Abstract
We reported previously that chromogranin A (Chga) knockout (KO) mice are hypertensive and hyperadrenergic. Here we sought to determine the basis of such alterations by probing physiological, biochemical, and pharmacological responses to perturbations of the autonomic nervous system. In the conscious state, KO mice had substantially elevated basal high blood pressure (BP) and heart rate (HR); immobilization stress caused increments in systolic BP and HR in both wild-type (WT) and KO mice, with higher maxima but blunted increments in the KO state. Catestatin (CST; CHGA352–372) selectively diminished stress-induced increments in BP and HR in KO mice, implicating CST as an antihypertensive peptide, even in stressful conditions. Heightened plasma catecholamines in KO mice returned to WT level after CST. Stress caused further increments in catecholamines in WT mice but no change in KO mice. KO mice displayed diminished baroreflex sensitivity in response to either phenylephrine or sodium nitroprusside, accounting for exaggerated pressor and depressor responses to these compounds; baroreceptor function was normalized by CST. To probe the relative roles of endogenous/basal sympathetic vs. parasympathetic tone in control of BP and HR, we used the muscarinic-cholinergic antagonist atropine or the β-adrenergic antagonist propranolol; HR and BP responses to each antagonist were exaggerated in KO animals. We conclude that ablation of Chga expression results in global disturbances in autonomic function, both sympathetic and parasympathetic, that can be abrogated (or rescued), at least in part, by replacement of CST. The results point to mechanisms whereby CHGA and its CST fragment act to control cardiovascular homeostasis.
Ablation of Chga gene expression results in global disturbances in sympathetic and parasympathetic autonomic function, that can be abrogated (or “rescued”) by replacement of catestatin.
Chromogranin A [human (CHGA), mouse (Chga)], a 48-kDa acidic secretory protein, is the index member of the chromogranin/secretogranin protein family. CHGA has been implicated in the genesis of systemic hypertension and consequent cardiac abnormalities (1). Although the plasma concentration of CHGA is elevated in established human essential hypertension (2,3), the catestatin (CST; human CHGA352–372) fragment of CHGA is diminished in not only hypertensive subjects but also normotensive subjects with a family history of hypertension (4), suggesting a mechanism whereby diminished CST might increase the risk for later development of hypertension. Humans with genetic variation in the CST region of CHGA have altered genetic risk for development of systemic hypertension (5). Consistent with the human findings, we detected high blood pressure (BP) in mice after targeted ablation of the Chga gene [knockout (KO)]. KO mice also displayed higher plasma catecholamine coupled with left ventricular hypertrophy and increased ventricular dilation (6). Furthermore, CST replacement rescued KO mice from the high resting BP. We have recently shown that CST inhibited both inotropic and lusitropic properties of the heart (7). In addition, CST inhibited isoproterenol and endothelin-1 induced positive inotropism and lusitropism (7). Besides being an antihypertensive peptide, CST is now emerging as a novel cardiac modulator, which would protect the heart against excessive sympathetic drive such as hypertensive cardiomyopathy.
Forced immobilization or restraint, characterized by a combination of physical and emotional stress, activates the sympathoadrenal medullary system (8). These stress-induced increases in heart rate (HR) and BP are largely due to enhanced sympathoadrenal system activation (9,10,11,12). Spontaneously hypertensive rats (SHRs), an experimental model of essential hypertension, present high plasma norepinephrine concentrations associated with increased sympathetic activation compared with normotensive rats (13). Additionally, SHRs are hyperactive in a novel environment and hyperresponsive to stress compared with normotensive animals (9).
Cardiovascular performance is controlled by the autonomic nervous system. Beat-to-beat fluctuation in the HR is a balanced consequence of autonomic nervous system tone to the heart, both sympathetic (increasing HR) and parasympathetic (decreasing HR). Abnormalities in baroreflex sensitivity (BRS) in experimental (14,15) and human hypertension have been demonstrated, with hypertensive subjects exhibiting diminished BRS compared with their normotensive counterparts (16,17,18). Additionally, the family history of hypertension is associated with lower BRS in both normotensive and hypertensive offspring (19). Humans with a genetic variation in the CST region, particularly Gly364Ser, displayed alterations in baroreceptor function, both parasympathetic and sympathetic, and seems to reduce risk of developing hypertension, especially in men (5). Our experimental objectives were 3-fold: 1) whether high BP coupled with dysregulated catecholamine storage and release in KO mice would make them more susceptible to stress, 2) whether such hyperresponsiveness to stress could be attenuated or abolished by pretreatment with CST, and 3) whether BRS is altered in hypertensive and hyperadrenergic KO mice.
Materials and Methods
Animals
Chga KO mice and their wild-type (WT) littermates were generated as described (6). All animals were housed on a 12-h light, 12-h dark cycle and fed a standard rodent chow. The Animal Care and Use Committee of the University of California, San Diego, approved all protocols for animal use and euthanasia in accordance with National Institutes of Health guidelines. Both WT and KO mice (5–6 months old) in this study were from mixed (129 SvJ and C57BL/6) genetic background.
Conscious mice
Measurement of BP and HR in immobilization stress-induced telemetered mice
BP and HR were measured by telemetry as described previously (6). The experiments were initiated 10 d after the surgery. Immobilization stress was initiated by placing mice in restrainer (Braintree Scientific Inc., Braintree, MA) at 1000 h and kept for 2 h for continuous recording of BP and HR by telemetry followed by recovery from stress in home cages for 2 h. CST was injected [4 μg/g body weight (bw) ip] 30 min before stress induction to explore the role of CST in stress response.
Catecholamine assay after immobilization stress
Mice were anesthetized by inhalation of isoflurane, USP (Baxter Healthcare Corp., Deerfield, IL) and blood was collected from the left ventricle in potassium EDTA tubes. Isoflurane anesthesia was found to be best for measurement of plasma catecholamines with minimal stress-induced variation. Plasma catecholamine was measured by HPLC connected to an electrochemical detector (model 600E multisolvent delivery system and model 2465 electrochemical detector, Waters, Milford, MA) in a group of restraint mice (nontelemetered). Separation was performed on an Atlantis dC18 column (2.1 × 150 mm, 3 μm) from Waters. The mobile phase (0.15 ml/min) consisted of phosphate-citrate buffer [2 mm NaH2PO4, 268 μm Na2EDTA, 50 mm sodium citrate, 10 mm diethylamine hydrochloride, 0.072% 1-octanesulfonic acid (pH was adjusted to 3.1 with phosphoric acid), and 2.2% N,N-dimethylacetamide] and acetonitrile at 95:5 (vol/vol). An internal standard 3,4-dihydroxybenzylamine (1 ng) and an antioxidant sodium metabisulfite (final concentrations of 0.125 mm) were added to 0.25 ml of plasma. After the addition of 15 mg of alumina (aluminum oxide, activity grade: Super I, type WA-4; Sigma-Aldrich, Allentown, PA), the pH of the solution was raised to pH 8.6 by adding Tris buffer [0.96 m Tris, 50 mm EDTA (pH 8.69)]. After a 30-min incubation and centrifugation (5000 rpm, 5 min at room temperature), the supernatant was discarded and the beads were washed with water. The catecholamine was eluted with 80 μl of 0.1 n HCl supplemented with 0.1 mm sodium metabisulfite. The data were analyzed using Empower software (Waters, Milford, MA). Catecholamine levels were normalized with the recovery of internal standard.
Unconscious mice
Surgical procedures for hemodynamic measurements
Hemodynamic evaluation in both WT and KO mice was performed under general anesthesia [ketamine (100 mg/kg) and xylazine (2.5 mg/kg)] while connected to a ventilator. Left ventricular pressure was monitored by Millar micromanometer catheter of size 1.4 French (0.46 mm; Millar Instruments, Houston, TX); it was inserted into the Left ventricle in which phasic and mean pressure was continuously monitored (Gould, Cleveland, OH). A venous catheter (MicroRenathane tube MRE-033; Braintree Laboratories) was implanted on the right femoral vein for injection of peptide and other reagents. Body temperature was controlled by a water-heating pad (K-MOD 100; Baxter Healthcare Corp., Toronto, Canada), which was kept underneath the mouse during the entire procedure, maintaining a temperature of 37 C. All physiological signals were acquired by WINDAQ (Dataq Instruments, Akron, OH) and analyzed by BP-Analysis software (developed for own laboratory).
Assessment of BRS
BRS (in milliseconds per millimeters of mercury) was assessed by both high-pressure [phenylephrine (PE) bolus] and low-pressure [sodium nitroprusside (SNP) bolus] stimuli in unconscious mice. Although conscious animals provide optimal physiological data, a host of undesirable signals are triggered by physical activities and sleep cycle alterations in behavioral state. Therefore, we chose to conduct the experiments in unconscious mice to avoid some of the pitfalls encountered in awake animals. The absolute changes of HR in response to changes in systolic blood pressure (SBP) induced by PE or SNP were subjected to linear regression analysis to determine BRS. To test the effect of CST on BRS in KO mice, CST was injected (2.5 μg/g bw iv) 30 min before the injection of PE or SNP through the catheter implanted into the femoral vein.
BRS to high-pressure stimulus
High pressure BRS was evaluated by recording diminution of HR in response to PE-induced hypertension. Changes in SBP and HR were recorded continuously for 0.5 min after a bolus injection of PE (0.005 μg/g bw iv). This dose was selected from a preliminary dose-response study (0.001–0.05 μg/g bw iv).
BRS to low-pressure stimulus
This was evaluated by recording increments in HR in response to SNP-induced fall in BP. Changes in SBP and HR were recorded continuously for 0.5 min after a bolus injection of SNP (0.05 μg/g bw iv). This dose was selected from a preliminary dose-response study (0.01–0.1 μg/g bw iv).
Measurements of cardiovascular autonomic tone
Cardiac parasympathetic tone was blocked by injection of muscarinic-cholinergic inhibitor atropine (0.5 μg/g bw iv) through the femoral vein. Cardiac sympathetic tone was blocked by injection of a β-adrenergic inhibitor propranolol (1 μg/g bw iv). Sympathetic tone was estimated by the decrease in basal HR after propranolol, and the parasympathetic tone was estimated by the increase in basal HR after atropine.
Statistical analysis
Data are expressed as the mean ± sem. Curve fitting and area under the curve (AUC) calculations (change in trait in response to stimulus, beginning with the basal state for each experiment) were accomplished in the program Kaleidagraph (Synergy Software, Reading, PA). Multiple comparisons were made using two-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance was concluded at P <0.05.
Results
Physiology: stress responses in conscious mice
SBP
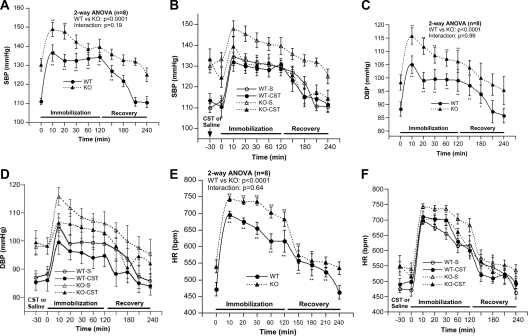
As reported previously (6), KO mice displayed higher SBP compared with WT mice (130.3 ± 3.3 vs. 111.0 ± 1.6 mm Hg; P < 0.0002) (Fig. 1A) under basal condition. SBP increased dramatically 10 min after immobilization stress in both WT (by 25.5 mm Hg) and KO (by 18.6 mm Hg) mice (Fig. 1A). In WT mice, the BP remained high during the 2 h of immobilization stress; in KO mice, BP returned to normal level 30 min after stress. SBP returned to normal level after 1 h of recovery in WT mice. In response to immobilization, SBP reached a higher maximum in KO mice, although the increment was diminished compared with WT (AUC: WT, 3462 ± 587 vs. KO, 1755 ± 231; P < 0.001).
Figure 1.
Immobilization stress-induced alterations of SBP, DBP, and HR and the effects of catestatin. A, SBP during stress and recovery period. B, SBP after supplementation with CST (hCHGA352–372; 4 μg/g bw ip) or saline 30 min before stress followed by stress and recovery. C, DBP during stress and recovery. D, DBP after supplementation with CST (4 μg/g bw ip) or saline 30 min before stress followed by stress and recovery. E, HR during stress and recovery period. F, HR after supplementation with CST (4 μg/g bw ip) or saline 30 min before stress followed by stress and recovery. *, P < 0.05; **, P < 0.01 (comparison with 0 time point). KO-S, Knockout mice treated with saline; WT-S, wild-type mice treated with saline; WT-CST, wild-type mice with catestatin; KO-CST, knockout mice with catestatin.
In WT mice, CST had no effect on basal SBP; CST attenuated (by 7.1 mm Hg) stress-induced increments in SBP (Fig. 1B). In contrast, in KO mice, CST decreased basal SBP (by 8.8 mm Hg), attenuated (by 12.6 mm Hg) stress-induced increments in SBP and brought SBP to WT level after 1 h of recovery from the stress (Fig. 1B). Overall, CST caused significant attenuation of stress-induced elevation of SBP in both WT (AUC: saline, 3462 ± 587 vs. CST, 1016 ± 330; P < 0.003) and KO mice (AUC: saline, 1755 ± 231 vs. CST, 501 ± 151; P < 0.001), resulting in similar increments in SBP in WT and KO mice (AUC: WT, 1016 + 330 vs. KO, 501 ± 151; P = 0.18), with respect to the control (saline treated) state.
Diastolic BP (DBP)
KO mice displayed significantly higher basal DBP compared with WT mice (98.2 ± 2.9 vs. 88.2 ± 2.7 mm Hg; P < 0.025) (Fig. 1C). DBP increased both in WT (by 16.8 mm Hg) and KO (by 17.6 mm Hg) mice after 10 min of stress (Fig. 1C). In both the groups, DBP remained high during 2 h of stress and returned to normal level 60 min (KO) or 90 min (WT) of recovery. Although KO animals reached a higher maximum DBP, the stress-induced increase in DBP was comparable in WT and KO mice (AUC: WT, 2128 ± 439 vs. KO, 1994 ± 337; P = 0.82).
Pretreatment with CST caused significant attenuation of stress-induced increments in DBP in both WT (AUC: saline, 2128 ± 439 vs. CST, 836 ± 304; P < 0.03) and KO mice (saline: 1994 ± 337 vs. CST, 253 ± 401; P < 0.01), compared with the control (saline treated) state (Fig. 1D).
HR
Resting HR was significantly higher (by 68 bpm) in KO mice compared with WT mice (Fig. 1E). Stress-induced increase in HR after 10 min of stress was comparable in WT (by 224 bpm) and KO (by 204 bpm) mice. In KO mice, HR remained high (by 48–87 bpm) during the stress period (Fig. 1E) and returned to basal level after 60 min. Overall, KO animals reached a higher maximum HR, although the stress-induced change in HR was comparable in WT and KO mice (AUC: WT, 25025 ± 2929 vs. KO, 22389 ± 2688; P = 0.52).
In WT mice, CST had no effect on the basal and stress-induced increase in HR (Fig. 1F); in KO mice, CST diminished the heightened HR responses to stress (AUC: saline, 22389 ± 2688 vs. CST, 9298 ± 4460; P < 0.03) compared with the control (saline treated) state.
Biochemistry: catecholamines in conscious mice
As reported previously (6), KO mice showed higher basal plasma norepinephrine (by 85%) and epinephrine (by 42%) (Fig. 2A), which returned to WT levels on CST replacement (Fig. 2A). In WT mice, immobilization stress (15 min) increased both plasma norepinephrine (by 92%) and epinephrine (by 61%); in KO mice, stress had no further effect on plasma catecholamines (Fig. 2B).
Figure 2.
Plasma catecholamine in WT and KO mice. A, Plasma catecholamines under basal and after supplementation with catestatin (4 μg/g bw ip) for 30 min. P values represent comparison between WT and KO mice. B, Plasma catecholamine under basal and after 15 min of immobilization stress. α, Comparison between control and stress; β, comparison between WT and KO mice.
Pharmacology: response to exogenous pressor/depressor agents in unconscious (anesthetized) mice
Effects of iv PE or SNP for 0.5 min
SBP.
In KO mice, anesthesia abolished heightened SBP (Fig. 3A) and HR (Fig. 3B). PE (0.005 μg/g bw iv) significantly increased SBP in both WT (by 34.1 mm Hg) and KO (by 45.6 mm Hg) mice (Fig. 4A; WT vs. KO: P < 0.014). SNP (0.05 μg/g bw iv) caused significant decrease in SBP in both the WT (by 40.1 mm Hg) and KO (by 61.6 mm Hg) mice (Fig. 4B; WT vs. KO: P < 0.0005).
Figure 3.
A, SBP in unconscious WT and KO mice. B, HR in unconscious WT and KO mice. P values represent comparison between WT and KO mice.
Figure 4.
Effects of PE or SNP on BRS. A, Effects of PE (0.005 μg/g bw iv) on SBP. B, Effects of SNP (0.05 μg/g bw iv) on SBP. C, Effects of PE (0.005 μg/g bw iv) on HR. D, Effects of SNP (0.05 μg/g bw iv) on HR. P values refer to comparison with WT mice.
HR.
In WT mice, PE (0.005 μg/g bw) caused significant decrease in HR (by 26 bpm) (Fig. 4C) as a result of reflex bradycardia. The HR changes were significantly different between WT and KO mice (P < 0.005). In contrast, in KO mice, PE-induced bradycardia was completely abolished (Fig. 4C), possibly indicating impairment in vagal tone. As expected, SNP (0.05 μg/g bw) increased HR in both the WT (by 19.9 bpm) and KO (by 13.9 bpm) mice (Fig. 4D). Reflex tachycardia in response to SNP was not significantly different between WT and KO mice.
Baroreceptor function
Baroreflex slope
Time-dependent cumulative effects of PE and SNP are shown in Fig. 5. Consistent with hypertensive subjects, the baroreflex slope in KO mice was decreased by about 3-fold (WT: 2.32 ± 0.42 vs. KO: 0.89 ± 0.29; P < 0.02) in response to PE (0.005 μg/g bw) and SNP (0.05 μg/g bw) (Fig. 5). In addition, the set point was increased in KO mice. CST replacement restored dampened baroreflex slope in KO mice (Fig. 5).
Figure 5.
BRS after treatment with PE (0.005 μg/g bw iv) or SNP (0.05 μg/g bw iv) in unconscious WT and KO mice or after supplementation of CST (4 μg/g bw iv) in KO mice. Slopes in line drawings are presented from one representative animal per group. The slope values presented at the top of the figure are the mean values ± one sem (milliseconds per millimeters of mercury; n = 8 animals/group). Set point refers to the initial/resting/starting point for each animal (for SBP, in millimeters of mercury, and beat-to-beat time interval of heart (R-R), in milliseconds), before administration of drugs. Actual values for SBP and HR set points are given in Fig. 3 (unconscious animals); those for KO animals after CST (in conscious animals) are given in Fig. 1B (SBP) and 1F (HR).
Cardiovascular autonomic tone: responses to parasympathetic and sympathetic antagonists
Chemical blockade of the parasympathetic tone by atropine increased SBP (Fig. 6A) and HR (Fig. 6C). The changes were more pronounced in KO mice, indicating impaired vagal tone. Chemical inhibition of sympathetic tone by propranolol caused modest increments in SBP in KO mice (Fig. 6B) and decrements in HR (Fig. 6D) compared with inhibition of the parasympathetic tone. Like atropine, HR and BP responses to propranolol were exaggerated in KO animals.
Figure 6.
Changes in SBP and HR in response to inhibition of cardiac autonomic tone. A, SBP in response to atropine. B, SBP in response to propranolol. C, Effect of atropine on HR. D, Effect of propranolol on HR.
Discussion
Overview
Previous studies indicated that genetic variation at the human CHGA locus results in alterations in BP in the population and that targeted ablation of the mouse Chga locus results in profound hypertension (6). Here we studied potential mechanisms of such changes in the autonomic nervous system, using physiological, biochemical, and pharmacological probes. Our results suggest widespread disturbances in autonomic function, both sympathetic and parasympathetic, that contribute to hypertension in this mouse model of human hypertension. Several features of autonomic dysfunction in the Chga−/− mouse were rescued by exogenous administration of the catecholamine release-inhibitory CHGA fragment CST, including resting and stress-induced increments of BP (Fig. 1), baroreceptor function (Fig. 5), and catecholamine secretion (Fig. 2). Endogenous CST is derived from the action of specific proteolytic enzymes upon CHGA within chromaffin granules, including the prohormone convertases (20), plasmin (21), and cathepsin L (22).
Immobilization stress-induced alterations of SBP, DBP, and HR
Stress is a major risk factor in the development of cardiovascular disease. Therefore, we determined the effects of acute immobilization stress on the hypertensive and hyperadrenergic KO mice (6). Because Chga−/− mice share many features with another rodent model of hereditary hypertension, the SHR, the present findings are discussed in light of the more widely investigated SHR. Basal SBP was higher in KO mice, and immobilization stress resulted in an even greater maximum in KO animals, although a diminished increment compared with WT mice (Fig. 1A), which is in congruence with the SHR in which restraint stress caused an increase in BP (10,23). As in the SHR, immobilization stress also caused an increase in DBP in KO mice (Fig. 1C). In contrast, DBP in Wistar-Kyoto (WKY) rats did not change in response to restraint stress (10). In C57/BL6 mice (24), α1-adrenergic receptor blockade with prazosin abolished shaker stress-induced pressor response, including the increase in BP variability in the low frequency domain; these findings support the idea that stress-induced pressor responses are mediated by sympathetic neural activation, and the low frequency range in the BP variability spectrum reflects sympathetic influences.
Whereas HR is comparable in SHR and WKY rats (10), HR in KO is higher than WT mice (Fig. 1E). The present study revealed heightened stress-induced maxima in HR in KO compared with WT mice (Fig. 1E). In Sprague Dawley rats, immobilization stress triggered a rapid increase in HR (∼200 bpm) (12). Like DBP, stress-induced increments in HR in KO mice persisted throughout the stress period, likely reflecting either an increase in sympathetic drive or inhibition of parasympathetic output to the heart. Of note, WKY rats did not display any change in HR in response to restraint stress (10). Acute shaker stress in C57/BL6 mice produced increases in BP and HR, accompanied by a decrease in BRS (24,25). In addition, autonomic blockade with atropine or atenolol in C57/BL6 mice decreased the magnitude of the stress-induced tachycardiac response as well as the increment in pulse interval variability (24). These findings support the concept that the cardiac response to stress in mice is due to activation of sympathetic and withdrawal of parasympathetic activity.
Exogenous CST rescues mice from immobilization stress-induced alteration of SBP, DBP, and HR
We have shown that exogenous CST rescued KO mice from stress-induced elevated SBP for up to 2 h (6). In the present study, pretreatment with CST attenuated stress-induced increments in SBP in both WT and KO mice (Fig. 1B). Remarkably, CST was able to sustain a reduction of elevated SBP in KO mice to WT level, even 1 h after recovery from stress (i.e. 3 h after CST administration) (Fig. 1B). These findings indicate that CST acts as an antihypertensive peptide under not only basal but also stressful conditions. Whereas CST pretreatment in KO mice reduced stress-induced increments in HR compared with the saline control (Fig. 1F) in WT mice CST did not exert this effect (Fig. 1, D and F).
CST rescues KO mice from elevated catecholamine secretion
Although we established CST as a potent nicotinic cholinergic antagonist in vitro in PC12 cells (26,27,28,29), ex vivo in rat adrenal gland (27) and in vivo in mice (30), CST’s effect on higher plasma catecholamine in KO mice are yet to be reported. Here CST administration (30 min) rescued KO mice from higher plasma catecholamine (Fig. 2A), indicating that the CST restoration of elevated BP in KO mice (6) likely resulted from CST inhibition of catecholamine secretion from chromaffin cells. Stress-induced increments in HR and BP are caused mainly by enhanced sympathoadrenal system activation (9,10,11,12). Consistent with previous finding in C57BL/6 mice (31), immobilization stress caused increments in plasma catecholamine in WT mice (Fig. 2B). In contrast to WT mice, plasma catecholamine did not change in KO mice after stress (Fig. 2B). This may reflect a ceiling effect, such that catecholamine cannot be further increased, which is consistent with the finding that stress-elevated plasma catecholamine in WT mice are comparable to that of the prestressed KO mice (Fig. 2B). In this context, it should be mentioned that the apparent quantal size of catecholamine release in KO mice was reduced to 66% of WT, which coincided with the reduction (by 41%) of the total amount of catecholamine secreted as revealed by our amperometric studies on primary chromaffin cells (32). Reduction in net catecholamine release observed in KO chromaffin cells was not caused by a lower rate of vesicle fusion, but by the lower content of catecholamine within each vesicle. We have also shown that the ability of chromaffin vesicles to accumulate catecholamine was impaired in KO mice (32).
Plasma catecholamine concentrations in the mouse are highly dependent on the method of blood sampling (33). Our plasma catecholamine values were obtained by cardiac puncture in unconscious, anesthetized mice; the values we measured are comparable with those found in other laboratories (33).
Anesthesia-induced alterations of SBP and HR
Anesthesia, in general, decreases BP in rodents. For example, in 129SvJ mice BP is higher than C57BL/6J in both conscious and unconscious states, but BP is reduced by about 6 mm Hg in both strains in unconscious state (34). Similar results were obtained in (mRen2) 27 renin transgenic rat in which heightened BP over the nontransgenic Sprague Dawley rat was retained in both conscious (by 109 mm Hg) and unconscious (by 26 mm Hg) conditions despite a drop of 83 mm Hg upon anesthesia (35). However, under chloralose-urethane anesthesia, the (mRen2) 27 rats displayed equivalent resting arterial BP to unconscious Sprague Dawley rats (36). In the present study, we found that anesthesia completely abolished heightened SBP (Fig. 3A) and HR (Fig. 3B) in KO mice when compared with the values in WT mice. Our findings are thus consistent with the idea that the activation of the central sympathetic nervous system is a major contributor to the development of hypertension in these mice. Despite the substantial (>50%) reduction in basal HR in both WT and KO mice after anesthesia (Fig. 3B), HR remained able to respond dynamically and in a predictable linear fashion (Fig. 5) to both pressor responses (with rise in R-R interval after phenylephrine) and depressor responses (with fall in R-R interval after nitroprusside).
Changes in baroreceptor responses to high- and low-pressure stimuli
The baroreceptor reflex plays a crucial role in the regulation of cardiovascular function, buffering acute variations in arterial pressure mainly through HR and changes in vessel resistance. Baroreflex dysfunction has been studied in rat (37,38,39) and transgenic mice (40,41,42). In the present study, both the reflex bradycardia caused by PE hypertension and the reflex tachycardia caused by SNP hypotension were abolished or attenuated in KO mice (Fig. 4). Experimental and clinical studies revealed that an increase in sympathetic activity is coupled to a decrease in BRS (43,44). Cardiac hypertrophy by itself can also lead to baroreflex dysfunction in both rats (45) and mice (46). Because the Chga−/− mice displayed cardiac hypertrophy (6), this myocardial alteration could also contribute to attenuation of the BRS (40). Other studies indicated that the impairment of BRS caused by hypertension and cardiac hypertrophy was mainly due to a reduced maximum capacity of the cardiac vagal component rather than to a change in the sympathetic component (45). Numerous studies in experimental animals including the SHR (14,47,48) and human hypertension (essential as well as secondary) (16,17,18) document diminished BRS compared with their normotensive counterparts. In addition, a positive family history of hypertension has been associated with lower BRS, even in still-normotensive individuals (19). Restoration (elevation) of BRS sensitivity after exogenous CST in KO mice indicates that CST resets the entire autonomic nervous reflex arc to restore normal cardiovascular function. In the Langendorff-perfused rat heart preparation, we have recently shown that CST inhibited both inotropic and lusitropic properties of the heart (7). In addition, CST inhibited isoproterenol and endothelin-1 induced positive inotropism and lusitropism (7). CST is thus emerging as a novel cardiac modulator, which would protect the heart against excessive sympathetic drive such as that found in hypertensive cardiomyopathy.
Changes in cardiovascular autonomic tone
To probe the relative roles of sympathetic vs. parasympathetic tone in control of BP and HR, we used atropine or propranolol. HR and BP changes to atropine were much higher compared with the values obtained after propranolol treatment (Fig. 6), indicating a predominance of cardiac vagal tone over sympathetic tone in regulation of HR. Of note, within the baroreflex circuitry, the parasympathetic system is considered to be the major pathway in baroreflex-mediated HR control (48). In agreement with our findings, it has been shown that murine HR under resting conditions is under joint control of the parasympathetic and sympathetic systems (49,50), as is also the case in the rat and other mammals. Of note, HR and BP responses to each antagonist were exaggerated in KO animals (Fig. 6), consistent with either diminished baroreceptor function, or heightened outflow from both the parasympathetic and sympathetic branches, or both. A perhaps more reliable method for estimating sympathetic vs. parasympathetic contributions to basal HR is to determine changes with reference to intrinsic heart rate, obtained by measuring HR after dual sympathetic/parasympathetic blockade (51); however, we did not obtain HR data after dual blockade.
Conclusion and perspectives
Genetic variation at the human CHGA locus has profound consequences for control of BP, and the Chga null mouse displayed profound hypertension and increased catecholamine secretion. We sought to understand such changes and probed autonomic function in this model by physiological, biochemical, and pharmacological means. Our results suggest global disruption of autonomic function in this model, both parasympathetic and sympathetic. Defects in baroreceptor function may underlie the cardiovascular instability and exaggerated sympathetic outflow observed in KO animals. At least some of these abnormalities can be rescued by administration of the CHGA catecholamine release-inhibitory fragment CST. Because the CHGA and CST mechanisms are altered in human hypertension, our results in this experimental animal model may provide insight into the pathogenesis of this common human disorder.
Footnotes
This work was supported by National Institutes of Health Grants R01 DA011311 (to S.K.M.), DK 60702 (to D.T.O.), and P01 HL58120 (to S.K.M. and D.T.O.) and the Department of Veterans Affairs (to S.K.M. and D.T.O.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: AUC, Area under the curve; BP, blood pressure; BRS, baroreflex sensitivity; bw, body weight; CST, catestatin; CHGA/CHGA, human chromogranin A protein/gene; Chga/Chga, mouse chromogranin A protein/gene; DBP, diastolic blood pressure; HR, heart rate; KO, Chga knockout; PE, phenylephrine; SBP, systolic blood pressure; SHR, spontaneously hypertensive rat; SNP, sodium nitroprusside; WKY, Wistar-Kyoto; WT, wild-type.
References
- Taupenot L, Harper KL, O'Connor DT 2003 Mechanisms of disease: the chromogranin-secretogranin family. N Engl J Med 348:1134–1149 [DOI] [PubMed] [Google Scholar]
- Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O'Connor DT 1990 Chromogranin A. Storage and release in hypertension. Hypertension 15:237–246 [DOI] [PubMed] [Google Scholar]
- Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, Lin MC, Li J, Grim CE, Wright FA, O'Connor DT 1995 Chromogranin A in human hypertension. Influence of heredity. Hypertension 26:213–220 [DOI] [PubMed] [Google Scholar]
- O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ 2002 Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345 [DOI] [PubMed] [Google Scholar]
- Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O'Connor DT 2007 Catecholamine release-inhibitory peptide catestatin [chromogranin A(352–372)]: naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation 115:2271–2281 [DOI] [PubMed] [Google Scholar]
- Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross Jr J, Mahata SK 2005 Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115:1942–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC 2008 The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnanský R, Pacák K, Fukuhara K, Viskupic E, Hiremagalur B, Nankova B, Goldstein DS, Sabban EL, Kopin IJ 1995 Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann NY Acad Sci 771:131–158 [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, McCarty R, Thoa NB, Lake CR, Kopin IJ 1979 Sympatho-adrenal responses of spontaneously hypertensive rats to immobilization stress. Am J Physiol 236:H457–H462 [DOI] [PubMed] [Google Scholar]
- Irvine RJ, White J, Chan R 1997 The influence of restraint on blood pressure in the rat. J Pharmacol Toxicol Methods 38:157–162 [DOI] [PubMed] [Google Scholar]
- McDougall SJ, Paull JR, Widdop RE, Lawrence AJ 2000 Restraint stress: differential cardiovascular responses in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 35:126–129 [DOI] [PubMed] [Google Scholar]
- Sabban EL, Schilt N, Serova LI, Masineni SN, Stier Jr CT 2009 Kinetics and persistence of cardiovascular and locomotor effects of immobilization stress and influence of ACTH treatment. Neuroendocrinology 89:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn HS, Park YN, Lee SR 2002 Effect of immobilization stress on brain polyamine levels in spontaneously hypertensive and Wistar-Kyoto rats. Brain Res Bull 57:575–579 [DOI] [PubMed] [Google Scholar]
- Aars H 1968 Aortic baroreceptor activity in normal and hypertensive rabbits. Acta Physiol Scand 72:298–309 [DOI] [PubMed] [Google Scholar]
- West MJ, Korner PI 1974 The baroreceptor-heart rate reflex in renal hypertension in the rabbit. Clin Exp Pharmacol Physiol 1:231–239 [DOI] [PubMed] [Google Scholar]
- Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS 1969 Diminished baroreflex sensitivity in high blood pressure. Circulation 39:48–54 [DOI] [PubMed] [Google Scholar]
- Goldstein DS 1983 Arterial baroreflex sensitivity, plasma catecholamines, and pressor responsiveness in essential hypertension. Circulation 68:234–240 [DOI] [PubMed] [Google Scholar]
- Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G 1998 Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension 31:68–72 [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Cervenka JH, Stone RA 1992 Baroreflex sensitivity and heredity in essential hypertension. Circulation 85:497–503 [DOI] [PubMed] [Google Scholar]
- Eskeland NL, Zhou A, Dinh TQ, Wu H, Parmer RJ, Mains RE, O'Connor DT 1996 Chromogranin A processing and secretion: specific role of endogenous and exogenous prohormone convertases in the regulated secretory pathway. J Clin Invest 98:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, Torpey JW, O'Connor DT, Mahata SK 2008 Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology 149:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas N, Rodriguez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O'Connor DT, Mahata SK 2009 Cathepsin L co-localizes with chromogranin a in chromaffin vesicles to generate active peptides. Endocrinology 150:3547–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SJ, Lawrence AJ, Widdop RE 2005 Differential cardiovascular responses to stressors in hypertensive and normotensive rats. Exp Physiol 90:141–150 [DOI] [PubMed] [Google Scholar]
- Farah VM, Joaquim LF, Morris M 2006 Stress cardiovascular/autonomic interactions in mice. Physiol Behav 89:569–575 [DOI] [PubMed] [Google Scholar]
- Farah VM, Joaquim LF, Bernatova I, Morris M 2004 Acute and chronic stress influence blood pressure variability in mice. Physiol Behav 83:135–142 [DOI] [PubMed] [Google Scholar]
- Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ 1997 Novel autocrine feedback control of catecholamine release. A discrete chromogranin A fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100:1623–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Wakade AR, O'Connor DT 2000 Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A344–364): Identification of amino acid residues crucial for activity. Mol Endocrinol 14:1525–1535 [DOI] [PubMed] [Google Scholar]
- Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT 2004 The catecholamine release-inhibitory “catestatin” fragment of chromogranin a: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol 66:1180–1191 [DOI] [PubMed] [Google Scholar]
- Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O'Connor DT, Hamilton BA 2004 Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet 74:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, O'Connor DT 2003 Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem 278:32058–32067 [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL 2002 Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 143:4520–4526 [DOI] [PubMed] [Google Scholar]
- Montesinos MS, Machado JD, Camacho M, Diaz J, Morales YG, Alvarez de la Rosa D, Carmona E, Castañeyra A, Viveros OH, O'Connor DT, Mahata SK, Borges R 2008 The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J Neurosci 28:3350–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouzmann E, Cavadas C, Grand D, Moratel M, Aubert JF, Brunner HR, Mazzolai L 2003 Blood sampling methodology is crucial for precise measurement of plasma catecholamines concentrations in mice. Pflugers Arch 447:254–258 [DOI] [PubMed] [Google Scholar]
- Lum C, Shesely EG, Potter DL, Beierwaltes WH 2004 Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension 43:79–86 [DOI] [PubMed] [Google Scholar]
- Diz DI, Westwood B, Bosch SM, Ganten D, Ferrario C 1998 NK1 receptor antagonist blocks angiotensin II responses in renin transgenic rat medulla oblongata. Hypertension 31:473–479 [DOI] [PubMed] [Google Scholar]
- Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB 2008 Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol 51:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RS, Cabral AM, Vasquez EC 1993 Evidence that the autonomic nervous system plays a major role in the L-NAME-induced hypertension in conscious rats. Am J Hypertens 6:806–809 [DOI] [PubMed] [Google Scholar]
- Veelken R, Hilgers KF, Ditting T, Leonard M, Mann JF, Geiger H, Luft FC 1994 Impaired cardiovascular reflexes precede deoxycorticosterone acetate-salt hypertension. Hypertension 24:564–570 [DOI] [PubMed] [Google Scholar]
- Moyses MR, Cabral AM, Bissoli N, Vasquez EC 1994 Time course of changes in sigmoidal-fitting baroreceptor curves in one-kidney, one clip hypertensive rats. Hypertension 23:I87–92 [DOI] [PubMed] [Google Scholar]
- Peotta VA, Vasquez EC, Meyrelles SS 2001 Cardiovascular neural reflexes in L-NAME-induced hypertension in mice. Hypertension 38:555–559 [DOI] [PubMed] [Google Scholar]
- Xue B, Pamidimukkala J, Hay M 2005 Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 288:H2177–H2184 [DOI] [PubMed] [Google Scholar]
- Carvalho TH, Lopes OU, Tolentino-Silva FR 2006 Baroreflex responses in neuronal nitric oxide synthase knockout mice (nNOS). Auton Neurosci 126–127:163–168 [DOI] [PubMed] [Google Scholar]
- Peotta VA, Gava AL, Vasquez EC, Meyrelles SS 2007 Evaluation of baroreflex control of heart rate in renovascular hypertensive mice. Can J Physiol Pharmacol 85:761–766 [DOI] [PubMed] [Google Scholar]
- Saleh TM, Connell BJ 1998 The parabrachial nucleus mediates the decreased cardiac baroreflex sensitivity observed following short-term visceral afferent activation. Neuroscience 87:135–146 [DOI] [PubMed] [Google Scholar]
- Head GA 1994 Cardiac baroreflexes and hypertension. Clin Exp Pharmacol Physiol 21:791–802 [DOI] [PubMed] [Google Scholar]
- Gava AL, Peotta VA, Cabral AM, Meyrelles SS, Vasquez EC 2004 Decreased baroreflex sensitivity in isoproterenol-treated mice with cardiac hypertrophy. Auton Neurosci 114:47–54 [DOI] [PubMed] [Google Scholar]
- Minami N, Head GA 1993 Relationship between cardiovascular hypertrophy and cardiac baroreflex function in spontaneously hypertensive and stroke-prone rats. J Hypertens 11:523–533 [DOI] [PubMed] [Google Scholar]
- Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan Jr R 2007 Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol Heart Circ Physiol 292:H593–H600 [DOI] [PubMed] [Google Scholar]
- Janssen BJ, Leenders PJ, Smits JF 2000 Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol Regul Integr Comp Physiol 278:R215–R225 [DOI] [PubMed] [Google Scholar]
- Just A, Faulhaber J, Ehmke H 2000 Autonomic cardiovascular control in conscious mice. Am J Physiol Regul Integr Comp Physiol 279:R2214–R2221 [DOI] [PubMed] [Google Scholar]
- Opthof T 2000 The normal range and determinants of the intrinsic heart rate in man. Cardiovasc Res 45:173–176 [PubMed] [Google Scholar]