Abstract
Interest in biological substrates of sex-related variations in psychological and physiological characteristics has led to a search for biomarkers of prenatal hormone exposure that can be measured postnatally. There has been particular interest in digit ratio, the relative lengths of the second and fourth fingers (2D:4D), but its validity as a measure of prenatal androgen has not been established. We report the strongest evaluation of the value of 2D:4D as a biomarker for early androgen exposure. Individuals with 46,XY karyotype but no effective prenatal androgen exposure due to complete androgen insensitivity syndrome had digit ratios that were feminized: they were higher than those of typical men and similar to those of typical women. Nevertheless, the effect was modest in size, and there was considerable within-group variability and between-group overlap, indicating that digit ratio is not a good marker of individual differences in prenatal androgen exposure.
Data from individuals with complete androgen insensitivity show that digit ratio (2D:4D) is not a good marker of individual differences in prenatal androgen exposure.
Sex-related psychological characteristics are increasingly being investigated from a neuroscience perspective, with particular interest in the permanent neural and behavioral effects of sex hormones present during prenatal development. Evidence from people with disorders of sex development, resulting in a discordance between prenatal hormone exposure and rearing sex, shows that several sex-related characteristics (e.g. child and adolescent activity interests, aggression, interest in babies, sexual orientation) are associated with the amount of prenatal androgen exposure (1), confirming studies in nonhuman species (2). Such findings are increasingly being verified in typical individuals, i.e. childhood-gendered interests have been related to testosterone levels in amniotic fluid (3).
Nevertheless, the methods currently available to study links between prenatal hormones and postnatal behavior are difficult and expensive to use, and studies require many years to complete. This has led to a search for biomarkers, physical characteristics that are shaped by prenatal androgen exposure and that can be measured postnatally to reflect that exposure.
A biomarker that has generated considerable interest is digit ratio, the ratio of the second (index) finger to the fourth (ring) finger, 2D:4D (4,5). The ratio is lower in boys and men than girls and women. Men have a longer fourth digit relative to the second digit than do women. [Other digit ratios have been investigated and some also show sex differences (6), but the 2D:4D ratio has been the most extensively studied.] Although most studies of digit ratio trace back to the work of Manning and colleagues (4,5,6), there is earlier work on this and related characteristics (e.g. Refs. 7 and 8). Digit ratio has been assumed to reflect testosterone exposure during the second trimester of gestation because of the sex difference detectable in childhood and because of postulated mechanisms regarding digit development (4,9).
Claims about androgen effects on digit ratio have led to its widespread use, and there is now an extensive literature examining links between digit ratio and sex-typed characteristics, both psychological (e.g. sexual orientation, spatial ability, risk taking, aggression, gendered interests, autism), and physical (e.g. body shape, fertility, cardiovascular risk). Digit ratios are assumed to mark individual differences in prenatal androgen exposure. An association between digit ratio and some characteristic is thus considered to result from between-person variations in prenatal androgen leading to between-person variations in that characteristic. The link between digit ratio and the characteristic is considered to be mediated by between-person variations in anatomical structures subserving that characteristic; in the case of psychological characteristics, the anatomical structure is the brain.
Given the assumptions about digit ratio and the direction of the sex difference, the expectation is that, within sex, individuals with a lower digit ratio will be more male typed (e.g. be more aggressive, have higher spatial ability) than individuals with a higher digit ratio. The findings are inconsistent (discussed, for example, in Refs. 1 and 10): although there are some expected associations between digit ratio and a variety of characteristics, there are also failures to find associations (even with large samples) and associations that are in the opposite direction to expectation. There are few systematic attempts to explore the inconsistent findings, with notable exceptions of a metaanalysis of associations between digit ratio and spatial ability (11) and a reanalysis of data on associations between digit ratio and sexual orientation (12).
There is little evidence to show that digit ratio is in fact an indicator of prenatal androgen exposure. Most cited evidence is indirect, e.g. an observed sex difference in children, and associations with characteristics also putatively associated with prenatal androgen (13). Only four studies provide a direct test of the link between prenatal androgen and digit ratio; two of the four studies found confirming evidence, and there are caveats about those findings. The strongest evidence comes from females with congenital adrenal hyperplasia (CAH), who are exposed to high levels of androgen during prenatal development because of a genetic disorder and who are consequently masculinized in several physical and psychological characteristics (1,14). Females with CAH had lower (masculinized) digit ratios than control females in two studies (15,16) but not in a third (17). Males with CAH also had lower digit ratios than control males (15,16), which is difficult to reconcile with their generally sex-typical androgen levels (18,19) and sex-typical behavior (1).
The other direct test of the validity of digit ratio comes from a study relating amniotic hormone concentrations during the second trimester of pregnancy to digit ratios in 2-yr-old children (20). Digit ratio was not significantly associated with amniotic testosterone, but it was negatively associated with the ratio of testosterone to estradiol across sex. This finding is difficult to interpret because the sexes were not considered separately, the sex difference in digit ratio was small and nonsignificant, and it is unclear why estradiol would have an effect.
Studies in nonhuman animals do not provide strong support for androgen effects on digit ratio. Sex differences are not consistently found, and the differences found vary across species in size and body location. Among primates, digit ratios measured in metacarpals and metatarsals of museum skeletons are larger in gorillas than chimpanzees (even adjusting for species differences in sex dimorphism in body size), with the sex differences in chimpanzees mostly not significant. Further complicating interpretation of primate data, sex differences in metacarpals in human skeletons do not parallel sex differences in finger-length ratios (21). Among mice, sex differences in digit ratios have been seen in small samples (e.g. Ref. 22) but not in a large-scale study of 20 inbred mouse strains (which revealed large strain differences) (23). Digit ratio in mice does not appear to be correlated with anogenital distance, a known marker of androgen exposure (24,25). In rats, testosterone treatment of pregnant mothers masculinized the 2D:4D ratio in male and female offspring, primarily by increasing the length of the fourth digit (26). The results are difficult to interpret, however, because the sex difference in control animals was not significant and was in the direction opposite to expectation (i.e. males had a higher ratio than females), and testosterone treatment appears to have reduced digit ratio to the same extent in both males and females.
Thus, there is a compelling need for additional evidence directly examining the link between prenatal androgen exposure and digit ratio. We report such evidence from a study of people with complete androgen insensitivity syndrome (CAIS), who provide the best natural experiment of prenatal androgen effects because they have no effective exposure in utero. Individuals with CAIS have a typical male 46,XY karyotype with normal testes and male-typical levels of testosterone, but absent or dysfunctional androgen receptors leave them unable to respond to endogenous or exogenous androgens. As a consequence, affected individuals have female-typical external genitalia, are raised as girls, undergo feminization at puberty (if their testes remain in situ) as a result of aromatization of testosterone to estradiol (27), and have female-typical gender identity and gender-related psychological characteristics (28,29). Because individuals with CAIS are physically and psychologically female, they are considered to be women.
If digit ratio is a reflection of androgen exposure during prenatal development, then women with CAIS should have extremely feminized (high) digit ratios. Furthermore, there should be minimal variability in digit ratio among women with CAIS because none has effective androgen exposure.
Materials and Methods
Participants
Women with CAIS (n = 16) were recruited at an annual meeting of the Androgen Insensitivity Syndrome Support Group of the United States of America (AISSG-USA). The comparison groups consisted of 90 women and 66 men unaffected by CAIS: control participants included unaffected parents (nine women, one man) of women with CAIS also attending the AISSG-USA meeting and unaffected adults (81 women, 65 men) participating in other studies [unpublished or described elsewhere (30)].
Women with CAIS were identified by self-report. Members of AISSG-USA are typically well educated about the specifics of their diagnosis, based on detailed research into their personal medical histories, including karyotype, hormonal profile, and clinical features. Women with conditions other than CAIS (e.g. partial androgen insensitivity syndrome, gonadal dysgenesis, and unknown causes of female sex differentiation in 46,XY individuals) also belong to AISSG-USA; nine such individuals also participated in this study, but the sample was too small and heterogeneous for meaningful analysis, so their data were excluded.
Participants ranged in age from 18 to 75 yr. Groups differed in age because of the recruitment methods [F (2, 169) = 10.62, P < 0.001]; mean (and sd) age in years: CAIS 41.94 (13.19), control women 30.64 (14.13), control men 26.00 (9.95). Nevertheless, age was not significantly associated with digit ratio (r = −0.05 and −0.03 for right and left hands, respectively), consistent with other data (5). Groups also differed in race; the control sample was more diverse than the CAIS sample. Although there are racial/ethnic differences in digit ratio, sex differences are similar across racial/ethnic groups (5). The pattern of results was unchanged when analyses were restricted to whites only.
The research was approved by the appropriate institutional review boards. Participants provided written consent.
Procedure
Data collection and scoring followed procedures used by others (5). Hands were photocopied, and the lengths of the right and left index and ring fingers were measured with a caliper from the most proximal crease to the finger tip by a single rater unaware of the participant’s group. Finger lengths were independently measured by a second rater for a subset of hand prints; the two sets of ratings were highly correlated: median r = 0.98 across right and left second and fourth digits.
The main analyses concerned group differences in mean and variance of 2D:4D. Mean differences were assessed by ANOVA with factors of group (CAIS, control men, control women) and hand (repeated measure: left, right), followed by post hoc comparisons (least significant difference) using one-tailed tests. Effect sizes are described by η2 for the overall group effect and Cohen’s d for pairwise differences. Variance differences were assessed by Levene’s test, with pairwise comparisons. A supplementary discriminant analysis was used to predict group membership from right and left hand 2D:4D. Effects significant at P < 0.05 are reported.
Results and Discussion
Comparison of right and left hand 2D:4D among groups of individuals with CAIS, control men, and control women showed that average 2D:4D differed across group [F (2, 169) = 4.23, P < 0.02, partial η2 = 0.05] but not hand [F (1,169) = 1.87, P = ns] or as an interaction between group and hand [F (2, 169) = 0.64, P = ns], as shown in Table 1. There was the expected sex difference among control participants: women had significantly higher 2D:4D than men (P < 0.003, d = 0.48, right hand; d = 0.33, left hand). Women with CAIS had feminized ratios: 2D:4D of women with CAIS were significantly higher than those of control men (P < 0.04, d = 0.61, right hand; d = 0.35, left hand) but not significantly different from those of control women.
Table 1.
Digit ratio in individuals with CAIS and controls: means and sds
| CAIS | Control women | Control men | |
|---|---|---|---|
| n | 16 | 90 | 66 |
| Right hand: means (sd) | 0.9715 (0.032) | 0.9685 (0.037) | 0.9517 (0.033) |
| Left hand: means (sd) | 0.9724 (0.041) | 0.9720 (0.037) | 0.9603 (0.033) |
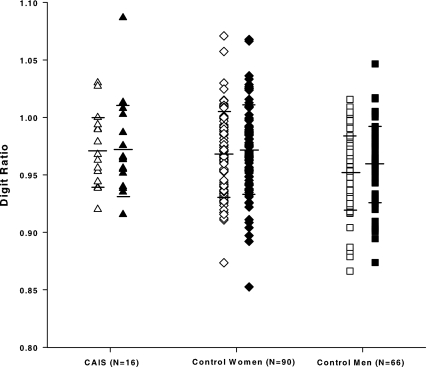
There was considerable variability in digit ratio in all groups, as shown in Fig. 1. Variability in 2D:4D did not differ significantly across groups for either right or left hand (pairwise Fs all <0.62, P = ns). Thus, women with CAIS were not less variable in 2D:4D than control women or control men, and there was considerable overlap among groups.
Figure 1.
Right hand 2D:4D (open symbols) and left hand 2D:4D (filled symbols) for women with CAIS, control women, and control men. Each point represents an individual. Horizontal lines represent group means and group sds.
Discriminant analyses to predict group membership from digit ratio yielded one significant function (Wilks’ λ = 0.94, χ2 (4, n = 172) = 9.70, P < 0.05), which was defined by 2D:4D in both hands but more by the right than the left; structure matrix loadings (correlations between the variables and the discriminant function) were 0.99 for the right hand and 0.65 for the left hand. As shown in Tables 2 and 3, accuracy of classification into groups (CAIS, control man, control woman) was not high. Group classification was 38% when the probability of group membership was equal for all groups, and 54% when the probability of group membership was based on group size; in the latter case, no participant was classified as CAIS and participants in all groups were most likely to be classified as control women.
Table 2.
Prediction of group membership from digit ratio: classification results with equal probability of membership in each group
| Actual group membership | Predicted group membership
|
||
|---|---|---|---|
| CAIS | Control women | Control men | |
| CAIS (n = 16) | 7 (43.8%) | 2 (12.5%) | 7 (43.8%) |
| Control women (n = 90) | 39 (43.3%) | 17 (18.9%) | 34 (37.8%) |
| Control men (n = 66) | 15 (22.7%) | 10 (15.2%) | 41 (62.1%) |
Overall correct classification was 37.8%. Table entries are numbers of participants correctly classified (and percentages in parentheses).
Table 3.
Prediction of group membership from digit ratio: classification results with probability of group membership based on group size
| Actual group membership | Predicted group membership
|
||
|---|---|---|---|
| CAIS | Control women | Control men | |
| CAIS (n = 16) | 0 (0%) | 15 (93.8%) | 1 (6.3%) |
| Control women (n = 90) | 0 (0%) | 71 (78.9%) | 19 (21.1%) |
| Control men (n = 66) | 0 (0%) | 44 (66.7%) | 22 (33.3%) |
Overall correct classification was 54.1%. Table entries are numbers of participants correctly classified (and percentages in parentheses).
Feminized digit ratios in 46,XY individuals with no effective prenatal androgen exposure provide the strongest evidence to date that androgens play a role in their formation. Nevertheless, the role is modest at best, as revealed in several ways. First, the mean difference in 2D:4D between women with CAIS and typical men was moderate in size (d = 0.48 averaged across hands). Second, 2D:4D was not significantly different between women with CAIS and typical women; 2D:4D should be more feminized in women with CAIS than typical women who have some effective androgen exposure. Third, there was considerable variability in 2D:4D among all groups, including those with CAIS who vary minimally in their effective androgen exposure. Fourth, group membership was not predicted with high accuracy from 2D:4D. Digit ratios did not even provide high discrimination of control men and women, despite the marked sex difference in prenatal androgen exposure.
It is important to note three qualifications about androgen action in individuals with CAIS. First, most individuals with CAIS do not completely lack androgen receptors but have a variety of missense mutations that impair receptor function; some women with CAIS may have a minimal degree of androgen responsiveness, as evidenced by small amounts of pubic and/or axillary hair beginning at puberty (31,32). It seems unlikely that this minimal degree of androgen response would account for our results, given that varying mutations are all associated with a complete female phenotype (32). Second, it is possible that individuals with CAIS are exposed to androgens via alternative pathways that do not involve classical androgen receptors. Thus, the variability among individuals with CAIS could reflect variability in androgen effects not due to androgen receptor sensitivity. This also seems unlikely, given the many ways in which individuals with CAIS are female typical (1,27,28,29). Third, effects of defective androgen receptors are not confined to prenatal development. It is therefore possible that feminized digit ratios in individuals with CAIS reflect a lack of androgen action at any point in development. The prenatal period is the most likely time in light of other data on digit ratio, e.g. early development of the sex difference, and masculinized ratios in females with CAH.
There are some methodological limitations that should be considered in interpreting the results. First, the sample of women with CAIS was small. It is thus noteworthy that results showed significant between-group differences and within-group variability. Second, individuals with CAIS were identified by self-report on the basis of their personal medical diagnoses, not by genetic analysis. But there is a good correlation between genotype and phenotype in CAIS (32), and the selection methods and results make it unlikely that the sample includes individuals with partial androgen insensitivity. Third, control participants were not matched in age or race to women with CAIS, but there is no reason to expect that differences would vary with other comparison groups. Fourth, type I error was increased with the use of one-tailed tests and uncorrected multiple comparisons. This was done because of clear directional predictions and to reduce type II error.
Our results show the subtlety of androgen effects on digit ratio, and thus both confirm and refute claims about the value of 2D:4D. On the one hand, the higher mean ratios in individuals with CAIS than in typical men support the claim that digit ratio is influenced by androgen exposure. On the other hand, the modest size of the difference and the considerable within-group variability challenge the claim that digit ratio serves as a marker of individual differences in prenatal androgen exposure that can be related to other physical and psychological characteristics. Androgens are clearly not a large contributor to variations in digit ratio.
An unanswered question concerns the mechanisms by which androgens affect digit ratio. Although androgens have been hypothesized to act directly on the formation of the fingers (4,9), the sex difference in digit ratios found in hand prints is not found in skeletons (21). Furthermore, measurement method can have an effect on 2D:4D; for example, photocopies produce lower ratios than direct finger measurements (33). Digit ratio thus appears to be heavily influenced by the tissue over the bones and the way that tissue is measured, including variations in the amount of pressure applied when hands are photocopied. This means that androgens could affect digit ratio in any number of ways, both direct (e.g. fat deposition) and indirect (e.g. social influences making individuals reared as girls more likely than those reared as boys to comply with test instructions in applying pressure during photocopying of hands). Thus, androgen effects on digit ratio cannot be assumed to reflect direct androgen effects on the brain.
In summary, our data show that digit ratio is related to effective androgen exposure but that the relation is too small to use digit ratio as a marker for individual differences in prenatal androgen exposure. It is wise to be cautious in interpreting the meaning of individual differences in 2D:4D.
Acknowledgments
We thank the participants; Dr. Arlene Baratz and members of the Androgen Insensitivity Syndrome Support Group USA for facilitating the study; Lauretta Brennan for assistance with data processing; and Kim Wallen and Emilie Rissman for valuable discussion of the meaning and measurement of digit ratio and helpful comments on an earlier version of the paper.
Footnotes
This work was supported in part by Grant HD044398 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
For editorial see page 4819
Abbreviations: AISSG-USA, Androgen Insensitivity Syndrome Support Group of the United States of America; CAH, congenital adrenal hyperplasia; CAIS, complete androgen insensitivity syndrome.
References
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA 2005 Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev 29:353–384 [DOI] [PubMed] [Google Scholar]
- Wallen K 2005 Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol 26:7–26 [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Ashwin E, Knickmeyer R, Taylor K, Hackett G, Hines M 2009 Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol Sci 20:144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI 1998 The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod 13:3000–3004 [DOI] [PubMed] [Google Scholar]
- Manning JT 2002 Digit ratio: a pointer to fertility, behavior and health. New Brunswick, NJ: Rutgers University Press [Google Scholar]
- Manning JT, Callow M, Bundred PE 2003 Finger and toe ratios in humans and mice: implications for the aetiology of diseases influenced by HOX genes. Med Hypotheses 60:340–343 [DOI] [PubMed] [Google Scholar]
- Ecker A 1875 Some remarks about a varying character in the hands of humans. Arch Anthropol 8:68–74 [Google Scholar]
- Voracek M, Dressler SG, Loibl LM 2008 The contributions of Hans-Dieter Rösler: pioneer of digit ratio (2D:4D) research. Psychol Rep 103:899–916 [DOI] [PubMed] [Google Scholar]
- McIntyre MH 2006 The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol 4:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putz DA, Gaulin SJC, Sporter RJ, McBurney DH 2004 Sex hormones and finger length. What does 2D:4D indicate? Evol Hum Behav 25:182–199 [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, Breedlove SM 2008 Spatial ability and prenatal androgens: meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Arch Sex Behav 37:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC, Breedlove SM, Lippa RA, Manning JT, Rahman Q 2005 A reanalysis of five studies on sexual orientation and the relative length of the 2nd and 4th fingers (the 2D:4D ratio). Arch Sex Behav 34:341–356 [DOI] [PubMed] [Google Scholar]
- Voracek M, Dressler SG, Manning JT 2007 Evidence for assortative mating on digit ratio (2D:4D), a biomarker for prenatal androgen exposure. J Biosoc Sci 39:599–612 [DOI] [PubMed] [Google Scholar]
- Speiser PW, ed. 2001 Congenital adrenal hyperplasia. Endocrinol Metab Clin North Am 30:i–244 [DOI] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM 2002 Masculinized finger length patterns in human males and females with congenital adrenal hyperplasia. Horm Behav 42:380–386 [DOI] [PubMed] [Google Scholar]
- Okten A, Kalyoncu M, Yariş N 2002 The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev 70:47–54 [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL 2003 In utero exposure and 2nd to 4th digit length ratio—comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod 18:976–979 [DOI] [PubMed] [Google Scholar]
- Pang S, Levine LS, Chow DM, Faiman C, New MI 1979 Serum androgen concentrations in neonates and young infants with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 11:575–584 [DOI] [PubMed] [Google Scholar]
- Pang S, Levine LS, Cederqvist LL, Fuentes M, Riccardi VM, Holcombe JH, Nitowsky HM, Sachs G, Anderson CE, Duchon MA, Owens R, Merkatz I, New MI 1980 Amniotic fluid concentrations of Δ5 and Δ4 steroids in fetuses with congenital adrenal hyperplasia due to 21 hydroxylase deficiency and in anencephalic fetuses. J Clin Endocrinol Metab 51:223–229 [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT 2004 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev 77:23–28 [DOI] [PubMed] [Google Scholar]
- McFadden D, Bracht MS 2009 Sex and race differences in the relative lengths of metacarpals and metatarsals in human skeletons. Early Hum Dev 85:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Finn CJ, Breedlove SM 2002 Sexual dimorphism in digit-length ratios of laboratory mice. Anat Rec 267:231–234 [DOI] [PubMed] [Google Scholar]
- Yan RH, Bunning M, Wahlsten D, Hurd PL 2009 Digit ratio (2D:4D) differences between 20 strains of inbred mice. PLoS One 4:e5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd PL, Bailey AA, Gongal PA, Yan RH, Greer JJ, Pagliardini S 2008 Intrauterine position effects on anogenital distance and digit ratio in male and female mice. Arch Sex Behav 37:9–18 [DOI] [PubMed] [Google Scholar]
- Manno 3rd FA 2008 Measurement of the digit lengths and the anogenital distance in mice. Physiol Behav 93:364–368 [DOI] [PubMed] [Google Scholar]
- Talarovicová A, Krsková L, Blazeková J 2009 Testosterone enhancement during pregnancy influences the 2D: 4D ratio and open field motor activity of rat siblings in adulthood. Horm Behav 55:235–239 [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Hughes IA, Conte FA 2003 Disorders of sex differentiation. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, eds. Williams textbook of endocrinology. 10th ed. Philadelphia: W. B. Saunders; 842–1002 [Google Scholar]
- Hines M, Ahmed SF, Hughes IA 2003 Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Arch Sex Behav 32:93–101 [DOI] [PubMed] [Google Scholar]
- Mazur T 2005 Gender dysphoria and gender change in androgen insensitivity or micropenis. Arch Sex Behav 34:411–421 [DOI] [PubMed] [Google Scholar]
- Nowak NT 2008 The relationship between second to fourth digit ratio (2D:4D), spatial cognition, and navigation in a virtual Morris water task. Master’s thesis, Wayne State University [Google Scholar]
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 16:271–321 [DOI] [PubMed] [Google Scholar]
- Cheikhelard A, Morel Y, Thibaud E, Lortat-Jacob S, Jaubert F, Polak M, Nihoul-Fekete C 2008 Long-term followup and comparison between genotype and phenotype in 29 cases of complete androgen insensitivity syndrome. J Urol 180:1496–1501 [DOI] [PubMed] [Google Scholar]
- Manning JT, Fink B, Neave N, Caswell N 2005 Photocopies yield lower digit ratios (2D:4D) than direct finger measurements. Arch Sex Behav 34:329–333 [DOI] [PubMed] [Google Scholar]