Abstract
Leucine-rich repeat-containing G protein-coupled receptor (LGR)-5 is a recently identified marker of stem cells in adult intestinal epithelium and hair follicles. Because of this characteristic, we studied the status of Lgr5 expression in the mouse uterus under various conditions. Lgr5 is highly expressed in the uterine epithelium of immature mice and is dramatically down-regulated after the mice resume estrous cycles. Surprisingly, whereas its expression is up-regulated in uteri of ovariectomized mice, the expression is down-regulated by estrogen and progesterone via their cognate nuclear receptors, estrogen receptor-α and progesterone receptor, respectively. Using a mouse endometrial cancer model, we also found that Lgr5 is highly expressed in the epithelium during the initial stages of tumorigenesis but is remarkably down-regulated in fully developed tumors. Lgr5 is a downstream target of Wnt signaling in the intestine. Genetic evidence shows that either excessive or absence of Wnt signaling dampens Lgr5 expression in the uterus. Collectively, our results show that Lgr5 expression in the mouse uterine epithelium is unique and dynamically regulated under various physiological and pathological states of the uterus, suggesting that this orphan receptor has important functions in uterine biology. However, identifying definitive uterine function of LGR5 will require further investigation using conditional deletion of uterine Lgr5 because systemic deletion of this gene is neonatally lethal.
Lgr5 is uniquely and dynamically expressed in the mouse uterus under changing conditions, suggesting its important role in uterine biology.
Leucine-rich repeat-containing G protein-coupled receptor (LGR)-5, an orphan G protein-coupled receptor, has recently been identified as a novel stem cell marker in intestinal epithelia (1) and hair follicles (2). Lgr5, also known as Gpr49, is specifically expressed in cycling columnar crypt cells, and lineage tracing experiments have shown that Lgr5-positive cells constitute multipotent stem cells that generate all cell types in the intestinal epithelium (1). However, lack of appropriate stem cell markers for many other tissues including the uterus precludes identification of stem cells in these tissues.
The uterus is a unique organ comprised of heterogeneous cell types. It undergoes maturation in puberty and serves as the womb in adult life to home and nurture the implanting embryo until birth ensues (3). The immature uterus is physiologically different from its mature counterpart. Although the immature uterus is acyclic, the adult uterus undergoes cyclic changes in response to ovarian steroids over a period of 4–5 d. The estrous cycle in mice is categorized into proestrus, estrus, metestrus, and diestrus. In adult mice, various cell types respond uniquely to estrogen and/or progesterone (P4) stimulation (4). During early pregnancy, expression of many genes changes in a spatiotemporal manner to prepare the uterus to receive the blastocyst for implantation and its subsequent development. In mice, initiation of implantation occurs in the evening of d 4 of pregnancy (d 1 = vaginal plug) (5).
Reproductive functions of the uterus are primarily coordinated by ovarian estrogen and P4. Most estrogen action in the uterus is mediated by two nuclear receptors, estrogen receptor (ER)-α and ERβ (6). ERα is the dominant form expressed in the mouse uterus (7). P4 function in the uterus is mediated by two nuclear progesterone receptor (PR) isoforms, PR-A and PR-B (8). Genetic deletion of PR-A, but not PR-B, results in female infertility, including failure in ovulation, fertilization, implantation, and decidualization (8). Female mice null for both PRs (PRKO) are completely infertile (9). Collectively, estrogen and P4 directly regulate uterine growth and maturation as well as most reproductive events including implantation, decidualization, and parturition via their cognate nuclear receptors.
Lgr5 is considered a Wnt target gene (10). Wnt signaling pathways play important roles in the regulation of tissue homeostasis, and β-catenin is a component of the canonical Wnt signaling pathway. In the absence of Wnt signaling, β-catenin is retained as a complex in the cytosol by adenomatous polyposis coli (Apc) (11,12) and undergoes ubiquitin-dependent degradation. Activation of Wnt signaling releases β-catenin from the complex, prompting its translocation to the nucleus in which it interacts with lymphoid enhancer factor/T cell factor (TCF) family members to activate the transcription of downstream target genes (13).
Stem cells reside in a confined microenvironment termed the stem cell niche. Signals generated by the niche regulate the initiation of stem cell self-renewal and differentiation of daughter cells to maintain tissue homeostasis. The down-regulation of canonical Wnt signaling results in the loss of stem cell proliferation in intestinal crypts (14,15,16) and adult hair follicles (17,18), whereas up-regulation of the signaling results in intestinal adenoma (19) and skin tumors (20). Although stem cell-specific deletion of Apc promotes more aggressive formation of adenoma within a month, expanding from the bottom of the crypt to higher up in the intestinal villi, Lgr5 expression is lost in most transformed cells in the upper part of the crypt, suggesting that Lgr5 expression is restricted (21). Lgr5-positive stem cells initiate cancers by amplifying rapid cycling of progenitor cells but not by enlarging the stem cell pool.
Information on the expression and regulation of Lgr5 and its function in the uterus remains totally unknown. To better understand the role of Lgr5 in uterine physiology and pathophysiology, we studied its spatiotemporal expression in the mouse uterus under different experimental conditions using genetic and molecular approaches. We found that Lgr5 is highly expressed in uterine epithelia of immature mice and in adult uteri deprived of ovarian hormone stimulation. However, its expression in ovariectomized mice is remarkably down-regulated after exogenous administration of estradiol-17β (E2) or P4. More interestingly, we observed elevated expression of Lgr5 in the epithelium during the initial stages of endometrial tumorigenesis resulting from conditional deletion of uterine Pten; the expression is totally absent in full-grown tumors. We also found that Lgr5 expression requires appropriate Wnt signaling. The persistent expression of uterine Lgr5 in ovariectomized mice may suggest its role in maintaining cell survival and integrity in a uterus deprived of growth stimulation.
Materials and Methods
Animals and treatments
Adult CD-1 mice were purchased from the Charles River Laboratories, Inc. (Raleigh, NC). Mice deficient of ERα (ERKO; 129/J/C57BL/6J) and PRKO mice (129SvEv/C57BL/6) were generated as previously described (9,22) and were kindly provided by Dennis Lubahn (University of Missouri, Columbia, MO) and Bert O'Malley (Baylor College of Medicine, Houston, TX), respectively. PtenloxP/loxP (23) and Ctnnb1loxP/loxP (24) mice were obtained from Jackson Laboratory. PR-Cre (25) (PRcre/+; C57BL6/129SV) mice were generously provided by John Lydon and Francesco DeMayo (Baylor College of Medicine). Ctnnb1f(ex3)/+) (26) mice were kindly provided by Mark Taketo (Kyoto University, Kyoto, Japan). PCR analysis of the genomic DNA determined specific genotypes. All mice were housed in the Animal Care Facility at the Cincinnati Children’s Hospital Medical Center (Cincinnati, OH) according to National Institutes of Health and institutional guidelines for laboratory animals. Females were mated with fertile males to induce pregnancy (vaginal plug = d 1 of pregnancy). Stages of the estrous cycle were determined by examining vaginal smear.
To examine the effects of E2 and/or P4 on uterine expression of Lgr5, adult mice (8–10 wk old) were ovariectomized and rested for 2 wk. Mice were injected with E2 (100 ng per 0.1 ml oil/mouse) or P4 (2 mg per 0.1 ml oil/mouse). All steroids were dissolved in sesame oil and injected sc. The control group of mice received sesame oil (0.1 ml/mouse). They were killed at different times as indicated. One horn of the uterus was stored frozen at −80 C and the other horn was fixed in 10% neutral buffered formalin for further processing.
RNA isolation and Northern hybridization
RNA was extracted from tissues using Trizol reagent (Invitrogen, Carlsbad, CA). Total RNA (6 μg) was denatured and separated by formaldehyde/agarose gel electrophoresis, transferred to nylon membranes, and UV cross-linked. Blots were prehybridized, hybridized, and washed as previously described (27). For quantification, all samples were normalized against a housekeeping gene Rpl7 using the same membrane. Band intensities were measured using the Scion Image system (Scion Corp., Frederick, MD).
In situ hybridization
In situ hybridization was performed as previously described (28). In brief, frozen sections (12 μm) were mounted onto poly-l-lysine-coated slides and fixed in cold 4% (wt/vol) paraformaldehyde in PBS. The sections were prehybridized and hybridized at 45 C for 4 h in 50% (vol/vol) formamide hybridization buffer containing the 35S-labeled antisense or sense cRNA probes. Ribonuclease A-resistant hybrids were detected by autoradiography. Sections were poststained with hematoxylin and eosin. Sections hybridized with the sense probes did not exhibit any positive signals and served as negative controls.
Hybridization probes
Lgr5 cDNA clone was kindly provided by Robert J. Coffey (Vanderbilt University, Nashville, TN). For in situ hybridization, sense and antisense 35S-labeled cRNA probes were generated using Sp6 and T7 polymerases, respectively. For Northern hybridization, antisense 32P-labeled cRNA probes for Lgr5 and Rpl7 (a housekeeping gene) were generated.
Immunohistochemistry
Immunolocalization was performed in 10% (vol/vol) neutral buffered formalin-fixed paraffin embedded sections using specific antibodies to Ki67 (SP-6, LabVision; NeoMarkers, Fremont, CA) or wide spectrum cytokeratin (Dako, Carpinteria, CA). A Histostain-Plus (diaminobenzidine) kit (Invitrogen) was used to visualize specific antigens.
LacZ staining
The expression of β-galactosidase was assessed by LacZ staining as previously described (29). In brief, small pieces of tissues were fixed in 0.2% (wt/vol) paraformaldehyde solution followed by infusion in 30% (wt/vol) sucrose at 4 C overnight. Tissues were embedded in OCT tissue freezing medium (Fisher Scientific, Pittsburgh, PA) and snap frozen. Frozen sections were mounted onto glass slides and stained overnight at 37 C using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside as a substrate. Sections were counterstained with eosin.
Results
Uterine Lgr5 expression is dynamic
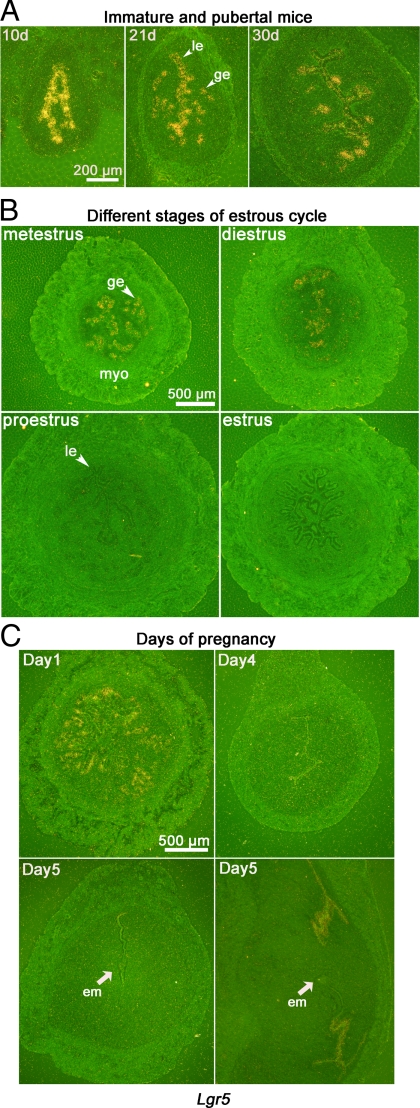
To better understand the role of LGR5, we examined its expression at different physiological states of the uterus (Fig. 1). Lgr5 is highly expressed in the luminal and glandular epithelia of mice at 10, 21, and 30 d after birth (Fig. 1A); the expression is remarkably down-regulated in the uterus after mice resume estrous cycles. Little or no Lgr5 expression is observed in the uterus in proestrus and estrus, whereas it is present in diestrus and metestrus (Fig. 1B).
Figure 1.
Lgr5 is spatiotemporally expressed in the uterus as determined by in situ hybridization. A, Lgr5 is highly expressed in luminal and glandular epithelia of immature and pubertal mice at 10, 21, and 30 d after birth. B, Lgr5 expression pattern is altered by the stage of estrous cycle. Positive signals are evident in metestrus and diestrus uteri. C, Lgr5 is expressed in epithelial cells on d 1 of pregnancy. On d 5, Lgr5 expression is absent at the implantation site (cross-section) but present in epithelial cells at the interimplantation site (longitudinal section). le, Luminal epithelium; ge, glandular epithelium; myo, myometrium; em, embryo.
During early pregnancy, Lgr5 is expressed in epithelial cells on d 1 of pregnancy after preovulatory ovarian estrogen surge (Fig. 1C). In contrast, its expression is not evident on d 4 when the uterus is under the influence of rising P4 levels superimposed with preimplantation ovarian estrogen secretion before embryo implantation. After implantation, luminal epithelial cells at interimplantation sites, but not at implantation sites, show Lgr5 expression. These data demonstrate that Lgr5 expression is dynamically regulated at altered physiological states of the uterus.
Lgr5 expression is not always correlated with epithelial growth in endometrial cancer
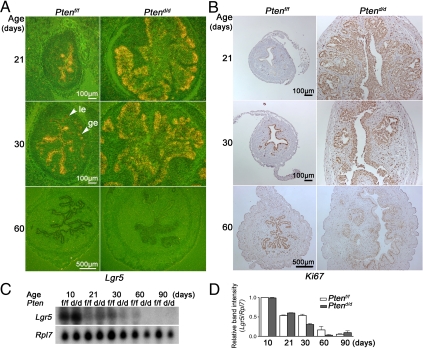
Previous studies showed that Lgr5 is overexpressed in primary colorectal and ovarian tumor tissues (30). Increased Lgr5 is also detected in skin carcinoma, and RNA interference knockdown of Lgr5 in vitro inhibits proliferation of a mouse skin carcinoma cell line (31). That Lgr5 is highly expressed in immature mouse uteri and uteri on d 1 of pregnancy with proliferating epithelial cells led us to surmise that this orphan receptor participates in epithelial cell proliferation. To address this issue, we examined Lgr5 expression in a mouse model of endometrial cancer. We crossed floxed Pten (Ptenf/f) mice with progesterone receptor-Cre (PR-Cre) mice to generate mice with conditional deletion of uterine Pten (Ptend/d) (32). We compared epithelial cell proliferation as examined by Ki67 immunostaining with Lgr5 expression by in situ hybridization (Fig. 2, A and B). Cytokeratin staining was used as an epithelial cell marker (supplemental Fig. S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). In Ptenf/f mice, most epithelial cells in 21-d-old uteri are negative for Ki67 staining with high levels of Lgr5 expression, whereas Ki67 signals overlap with Lgr5 expression pattern in 30-d-old uteri. In 60-d-old mature mice, proliferating epithelial cells are also negative for Lgr5 expression.
Figure 2.
Lgr5 expression is not correlated with uterine cell proliferation. A, In situ hybridization of Lgr5. Lgr5 expression pattern is similar in Ptend/d and Ptenf/f uteri. B, Immunostaining of Ki67. Ki67-positive cells are more abundant in Ptend/d compared with Ptenf/f uteri. C, Northern hybridization of Lgr5. Lgr5 mRNA levels decrease in an age-dependent manner. Its expression pattern and levels in Ptenf/f and Ptend/d uteri are comparable. le, Luminal epithelium; ge, glandular epithelium. D, Quantitation of Northern hybridization. Quantitation was performed from two independent RNA samples pooled from two to five mice at each time point. All signals were normalized against Rpl7. Results are presented as mean ± sem.
The results suggest that Lgr5 is not always accompanied by uterine epithelial cell proliferation in Ptenf/f mice. Ten-day-old Ptend/d mice rapidly develops uterine epithelial hyperplasia, leading to endometrial cancer in all Ptend/d mice by 30 d of age; tumorigenesis progresses further with time ultimately showing myometrial invasion (32) (supplemental Fig. S1). As shown in Fig. 2A, in situ hybridization shows high levels of Lgr5 expression in uterine epithelia of Ptend/d mice at 21 and 30 d of age which correlate with heightened cell proliferation (Fig. 2B). However, although epithelial cell proliferation and growth continue with tumor progression at 60 d of age, Lgr5 expression becomes undetectable. We also performed Northern hybridization of uterine RNA samples isolated from Ptend/d and Ptenf/f mice at different ages and quantified the results against Rpl7 (Fig. 2, C and D). These findings are consistent with in situ hybridization results and show that Lgr5 expression does not always correlate with uterine epithelial cell proliferation; rather, this orphan receptor may function as a first responder for the initiation of cell proliferation under specific conditions.
Lgr5 expression is down-regulated by estrogen and P4
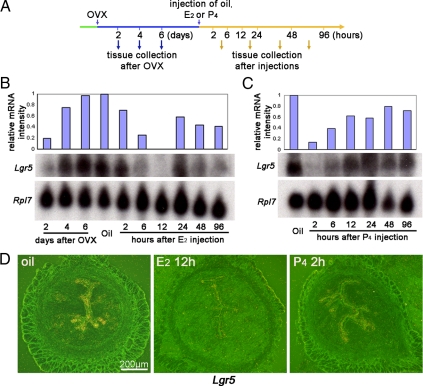
Fluctuation of Lgr5 expression in the adult uterus suggests that ovarian hormones regulate this gene. Estrogen and P4 levels are higher in proestrus (33), coincident with decreased Lgr5 expression. Vaginal cornification in mice starts around 5 wk of age, and regular estrous cycles normally resume around 7 wk of age (34); these characteristics coincide with down-regulation of Lgr5 expression. These results suggest that Lgr5 is regulated by ovarian hormones. To address this issue more definitively, uterine Lgr5 expression was examined at different times after ovariectomy and after treatment with ovarian steroid hormones (Fig. 3A). After ovariectomy, uterine Lgr5 expression showed gradual increases with time (Fig. 3B). Eight days after ovariectomy, the uterus received an injection of E2, P4, or vehicle (oil) as shown in Fig. 3A. Lgr5 mRNA levels started declining 2 h after E2 treatment, becoming almost undetectable at 12 h (Fig. 3B). However, Lgr5 expression again became detectable at 24 h, albeit at reduced levels. This latter expression coincides with epithelial cell proliferation. These results show that whereas Lgr5 is reexpressed with declining estrogen influence, Lgr5 expression persists in ovariectomized mice treated with the vehicle alone. An injection of P4 rapidly down-regulated the expression of Lgr5 in ovariectomized uteri (Fig. 3C); low levels were maintained through 2–24 h after which the levels bounced back to ovariectomized levels with the waning of hormonal stimulation. In situ hybridization results are consistent with those of Northern hybridization. Two representative uterine sections at two time points with low Lgr5 expression are shown in Fig. 3D. The results of the remaining time points are presented in supplemental Figs. S2 and S3. Collectively, the data show that withdrawal of ovarian hormones induces Lgr5, which can be rapidly suppressed by administration of E2 or P4.
Figure 3.
Lgr5 expression is down-regulated by ovarian hormones. A, The scheme of treatment schedules and tissue collection. Uteri were collected 2, 4, and 6 d after ovariectomy. Eight days after ovariectomy, mice were treated with oil, E2 (100 ng/mouse), or P4 (2 mg/mouse), and tissues were collected at the indicated times. OVX, Ovariectomy. B, Lgr5 mRNA levels in ovariectomized and E2-treated uteri. Lgr5 expression levels gradually increased over a period of 2–6 d after ovariectomy. After E2 treatment, the levels dramatically decreased. Lgr5 reappeared 24 h after E2 treatment. Lgr5 mRNA levels as detected by Northern blotting were quantified against Rpl7, a housekeeping gene and normalized to vehicle (oil)-treated ovariectomized mice. C, Lgr5 mRNA levels in ovariectomized P4-treated uteri. Lgr5 levels dramatically decreased as early as 2 h after P4 injection and began to reappear thereafter. D, In situ hybridization of Lgr5. The results are consistent with those of Northern hybridization.
Ovarian hormones differentially regulate Lgr5 expression via ERα and PR
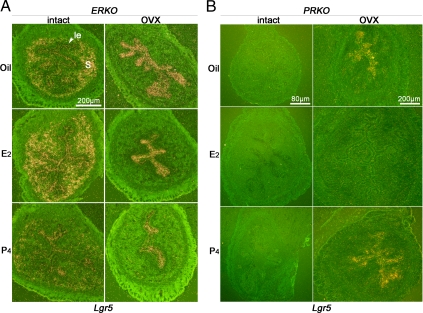
To examine whether Lgr5 expression by E2 or P4 is mediated by ERα and PR, respectively, we examined uterine Lgr5 expression in ERKO or micePRKO. As stated above, estrogen signaling in the uterus is primarily mediated by ERα. Surprisingly, we found for the first time that Lgr5 expression was detected in not only epithelial cells but also stromal cells of intact ERKO mice (Fig. 4A). As expected, Lgr5 expression was down-regulated after an injection of P4, similar to that seen in the wild-type uterus. However, the repressive effect of E2 on Lgr5 expression was abrogated in ERKO mice, suggesting that estrogen’s effects on Lgr5 expression are mediated by ERα. After ovariectomy, Lgr5 expression was detected only in the epithelium but not the stroma, similar to that seen in ovariectomized wild-type mice. Modest suppression of Lgr5 was observed after injection of P4, but not E2, in ovariectomized ERKO mice. Because PR expression is low in ERKO uteri (35), it is reasonable to assume that the modest reduction was due to suboptimal P4-PR signaling. Nonetheless, the results show that the repression of Lgr5 by estrogen requires ERα.
Figure 4.
E2 and P4 suppress Lgr5 expression via ERα and PR, respectively. A, In situ hybridization in intact or ovariectomized ERKO uteri with different hormone treatments. le, Luminal epithelium; s, stroma. B, In situ hybridization in intact or ovariectomized PRKO uteri after E2 or P4 treatment.
Because PRKO mice lack both PR-A and PR-B receptor isoforms, their uteri are dominated by unopposed estrogen action with high extracellular edema (9). We found no evidence of Lgr5 expression in these hyperplasmic PRKO uteri. Treatment with E2 or P4 did not show any alteration in Lgr5 expression (Fig. 4B). After ovariectomy, PRKO uteri underwent atrophy with reappearance of Lgr5 expression in both luminal and glandular epithelia (Fig. 4B); the expression pattern was indistinguishable from that of ovariectomized wild-type uteri. An injection of E2 significantly induced uterine growth with suppressed Lgr5 expression. In contrast, Lgr5 expression remained at comparable levels in P4 or oil-treated PRKO mice. These results demonstrate that P4’s suppressive effects on Lgr5 are mediated by PR.
Aberrant Wnt signaling interferes with Lgr5 expression
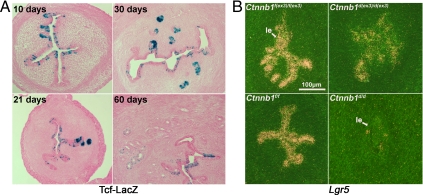
In the small intestine, Lgr5-positive stem cells are regulated by Wnt signaling (21). Previous studies have shown that Wnt signaling is involved in uterine growth and fertility (36,37). To study the role of β-catenin on uterine Lgr5 expression, we first examined uterine canonical Wnt signaling using a Tcf-lacZ reporter mouse line in which a LacZ reporter is activated by a nuclear β-catenin-TCF complex (38). Uterine tissues were collected from Tcf-LacZ reporter mice at different ages. We found that LacZ-stained cells are located in uterine epithelia at all ages examined. In 10-d-old uteri, LacZ-positive cells are randomly distributed along the epithelium (Fig. 5A). In 1-month-old mice with the development of more glands, more cells in glandular epithelia showed LacZ staining than in the luminal epithelium. However, the LacZ signals declined in the adult uterus. These data suggest that canonical Wnt signaling is active in both immature and mature uteri.
Figure 5.
Aberrant Wnt signaling disrupts Lgr5 expression. A, Canonical Wnt signaling is activated in uteri at different ages. Blue color represents expression of LacZ triggered by canonical Wnt signaling. B, Persistent or diminished Wnt signaling down-regulates Lgr5 expression. Lgr5 mRNA levels are down-regulated by heightened Wnt signaling in Ctnnb1d(ex3)/d(ex3) uteri. Elimination of canonical Wnt signaling by conditional deletion of uterine Ctnnb1 dramatically down-regulates Lgr5 mRNA levels. le, Luminal epithelium.
To investigate the role of Wnt signaling in regulating uterine Lgr5 expression, two mouse lines with aberrant Wnt signaling were generated by crossing floxed dominant stabilized β-catenin (Ctnnb1f(ex3)/f(ex3)) (26) or floxed β-catenin (Ctnnb1f/f) (24) mice with PR-Cre mice (25,37). In Ctnnb1d(ex3)/d(ex3) mice, the exon 3 of β-catenin, which is phosphorylated by glycogen synthase kinase 3b (Gsk3b), are deleted (26). The stabilized β-catenin results in an elevated canonical Wnt signaling. In contrast, β-catenin is conditionally deleted in Ctnnb1d/d uterus. Because Lgr5 expression was high in 10-d-old uteri and because the uterus at this stage is least exposed to ovarian hormone stimulation, we chose 10-d-old uteri to study Lgr5 regulation by Wnt signaling. We examined Lgr5 expression by in situ hybridization in 10-d-old uteri from Ctnnb1d(ex3)/d(ex3) mice, Ctnnb1d/d mice, or wild-type litter mates. In agreement with previous studies (14,16), Lgr5 expression was very low to undetectable in the absence of β-catenin, suggesting Lgr5 expression requires Wnt signaling (Fig. 5B). But to our surprise, sustained Wnt signaling in Ctnnb1d(ex3)/d(ex3) mice also down-regulated uterine Lgr5 expression (Fig. 5B), suggesting chronically elevated Wnt signaling down-regulates Lgr5 expression. Collectively, these results strongly suggest that Lgr5 expression in the uterus requires appropriate Wnt signaling because either heightened or absence of Wnt signaling disrupts Lgr5 expression.
Discussion
LGR5 is an orphan G protein-coupled receptor that belongs to a family of glycoprotein hormone receptors. The large extracellular domain with leucine-rich repeats suggests that the LGR5 ligand is likely to be a glycoprotein. In adult mice, Lgr5 expression is restricted to crypt cells in the intestine and a few scattered cells in the eye, brain, mammary gland, and reproductive organs. Mice lacking Lgr5 exhibit ankyloglossia, resulting in neonatal lethality (39). Because its ligand and signaling pathway have not yet been identified, exact functions of Lgr5 still remain elusive. A recent report shows that Lgr5 deficiency does not affect normal intestinal development, although null mice show premature Paneth cell differentiation (40).
Here we provide new information regarding regulation of Lgr5 expression in the mouse uterus at different physiological, pathological, and experimental conditions. The highlights of our findings are that: 1) Lgr5 is expressed primarily in the uterine epithelium under various physiological and experimental conditions, except in ERKO uteri in which it is expressed in both epithelial and stromal cells; 2) persistent expression of uterine Lgr5 requires withdrawal of steroid hormonal stimulation and suppressive effects of estrogen and progesterone on Lgr5 are mediated by their nuclear receptors, and 3) there is no long-term Lgr5-positive cell pool like that in the small intestine; even full-blown endometrial cancer lacks Lgr5 expression.
The uterine expression pattern of Lgr5 is unlike that of the small intestine. Whereas Lgr5 expression is restricted to crypt cells in the small intestine (1), its expression is spatiotemporally dynamic in the uterus. Lgr5 is mostly expressed in luminal and glandular epithelial cells in immature uteri and is greatly attenuated in adult uteri. It is primarily detected in the epithelium on d 1 of pregnancy and in epithelia of interimplantation sites on d 5, the significance of which is not clearly understood at this time. It is possible that Lgr5-positive epithelial cells at the interimplantation sites are involved in the formation of the secondary lumen during pregnancy. Collectively Lgr5 expression is not restricted to a certain epithelial cell population, and most, if not all, epithelial cells can express Lgr5 under certain but not under all conditions.
Estrogen and P4 are considered the master regulators of uterine growth and differentiation. Removal of ovaries leads to uterine atrophy with down-regulation of RNA transcription (41,42). The ovariectomized uterus remains quiescent but maintains its morphological and histological integrity, albeit with a reduced number of various cell types. Our observations of high expression of Lgr5 in immature or ovariectomized uterine epithelial cells suggest that Lgr5 preserves these quiescent cells and keeps them ready to respond to the growth stimulation. It is also possible that the unique induction of Lgr5 in ovariectomized uteri confers long-term survival of quiescent epithelial cells in the absence of ovarian hormones.
After Lgr5 was identified as a stem cell marker in the small intestine and hair follicle, Lgr5-positive cells were considered stem cell pools in the mammary gland and the stomach epithelium, promoting the idea that Lgr5 is a general marker of adult epithelial stem cell populations (43). Our results raise doubt about Lgr5’s identity as a stem cell marker in the uterus. The uterus is a very dynamic organ in that its morphological and histological characteristics are changing through out the estrous cycles and during pregnancy. That mouse uterine epithelial cells undergo cyclic changes every 4–5 d suggests the existence of fast amplifying cells. Moreover, the uterine bed undergoes drastic remodeling and repair with significant loss of resident stromal and epithelial cells after parturition. These characteristics may suggest that there is no sanctuary for stem cell niches to survive these dramatic structural changes in the uterus. However, uniform expression of Lgr5 in the ovariectomized uterine epithelium suggests that most epithelial cells have the potential to proliferate necessary for uterine growth.
Wnt signaling is known to play roles in epithelial-mesenchymal interactions and cellular organization, the processes that are also important for appropriate functioning of the female reproductive system (44). Wnt genes are expressed in developing and adult female reproductive tracts (45). Wnt4, Wnt5a, and Wnt7a are crucial for the formation of the Müllerian duct and uterus (46,47,48). Previous studies have shown that estrogen can induce the expression of Wnt family members Wnt4 and Wnt5a as well as frizzled homolog 2 (Fzd2), a member of the Wnt receptor family, in the uterus in an ERα-independent manner (36). In contrast, a negative regulator of Wnt signaling, secreted frizzled-related protein 2 (Sfrp2), is suppressed by estrogen (49). Collectively these results suggest that estrogen influences Wnt signaling in the uterus. Our present study showing suppression of Lgr5 expression by P4 and estrogen via PR and ER, respectively, suggests that these hormones regulate this Wnt target gene via their nuclear receptors. In addition, our data show that like in the small intestine (21), Lgr5 expression is also influenced by the degree of Wnt signaling in the uterus because the expression is disrupted by either amplified or silenced Wnt signaling. This suggests that an appropriate Wnt signaling is needed to regulate uterine Lgr5 expression. However, further studies are warranted to reveal definitive roles of Wnt signaling in regulating Lgr5 in the uterus.
The presence of Lgr5-positive stromal cells in intact, but not ovariectomized ERKO mice, is an intriguing observation (Fig. 4A). In the ERKO uterus, E2 may function through an ERβ- or ER-independent pathway. As discussed above, E2 up-regulates Wnt signaling in an ERα-independent manner (43). Because Lgr5 expression is normally suppressed by E2 via ERα, it is possible that E2 induces Lgr5 in both the stroma and epithelium by up-regulating Wnt signaling in an ERα-independent manner. In ERKO mice, P4 fails to suppress Lgr5 expression efficiently (Fig. 4A). PR is an estrogen-regulated gene and is widely recognized as a marker for estrogen action (50,51). Although the ERKO uterus can undergo a P4-dependent decidual response, uterine levels of PR in ERKO mice are 60% of those seen in wild-type mice (35). This suggests that the degree of suppression of Lgr5 expression by P4 is compromised in ERKO mice. Although our data show that Lgr5 is dynamically regulated in the mouse uterus, defining the function of Lgr5 in uterine biology will require conditional deletion of this gene in the uterus.
Supplementary Material
Acknowledgments
We thank Erin L. Adams for editing the manuscript.
Footnotes
This work was supported by National Institutes of Health Grants P01-CA-77839/HD12304, and DA06668 (to S.K.D.), and Concern Foundation grant (to T.D.). S.K.D. is a recipient of a Method to Extend Research in Time Award from the National Institute of Child Health and Human Development and the National Institute on Drug Abuse.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 1, 2009
Abbreviations: E2, Estradiol-17β; ER, estrogen receptor; ERKO, deficient of ERα; LGR, leucine-rich repeat-containing G protein-coupled receptor; P4, progesterone; PR, progesterone receptor; PRKO, mice null for both PRs; TCF, T cell factor.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H 2007 Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449:1003–1007 [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R 2008 Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40:1291–1299 [DOI] [PubMed] [Google Scholar]
- Hess AP, Nayak NR, Giudice LC 2005 Oviduct and endometrium: cyclic changes in the primate oviduct. In: Neill JD, Richards JS, eds. Knobil and Neill’s physiology of reproduction. 3rd ed. San Diego, CA: Academic Press; 337–382 [Google Scholar]
- Burroughs KD, Fuchs-Young R, Davis B, Walker CL 2000 Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod 63:1322–1330 [DOI] [PubMed] [Google Scholar]
- Wang H, Dey SK 2006 Roadmap to embryo implantation: clues from mouse models. Nat Rev 7:185–199 (Review) [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA 1997 Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138:863–870 [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O'Malley BW 2002 Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355 [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW 1995 Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- Van der Flier LG, Sabates-Bellver J, Oving I, Haegebarth A, De Palo M, Anti M, Van Gijn ME, Suijkerbuijk S, Van de Wetering M, Marra G, Clevers H 2007 The intestinal Wnt/TCF signature. Gastroenterology 132:628–632 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Müller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P 1993 Association of the APC gene product with β-catenin. Science 262:1731–1734 [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW 1993 Association of the APC tumor suppressor protein with catenins. Science 262:1734–1737 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H 1997 Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 275:1784–1787 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H 1998 Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19:379–383 [DOI] [PubMed] [Google Scholar]
- Pinto D, Gregorieff A, Begthel H, Clevers H 2003 Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17:1709–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ 2004 Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA 101:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W 2001 β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105:533–545 [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, Millar SE 2002 WNT signals are required for the initiation of hair follicle development. Dev Cell 2:643–653 [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ 2004 Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev 18:1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E 1998 De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95:605–614 [DOI] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H 2009 Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457:608–611 [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O 1993 Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, Liu X, Wu H 2002 Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis 32:148–149 [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R 2001 Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264 [DOI] [PubMed] [Google Scholar]
- Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP 2005 Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41:58–66 [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM 1999 Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J 18:5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK 1994 Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120:1071–1083 [DOI] [PubMed] [Google Scholar]
- Tan J, Paria BC, Dey SK, Das SK 1999 Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 140:5310–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Tan J, Matsumoto H, Robert B, Abrahamson DR, Das SK, Dey SK 2001 Adult tissue angiogenesis: evidence for negative regulation by estrogen in the uterus. Mol Endocrinol 15:1983–1992 [DOI] [PubMed] [Google Scholar]
- McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA 2006 Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther 5:419–426 [DOI] [PubMed] [Google Scholar]
- Tanese K, Fukuma M, Yamada T, Mori T, Yoshikawa T, Watanabe W, Ishiko A, Amagai M, Nishikawa T, Sakamoto M 2008 G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am J Pathol 173:835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK 2008 Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 68:5619–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW 1974 Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology 94:1704–1708 [DOI] [PubMed] [Google Scholar]
- Nelson JF, Karelus K, Felicio LS, Johnson TE 1990 Genetic influences on the timing of puberty in mice. Biol Reprod 42:649–655 [DOI] [PubMed] [Google Scholar]
- Curtis SW, Clark J, Myers P, Korach KS 1999 Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor α knockout mouse uterus. Proc Natl Acad Sci USA 96:3646–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK 2004 Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ 2009 β-Catenin mediates glandular formation and dysregulation of β-catenin induces hyperplasia formation in the murine uterus. Oncogene 28:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA 2002 β-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci USA 99:6973–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H, Mazerbourg S, Bouley DM, Luo CW, Kawamura K, Kuwabara Y, Baribault H, Tian H, Hsueh AJ 2004 Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol 24:9736–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G 2009 LGR5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev Biol 331:58–67 [DOI] [PubMed] [Google Scholar]
- Helvering LM, Adrian MD, Geiser AG, Estrem ST, Wei T, Huang S, Chen P, Dow ER, Calley JN, Dodge JA, Grese TA, Jones SA, Halladay DL, Miles RR, Onyia JE, Ma YL, Sato M, Bryant HU 2005 Differential effects of estrogen and raloxifene on messenger RNA and matrix metalloproteinase 2 activity in the rat uterus. Biol Reprod 72:830–841 [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS 2003 Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 17:2070–2083 [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H 2009 Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol 174:715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä M, Peltoketo H, Vainio S 2001 Wnts and the female reproductive system. J Exp Zool 290:616–623 [DOI] [PubMed] [Google Scholar]
- Miller C, Pavlova A, Sassoon DA 1998 Differential expression patterns of Wnt genes in the murine female reproductive tract during development and the estrous cycle. Mech Dev 76:91–99 [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkilä M, Kispert A, Chin N, McMahon AP 1999 Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409 [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S 1999 A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223 [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP 1998 Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707–710 [DOI] [PubMed] [Google Scholar]
- Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK 2000 Estrogen targets genes involved in protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-α and -β. J Biol Chem 275:28834–28842 [DOI] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990 Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E 1991 Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J 10:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.