Abstract
Nesfatin-1, derived from nucleobindin2, is expressed in the hypothalamus and reported in one study to reduce food intake (FI) in rats. To characterize the central anorexigenic action of nesfatin-1 and whether gastric emptying (GE) is altered, we injected nesfatin-1 into the lateral brain ventricle (intracerebroventricular, icv) or fourth ventricle (4v) in chronically cannulated rats or into the cisterna magna (intracisternal, ic) under short anesthesia and compared with ip injection. Nesfatin-1 (0.05 μg/rat, icv) decreased 2–3 h and 3–6 h dark-phase FI by 87 and 45%, respectively, whereas ip administration (2 μg/rat) had no effect. The corticotropin-releasing factor (CRF)1/CRF2 antagonist astressin-B or the CRF2 antagonist astressin2-B abolished icv nesfatin-1’s anorexigenic action, whereas an astressin2-B analog, devoid of CRF-receptor binding affinity, did not. Nesfatin-1 icv induced a dose-dependent reduction of GE by 26 and 43% that was not modified by icv astressin2-B. Nesfatin-1 into the 4v (0.05 μg/rat) or ic (0.5 μg/rat) decreased cumulative dark-phase FI by 29 and 60% at 1 h and by 41 and 37% between 3 and 5 h, respectively. This effect was neither altered by ic astressin2-B nor associated with changes in GE. Cholecystokinin (ip) induced Fos expression in 43% of nesfatin-1 neurons in the paraventricular hypothalamic nucleus and 24% of those in the nucleus tractus solitarius. These data indicate that nesfatin-1 acts centrally to reduce dark phase FI through CRF2-receptor-dependent pathways after forebrain injection and CRF2-receptor-independent pathways after hindbrain injection. Activation of nesfatin-1 neurons by cholecystokinin at sites regulating food intake may suggest a role in gut peptide satiation effect.
Nesfatin-1’s anorectic effect on dark phase food intake is CRF2-receptor dependent after injection into the forebrain ventricular site but CRF2-independent after injection into hindbrain sites, and is independent from a decrease in gastric emptying.
Nesfatin-1 is an 82-amino-acid peptide derived from posttranslational processing of the N-terminal fragment of nucleobindin2 (NUCB2), a 396-amino-acid protein highly conserved across mammalian species (1). Posttranslational cleavage of NUCB2 results in the expression of nesfatin-2 (85–163) and nesfatin-3 (166–396) in addition to nesfatin-1 (1). Oh-I et al.’s (1) initial report indicates that nesfatin-1 (named as acronym for NEFA/nucleobindin2-encoded satiety- and fat-influencing protein) may have physiological relevance in regulating food intake. This was based on convergent observations that nesfatin-1 injected acutely into the third brain ventricle reduced food consumption occurring during the dark phase, whereas nesfatin-2 or nesfatin-3 had no effect (1). Likewise, continuous infusion of nesfatin-1 (5 pmol/d for 10 d into the third brain ventricle) significantly decreased food intake and body weight gain in rats (1). Conversely, third ventricle infusion of a NUCB2 antisense oligonucleotide increased food intake and body weight gain compared with a missense NUCB2 oligonucleotide (1). In addition, a 24-h fast decreased the expression of NUCB2 in the paraventricular nucleus (PVN) (1). Lastly, HPLC of rat cerebrospinal fluid yields a peak corresponding to nesfatin-1, suggestive of occurrence as secreted fragment (1), although the nesfatin-1 receptor remains to be identified. Several laboratories reproducibly showed abundant NUCB2/nesfatin-1 expression in relevant hypothalamic and medullary sites involved in feeding regulation in rats, including the arcuate nucleus, PVN, and the nucleus of the solitary tract (NTS) (1,2,3,4,5), further supporting the assumption that nesfatin-1 is involved in food intake regulation.
However, there is still a paucity of information on the central action of nesfatin-1 to influence food intake outside the initial report (1). In the present study, we evaluated nesfatin-1 action to modulate food intake response upon injection into the forebrain at the level of the lateral brain ventricle (intracerebroventricularly, icv) or the hindbrain into the fourth ventricle (4v) or cisterna magna (intracisternally, ic) in freely fed rats during the dark phase or in response to fasting-refeeding during the light phase. A recent in vitro study indicates that nesfatin-1 influences the activity of a large proportion of PVN neurons including those containing corticotropin-releasing factor (CRF) (6). Activation of brain CRF signaling pathways by CRF acting on CRF1 and CRF2 receptors and by selective endogenous CRF2 agonists urocortin 2 or 3 (7) inhibits food intake (8). Therefore, we investigated whether the central action of nesfatin-1 on food intake may be mediated by the activation of brain CRF receptors using astressin-B, a CRF1 and CRF2 receptor antagonist, and astressin2-B, a selective CRF2 receptor antagonist, compared with a structurally related astressin2-B analog, devoid of CRF antagonist activity (9). Because alterations of gastric transit influence the degree of fullness and can convey satiety signals to reduce food intake (10,11), we also examined whether nesfatin-1 injected icv, into the 4v, or ic influences gastric emptying (GE). Changes in circulating levels of glucose are reflected in brain extracellular glucose levels (12), which influence food intake and gastric motility (13,14). Therefore, next we assessed whether the nesfatin-1 action is associated with variations in blood glucose levels. To gain insight into the responsiveness of brain nesfatin-1 neurons to a stimulus known to inhibit food intake, we explored whether an ip injection of sulfated cholecystokinin-8 (CCK-8S) (15) activates neurons in the PVN and NTS known to express nesfatin-1 immunoreactivity (1,2,3,16) and to be involved in CCK satiety actions (17,18). Because a recent report showed a reduction of dark-phase food intake after ip injection of nesfatin-1 in mice (19) and we recently reported the expression of NUCB2/nesfatin-1 in rat gastric endocrine cells (20), we also assessed whether the orexigenic drive induced by fasting (21) influences both the activity of nesfatin-1-containing neurons in the brain and nesfatin-1 plasma levels.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, San Diego, CA) weighing 280–350 g were housed under controlled illumination (0600–1800 h) and temperature (21–23 C). Rats had free access to standard rodent chow (Prolab RMH 2500 LabDiet; PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Veterans Administration Institutional Animal Care and Use Committee (99-127-07).
Peptides
Rat nesfatin-1 (1-82; Phoenix Pharmaceuticals, Burlingame, CA) and CCK-8S (Bachem, Torrance, CA) were aliquoted in distilled water and stored at −80 C. Astressin-B, astressin2-B, the astressin2-B analog cyclo(33–36) [DPhe11, His12, Nle17, CαMeLeu13, 39, Glu33, Lys36] Ac-Sau (8–40) devoid of CRF receptor binding affinity (9), and amidated rat Ac-nesfatin-11–44-NH2, (Salk Institute, La Jolla, CA) were synthesized as previously described (9,22) and kept in powder form at −80 C. Peptides were dissolved in distilled water immediately before the experiments.
Brain injections
Implanting cannulas into the right lateral (icv) or 4v and ic injections in 5 μl were performed as previously described (23,24). Stereotaxic coordinates were obtained from Paxinos and Watson’s brain atlas (25). After surgery, animals were housed one per cage, had a 7-d recovery period, and were handled for another 7 d to become accustomed to the injection procedure. At the end of the experiments, the correct placement of the cannula was verified by injecting dye (0.1% toluidine blue). Rats with misplaced cannulas (five of 122 after icv and three of 20 after 4v injection) were excluded retrospectively. The ic injection in 5 μl was performed as previously reported (26) in rats under short isoflurane anesthesia (2–3 min, 4.5% vapor concentration in oxygen; VSS, Rockmart, GA). The accuracy of the ic injection was ascertained before the injection by withdrawal of cerebrospinal fluid into the Hamilton syringe. On average, rats completely recovered from anesthesia within 7 min.
Food intake
Dark-phase food intake in conscious rats with chronic cannula into the lateral and fourth brain ventricle
Rats maintained one per cage were injected with vehicle (pyrogen-free distilled water) or nesfatin-1 either icv or into the 4v (0.05 μg/rat), and preweighed rat chow was made available. Food intake was monitored up to 24 h and calculated as g/300 g body weight. The 24-h body weight change was also determined. The dose of nesfatin-1 was based on previous dose-response studies performed after third brain ventricle injection in rats (1). In other studies, rats were injected icv with vehicle, astressin-B (30 μg/rat), astressin2-B (30 μg/rat), or astressin2-B analog (30 μg/rat) immediately before the icv injection of vehicle or nesfatin-1 (0.05 μg/rat), and cumulative food intake was measured for 6 h. The dose of CRF antagonists was selected based on our previous dose-response studies showing blockade of icv CRF or acute stress on gut function (23,27). All injections were performed at the onset of the dark phase in freely fed rats maintained in their familiar housing cages, except a reduced amount of bedding.
Dark-phase food intake in response to nesfatin-1 injected ic or ip in noncannulated rats
In freely fed rats housed two per cage until the start of the experiment, nesfatin-1 was injected at the onset of the dark phase either ic (0.5 μg/rat) under short isoflurane anesthesia or ip (2 μg/rat) in conscious rats, or vehicle (pyrogen-free distilled water) was injected under similar conditions. Afterward, rat chow was made available and food intake monitored up to 24 h. In another study, freely fed rats were injected ic at the onset of the dark phase with vehicle, astressin-B (30 μg/rat), or astressin2-B (30 μg/rat) before the ic injection of vehicle or nesfatin-1 (0.5 μg/rat), and cumulative food intake was monitored for 6 h. Feeding experiments were repeated in a crossover design, and rats were habituated to the experimental conditions by training them for single housing for 8 h/d three times before the experiments.
Blood glucose
Freely fed singly housed rats were injected icv with vehicle or nesfatin-1 (0.05 μg/rat) at the onset of the dark phase. Blood was collected by tail prick in conscious rats at 20 min, 1 h, and 2 h after injection, and blood glucose levels were measured (One-Touch Ultra; LifeScan, Milpitas, CA).
Behavior
Freely fed singly housed rats were injected icv with nesfatin-1 (0.05 μg/rat) or vehicle (n = 8 per group) at the onset of the dark phase and placed back in their home cage with paper under the cage divided into six equal squares. Locomotor activity (total number of squares crossed) and grooming (washing, licking, and/or scratching) were monitored throughout the third hour after injection. The investigator was blinded to the treatment.
Gastric Emptying (GE)
GE of a nonnutrient viscous solution was determined by the phenol red/methyl cellulose method as previously described (27). Rats were food but not water deprived for 8 h before the dark phase. Chronically cannulated conscious rats were injected with nesfatin-1 icv (0.05 or 0.5 μg/rat) or into the 4v (0.05 μg/rat) at the beginning of the dark phase, and water bottles were removed. Likewise, noncannulated rats were injected with nesfatin-1 ic (0.5 μg/rat) under short isoflurane anesthesia. At 150 min after icv or 30 min after 4v or ic injection, conscious rats received an orogastric gavage of 1.5 ml viscous solution, and GE was monitored 20 min later. In another study, 8-h fasted rats received two consecutive icv injections of vehicle or astressin2-B (30 μg/rat) followed by vehicle or nesfatin-1 (0.05 μg/rat), and the 20-min GE was assessed at 170 min after injection. The time of GE monitoring was selected based on the peak hourly inhibition of food intake upon icv, 4v, and ic injection of nesfatin-1 established in the studies described above.
Fos and nesfatin-1 immunohistochemistry
Freely fed rats were injected ip (300 μl) with CCK-8S (3 μg/kg, n = 3) or vehicle (saline, n = 3; group size was based on a power analysis) during the dark phase. Ninety minutes later, rats were anesthetized with sodium pentobarbital (70 mg/kg, ip, Nembutal; Abbott Laboratories, Chicago, IL). Transcardial perfusion and brain processing were performed as described before (28). Brain sections were incubated in rabbit anti-c-Fos (1:10,000; Oncogene, Cambridge, MA) followed by the avidin-biotin-peroxidase complex method and visualized with 3,3′-diaminobenzidine tetrachloride and nickel ammonium sulfate. Sections were thereafter incubated in rabbit anti-nesfatin-1 (1:10,000, catalog no. H-003-22; Phoenix Pharmaceuticals) followed by the avidin-biotin-peroxidase complex method and 3,3′-diaminobenzidine tetrachloride. Cells with dark blue nuclear staining were Fos positive, and cells with strong brown cytoplasmic staining were nesfatin-1 immunoreactive (ir). The anti-nesfatin-1 antibody used, raised in rabbits against rat nesfatin-1 (1-82 aa), shows rat nesfatin-1 as a 9.7-kDa band on the Western blot (20). Specificity of the staining as previously stipulated (29) was assessed by preabsorption of the antibody (1 ml, 1:10,000) with rat nesfatin-1 (10 μg; Phoenix Pharmaceuticals). After 24 h incubation, the solution was centrifuged and the supernatant used for immunostaining. Fos-ir and nesfatin-1-ir cells were counted unilaterally as previously described (28) in the anterior parvocellular part of the PVN (apPVN, −1.08 to −1.32 mm from bregma, four sections) and NTS at the level of the area postrema (−13.68 to −14.28 mm from bregma, eight sections) (25). The average number of single- or double-labeled Fos-ir and nesfatin-1-ir cells per section for each animal was calculated. The investigator was blinded to the treatment.
In another study, rats freely fed, 24-h fasted, or 24-h fasted followed by refeeding for 2 h (n = 3 per group) were anesthetized at the beginning of the dark phase, transcardially perfused, and brains processed for Fos and nesfatin-1 as described above. The number of Fos-ir and nesfatin-1-ir cells was counted in the supraoptic nucleus (SON, −0.6 to −1.56 mm from bregma, 12 sections), PVN (−1.08 to −2.0 mm from bregma, 12 sections), and NTS (−13.32 to −14.6 mm from bregma, 13 sections). The average number of Fos-ir and nesfatin-1-positive cells per section per brain nucleus was assessed.
Nesfatin-1 plasma levels
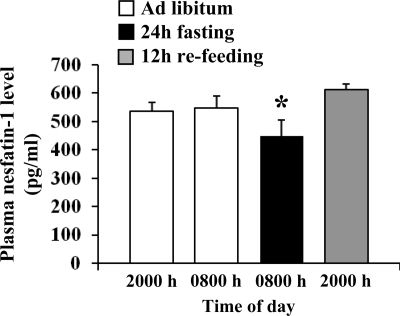
Rats (n = 5) were chronically equipped with a jugular vein catheter and allowed to recover for 4 d after surgery until the body weight started to increase again. Blood was withdrawn serially from conscious lightly hand-restrained rats first under ad libitum feeding during the dark phase (between 2000 and 2100 h), 12 h later during the light phase (between 0800 and 0900 h), and then after a 24-h fast (between 0800 and 0900 h) followed by a 12-h refeeding period (between 2000 and 2100 h). Blood was collected in tubes containing EDTA (7.5%, 10 μl/0.5ml blood; Sigma Chemical Co., St. Louis, MO) and aprotinin (0.6 U trypsin inhibitor/0.5 ml blood; ICN Pharmaceuticals, Costa Mesa, CA). Plasma nesfatin-1 was measured by RIA (rat nesfatin-1, detection range 125–16,000 pg/ml; Phoenix Pharmaceuticals).
Statistical analysis
Data are expressed as mean ± sem and analyzed by one way ANOVA followed by Tukey post hoc test. The RIA data were evaluated by paired t test. Differences between groups were considered significant when P < 0.05.
Results
Intracerebroventricular nesfatin-1 inhibits dark-phase feeding and GE without altering blood glucose in rats chronically implanted with a cannula into the lateral brain ventricle
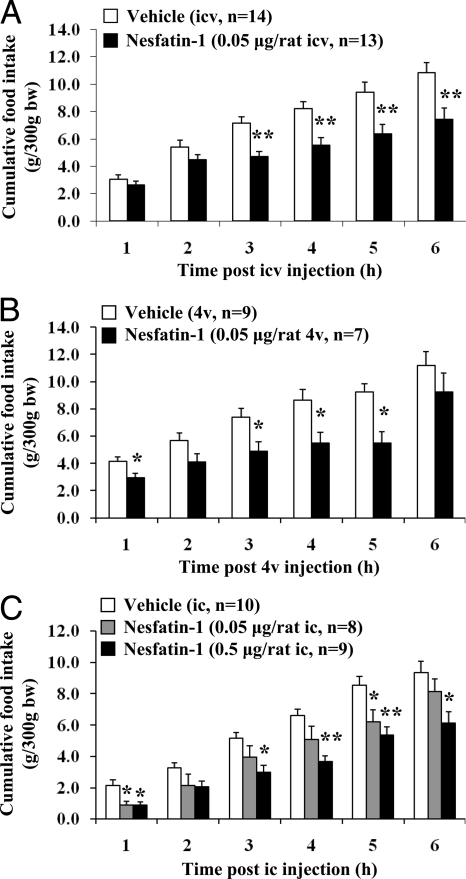
In freely fed chronically icv cannulated rats, nesfatin-1 (0.05 μg/rat = 5 pmol) injected icv at the beginning of the dark phase significantly reduced cumulative food intake from the third to the sixth hour after injection compared with vehicle-injected controls (P < 0.01; Fig. 1A) without altering blood glucose (supplemental Table 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) or inducing behavioral changes relative to controls (supplemental Table 2). The hourly food intake after nesfatin-1 showed an 87% reduction compared with vehicle-treated animals at the 2- to 3-h period after injection (P < 0.001). Although the 24-h cumulative food intake was not significantly changed (nesfatin-1 vs. vehicle, 17.4 ± 1.4 vs. 20.4 ± 0.7 g/300 g body weight, P > 0.05), the body weight was reduced at 24 h after nesfatin-1 injection by 1.8 ± 0.5% compared with vehicle (P < 0.001). The shorter fragment, Ac-nesfatin-11–44-NH2 (0.025 or 0.075 μg/rat = 5 or 15 pmol, icv), by contrast, did not influence the 3-h dark phase feeding response (supplemental Fig. 1).
Figure 1.
Nesfatin-1 injected into the cerebrospinal fluid at different levels of the brain decreases dark-phase food intake in freely fed rats. A and B, Nesfatin-1 or vehicle was injected at the beginning of the dark phase in ad libitum fed rats chronically implanted with cannula either into the lateral brain ventricle, icv (A), or the 4v (B); C, injection ic was performed under short anesthesia in noncannulated rats. Cumulative food intake was monitored for 6 h after injection. Each bar represents the mean ± sem. *, P < 0.05; **, P < 0.01 vs. vehicle.
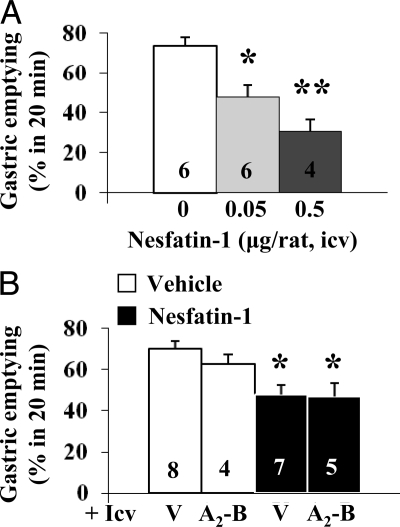
Nesfatin-1 (0.05 or 0.5 μg/rat, icv) also dose-dependently reduced the 20-min GE of a nonnutrient viscous solution to 47.9 ± 5.6% (P = 0.008) and 30.8 ± 5.8% (P < 0.001), respectively, compared with 73.5 ± 4.4% in the vehicle group as measured during the dark phase at 150–170 min after icv injection (Fig. 2A).
Figure 2.
Nesfatin-1 injected icv dose-dependently inhibits the dark-phase GE of a nonnutrient solution in fasted rats: lack of reversal by icv CRF2 antagonist. A, Chronically icv implanted rats fasted for 8 h were injected icv with vehicle or nesfatin-1 at the beginning of the dark phase and 150 min later gavaged; GE was monitored 20 min later. *, P < 0.01; **, P < 0.001 vs. vehicle. B, Astressin2-B (A2-B, 30 μg/rat) or vehicle (V) was injected icv before nesfatin-1 (0.05 μg/rat) or vehicle. *, P < 0.05 vs. vehicle/vehicle. Each column represents the mean ± sem of number of rats indicated at the bottom.
Nesfatin-1 (0.5 μg/rat) injected icv during the light phase in overnight fasted rats did not reduce the refeeding response compared with vehicle (P > 0.05) when food was given either immediately (supplemental Fig. 2A) or 2 h after injection (supplemental Fig. 2B).
CRF2 receptor antagonist injected into the lateral brain ventricle (icv) selectively blocks icv nesfatin-1-induced reduction of dark-phase food intake but not GE inhibition
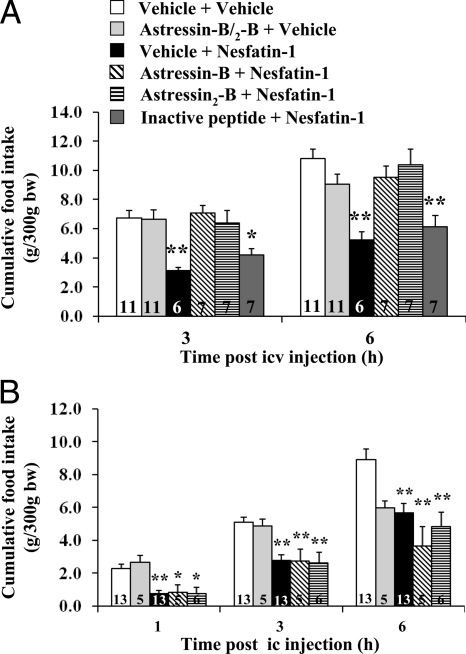
Nesfatin-1 (0.05 μg/rat, icv) reduced the 3- and 6-h cumulative food intake by 53.7 and 51.8% in icv vehicle-pretreated rats compared with vehicle alone (Fig. 3A). Nesfatin-1 inhibitory action was blocked by astressin-B and astressin2-B (30 μg/rat, icv) whereas the astressin2-B analog devoid of CRF antagonist activity (30 μg/rat, icv) had no effect (Fig. 3A). By contrast, astressin2-B (30 μg/rat, icv) did not modify the delayed GE induced by icv nesfatin-1 (0.05 μg/rat, Fig. 2B). The CRF antagonists injected icv alone under the same conditions modified neither basal food intake nor basal GE (Figs. 3A and 2B).
Figure 3.
A, CRF receptor antagonists injected icv prevent icv nesfatin-1-induced reduction of dark-phase food intake in freely fed rats. Chronically icv implanted rats were injected icv with 30 μg/rat astressin-B, astressin2-B, astressin2-B analog devoid of CRF2 binding affinity (inactive peptide), or vehicle before icv nesfatin-1 (0.05 μg/rat) or vehicle; cumulative food intake was monitored for 6 h. *, P < 0.02; **, P < 0.001 vs. vehicle/vehicle. B, CRF receptor antagonists injected ic do not prevent ic nesfatin-1-induced reduction of dark-phase food intake. Rats under brief anesthesia were injected ic with 30 μg/rat astressin-B, astressin2-B, or vehicle before ic injection of nesfatin-1 (0.5 μg/rat) or vehicle, and cumulative food intake monitored for 6 h. *, P < 0.05; **, P < 0.005 vs. vehicle/vehicle. Each bar in A and B represents the mean ± sem of number of rats indicated at the bottom. bw, Body weight.
Nesfatin-1 injected into the 4v or cisterna magna inhibits dark-phase food intake through CRF2-independent mechanisms while not altering GE
Nesfatin-1 injected into the 4v (0.05 μg/rat) in freely fed conscious rats chronically implanted with a cannula significantly decreased dark-phase cumulative food intake during the first hour (29%, P < 0.05) and up to 5 h (41%, P < 0.05) after injection (Fig. 1B) while not reducing GE (74.3 ± 5.3 vs. vehicle 75.1 ± 6.8%, n = 4 per group; P > 0.05) as measured in the dark period during the 30–50 min after injection. Likewise, when nesfatin-1 (0.05 or 0.5 μg/rat) was injected under brief anesthesia into the cisterna magna of noncannulated freely fed rats, there was a reduction of dark-phase food intake by 59–60% at 1 h after injection and a dose-related inhibition of cumulative food intake over 6 h after injection (Fig. 1C and supplemental Table 3) without influencing GE monitored for the 30- to 50-min period after injection (nesfatin-1 0.5 μg/rat: 51.6 ± 3.4%, n = 8 vs. vehicle 59.2 ± 3.8%, n = 4; P > 0.05). In addition, ic (0.5 μg/rat) nesfatin-1-induced suppression of dark-phase food intake was not altered by astressin-B or astressin2-B (30 μg/rat) (Fig. 3B).
Nesfatin-1 injected ip (2 μg/rat) had no effect on the dark-phase food intake (supplemental Fig. 3) as well as when injected into the cisterna magna during the light phase in overnight fasted rats (supplemental Fig. 4).
CCK-8S injected peripherally activates nesfatin-1 immunopositive neurons in the PVN and NTS
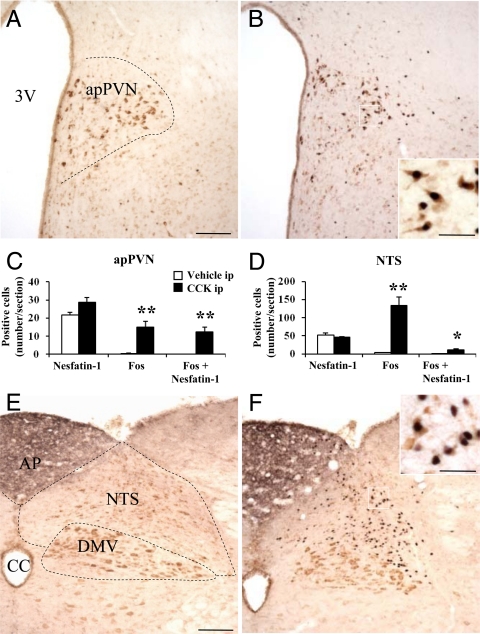
CCK-8S (3 μg/kg, ip) significantly increased the number of Fos-positive neurons in the apPVN (P < 0.01; Fig. 4, B and C) and the NTS (P < 0.01; Fig. 4, D and F) compared with ip vehicle (Fig. 4, A and C–E). Double labeling revealed that after CCK-8S, 43% of all nesfatin-1-ir cells in the apPVN and 24% in the NTS were activated as shown by double labeling of Fos and nesfatin-1 immunoreactivity (Fig. 4, C and D). After preabsorption of the anti-nesfatin-1 antibody, no immunostaining could be detected (data not shown).
Figure 4.
CCK injected ip induces Fos expression in nesfatin-1-ir neurons of the apPVN and NTS. Nonfasted rats were injected ip with vehicle (A and E) or CCK-8S (3 μg/kg) (B and F) and at the beginning of the dark phase euthanized 90 min later. CCK increased the number of Fos-positive neurons (black) in the apPVN (B and C) and NTS (D and F). The higher magnification shows that a proportion of these activated neurons colocalized with nesfatin-1 (brown) (B and F). Scale bars (A and E) 100 μm; scale bars in insets of B and F, 25 μm. Each bar represents the mean ± sem of three rats. *, P < 0.05; **, P < 0.01 vs. ip vehicle (C and D). AP, Area postrema; CC, central canal; DMV, dorsal motor nucleus of the vagus nerve; 3V, third ventricle.
Changes in Fos expression in nesfatin-1-ir neurons in the hypothalamus and NTS and plasma nesfatin-1 levels in response to fasting and refeeding
Fasting for 24 h did not significantly change the low Fos expression in the SON, PVN, and NTS (fewer than five neurons per section) compared with ad libitum fed rats (P > 0.05). A 2-h refeeding period after 24 h food deprivation significantly increased the number of Fos-positive neurons in the SON (P < 0.001, supplemental Fig. 5, C and D) and the NTS (P < 0.001, supplemental Fig. 6, C and D), whereas there was no change in the PVN (data not shown). In the SON, 99% of all activated cells induced by refeeding after a fast were nesfatin-1-ir (supplemental Fig. 5, C and D), whereas no significant difference in the number of double-labeled nesfatin-1/Fos-immunopositive neurons was detected in the NTS (supplemental Fig. 6).
Plasma levels of nesfatin-1 under ad libitum feeding conditions showed no significant difference between dark and light phase (2000 h 537 ± 32 vs. 0800 h 548 ± 42 pg/ml, P > 0.05, n = 5; Fig. 5). Fasting for 24 h significantly decreased plasma nesfatin-1 levels compared with ad libitum feeding conditions (448 ± 57 vs. 548 ± 42 pg/ml, P < 0.05), whereas 12 h refeeding restored circulating nesfatin-1 levels similar to ad libitum feeding (Fig. 5).
Figure 5.
Fasting for 24 h reduces plasma levels of nesfatin-1 compared with fed rats. Serial blood collections from a chronically implanted intrajugular catheter were performed at the onset of the dark phase (2000 h) and 12 h later (0800 h) in rats fed ad libitum and then after a 24-h fast and after a 12-h refeeding period. Data are expressed as mean ± sem (n = 5). *, P < 0.05 vs. ad libitum and 12-h refeeding, respectively.
Discussion
NUCB2 or nesfatin-1 (5 or 25 pmol) injected into the third brain ventricle was initially reported to inhibit nocturnal feeding in Wistar rats, whereas nesfatin-2 and -3 had no effect (1). In the present study, we extend these observations by showing that the low dose of nesfatin-1 (0.05 μg/rat = 5 pmol) injected into the lateral brain ventricle before the onset of the dark period significantly reduced the 3- to 6-h cumulative food intake by 45% in freely fed Sprague Dawley rats without altering blood glucose or inducing unusual changes in behavior. Although nesfatin-1 did not modify the 24-h cumulative food intake, body weight at 24 h after injection was significantly decreased indicative of increased energy expenditure. Whether nesfatin-1 induces changes in respiratory exchange ratio, body temperature, and uncoupling protein levels in brown adipose tissue requires further investigations. Next, we assessed whether a shorter fragment of nesfatin-1 may carry some biological activity. We hypothesized that in the absence of double basic residues, the Asp43-Pro45 bond may be more labile than any other bond. We synthesized the acetylated and amidated nesfatin-11–44 to mimic the amide bond of the extended molecule at both the N and C termini and therefore eliminating the possible charge effect. However, Ac-nesfatin-11–44-NH2, injected icv at 5 or 15 pmol, failed to influence the dark-phase food intake in freely fed rats, showing the specificity of nesfatin-1 action and indicating that the full-length peptide is required for biological activity. However, a recent study showed that a middle fragment of nesfatin-1 consisting of amino acids 24–53 reduced food intake after peripheral injection in mice whereas the N-terminal and C-terminal fragments were without effect (19). Yet to be established, as pointed out by the authors, is whether this middle fragment represents an endogenous form of nesfatin-1 (19).
Present data and existing evidence supports that icv nesfatin-1-induced reduction of nocturnal feeding involves the activation of hypothalamic CRF2 receptors. First, there is similarity between nesfatin-1 and CRF2 agonists, urocortins, in the temporal pattern of food reduction. We found that icv nesfatin-1 inhibited nocturnal feeding in freely fed rats with a 3-h delay without influencing the refeeding response to a fast in the light phase. Likewise, several reports indicate that urocortin 2 or 3 injected icv or microinjected into the PVN or ventromedial hypothalamic nucleus reduced nocturnal food intake with a 2- to 3-h delayed onset in freely fed rats through activation of hypothalamic CRF2 receptors while not altering the food intake in response to a fast (30,31,32). Second, astressin-B, which blocks both CRF receptors, and the selective CRF2 antagonist astressin2-B (9) injected icv completely prevented the nesfatin-1-induced inhibition of nocturnal feeding. Injected alone, the CRF antagonists had no effect as previously reported (33,34). Nonspecific potential peptide-peptide interaction was ruled out by showing the unchanged icv nesfatin-1 anorexic action in the presence of an astressin analog that bears structural similarity with astressin2-B but is devoid of CRF1 and CRF2 receptor affinity (>100 nm) (9). Taken together, these data are consistent with the hypothalamic CRF2 signaling system being part of underlying mechanisms of nesfatin-1-induced reduction of dark-phase food intake. In addition, we demonstrated that low doses of nesfatin-1 injected at the hindbrain level into the 4v (0.05 μg/rat) through a chronic cannula or into the cisterna magna (0.5 μg/rat) under light anesthesia inhibited the first hour of dark-phase food intake by 29 and 60%, respectively, with a cumulative food intake reduction still maintained for 5 h after injection. However, ic astressin-B and astressin2-B did not influence ic nesfatin-1-induced decrease in food intake. It is unlikely that the lack of CRF antagonist effect injected ic relates to the 10-fold higher dose of nesfatin-1 given ic vs. icv because astressin-B and astressin2-B were injected ic at a 10-fold higher dose than the maximally effective dose to block ic CRF inhibitory action on gastric motor function (27). CRF2-independent inhibition of the dark-phase food intake along with the short vs. delayed onset of the inhibitory effect induced by ic or 4v vs. icv nesfatin-1, respectively, provide compelling indication that icv and hindbrain (4v and cisterna magna) injections represent distinct sites of nesfatin-1 action. The hindbrain action site upon 4v and ic injections is also supported by the demonstration that compounds injected into the 4v are not seen rostrally to the caudal brainstem (35). The abundant distribution of nesfatin-1 in several hypothalamic as well as medullary nuclei such as the NTS and dorsal motor nucleus of the vagus (1,2,3,36) (present study) linked with food intake regulation (37) also gives neuroanatomical support for differential action sites of nesfatin-1. Distinct forebrain and hindbrain sites of action were established previously for several peptides (melanocortin, oxytocin, and bombesin) based on comparison between injection into the lateral cerebroventricle vs. 4v (38,39,40). Intraparenchymal microinjection studies will be required to identify specific hypothalamic and brainstem nuclei responsive to nesfatin-1.
The present study also demonstrates that the anorexic effect of icv or ic nesfatin-1 was specific to the physiological feeding occurring in the dark phase while not influencing the feeding response to a fast during the light phase. The lack of effect is not related to the delayed onset of icv nesfatin-1 inhibitory action, which may have occurred at a time when the drive to eat is low after the initial binge eating. When food was given to fasted rats at 2 h after icv nesfatin-1 injection, the peptide still did not decrease food intake. Moreover, the ic injection of nesfatin-1, which showed a rapid food intake-suppressing effect during the dark phase, did not influence food intake of overnight fasted rats during the light phase. This segregation of nesfatin-1 effects under ad libitum feeding during the dark phase vs. fasted conditions during the light phase may be linked with the interaction of nesfatin-1 with specific brain neuropeptide circuitries recruited during nighttime feeding as shown in the abrupt rise or fall of several neuropeptide genes at the onset of the dark phase before and during the feeding period (41,42,43).
Most of the centrally acting peptides reducing food intake also influence the process of digestion (44). Here we show for the first time that icv nesfatin-1 dose-dependently suppresses GE of a nonnutrient viscous solution. In addition, we demonstrate distinct mechanisms for icv nesfatin-1-induced inhibition of dark-phase food intake and gastric motor function because icv astressin2-B blocked the reduction of food intake without influencing the delayed GE. Moreover, we show that nesfatin-1 injected ic or into the 4v did not influence GE, supporting a forebrain site of action for icv nesfatin-1 to regulate gastric propulsive motor function. These data also indicate that delayed GE is unlikely to contribute to icv, ic, or 4v nesfatin-1-induced food intake reduction. Nesfatin-1 is colocalized with a large proportion of oxytocin neurons in the PVN (2,16,36) and also modulates the activity of PVN oxytocin neurons (6). In a previous study, we showed a prominent expression of nesfatin-1-ir in the magnocellular part of the PVN (3), and more than 70% of those neurons have been shown to be oxytocin-ir (16). Other neuroanatomical and functional studies established that oxytocinergic neurons of the PVN project to gastric subregions of the NTS (45) and inhibit GE in rats (46).
The present studies also revealed the activation of 43% of nesfatin-1 immunoreactivity neurons in the apPVN, a region where many neurons also contain CRF (47), and 24% of nesfatin-1-ir neurons in the NTS induced by ip injection of CCK-8S at a dose known to reduce food intake (15) in ad libitum fed rats in the dark phase under conditions where nesfatin-1 decreased food intake. These data suggest a possible implication of nesfatin-1 in the mediation of gut hormone-related reduction of food intake. Moreover, 2 h refeeding after 24 h food deprivation, a condition associated with satiety, induced Fos expression in SON and NTS neurons. The activation of neurons in the SON during refeeding after 48 h fasting has been reported before (36). In the SON, a region recently implicated in food intake regulation (48), the majority (99%) of activated neurons were nesfatin-1-ir, whereas in the NTS other than nesfatin-1-immunopositive neurons were activated by refeeding.
In addition to the brain, our recent report characterized the expression of NUCB2/nesfatin-1 in the stomach, most prominently within ghrelin cells of the rat gastric oxyntic mucosa (20). In particular, we demonstrated a significant down-regulation of NUCB2 in rat gastric small endocrine cells after 24 h fasting (20). In the present study, we showed that nesfatin-1 plasma levels decreased significantly after 24 h fasting and returned to baseline after refeeding, providing the first evidence of circulating NUCB2/nesfatin-1 and its modulation by changes in feeding status. However, circulating NUCB2/nesfatin-1 levels did not change between dark phase and light phase under ad libitum feeding conditions, suggesting the absence of circadian changes. Collectively, these data suggest that fasting inhibits both the synthesis and release of gastric nesfatin-1/NUCB2. The present findings add to a recent report showing that ip injection of nesfatin-1 at approximately 60 μg/mouse decreases nocturnal food intake in mice (19), which would suggest peripheral signaling in addition to the central signaling by nesfatin-1 to suppress food intake. However, we found that nesfatin-1 injected ip at a dose 40-fold higher than the icv effective dose did not alter the nocturnal food consumption in rats, indicative that the anorexic effect of nesfatin-1 is more readily observed upon brain than ip injection. Likewise in mice, nesfatin-1 injected ip at low doses (2 and 12 μg/mouse) did not influence food intake (19). Whether the lack of effect of nesfatin-1 upon ip injection at low doses in rodents reflects poor pharmacokinetics to reach the site of action under these conditions compared with direct systemic level changes will need to be ascertained.
In conclusion, we demonstrate that 5 pmol nesfatin-1 injected icv in freely fed rats induce a delayed inhibition of dark-phase feeding that involves the activation of CRF2 receptors. We also established a responsiveness of 4v or cisterna magna to nesfatin-1, resulting in a rapid onset reduction of dark-phase food intake that is CRF2 independent. These data point to distinct forebrain and hindbrain sites of nesfatin-1 consistent with the presence of nesfatin-1-immunopositive cells in both hypothalamic and hindbrain nuclei regulating ingestive behavior. Nesfatin-1 icv action on food intake is not linked with changes in blood glucose levels, locomotor activity, or grooming behavior. In addition, we established a novel central action of nesfatin-1 to influence visceral function as shown by the inhibition of GE selectively occurring upon icv injection and mediated by mechanisms distinct from those influencing food intake because they are CRF2 independent. A role of hypothalamic nesfatin-1 in satiety signaling induced by exogenous or endogenous gut peptides released by eating is suggested by the activation of nesfatin-1-ir neurons primarily within the apPVN by ip CCK and SON after 2 h refeeding after a fast. Our observations of a functional relationship between nesfatin-1 and the CRF2 signaling system after lateral brain ventricle injection to inhibit feeding may have implications in the understanding of stress-related eating disorders.
Supplementary Material
Acknowledgments
We thank Mrs. Honghui Liang for her excellent technical support, and we thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.), Veterans Administration Research Career Scientist Award, Veterans Administration Merit Award, NIHDK 33061, Center Grant DK-41301 (Animal Core) (Y.T.), and DK PO1-26741 (J.R.).
Disclosure Summary: A.S., M.G., L.W., P.K., H.M., N.W.G.L., and Y.T. have nothing to disclose. J.R. is Founder of Sentia Medical Sciences, Inc. No conflicts of interest exist.
First Published Online October 1, 2009
Abbreviations: apPVN, Anterior parvocellular part of the PVN; CCK-8S, sulfated cholecystokinin-8; CRF, corticotropin-releasing factor; GE, gastric emptying; ic, intracisternal; icv, intracerebroventricular; ir, immunoreactive; NTS, nucleus of the solitary tract; NUCB2, nucleobindin2; PVN, paraventricular nucleus; SON, supraoptic nucleus; 4v, fourth ventricle.
References
- Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M 2006 Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443:709–712 [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ 2007 Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148:5088–5094 [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Lambrecht NW, Taché Y 2009 Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett 452:241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB 2005 Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 493:63–71 [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C 2008 The brain, appetite, and obesity. Annu Rev Psychol 59:55–92 [DOI] [PubMed] [Google Scholar]
- Price CJ, Hoyda TD, Samson WK, Ferguson AV 2008 Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol 20:245–250 [DOI] [PubMed] [Google Scholar]
- Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM 2003 International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev 55:21–26 [DOI] [PubMed] [Google Scholar]
- Richard D, Lin Q, Timofeeva E 2002 The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol 440:189–197 [DOI] [PubMed] [Google Scholar]
- Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Taché Y, Vale W 2002 Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med Chem 45:4737–4747 [DOI] [PubMed] [Google Scholar]
- Moran TH, McHugh PR 1982 Cholecystokinin suppresses food intake by inhibiting gastric emptying. Am J Physiol 242:R491–R497 [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL 1996 Gastric volume rather than nutrient content inhibits food intake. Am J Physiol 271:R766–R769 [DOI] [PubMed] [Google Scholar]
- Silver IA, Ereciñska M 1994 Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci 14:5068–5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA 2004 Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- Shi M, Jones AR, Ferreira Jr M, Sahibzada N, Gillis RA, Verbalis JG 2005 Glucose does not activate nonadrenergic, noncholinergic inhibitory neurons in the rat stomach. Am J Physiol Regul Integr Comp Physiol 288:R742–R750 [DOI] [PubMed] [Google Scholar]
- Smith GP, Gibbs J 1994 Satiating effect of cholecystokinin. Ann NY Acad Sci 713:236–241 [DOI] [PubMed] [Google Scholar]
- Foo KS, Brismar H, Broberger C 2008 Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156:563–579 [DOI] [PubMed] [Google Scholar]
- Crawley JN, Kiss JZ 1985 Paraventricular nucleus lesions abolish the inhibition of feeding induced by systemic cholecystokinin. Peptides 6:927–935 [DOI] [PubMed] [Google Scholar]
- Edwards GL, Ladenheim EE, Ritter RC 1986 Dorsomedial hindbrain participation in cholecystokinin-induced satiety. Am J Physiol 251:R971–R977 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M 2009 Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology 150:662–671 [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Taché Y, Sachs G, Lambrecht NW 2009 Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150:232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Dallman MF, Woods SC 1995 Hypothalamic response to starvation: implications for the study of wasting disorders. Am J Physiol 269:R949–R957 [DOI] [PubMed] [Google Scholar]
- Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL 1999 Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem 42:3175–3182 [DOI] [PubMed] [Google Scholar]
- Martínez V, Taché Y 2001 Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res 893:29–35 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG 2002 Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143:239–246 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 2007 The rat brain in stereotaxic coordinates. San Diego: Academic Press [Google Scholar]
- Martinez V, Barquist E, Rivier J, Taché Y 1998 Central CRF inhibits gastric emptying of a nutrient solid meal in rats: the role of CRF2 receptors. Am J Physiol 274:G965–G970 [DOI] [PubMed] [Google Scholar]
- Czimmer J, Million M, Taché Y 2006 Urocortin 2 acts centrally to delay gastric emptying through sympathetic pathways while CRF and urocortin 1 inhibitory actions are vagal dependent in rats. Am J Physiol Gastrointest Liver Physiol 290:G511–G518 [DOI] [PubMed] [Google Scholar]
- Wang L, Martínez V, Vale W, Taché Y 2000 Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res 855:47–57 [DOI] [PubMed] [Google Scholar]
- Saper CB 2005 An open letter to our readers on the use of antibodies. J Comp Neurol 493:477–478 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Taché Y, Koob GF 2003 Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci 24:421–427 [DOI] [PubMed] [Google Scholar]
- Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szücs A, Koob GF, Zorrilla EP 2007 Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology 32:1052–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohata H, Shibasaki T 2004 Effects of urocortin 2 and 3 on motor activity and food intake in rats. Peptides 25:1703–1709 [DOI] [PubMed] [Google Scholar]
- Sekino A, Ohata H, Mano-Otagiri A, Arai K, Shibasaki T 2004 Both corticotropin-releasing factor receptor type 1 and type 2 are involved in stress-induced inhibition of food intake in rats. Psychopharmacology 176:30–38 [DOI] [PubMed] [Google Scholar]
- Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H 2005 Glucagon-like peptide-1, corticotropin-releasing hormone, and hypothalamic neuronal histamine interact in the leptin-signaling pathway to regulate feeding behavior. FASEB J 19:1131–1133 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Donahey JC, King L, Kaplan JM 1997 Contribution of caudal brainstem to d-fenfluramine anorexia. Psychopharmacology 130:375–381 [DOI] [PubMed] [Google Scholar]
- Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T 2008 Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149:1295–1301 [DOI] [PubMed] [Google Scholar]
- Gao Q, Horvath TL 2007 Neurobiology of feeding and energy expenditure. Annu Rev Neurosci 30:367–398 [DOI] [PubMed] [Google Scholar]
- Blevins JE, Schwartz MW, Baskin DG 2004 Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol 287:R87–R96 [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM 1998 Brainstem application of melanocortin receptor ligands produces long-lasting effects on feeding and body weight. J Neurosci 18:10128–10135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenheim EE, Ritter RC 1988 Low-dose fourth ventricular bombesin selectively suppresses food intake. Am J Physiol 255:R988–R993 [DOI] [PubMed] [Google Scholar]
- Xu B, Kalra PS, Farmerie WG, Kalra SP 1999 Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology 140:2868–2875 [DOI] [PubMed] [Google Scholar]
- Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, Bloom S 2000 Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett 279:109–112 [DOI] [PubMed] [Google Scholar]
- Lu XY, Shieh KR, Kabbaj M, Barsh GS, Akil H, Watson SJ 2002 Diurnal rhythm of agouti-related protein and its relation to corticosterone and food intake. Endocrinology 143:3905–3915 [DOI] [PubMed] [Google Scholar]
- Wren AM, Bloom SR 2007 Gut hormones and appetite control. Gastroenterology 132:2116–2130 [DOI] [PubMed] [Google Scholar]
- Rinaman L 1998 Oxytocinergic inputs to the nucleus of the solitary tract and dorsal motor nucleus of the vagus in neonatal rats. J Comp Neurol 399:101–109 [DOI] [PubMed] [Google Scholar]
- Wu CL, Hung CR, Chang FY, Pau KY, Wang JL, Wang PS 2002 Involvement of cholecystokinin receptor in the inhibition of gastric emptying by oxytocin in male rats. Pflugers Arch 445:187–193 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovács K, Imaki J, Vale W 1993 The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp 172:5–21; discussion 21–29 [DOI] [PubMed] [Google Scholar]
- Johnstone LE, Fong TM, Leng G 2006 Neuronal activation in the hypothalamus and brainstem during feeding in rats. Cell Metab 4:313–321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.