Fig. 2.
Changes in tissue structure in nodes at three different stages. Nodes of wild-type (A–C, G and H) and bc5 mutant (D–F, I and J) plants are shown. Longitudinal (A–F) and transverse (G–J) sections were double-stained with Safranin O and Fast Green FCF. The morphology was observed BH (A and D), and at 1 (B and E) and at 2 WAH (C, F and G–J). The sclerenchyma tissues in (G) and (I) are shown at higher magnification in (H) and (J), respectively. The arrows indicate sclerenchyma tissues in the nodes. Sections of 10 μm thickness were used. Scale bars indicate 100 μm for A–G and I, and 50 μm for H and J.
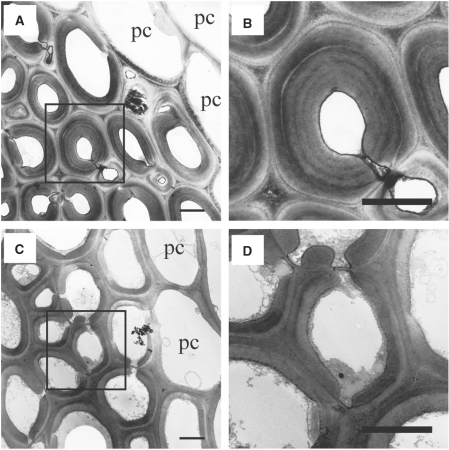
We next observed the sclerenchyma cell walls in nodes by electron microscopy. Consistent with the observations presented in Fig. 2, the cell walls in the sclerenchyma tissues of the bc5 mutant were apparently thinner than those in wild-type nodes (Fig. 3). Additionally, the multilayered walls, which were seen in the sclerenchyma tissues of the wild-type plant (Fig. 3A, B), were not observed in the bc5 mutant (Fig. 3C, D). On the other hand, the bc5 mutant had parenchyma cell walls in the nodes, similar to those in the wild-type nodes (Fig. 3A, C). These results demonstrate that the bc5 mutation causes an aberrant deposition of secondary cell walls specifically in node sclerenchyma tissues, resulting in reduced thickness of cell walls.
Fig. 3.
Transmission electron micrographs of sclerenchyma tissues in nodes. Transverse sections of wild-type (A and B) and bc5 (C and D) nodes at 2 WAH were observed with transmission electron microscopy. In (B) and (D), the boxed regions in (A) and (C), respectively, are observed at higher magnification. Scale bars indicate 3 μm. pc, parenchyma cell.
Accumulation of lignin in nodes
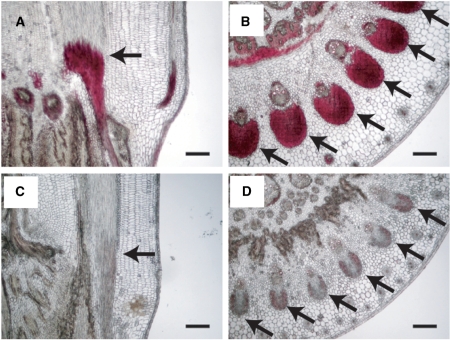
As lignin is stained with Safranin O (Srebotnik and Messner 1994), it is probable that the bc5 mutation affects the deposition of lignin in the sclerenchyma tissue of nodes, causing the brittle phenotype. To examine the deposition of lignin in sclerenchyma tissues, sections of bc5 nodes were treated with phloroglucinol-HCl, which selectively stains lignified cell walls, such as sclerenchyma tissues and mature xylem elements (Li et al. 2003), and were then observed by microscopy. In transverse sections, while sclerenchyma tissues in wild-type nodes at 2 WAH showed substantial staining with phloroglucinol-HCl (Fig. 4A, B), the tissues in the bc5 nodes were not stained at all (Fig. 4C, D). These results suggest that the bc5 mutation affects the deposition of lignin in the sclerenchyma tissues of nodes.
Fig. 4.
Accumulation of lignin in nodes. Nodes of wild-type (A and B) and bc5 mutant (C and D) plants are shown. Longitudinal (A and C) and transverse (B and D) sections were stained with phloroglucinol-HCl, which selectively stains lignified cell walls. Morphology was observed at 2 WAH. The arrows indicate sclerenchyma tissues in nodes. Sections of 30 μm thickness were used. Scale bars indicate 100 μm.
Levels of cellulose and hemicellulose in nodes
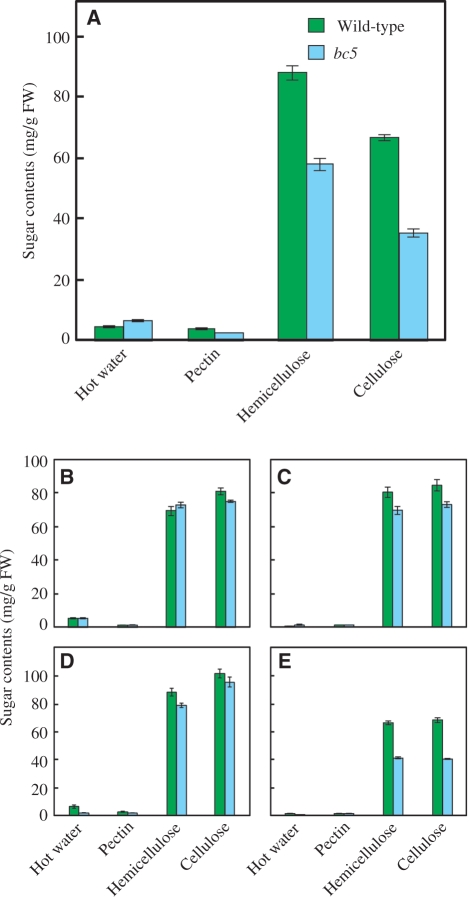
In general, bc mutants of grasses have decreased amounts of cellulose, whereas the amounts of hemicellulose are barely affected by these mutations (Kokubo et al. 1989, Kokubo et al. 1991, Li et al. 2003). To examine the influence of the bc5 mutation on the deposition of cell wall components, we fractionated the cell wall polysaccharides from various parts of the bc5 mutant plant and compared the levels with those of wild-type controls. In this experiment, the cell wall polysaccharides were extracted from the uppermost nodes, uppermost culms and mature leaves and roots at 2 WAH. The analysis indicated that the bc5 mutation reduced the amounts of cellulose and hemicellulose in the nodes to 53 and 65% of those in wild-type plants, respectively (Fig. 5A). Importantly, the proportion of the amounts of cellulose and hemicellulose was not obviously altered by the mutation, suggesting that the bc5 mutation affects the accumulation of various components, rather than a specific one, of the secondary cell walls. Consistent with the brittle phenotype, the only small alteration was observed in the amounts of cellulose and hemicellulose in the culms and leaf blades and sheaths of the bc5 mutant (Fig. 5B–D). Such features of bc5 mutant were obviously different from those of the other rice bc mutants (i.e. bc1, 2, 3, 4, 6 and 7). The bc5 mutant also exhibited reduced amounts of hemicellulose and cellulose in the roots (Fig. 5E); however, no brittle phenotype was observed. It is likely that the BC5 gene participates in secondary cell wall formation not only in nodes but also in roots.
Fig. 5.
Sugar content of cell wall fractions in different tissues. (A) Sugar content of cell wall fractions in nodes at 2 WAH. Cell wall components were fractionated into four fractions: hot water-soluble polysaccharides, pectin, hemicellulose and cellulose. Green and blue bars indicate the sugar content of wild-type and bc5 mutant plants, respectively. (B–E) Sugar content of cell wall fractions in culm (B), leaf blade (C), leaf sheath (D) and root (E) at 2 WAH. Vertical bars show standard errors (n = 3).
Reduction in glucuronosyl arabinoxylan in the bc5 mutant
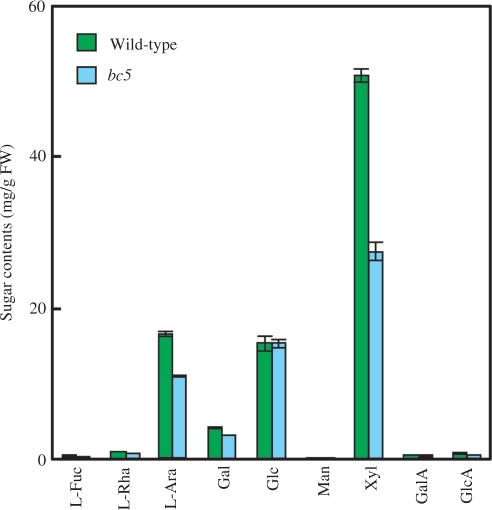
To identify the hemicellulosic polysaccharides affected by the bc5 mutation in the nodes, we analyzed the sugar composition of hemicellulose extracted from the nodes at 2 WAH, by high-performance anion-exchange chromatography and pulsed amperometric detection (HPAEC-PAD). In the bc5 nodes, the levels of xylose (Xyl) and l-arabinose (l-Ara), major constituents of glucuronosyl arabinoxylan, were reduced to 53 and 66% of those of wild-type plants, respectively, based on per g fresh weight (Fig. 6). On the other hand, the amount of glucose (Glc) was unaffected by the bc5 mutation in the nodes. These results indicate that the decrease in the amount of hemicellulose in the bc5 nodes was due to a reduced accumulation of glucuronosyl arabinoxylan.
Fig. 6.
Sugar composition of hemicellulose extracted from nodes at 2 WAH. Green and blue bars indicate the sugar content in wild-type and bc5 mutant plants, respectively. The amounts of each monosaccharide were estimated based on the ratio of monosaccharides in hydrolysate of hemicellulose determined by HPAEC-PAD, and on the amount of hemicellulose in nodes. Vertical bars show standard errors (n = 3).
To address further the effects of the bc5 mutation on glucuronosyl arabinoxylan in bc5 nodes, a glycosyl residue linkage analysis was performed on the hemicellulose fractions. The proportions of non-reducing terminal l-Araf, non-reducing terminal Xylp, (1→4)-linked Xylp and (1→2, 4)-linked Xylp residues in bc5 nodes were found to have decreased to 68, 58, 48 and 66% of those of wild-type plants, respectively (Table 1). These results clearly indicate that BC5 regulates the accumulation of not only cellulose but also glucuronosyl arabinoxylan in the secondary cell walls of nodes. We also compared the molecular mass of glucuronosyl arabinoxylan in the bc5 mutant with that of the wild-type plant by size exclusion chromatography, but no significant difference was observed (Supplementary Fig. S2). It was suggested that BC5 affects the amount of glucuronosyl arabinoxylan rather than the structure and molecular weight in developing nodes.
Table 1.
Methylation analysis of hemicellulose in nodes at 2 WAH
| Sugar componenta | Mode of linkage | Proportion (mol%) |
%c | |
|---|---|---|---|---|
| Wild-type | bc5b | |||
| 2, 3, 5-Me3-Ara | Araf1→ | 14.6 ± 0.8 | 9.90 ± 0.50 | 67.8 |
| 2, 3, 4-Me3-Xyl | Xylp1→ | 2.50 ± 0.09 | 1.46 ± 0.07 | 58.4 |
| 3, 5-Me2-Ara | →2Araf1→ | 0.54 ± 0.07 | 0.48 ± 0.08 | 88.9 |
| 2, 3, 4, 6-Me4-Glc | Glcp1→ | 0.84 ± 0.16 | 0.65 ± 0.04 | 77.4 |
| 2, 5-Me2-Ara | →3Araf1→ | 1.15 ± 0.07 | 1.15 ± 0.12 | 100 |
| 2, 3, 4, 6-Me4-Gal | Galp1→ | 1.42 ± 0.05 | 1.11 ± 0.08 | 78.2 |
| 2, 3-Me2-Ara | →5Araf1→ | 1.55 ± 0.06 | 1.33 ± 0.07 | 85.8 |
| 2, 3-Me2-Xyl | →4Xylp1→ | 43.7 ± 1.9 | 21.1 ± 1.2 | 48.3 |
| 2, 4, 6-Me3-Glc | →3Glcp1→ | 2.37 ± 0.08 | 2.30 ± 0.14 | 97.0 |
| 2, 4, 6-Me3-Gal | →3Galp1→ | 0.90 ± 0.21 | 0.50 ± 0.07 | 55.6 |
| 2-Me-Ara | →3, 5Araf1→ | 0.57 ± 0.23 | 0.42 ± 0.10 | 73.7 |
| 2, 3, 6-Me3-Glc | →4Glcp1→ | 12.1 ± 0.3 | 12.6 ± 0.44 | 104 |
| 3-Me-Xyl | →2, 4Xylp1→ | 16.7 ± 0.7 | 11.0 ± 0.49 | 65.9 |
| 2, 3-Me2-Glc | →4, 6Glcp1→ | 1.08 ± 0.37 | 1.10 ± 0.04 | 102 |
aDetermined as O-acetyl alditol derivatives.
bIn the bc5 mutant, the total amount of sugar residues was adjusted to 65% based on the hemicellulose fraction compared with wild-type plants (see Fig. 5).
cAmounts of each component in the bc5 mutant are expressed as proportions relative to those of wild-type plants taken as 100%.
Data are presented as means ± SE, n = 3.
Expression levels of CesA, the GT 43 family and lignin biosynthetic genes
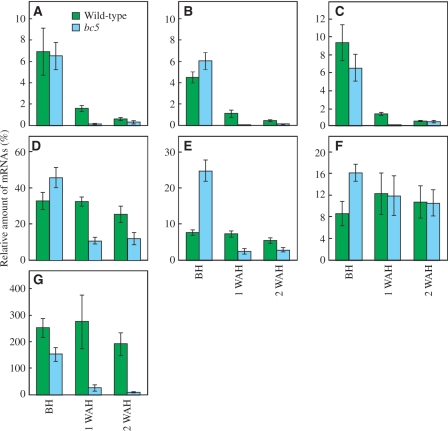
CesA genes encode catalytic subunits of cellulose synthase, and are required for the synthesis of β-1,4-glucan (Doblin et al. 2002). A GT 43 family gene, IRX9, has been shown to be involved in the synthesis of β-1,4-xylan main chains in Arabidopsis (Lee et al. 2007). An expression pattern of IRX9 highly related to those of the secondary cell wall-specific CesA genes, IRX1 and IRX3, has been reported (Brown et al. 2007). It has been reported that the phenylalanine ammonia-lyase (PAL) in the phenylpropanoid pathway is important for lignin accumulation (Vanholme et al. 2008). To characterize the functions of the BC5 gene in regulating the synthesis of secondary cell wall components in nodes, we examined the expression levels of CesA, the GT 43 family and PAL genes by quantitative reverse-transcriptase–PCR (RT–PCR). Based on the expression profiles provided by the GENEVESTIGATOR (Hruz et al. 2008; https://www.genevestigator.com/gv/index.jsp), we selected three Oryza sativa (Os) CesA genes, OsCesA4, OsCesA7 and OsCesA9, out of nine CesA genes (Tanaka et al. 2003), three GT 43 family genes, Os06g47340, Os05g03174 and Os05g48600, and a lignin biosynthetic gene, OsPAL (Os02g41630), for the analysis. Os06g47340 and Os05g03174 share high similarities with Arabidopsis IRX14 (48% identity) and IRX9 (43% identity), respectively. The expression levels of the OsCesA4, OsCesA7 and OsCesA9 genes in the bc5 nodes at 1 WAH were reduced to 9.6, 5.3 and 11% of those of wild-type nodes, respectively (Fig. 7A–C). Reduced expression levels in the OsCesA4 and OsCesA7 genes were also observed in bc5 nodes at 2 WAH (Fig. 7A, B). The expression level of Os06g47340 was decreased to 33 and 48% of that of wild-type plants at 1 and 2 WAH, respectively (Fig. 7D). The expression level of Os05g03174 was significantly lowered in bc5 nodes at 1 and 2 WAH; on the contrary, it was increased in bc5 nodes BH (Fig. 7E). We found no significant difference in the expression level of Os05g48600 between the bc5 mutant and wild-type plants at 1 and 2 WAH, whereas Os05g48600 expression was higher in the bc5 mutant than in the wild-type plants BH (Fig. 7F). The expression level of the OsPAL gene was decreased to 9.1 and 4.4% of that of wild-type plants at 1 and 2 WAH, respectively (Fig. 7G). These results indicate that BC5 positively regulates the expression of genes involved in the synthesis of cellulose, glucuronosyl arabinoxylan and lignin, except for Os05g48600, in nodes AH.
Fig. 7.
The relative expression levels of OsCesA, the GT 43 family and OsPAL genes in nodes. The amounts of OsCesA4 (A), OsCesA7 (B), OsCesA9 (C), Os06g47340 (D), Os05g03174 (E), Os05g48600 (F) and OsPAL (G) mRNAs relative to that of ACT1 at three different developmental stages in nodes were measured by quantitative RT–PCR. Green and blue bars indicate the relative expression levels in wild-type and bc5 mutant plants, respectively. Vertical bars show standard errors (n = 3).
Mapping of the bc5 gene
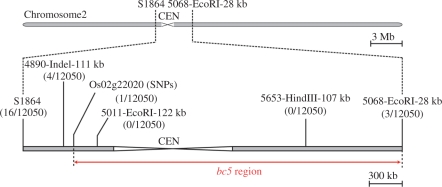
We also performed map-based cloning of the bc5 causal gene using an F2 population of 6,025 plants. In preliminary experiments, the bc5 gene locus was mapped as being roughly proximate to the S1864 marker (Yamamoto and Sasaki 1997) on chromosome 2 (data not shown). Using DNA markers designed on the basis of the genomic database (Supplementary Table S2), we narrowed the bc5 locus to an approximately 3.1 Mb region (Fig. 8). However, we have been unable to narrow the locus any further, owing to its chromosomal position near the centromere.
Fig. 8.
Map position of the bc5 gene on chromosome 2. The bc5 gene locus was narrowed to an approximately 3.1 Mb region between DNA markers Os02g22020 [single nucleotide polymorphisms (SNPs)] and 5068-EcoRI-28 kb. CEN indicates the centromeric region.
Discussion
Although a number of cell wall mutants have been identified in grasses, including rice, barley, wheat and maize, bc5 is unique in that it shows the brittle phenotype in nodes. In this study, we showed that the bc5 mutation leads to a reduced thickness of sclerenchyma cell walls and amounts of cell wall components in the nodes, but not in culms and leaves. This observation suggests that the mechanism for secondary cell wall formation in nodes is distinct from those for other organs such as culms and leaves. This is the first report that the reduced strength of the nodes of the bc mutant is derived from a defect in the formation of secondary cell walls.
The cell walls of the bc5 nodes have several features in common with those of the Arabidopsis fra8 mutant, which shows reduced mechanical strength in the inflorescence stems. First, the decreased thickness of the sclerenchyma cell walls reported in fra8 inflorescence stems was also observed in the bc5 nodes (Figs. 2, 3). Secondly, the fra8 mutation causes a reduced accumulation of both cellulose and xylan in inflorescence stems (Zhong et al. 2005), and the bc5 mutant exhibited decreased levels of both cellulose and glucuronosyl arabinoxylan in the nodes (Fig. 5; Table 1). Genetic analyses indicated that IRX7/FRA8 encodes a putative GT belonging to the GT 47 family possibly required for xylan synthesis in secondary cell walls. The reduced level of xylan has been suggested to cause an aberrant assembly of nascent cellulose microfibrils at the plasma membrane, which in turn affects further synthesis of cellulose microfibrils (Zhong et al. 2005). The bc5 mutant may have a defect in the synthesis of glucuronosyl arabinoxylan of secondary cell walls in the sclerenchyma tissues of the nodes. On the other hand, unlike the fra8 mutant, the bc5 nodes did not exhibit increased levels of other cell wall polysaccharides.
We also showed that the bc5 mutation reduced the accumulation not only of cellulose and glucuronosyl arabinoxylan but also that of lignin in the sclerenchyma tissues of the nodes (Figs. 4–6; Table 1). Moreover, several genes involved in the synthesis of secondary cell wall components showed decreased expression levels in bc5 nodes AH (Fig. 7). These facts suggest another possibility that the bc5 mutation leads to a defect in the regulation of secondary cell wall formation in the nodes. In Arabidopsis, NAC transcription factors, VND6, VND7, NST1, NST2 and NST3, and a MYB transcription factor, MYB46, appear to control the formation of secondary cell walls (Stracke et al. 2001, Kubo et al. 2005, Mitsuda et al. 2007, Olson et al. 2005). Recently, the expression levels of the CesA7/IRX3 and CesA8/IRX1 genes, a xylan biosynthesis-related gene, IRX7/FRA8, and genes for hydroxycinnamate CoA ligase and caffeoyl CoA O-methyltransferase involved in lignin synthesis were shown to be drastically reduced by NST1 NST3/SND1 RNA interference in Arabidopsis, causing a decrease in cell wall thickness among interfascicular fiber cells in the inflorescence stems (Zhong et al. 2007b). The rice genome encodes several proteins closely related to these transcription factors. However, the homologous genes do not exist in the bc5 region on chromosome 2 (Fig. 8), i.e. the closest homologs of Arabidopsis VND6 (Os06g01480), VND7 (Os08g01330), NST1 (Os08g02300; NST2 also showed the highest similarity to the sequence), NST3/SND1 (Os06g04090), MYB46 (Os12g33070) and IFL1 (Os10g33960) are located on chromosomes 6, 8, 8, 6, 12 and 10, respectively (http://rice.plantbiology.msu.edu/blast .shtml). In addition, we did not find close homologs of Arabidopsis IRX7/FRA8 (Os03g01760), IRX8 (Os12g38930), IRX9 (Os05g03174), IRX14 (Os06g47340) and PARVUS (Os03g47530) in the bc5 region. None of the rice CesA genes identified to date (OsCesA1-9, Os05g08370, Os03g59340, Os07g24190, Os01g54620, Os03g62090, Os07g14850, Os10g32980, Os07g10770 and Os09g25490) is located in the bc5 region. These observations suggest that the protein encoded by the BC5 gene is distinct from those that have been implicated in the formation of secondary cell walls in Arabidopsis.
Out of seven bc mutants identified in rice to date, the genes responsible for five, including bc5, remain to be identified. BC1 has been shown to encode a COBRA-like protein (Li et al. 2003). Although the molecular function of the BC1 protein is still unclear, COBRA and COBRA-like proteins are presumed to participate in cellulose synthesis in Arabidopsis (Brown et al. 2005, Roudier et al. 2005). Recently, the BC7 gene was shown to encode a CesA protein, OsCesA4, with high sequence similarity to Arabidopsis CesA8/IRX1 (Yan et al. 2007). These observations suggest that the mechanism of secondary cell wall formation, particularly cellulose synthesis, is at least partially conserved between rice and Arabidopsis. However, there must be an as yet unknown mechanism underlying the cell wall properties that differs between Gramineae and dicotyledonous plants. Identification of the BC5 gene and an elucidation of its function will clarify the mechanism of secondary cell wall formation in rice nodes. It is also important to understand the relationship between BC5 and other BC genes in the formation of secondary cell walls.
Materials and Methods
Plant materials and growth conditions
The rice bc5 mutant (O. sativa L. ssp. japonica cv. GR: 0060072) was provided by the International Rice Research Institute, Manila, Philippines (Khush et al. 1993). As the bc5 mutant showed the japonica genotype on chromosome 2, where bc5 is located, Taichung 65 (japonica cv.) was used as the corresponding wild-type control. Rice plants were grown under field conditions at the National Institute of Agrobiological Sciences, Tsukuba, Ibaraki. For analysis of cell wall polysaccharides and histological staining, the uppermost nodes, culms and leaf blades, leaf sheaths and roots BH and at 1 and 2 WAH were used.
Extraction of cell wall polysaccharides
Plant tissues (∼0.5 g) were homogenized into a fine powder using a mortar and pestle in liquid nitrogen, and then washed twice with cold water. The cell walls were treated with 80% (v/v) ethanol at 100°C for 15 min and then digested with 100 U of porcine pancreatic α-amylase (type VII-A; Sigma, St Louis, MO, USA) in 50 mM MOPS-NaOH buffer (pH 6.5) at 37°C for 4 h, to remove starch. After removal of solubilized starch by centrifugation, hot water-soluble pectin and hemicellulose fractions were extracted from the cell wall materials with hot water, 50 mM EDTA (pH 6.8) and 17.5% sodium hydroxide containing 0.04% sodium borohydride, by heating in a boiling water bath for 10 min, respectively. The hemicellulose fractions were neutralized with acetic acid, dialyzed against water at 4°C for 1 d, and lyophilized. The residual precipitate was washed with water, ethanol and diethylether and collected as the cellulose fraction. The total sugar content of the fractions was determined by the phenol–sulfuric acid method (Dubois et al. 1956) using Glc and Xyl as standards.
Analysis of carbohydrate
For sugar composition analysis, samples of approximately 1 mg were hydrolyzed with 72% (v/v) sulfuric acid (0.2 ml) at 4°C for 1 h, followed by heating in diluted (8%, v/v) sulfuric acid solution at 100°C for 4 h. The hydrolysate was neutralized with barium carbonate and desalted with Dowex 50W (H+) resin. Quantification of monosaccharides was carried out by HPAEC-PAD using Dionex DX-500 liquid chromatography (Dionex, Osaka, Japan) fitted with a CarboPac PA-1 column (250 mm in length, 4 mm i.d.) as described previously (Ishikawa et al. 2000). Samples were chromatographed on a column equilibrated with 20 mM sodium hydroxide at a flow rate of 1.0 ml min–1 and separated by a linear gradient of 20–35 mM sodium hydroxide (0–13.2 min) and 100 mM sodium hydroxide (13.2–25.0 min), followed by 250 mM sodium acetate in 50 mM sodium hydroxide (25.0–31.25 min).
Glycosidic linkage analysis of hemicellulose
Hemicellulose (∼1 mg) was dissolved in dimethylsulfoxide (2 ml) under a nitrogen atmosphere, it was then methylated by the Hakomori method (Hakomori 1964), with potassium methylsulfinyl carbanion (0.5 ml) (Harris et al. 1984) and methyl iodide (1.5 ml). The methylated polysaccharides were dialyzed against water for 1 d, extracted with chloroform and dried. The methylated polysaccharides were hydrolyzed in 72% (v/v) sulfuric acid (0.1 ml) for 1 h at room temperature and then in 8% (v/v) sulfuric acid (0.9 ml) at 100°C for 4 h. The resulting methylated monosaccharides obtained were converted to the corresponding alditol acetates. The samples were analyzed by gas–liquid chromatography using a Shimadzu gas chromatograph GC-8A fitted with a Silar-10C glass capillary column (50 m in length, 0.28 mm i.d.).
Histological staining of cell wall components
The uppermost nodes excised from rice plants at three developmental stages, BH and at 1 and 2 WAH, were fixed in a formalin–aceto-alcohol solution [45% (v/v) ethanol, 5% (v/v) acetic acid and 5% (v/v) formaldehyde], dehydrated through a graded series of ethanol and t-butyl alcohol, and embedded in paraffin (Palaplast, Oxford, UK). For cell wall staining, sections were cut at a thickness of 10 μm with a microtome (RM2125RT; Leica, Tokyo, Japan) equipped with a disposable steel blade (C35; Feather, Osaka, Japan), stained successively in 1% (w/v) Safranin O (Nacalai Tesque, Kyoto, Japan) and 0.5% (w/v) Fast Green FCF (Wako, Osaka, Japan) solution, and then viewed under a light microscope (Eclipse E400; Nikon, Tokyo, Japan). For lignin staining, paraffin-embedded sections of 30 μm thickness were stained with 2% (w/v) phloroglucinol (Wako) in 95% ethanol for 5 min, rinsed briefly with distilled water and then treated in 18% HCl for 5 min. The stained sections were visualized immediately under a light microscope (Eclipse E400).
Electron microscopy
Rice plants were harvested at 2 WAH and nodes were excised with a razor blade. The node sections were fixed with 2% glutaraldehyde in 50 mM potassium phosphate buffer (pH 6.8) for 2 h at room temperature and at 4°C for 1 d. They were rinsed with the same buffer and post-fixed in 2% osmium tetroxide in 50 mM potassium phosphate buffer (pH 6.8) for 2 h at room temperature, dehydrated in an acetone series and embedded in Spurr’s resin (Polysciences Inc., Warrington, PA, USA). For transmission electron microscopy, ultrathin sections (silver–gold) were stained with aqueous 2% uranyl acetate for 10 min, followed by lead citrate for 2 min. The sections were then observed with a Hitachi H-7500 transmission electron microscope (Tokyo, Japan) at an accelerating voltage of 100 kV.
Quantitative analysis of OsCesA, the GT 43 family and lignin biosynthetic genes
Nodes of rice plants harvested at three developmental stages (i.e. BH and at 1 and 2 WAH) were frozen in liquid nitrogen and homogenized with a mortar and pestle. Total RNA was extracted with an Isogen kit (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions. Single-strand cDNA was synthesized from approximately 1 μg of total RNA from the rice nodes using a reverse transcriptase, ReverTra Ace-α- (Toyobo, Osaka, Japan) and an oligo(dT12–18) primer (Invitrogen, Carlsbad, CA, USA). The relative amounts of mRNAs for OsCesAs, the GT 43 family and PAL genes were determined by quantitative RT–PCR. Sets of specific primers were designed with the Primer 3.0 program (http://frodo .wi.mit.edu/primer3/) (Supplementary Table S1). PCR was performed with a SYBR Premix Ex Taq kit (TAKARA BIO INC., Otsu, Japan) under the following conditions: 10 s denaturation at 95°C, 30 s annealing at 60°C and 20 s extension at 72°C, 40 cycles. PCR product was detected with Opticon 2 (Bio-Rad, Hercules, CA, USA) and the amounts of mRNAs for OsCesA, the GT 43 family and PAL genes relative to OsACT1 mRNA were calculated.
Mapping of the bc5 gene
The F2 population of 6,025 plants was generated from a cross between bc5 and a wild-type rice, co39 (indica cv.). To narrow the bc5 gene region, DNA markers were designed based on the genomic database (Supplementary Table S2). Genomic DNA was extracted from leaves of 0.5 cm in length. Leaves were ground twice in 96-well collection microtubes (QIAGEN, Tokyo, Japan) in liquid nitrogen for 2 min using a Shake Master BMS-12 (Inter Bio Techno, Tokyo, Japan), supplied with 100 μl of TE buffer (pH 8.0) and then heated at 100°C for 15 min. The homogenates were centrifuged at 1,660 × g for 10 min at room temperature and the supernatant was used for genotyping by PCR. PCR was performed with Phusion DNA polymerases (Finnzymes, Espoo, Finland) under the following conditions: 10 s denaturation at 98°C, 20 s annealing at 60°C and 30 s extension at 72°C, 35 cycles.
Supplementary data
Supplementary data are available at PCP online.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Xu Xin and Dr. Raman Babu (National Institute of Agrobiological Sciences) for their kind and skillful technical supports and valuable advices, especially on fine mapping of the bc5 gene.
Glossary
Abbreviations
- AH
after heading
- l-Ara
l-arabinose
- bc
brittle culm
- BH
before heading
- CesA
cellulose synthase catalytic subunit
- fra
fragile fiber
- l-Fuc
l-fucose
- Gal
galactose
- GalA
galacturonic acid
- Glc
glucose
- GlcA
glucuronic acid
- GT
glycosyltransferase
- HPAEC-PAD
high-performance anion-exchange chromatography with pulsed amperometric detection
- irx
irregular xylem
- Man
mannose
- NST
NAC SECONDARY WALL THICKENING PROMOTING FACTOR
- Os
Oryza sativa
- PAL
phenylalanine ammonia-lyase
- l-Rha
l-rhamnose
- RT–PCR
reverse transcription–PCR
- SND
SECONDARY WALL-ASSOCIATED NAC DOMAIN
- VND
VASCULAR-RELATED NAC DOMAIN
- WAH
week after heading
- Xyl
xylose.
References
- Bourne Y, Henrissat B. Glycoside hydrolases and glycosyltransferases: families and functional modules. Curr. Opin. Struct. Biol. 2001;11:593–600. doi: 10.1016/s0959-440x(00)00253-0. [DOI] [PubMed] [Google Scholar]
- Brown DM, Goubet F, Wong VW, Goodacre R, Stephens E, Dupree P, et al. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J. 2007;52:1154–1168. doi: 10.1111/j.1365-313X.2007.03307.x. [DOI] [PubMed] [Google Scholar]
- Brown DM, Zeef LA, Ellis J, Goodacre R, Turner SR. Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell. 2005;17:2281–2295. doi: 10.1105/tpc.105.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk DH, Liu B, Zhong R, Morrison HW, Ye ZH. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–827. [PMC free article] [PubMed] [Google Scholar]
- Carpita NC. Structure and biogenesis of the cell walls of grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:445–476. doi: 10.1146/annurev.arplant.47.1.445. [DOI] [PubMed] [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Demura T, Tashiro G, Horiguchi G, Kishimoto N, Kubo M, Matsuoka N, et al. Visualization by comprehensive microarray analysis of gene expression programs during transdifferentiation of mesophyll cells into xylem cells. Proc. Natl Acad. Sci. USA. 2002;99:15794–15799. doi: 10.1073/pnas.232590499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP. Cellulose biosynthesis in plants: from genes to rosettes. Plant Cell Physiol. 2002;43:1407–1420. doi: 10.1093/pcp/pcf164. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Robers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Gibeaut DM, Carpita NC. Biosynthesis of plant cell wall polysaccharides. FASEB J. 1994;8:904–915. doi: 10.1096/fasebj.8.12.8088456. [DOI] [PubMed] [Google Scholar]
- Hakomori S. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 1964;55:205–208. [PubMed] [Google Scholar]
- Harris PJ, Henry RJ, Blakeney AB, Stone BA. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr. Res. 1984;127:59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, et al. Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics. 2008;2008:420747–420751. doi: 10.1155/2008/420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Kuroyama H, Takeuchi Y, Tsumuraya Y. Characterization of pectin methyltransferase from soybean hypocotyls. Planta. 2000;210:782–791. doi: 10.1007/s004250050680. [DOI] [PubMed] [Google Scholar]
- Iwata N, Omura T. Linkage studies in rice (Oryza sativa L.). On some mutants derived from chronic gamma irradiation. J. Fac. Agric. Kyushu Univ. 1977;21:117–127. [Google Scholar]
- Khush GS, Sanchez AC. Brittle node, a new marker for chromosome 2. Rice Genet. Newslett. 1993;10:75–77. [Google Scholar]
- Kokubo A, Kuraishi S, Sakurai N. Culm strength of barley: correlation among maximum bending stress, cell wall dimensions, and cellulose content. Plant Physiol. 1989;91:876–882. doi: 10.1104/pp.91.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubo A, Sakurai N, Kuraishi S, Takeda K. Culm brittleness of barley (Hordeum vulgare L.) mutants is caused by smaller number of cellulose molecules in cell wall. Plant Physiol. 1991;97:509–514. doi: 10.1104/pp.97.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, et al. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005;19:1855–1860. doi: 10.1101/gad.1331305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, O’Neill MA, Tsumuraya Y, Darvill AG, Ye ZH. The irregular xylem9 mutant is deficient in xylan xylosyltransferase activity. Plant Cell Physiol. 2007;48:1624–1634. doi: 10.1093/pcp/pcm135. [DOI] [PubMed] [Google Scholar]
- Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, et al. BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell. 2003;15:2020–2031. doi: 10.1105/tpc.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librojo AL, Khush GS. Rice Genetics. Manila, Philippines: IRRI; 1986. Chromosomal location of some mutant genes through the use of primary trisomics in rice; pp. 249–255. [Google Scholar]
- Mitsuda N, Iwase A, Yamamoto H, Yoshida M, Seki M, Shinozaki K, et al. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell. 2007;19:270–280. doi: 10.1105/tpc.106.047043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence. Plant Cell. 2005;17:2993–3006. doi: 10.1105/tpc.105.036004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S, Takahashi M. Trial construction of twelve linkage groups in Japanese rice. Genetical studies on rice plant, 28. J. Fac. Agric. Hokkaido Univ. 1963;53:72–130. [Google Scholar]
- Olson AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10:79–87. doi: 10.1016/j.tplants.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Peña MJ, Zhong R, Zhou GK, Richardson EA, O’Neill MA, Darvill AG, et al. Arabidopsis irregular xylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell. 2007;19:549–563. doi: 10.1105/tpc.106.049320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Caffall KH, Freshour G, Hilley MT, Bauer S, Poindexter P, et al. The Arabidopsis irregular xylem8 mutant is deficient in glucuronoxylan and homogalacturonan, which are essential for secondary cell wall integrity. Plant Cell. 2007;19:237–255. doi: 10.1105/tpc.106.047720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD. Biosynthesis and properties of the plant cell wall. Curr. Opin. Plant Biol. 2002;5:536–542. doi: 10.1016/s1369-5266(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Roudier F, Fernandez AG, Fujita M, Himmelspach R, Borner GH, Schindelman G, et al. COBRA, an Arabidopsis extracellular glycosyl-phosphatidyl inositol-anchored protein, specifically controls highly anisotropic expansion through its involvement in cellulose microfibril orientation. Plant Cell. 2005;17:1749–1763. doi: 10.1105/tpc.105.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Multani DS, Khush GS. A new brittle culm mutant in rice. RGN. 1994;11:91–92. [Google Scholar]
- Srebotnik E, Messner K. A simple method that uses differential staining and light microscopy to assess the selectivity of wood delignification by white rot fungi. Appl. Environ. Microbiol. 1994;60:1383–1386. doi: 10.1128/aem.60.4.1383-1386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001;4:447–456. doi: 10.1016/s1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Kinoshita T, Takeda K. Character expression and causal genes of some mutants in rice plant. Genetical studies on rice plant, 33. J. Fac. Agric. Hokkaido Univ. 1968;55:496–512. [Google Scholar]
- Tanaka K, Murata K, Yamazaki M, Onosato K, Miyao A, Hirochika H. Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol. 2003;133:73–83. doi: 10.1104/pp.103.022442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Howells RM, Huttly AK, Vickers K, Turner SR. Interactions among three distinct CesA proteins essential for cellulose synthesis. Proc. Natl Acad. Sci. USA. 2003;100:1450–1455. doi: 10.1073/pnas.0337628100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Laurie S, Turner SR. Multiple cellulose synthase catalytic subunits are required for cellulose synthesis in Arabidopsis. Plant Cell. 2000;12:2529–2540. doi: 10.1105/tpc.12.12.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NG, Scheible WR, Cutler S, Somerville CR, Turner SR. The irregular xylem3 locus of Arabidopsis encodes a cellulose synthase required for secondary cell wall synthesis. Plant Cell. 1999;11:769–80. doi: 10.1105/tpc.11.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Somerville CR. Collapsed xylem phenotype of Arabidopsis identifies mutants deficient in cellulose deposition in the secondary cell wall. Plant Cell. 1997;9:689–701. doi: 10.1105/tpc.9.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Morreel K, Ralph J, Boerjan W. Lignin engineering. Curr. Opin. Plant Biol. 2008;11:278–85. doi: 10.1016/j.pbi.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sasaki T. Large-scale EST sequencing in rice. Plant Mol. Biol. 1997;35:135–144. [PubMed] [Google Scholar]
- Yan C, Yan S, Zeng X, Zhang Z, Gu M. Fine mapping and isolation of Bc7(t), allelic to OsCesA4. J. Genet. Genomics. 2007;34:1019–1027. doi: 10.1016/S1673-8527(07)60115-5. [DOI] [PubMed] [Google Scholar]
- Zhong R, Burk DH, Morrison W.H., III, Ye, Z H. A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell. 2002;14:3101–3117. doi: 10.1105/tpc.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Burk DH, Morrison W.H., III, Ye, Z H. FRAGILE FIBER3, an Arabidopsis gene encoding a type II inositol polyphosphate 5-phosphatase, is required for secondary wall synthesis and actin organization in fiber cells. Plant Cell. 2004;16:3242–3259. doi: 10.1105/tpc.104.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Demura T, Ye ZH. SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell. 2006;18:3158–3170. doi: 10.1105/tpc.106.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Peña MJ, Zhou GK, Nairn CJ, Wood-Jones A, Richardson EA, et al. Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell. 2005;17:3390–33408. doi: 10.1105/tpc.105.035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis. Plant Cell. 2007a;19:2776–2792. doi: 10.1105/tpc.107.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Richardson EA, Ye ZH. Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta. 2007b;225:1603–1611. doi: 10.1007/s00425-007-0498-y. [DOI] [PubMed] [Google Scholar]
- Zhong R, Ye YH. IFL1, a gene regulating interfascicular fiber differentiation in Arabidopsis, encodes a homeodomain–leucine zipper protein. Plant Cell. 1999;11:2139–2152. doi: 10.1105/tpc.11.11.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.