Abstract
The correct identification of circulating molecular forms and measurement of peptide levels in blood entails that the endocrine peptide being studied is stable and recovered in good yields during blood processing. However, it is not clear whether this is achieved in studies using standard blood processing. Therefore, we compared peptide concentration and form of 12 125I-labeled peptides using the standard procedure (EDTA-blood on ice) and a new method employing Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls, and Dilution (RAPID). During standard processing there was at least 80% loss for calcitonin-gene-related peptide and cholecystokinin-58 (CCK-58) and more than 35% loss for amylin, insulin, peptide YY forms (PYY(1–36) and PYY(3–36)), and somatostatin-28. In contrast, the RAPID method significantly improved the recovery for 11 of 12 peptides (P < 0.05) and eliminated the breakdown of endocrine peptides occurring after standard processing as reflected in radically changed molecular forms for CCK-58, gastrin-releasing peptide, somatostatin-28, and ghrelin. For endogenous ghrelin, this led to an acyl/total ghrelin ratio of 1:5 instead of 1:19 by the standard method. These results show that the RAPID method enables accurate assessment of circulating gut peptide concentrations and forms such as CCK-58, acylated ghrelin, and somatostatin-28. Therefore, the RAPID method represents an efficacious means to detect circulating variations in peptide concentrations and form relevant to the understanding of physiological function of endocrine peptides.
The RAPID method allows correct assessment of circulating peptide levels and more importantly their correct bioactive molecular forms.
Endocrine peptides have been studied for over 100 yr (1). Analytical methods were developed that could sequence linear peptides purified from bioassays that quickly led to the determination of the sequences of insulin, secretin, gastrin, and cholecystokinin (CCK) (2). The bioassays to purify these peptides were expensive and time-consuming, and peptide levels were often difficult to accurately measure.
The study of peptides took a gigantic leap forward when Berson and Yalow (3) developed a sensitive and specific detection method for blood and tissue extracts now known as RIA. For accurate determination of peptide concentration by RIA, the endocrine peptide being studied should be stable and recovered in good yields during blood processing. An often unstated hypothesis underlying reports on forms and concentrations of circulating peptides is that they are stable and well recovered during plasma formation.
However, we showed that both aspects of this hypothesis are false for CCK when plasma is formed (4). Several endocrine forms of CCK have been reported: CCK-58 (4,5), CCK-33/39 (6), CCK-22 (7), and CCK-8 (8,9). We hypothesized that this diversity of observations was due to differences in blood processing and not to actual differences of circulating forms. To test this, CCK-58 was radiolabeled to produce [125I]CCK-58 and added to blood. After plasma was formed as commonly done by others when evaluating the molecular forms of CCK (6,7,8,9), the recovery was determined by counting supernatant and pellet and the stability evaluated by the elution position of radioactivity during HPLC. Surprisingly, during plasma formation, most of [125I]CCK-58 was associated with the pellet and only 20% observed in the plasma, indicating that recovery could be improved if methods were developed that prevented peptides from associating with plasma. Furthermore, most of the radiolabeled peptide present in the plasma eluted as several peaks in earlier positions than the one observed for intact [125I]CCK-58. These data indicated that the differences in the observed CCK forms in previous studies (4,5,6,7,8,9) may involve various amounts of ex vivo degradation and are probably not due to actual differences in circulating forms of CCK.
The hypothesis that loss or degradation of endocrine peptides can occur during plasma formation was tested for twelve bioactive peptides: amylin, calcitonin, calcitonin-gene-related peptide (CGRP), CCK, gastrin, ghrelin (Ghr), glucagon-like peptide (GLP)-1, gastrin-releasing peptide (GRP), insulin, peptide YY forms (PYY(1–36), PYY(3–36)), and somatostatin-28. Processing according to standard plasma formation was compared with new processing combining Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls, and Dilution that we termed the RAPID method.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Harlan, San Diego, CA), weighing 280–350 g, were housed four per cage under controlled illumination (0600–1800 h) and temperature (21–23 C). Rats had free access to rodent chow (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Animal Research Committee at Veterans Affairs Greater Los Angeles Healthcare System (no. 99-059-04).
Radiolabeling CCK-58, PYY(1–36), or PYY(3–36)
CCK-58, PYY(1–36), and PYY(3–36) were synthesized in the University of California, Los Angeles, Peptide Synthesis Facility, purified by reverse-phase HPLC and characterized as previously described (5). All peptides had the correct mass, CCK-58 had a purity greater than 90% and PYY peptides greater than 97%. Ten micrograms of CCK-58, PYY(3–36), or PYY(1–36) were dissolved in 20 μl PBS (pH 7.4). Radiolabel Na125I (500 μCi) in 5 μl PBS was added to the peptide solution. Chloramine-T (10 μg/10 μl PBS) was added to the mixture. After 20 sec, the reaction was quenched by adding an equal volume of 50% acetic acid. The labeled peptide was separated from free 125I by G-10 gel-permeation chromatography. Tubes containing the first peak of radioactivity from the G-10 column were pooled, diluted 3-fold with 0.1% trifluoroacetate (TFA) and loaded onto a C-18 reverse-phase column. The 125I-labeled peptides were eluted with a gradient of 22.5–37.5% acetonitrile. Other iodinated peptides were purchased (Bachem, Inc., Torrance, CA). All radioactive peptides eluted as a single major radioactive peak during reverse-phase HPLC of the label before it was added to blood. Calcitonin, CGRP, and GLP-1 needed to be purified to a single radioactive peak before use in the experiments. The specific activity was 500-2000 cpm/fmol peptide.
Recovery after standard and RAPID method for blood processing
For each of the 12 peptides, blood from six naive rats was used to compare recovery by the two processing methods. Rats were anesthetized with pentobarbital (10 mg/kg, ip) between 0800 and 0900 h, and blood (10 ml) was collected by cardiac puncture into EDTA-rinsed syringes, and 1-ml aliquots added into tubes containing the respective radiolabeled analog (20,000–50,000 cpm in 10–20 μl 0.1% acetic acid). Thereafter, the two methods were performed separately to compare recovery and forms.
For the standard method, blood was kept on ice (10 min maximum) until centrifugation. The RAPID method used blood diluted 1:10 in ice-cold buffer (pH 3.6) containing radiolabel, 0.1 m ammonium acetate, 0.5 m NaCl, and enzyme inhibitors diprotin A, E-64-d, antipain, leupeptin, chymostatin (1 μg/ml; Peptide International, Louisville, KY). All samples were centrifuged at 3000 × g for 10 min at 4 C.
The supernatants were collected, counted for radioactivity, and frozen at −80 C for 24–72 h. The pellets were also counted for radioactivity and then discarded. Frozen supernatants were thawed at room temperature for 30 min, chromatographed by Sep-Pak C18 cartridges (360 mg, 55–105 μm, product no. WAT051910; Waters Corp., Milford, MA) and characterized by reverse-phase HPLC.
Stepwise Sep-Pak chromatography of standard or RAPID samples
Sep-Pak cartridges were charged with 2 ml 100% acetonitrile and equilibrated with 4 ml 0.1% TFA. The equilibrated columns were loaded with sample, rinsed with 5 ml 0.1% TFA, and eluted with 70% acetonitrile containing 0.1% TFA. Fractions (2 ml) were collected starting with sample loading. All fractions were counted, and those containing radioactivity were pooled for HPLC analysis of peptide stability. For RIA, samples were diluted, centrifuged, Sep-Pak chromatographed, and dried by vacuum centrifugation. Peptide powder was stored at −80 C until further processing (Fig. 1).
Figure 1.
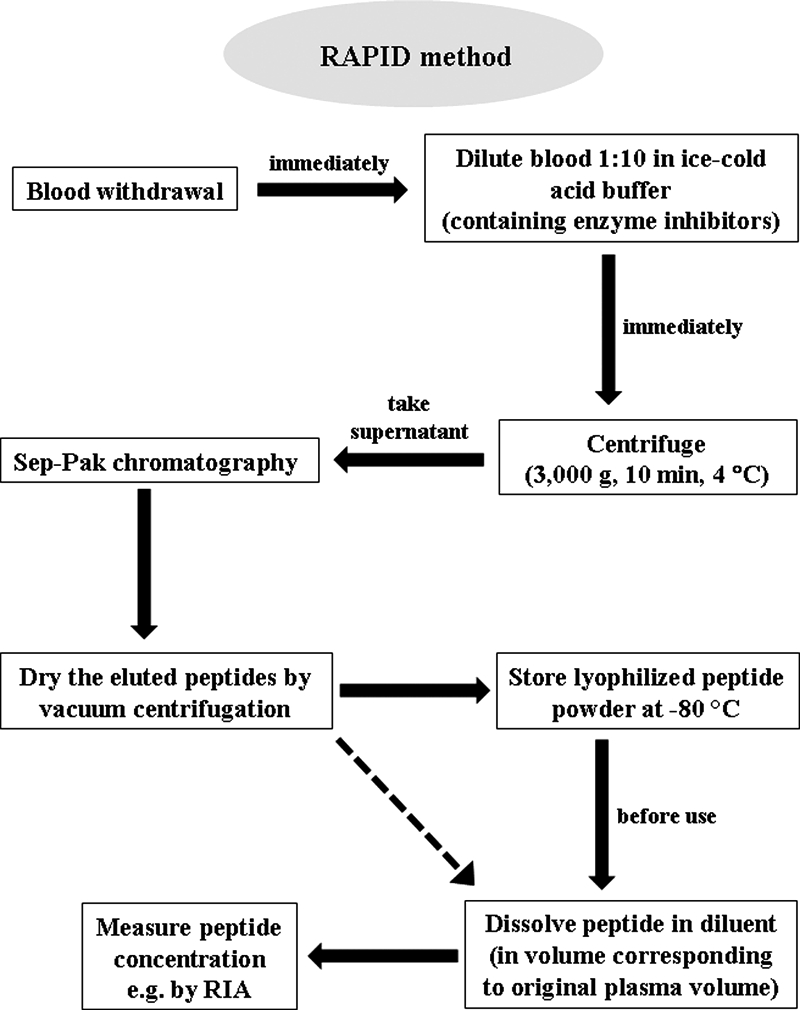
Flow chart for blood processing using the RAPID method combining reduced temperatures, acidification, protease inhibition, isotopic exogenous controls, and dilution. The different steps should be performed rapidly until peptide powder is obtained. For simplification purposes, the comparison of recovery between standard and RAPID methods using radiolabeled peptide analogs is not shown in the figure. The plasma volume required for detection of different circulating peptides by RIA may vary and depends on the plasma concentration of the respective peptide.
Reverse-phase HPLC evaluation of peptide stability during standard and RAPID methods
For all 12 peptides, all radioactive fractions from the Sep-Pak chromatography were pooled, diluted 1:3, and chromatographed on an analytical Vydac C-18 HPLC column (10 μm, 4.6 × 250 mm; Western Analytical, Hesperia, CA) equilibrated in 0.1% TFA. The diluted sample was injected in 7-ml aliquots with a 10-ml injection loop. The sample was eluted with a 10 min gradient to 20% acetonitrile, then 120 min gradient to 35% acetonitrile at a flow rate of 1 ml/min. Fractions (2 ml) were collected from the start of loading and counted to determine recovery of radioactivity. No radioactivity was observed before the 120-min gradient.
RIA for acyl Ghr (A-Ghr) and total Ghr (T-Ghr)
Rats received a jugular vein catheter and were allowed to recover for 4 d as in previous studies (10). Blood (1 ml) was withdrawn from conscious lightly hand-restrained ad libitum-fed and 24-h fasted rats between 0800 and 0900 h and processed according to the standard or RAPID method. Samples were resuspended in ddH2O before use. Plasma T- and A-Ghr was measured using separate RIA kits (Linco Research, St. Charles, MO).
Data analysis
Data are expressed as mean ± sd and were analyzed by ANOVA followed by Tukey’s post hoc test, and P < 0.05 was considered significant.
Results
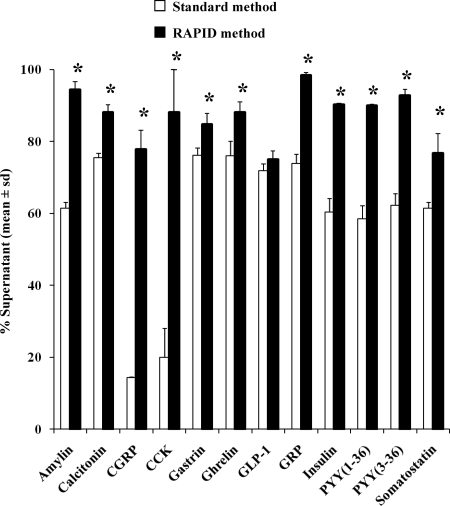
The recovery of 12 radiolabeled peptides (supplemental Fig. 1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) was studied, selected for diversity in net ionic charge (−6 to +6), size (17-58 amino acids), end groups (NH3+ and pyroglutamyl amino termini and free acid and amide carboxyl termini), and posttranslational modification (acylation and sulfation). Only three peptides, [125I]calcitonin, [125I]gastrin, and [125I]Ghr (T-Ghr), were recovered with higher than 75% yields after standard processing, but still significantly less compared with the RAPID method (P < 0.05, Fig. 2). Importantly, [125I]CGRP and [125I]CCK-58 showed a recovery in plasma of only 14 and 20%, respectively (Fig. 2). More than half of the peptides, namely amylin, CGRP, CCK-58, insulin, PYY(1–36), PYY(3–36), and somatostatin-28 were recovered at 60% yield or less, indicating a loss of 40% by standard processing (Fig. 2). Plasma acidification alone did not prevent the breakdown of CCK-58 (supplemental Fig. 2). The RAPID method improved the yields to 75% or above for all 12 endocrine peptides (Fig. 2).
Figure 2.
Recovery of iodinated peptides using the standard method (white bars) compared with the RAPID method (black bars). After counting, the samples were processed with stepwise Sep-Pak chromatography and chromatographed by reverse-phase HPLC to determine the stability of the label. *, P < 0.05.
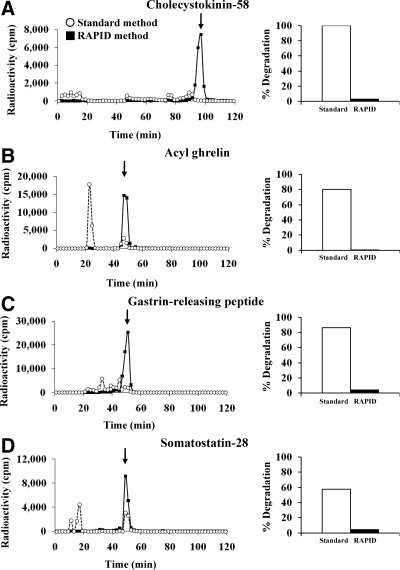
During reverse-phase HPLC, degraded peptides elute earlier than the intact peptide. After standard processing, radiolabel eluted before intact label for CCK-58, Ghr, GRP, and somatostatin-28, indicating substantial degradation (>80% for three peptides) resulting in radically changed molecular forms (Fig. 3, A–D). The RAPID method eliminated this degradation (Fig. 3, A–D). No degradation was observed for the other eight peptides when blood was processed by either method.
Figure 3.
Elution profile of radioactive peptides from reverse-phase HPLC after the standard (○) or RAPID (▪) method for blood processing. Degraded peptides elute earlier than the intact peptide. The degradation seen in the plasma sample can be avoided by using the RAPID method for CCK-58 (A), A-Ghr (B), GRP (C), and somatostatin-28 (D). The arrows show the elution position of labeled peptides not added to blood and chromatographed separately. These elution positions correspond to the elution position of peptides added to blood and processed by the RAPID method. The right panel shows the percentage of degradation observed after the standard and the RAPID method, respectively. cpm, Counts per minute.
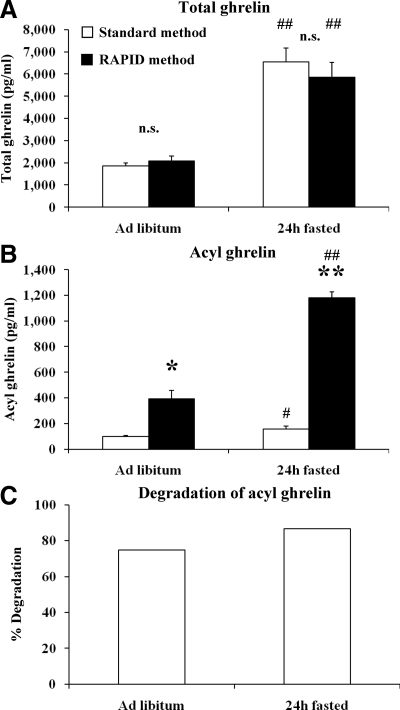
Changes in total T-Ghr and A-Ghr plasma levels assessed after RAPID method showed fasting-induced increases of T-Ghr and A-Ghr compared with freely fed rats (P < 0.001; Fig. 4) with an A/T-Ghr ratio remaining 1:5 [A-Ghr to des-acyl Ghr (D-Ghr) ratio 1:4] under both metabolic conditions. Standard processing also showed a fasting-induced increase in T-Ghr and A-Ghr compared with ad libitum-fed rats (P < 0.05; Fig. 4); however, the A/T-Ghr ratio was 1:41 and differed from freely fed conditions, which was 1:19. T-Ghr did not differ between the two methods (P > 0.05, Fig. 4A). With the standard method, about 80% of the endogenous A-Ghr was degraded (Fig. 4, B and C), which was inhibited by the RAPID method (P < 0.001, Fig. 4B).
Figure 4.
Circulating A- and T-Ghr levels after the standard method (white bars) compared with the RAPID method (black bars). Blood was withdrawn from ad libitum-fed and 24-h fasted rats and processed according to the standard or the RAPID method, and plasma T-Ghr (A) and A-Ghr (B) levels were measured by RIA. Standard blood processing results in degradation of 75 and 87% of A-Ghr under ad libitum and fasting conditions, respectively (C). n.s., Not significant; *, P < 0.05; **, P < 0.001 vs. standard method; #, P < 0.05; ##, P. < 0.001 vs. ad libitum feeding.
Discussion
Our study provides converging evidence that standard blood processing can hinder accurate measurement of amylin, calcitonin, gastrin, Ghr, GRP, insulin, PYY(1–36), PYY(3–36), and somatostatin-28 and is unsuitable for estimating blood levels of CGRP and CCK-58. It also leads to misidentification of molecular forms of CCK-58, Ghr, GRP, and somatostatin-28 due to their ex vivo degradation. The improvement in determination of levels and molecular forms is likely to have a major impact on endocrine peptide physiology.
In clinical routines, and often in research, hematological analysis commonly uses blood collected in EDTA tubes to inhibit peptide degradation occurring via proteolytic enzymes with metal cofactors. For investigating plasma proteins and peptides, EDTA sampling and storage at 4 C is widely recommended (11). However, this procedure shows significantly inferior results for 11 of 12 peptides compared with the RAPID method. The rates of most chemical reactions strongly depend on temperature (12,13) and pH (14,15). Reducing pH also protonates aspartate, glutamate, histidine, and C-terminal carboxylic acid groups on endocrine peptides and other blood components and may decrease adsorption of peptides to surfaces or proteins in the blood. Protease inhibitors used in this study inhibit a broad spectrum of peptidases. We have shown before that, e.g. the use of a dipeptidyl aminopeptidase IV inhibitor, diprotin A, is crucial to investigate PYY (16) and other peptides bearing the consensus sequence for dipeptidyl aminopeptidase IV (EC 3.4.14.5) such as GRP. The Michaelis-Menten hypothesis suggests that the rate of enzyme-substrate complex formation depends on the concentration of enzyme (peptidase) and substrate (peptide) (12). Dilution will decrease the rate of this formation by the square of the dilution ratio (12). Therefore, diluting plasma 10-fold will decrease this rate 100-fold. Isotopic recovery standards were used to monitor the effect of the above mentioned parameters and to reflect potential degradation of peptides.
Correct identification of circulating peptides’ forms is essential for studying the physiology of an endocrine peptide. Examples include somatostatin, CCK, and Ghr. Somatostatin exists as two major forms, somatostatin-28 and somatostatin-14 (17). Somatostatin-28 binds and activates the SST-5 receptor with higher affinity than somatostatin-14 (18), so the blood concentration of both forms is important for actions uniquely mediated by this receptor.
In rats, molecular forms of CCK have been reported as CCK-8 (8,9), CCK-22 (7), CCK-33/39 (6), or CCK-58 (4,5). However, we showed that with proper blood processing the only detectable form of CCK is CCK-58 (4). Moreover, CCK-58 has a markedly altered physiology from the most studied but artificial circulating form, CCK-8, e.g. for stimulation of pancreatic fluid secretion (19) and feeding pattern (20). Therefore, the RAPID method can greatly impact our understanding of CCK’s physiology.
Ghrelin has a unique octanoyl group essential for activating its receptor (21) and circulates in A-Ghr and D-Ghr forms (22). A-Ghr was implicated in food intake regulation (21) whereas D-Ghr may modulate A-Ghr’s orexigenic action (23). Studies describe that the major circulating form is D-Ghr, accounting for more than 90% of T-Ghr (22). The ratio of A/T-Ghr was shown to vary from 1:15 to 1:55 (22,24). This could be attributed to different blood sampling and evaluation methods. However, most studies show a very low percentage of A-Ghr compared with T-Ghr. Because A-Ghr is rapidly degraded in the plasma (present study), appropriate blood processing is crucial for obtaining correct A-Ghr levels. Hosoda and colleagues (25) investigated different processing conditions before measuring A-Ghr and T-Ghr. Use of EDTA and aprotinin combined with plasma acidification resulted in higher yield of A-Ghr (25). However, after this treatment. only 2–5% of T-Ghr in rodents and 10% in humans were found to be A-Ghr (25). In our study, the RAPID method resulted in 80% greater recovery of A-Ghr compared with standard processing and also showed a greater yield of A-Ghr compared with the previously proposed conditions as reflected by a proportion of A-Ghr of 20% of T-Ghr. Furthermore, after RAPID processing, the A/T-Ghr ratio (1:5) did not change under different metabolic conditions, and therefore this ratio does not seem to be important in food intake regulation. Thus, proper blood sampling and processing is essential when assessing the levels of labile peptides like A-Ghr.
Several reports indicated that GRP is not normally present in the circulation (26) or that the circulating form remains to be characterized (27). However, Brown et al. (28) showed that acidification and EDTA treatment followed by lyophilization results in markedly higher levels for bombesin-like immunoreactivity compared with untreated plasma samples. Moreover, our data show most of the GRP is degraded during standard processing, but no degradation occurred using the RAPID method. Therefore, acidification of blood may be required for accurate GRP measurements.
Proper blood processing will be critical to understand how PYY, GLP-1, CCK, Ghr, and GRP regulate food intake. This is even more important in light of the increasing prevalence of human obesity accompanied by increasing research in this field and concomitantly higher frequency of hunger and satiety peptide measurements. Because ex vivo degradation was shown for circulating peptides in human blood samples (25,29,30), the RAPID method may also have relevance to human blood processing.
The RAPID method is an important advance over the standard method because 1) it can improve recovery over 5-fold and 2) it yields correct identification of endocrine molecular peptide forms. The RAPID method should facilitate the study of various endocrine peptides not included here. Our results demonstrate that the RAPID method is essential for correct assessment of CGRP, CCK, Ghr, GRP, and somatostatin.
Supplementary Material
Acknowledgments
We thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.) and GO 1718/1-1 (M.G.), National Institutes of Health Center Grant DK-41301 (Animal Core, Peptidomic RIA Proteomic Core) (Y.T. and J.R.R.), RO1 Grants DK 33850 and DK 56805 (J.R.R.), and Veterans Administration Research Services.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: A-Ghr, Acyl Ghr; CCK, cholecystokinin; CGRP, calcitonin-gene-related peptide; D-Ghr, des-acyl Ghr; Ghr, ghrelin; GLP, glucagon-like peptide; GRP, gastrin-releasing peptide; PYY, peptide YY; RAPID, Reduced temperatures, Acidification, Protease inhibition, Isotopic exogenous controls, and Dilution; TFA, trifluoroacetate; T-Ghr, total Ghr.
References
- Bayliss WM, Starling EH 1902 The mechanism of pancreatic secretion. J Physiol 28:325–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan JE 1978 Gastrointestinal hormones. Annu Rev Med 29:307–318 [DOI] [PubMed] [Google Scholar]
- Berson SA, Yalow RS 1968 General principles of radioimmunoassay. Clin Chim Acta 22:51–69 [DOI] [PubMed] [Google Scholar]
- Reeve Jr JR, Green GM, Chew P, Eysselein VE, Keire DA 2003 CCK-58 is the only detectable endocrine form of cholecystokinin in rat. Am J Physiol Gastrointest Liver Physiol 285:G255–G265 [DOI] [PubMed] [Google Scholar]
- Reeve Jr JR, Wu SV, Keire DA, Faull K, Chew P, Solomon TE, Green GM, Coskun T 2004 Differential bile-pancreatic secretory effects of CCK-58 and CCK-8. Am J Physiol Gastrointest Liver Physiol 286:G395–G402 [DOI] [PubMed] [Google Scholar]
- Izzo RS, Brugge WR, Praissman M 1984 Immunoreactive cholecystokinin in human and rat plasma: correlation of pancreatic secretion in response to CCK. Regul Pept 9:21–34 [DOI] [PubMed] [Google Scholar]
- Liddle RA, Goldfine ID, Williams JA 1984 Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology 87:542–549 [PubMed] [Google Scholar]
- Lindén A, Carlquist M, Hansen S, Uvnäs-Moberg K 1989 Plasma concentrations of cholecystokinin, CCK-8, and CCK-33, 39 in rats, determined by a method based on enzyme digestion of gastrin before HPLC and RIA detection of CCK. Gut 30:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölsch UR, Cantor P, Wilms HM, Schafmayer A, Becker HD, Creutzfeldt W 1987 Role of cholecystokinin in the negative feedback control of pancreatic enzyme secretion in conscious rats. Gastroenterology 92:449–458 [DOI] [PubMed] [Google Scholar]
- Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St-Pierre DH, Taché Y 2006 LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am J Physiol Gastrointest Liver Physiol 291:G611–G620 [DOI] [PubMed] [Google Scholar]
- Banfi G, Salvagno GL, Lippi G 2007 The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin Chem Lab Med 45:565–576 [DOI] [PubMed] [Google Scholar]
- White A, Handler P, Smith EL 1973 Principles of biochemistry. 5th ed. New York: McGraw-Hill [Google Scholar]
- Evans MJ, Livesey JH, Ellis MJ, Yandle TG 2001 Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem 34:107–112 [DOI] [PubMed] [Google Scholar]
- Strickley RG, Brandl M, Chan KW, Straub K, Gu L 1990 High-performance liquid chromatographic (HPLC) and HPLC-mass spectrometric (MS) analysis of the degradation of the luteinizing hormone-releasing hormone (LH-RH) antagonist RS-26306 in aqueous solution. Pharm Res 7:530–536 [DOI] [PubMed] [Google Scholar]
- Nabuchi Y, Fujiwara E, Kuboniwa H, Asoh Y, Ushio H 1997 The stability and degradation pathway of recombinant human parathyroid hormone: deamidation of asparaginyl residue and peptide bond cleavage at aspartyl and asparaginyl residues. Pharm Res 14:1685–1690 [DOI] [PubMed] [Google Scholar]
- Keire DA, Whitelegge JP, Bassilian S, Faull KF, Wiggins BW, Mehdizadeh OB, Reidelberger RD, Haver AC, Sayegh AI, Reeve Jr JR 2008 A new endogenous form of PYY isolated from canine ileum: Gly-extended PYY(1–36). Regul Pept 151:61–70 [DOI] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R 1973 Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179:77–79 [DOI] [PubMed] [Google Scholar]
- Srikant CB, Patel YC 1981 Receptor binding of somatostatin-28 is tissue specific. Nature 294:259–260 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Reeve Jr JR, Keire DA, Green GM 2005 Water and enzyme secretion are tightly coupled in pancreatic secretion stimulated by food or CCK-58 but not by CCK-8. Am J Physiol Gastrointest Liver Physiol 288:G866–G879 [DOI] [PubMed] [Google Scholar]
- Glatzle J, Raybould HE, Kueper MA, Reeve Jr JR, Zittel TT 2008 Cholecystokinin-58 is more potent in inhibiting food intake than cholecystokinin-8 in rats. Nutr Neurosci 11:69–74 [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K 2005 Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K 2000 Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 279:909–913 [DOI] [PubMed] [Google Scholar]
- Inhoff T, Mönnikes H, Noetzel S, Stengel A, Goebel M, Dinh QT, Riedl A, Bannert N, Wisser AS, Wiedenmann B, Klapp BF, Taché Y, Kobelt P 2008 Desacyl ghrelin inhibits the orexigenic effect of peripherally injected ghrelin in rats. Peptides 29:2159–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H 2003 Total and active ghrelin in developing rats during hypoxia. Endocrine 21:159–161 [DOI] [PubMed] [Google Scholar]
- Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K 2004 Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 50:1077–1080 [DOI] [PubMed] [Google Scholar]
- Giraud A, Parker L, Taupin D, Hardy K, Shulkes A 1993 Mammalian bombesin as a hormone in ovine pregnancy: ontogeny, origin, and molecular forms. Am J Physiol 265:E866–E873 [DOI] [PubMed] [Google Scholar]
- Ischia J, Patel O, Shulkes A, Baldwin GS 2009 Gastrin-releasing peptide: different forms, different functions. Biofactors 35:69–75 [DOI] [PubMed] [Google Scholar]
- Brown M, Allen R, Villarreal J, Rivier J, Vale W 1978 Bombesin-like activity: radioimmunologic assessment in biological tissues. Life Sci 23:2721–2728 [DOI] [PubMed] [Google Scholar]
- Eberlein GA, Eysselein VE, Hesse WH, Goebell H, Schaefer M, Reeve Jr JR 1987 Detection of cholecystokinin-58 in human blood by inhibition of degradation. Am J Physiol 253:G477–G482 [DOI] [PubMed] [Google Scholar]
- Springer CJ, Eberlein GA, Eysselein VE, Schaeffer M, Goebell H, Calam J 1991 Accelerated in vitro degradation of CCK-58 in blood and plasma of patients with acute pancreatitis. Clin Chim Acta 198:245–253 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.