Abstract
Colony-stimulating factor-1 (CSF-1), released by osteoblasts, stimulates the proliferation of osteoclast progenitors via the c-fms receptor (CSF-1R) and, in combination with receptor activator of nuclear factor-κB ligand (RANKL), leads to the formation of mature osteoclasts. Whether the CSF-1R is expressed by osteoblasts and mediates specific biological effects in osteoblasts has not been explored. Wild-type primary calvaria osteoblasts (OB) were analyzed for CSF-1R expression (RT-PCR and Western blot) and functionality (immunocomplex kinase assay). OB were serum starved for 24 h, and the effect of CSF-1 (0–100 ng/ml) on OB biological activities was determined at 48 h. In wild-type mouse bone marrow cultures, CSF-1 was tested for its effect on RANKL mRNA and osteoclast formation. Because ROS influence osteoblast RANKL expression, studies analyzed the effect of CSF-1 on reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and Nox1 and Nox4 proteins. Results indicate that OB express CSF-1R mRNA and protein and that CSF-1R could be phosphorylated in the presence of CSF-1. In osteoblasts, CSF-1 decreased RANKL mRNA in a dose- and time-dependent manner. Incubation of bone marrow cultures with CSF-1 resulted in a significant decline in tartrate-resistant acid phosphatase (TRACP) activity and CTR expression. RANKL-decreased expression by CSF-1 was correlated with a decrease of NADPH oxidase activity as well as Nox1 and Nox4 protein levels. These findings provide the first evidence that osteoblasts express CSF-1R and are a target for CSF-1 ligand. CSF-1-mediated inhibition of RANKL expression on osteoblasts may provide an important mechanism for coupling bone formation/resorption and preventing excessive osteoclastogenesis during normal skeletal growth.
Osteoblasts express CSF-1 receptor, and CSF-1-mediated inhibition of RANKL in osteoblasts is associated with decreased Nox oxidases.
Macrophage colony-stimulating factor (CSF-1), released by osteoblasts, stimulates the proliferation of osteoclast progenitors via the c-fms receptor (CSF-1R) and, in combination with receptor activator of nuclear factor-κB ligand (RANKL), leads to the formation of mature osteoclasts. Osteoblasts produce membrane-bound RANKL in response to osteotropic factors including PTH, prostaglandin E2, 1,25-dihydroxyvitamin D3 (VD3), and TGF-β (1). Previous studies indicate that high levels of CSF-1 alter osteoblast growth and inhibit osteoclast formation.
The expression of CSF-1R in osteoblasts has not been reported, and little is known about the biological effect of CSF-1 on osteoblasts. We hypothesized that osteoblasts express the CSF-1R and CSF-1 alters osteoblast gene expression that, in turn, may influence osteoclastogenesis.
The CSF-1R is expressed by cells of the mononuclear phagocytic lineage including osteoclast progenitors and mature osteoclasts as well as placental trophoblasts (2,3), uterine decidual cells, smooth muscle cells (4), microglia (5), and renal mesangial cells (6). In contrast, analysis of bone tissue and cell cultures using in situ hybridization, immunohistochemistry, or Western blotting (7,8) failed to detect CSF-1R in osteoblasts. Despite this observation, CSF-1 has been reported to influence osteoblast maturation and gene expression (9,10). In CSF-1-deficient tl rats, whole bone preparations showed increased alkaline phosphatase and decreased type I collagen and osteocalcin mRNA levels. Injection of recombinant CSF-1 resulted in normalization of osteoblast gene expression (10), and due to the postulated lack of CSF-1 receptors on osteoblasts, this effect was attributed to induction of osteoclastogenesis that directly or indirectly restored the osteoblast phenotype. In our laboratory, we observed that overexpression of soluble CSF-1 (sCSF-1) in transgenic mice led to increased cortical bone remodeling with a net increase in bone formation, suggesting that CSF-1 may directly influence osteoblastogenesis.
The CSF-1R, a tyrosine kinase that mediates all known effects of CSF-1, contains an extracellular domain of five Ig-like domains, a transmembrane domain, and an intracellular tyrosine kinase domain (11). CSF-1 binding results in tyrosine phosphorylation of the receptor and activation of phosphatidylinositol-3-kinase in macrophage/osteoclast cells (12). Reactive oxygen species (ROS) such as superoxide anions (O2−), hydroxyl radicals, and hydrogen peroxide (H2O2) may act as second messengers in various signal transductions and elicit a wide spectrum of cellular responses ranging from proliferation to senescence. Reduced nicotinamide adenine dinucleotide (phosphate) [NAD(P)H] oxidase systems including the Nox family of NAD(P)H oxidases are major sources of ROS (13,14). Recent studies indicate that ROS is crucial for RANKL-induced osteoclastogenesis (15,16,17). ROS produced in bone marrow macrophage cells stimulate osteoclast differentiation, whereas decreasing the level of ROS reverses RANKL responses (18,19). Importantly, in primary calvarial osteoblast and stromal cultures, Bai et al. (20) demonstrated that superoxide anion generated in response to H2O2 and xanthine/xanthine oxidase stimulated RANKL mRNA and protein expression.
In the present study, primary calvarial osteoblasts were analyzed for CSF-1R expression and phosphorylation. Mouse bone marrow cultures, incubated with increasing doses of CSF-1, were assessed for RANKL expression and osteoclastogenesis. To determine the potential mechanisms by which CSF-1 regulates RANKL, osteoblasts were analyzed for NADPH oxidase activity, Nox1 and Nox4 expression, and cells were transduced with Nox1 or Nox4 small interfering RNA (siRNA). Our findings show, for the first time, that osteoblasts express a functional CSF-1R. High as well as low levels of CSF-1 inhibited RANKL expression in osteoblasts and, in bone marrow cultures, led to decreased osteoclast formation. Our data provide the first evidence that osteoblasts express Nox1 and Nox4 proteins. Inhibition of RANKL by CSF-1 was associated with a decline in NADPH superoxide generation that paralleled the decrease in Nox1 and Nox4 protein expression.
Materials and Methods
Primary calvarial osteoblast cultures
Primary mouse osteoblasts were prepared from calvariae of newborn wild-type (wt) C57/BL mice by five sequential digestions using 0.1% collagenase and 0.2% dispase as previously described (21). Mice used in this study were approved by the University of Texas Health Science Center, San Antonio, Animal Care and Use Committee. Calvarial osteoblasts isolated in fractions 2–5 were cultured for 3 d, and cells from passage 2 were used for experiments. Cultures were analyzed for expression of osteoblast markers including osterix, type I collagen, and osteocalcin. Cultures were also examined for the presence of monocytes or macrophages using flow cytometry. Briefly, cells were detached from the plate by adding trypsin for 4 min at 37 C. Single-cell suspensions (5 × 105) were incubated on ice for 35 min with the fluorescein isothiocyanate-conjugated F4/80 (1:100) (eBioscience, San Diego, CA), PE-labeled Mac-1 (CD11b, 1:200) or APC-labeled CD45.2 (1:40) (PharMingen, San Diego, CA). As a positive control, bone marrow cells were harvested from a wt C57/BL mouse by flushing the marrow from femurs. To assess the effect of trypsin on monocyte/macrophage cell surface markers, a portion of marrow cells were trypsinized in parallel with osteoblasts before staining. Cells were then washed twice and during the last wash, 7-amino-actinomycin D (7-AAD) (eBioscience) was added to stain the dead cells. Analysis was carried out using a FACS Aria (Becton Dickinson, Franklin Lakes, NJ). Cells were seeded at a density of 1 × 104/cm2 in α-MEM supplemented with 10% fetal calf serum (FCS) and incubated at 37 C in 5% CO2. At 80% confluence, cells were harvested to assess CSF-1R mRNA expression. To determine the effect of CSF-1 on RANKL expression, cells were placed in serum-free (SF) medium for 24 h and then incubated in presence or absence of recombinant murine CSF-1 (rmCSF-1, 10–100 ng/ml; R&D Systems, Minneapolis, MN) before harvesting for total RNA at 48 h. In parallel studies, cultures incubated with or without 100 ng/ml rmCSF-1 were treated or not treated with the flavoprotein inhibitor diphenyleneiodonium chloride (DPI, 1 μm; Calbiochem, La Jolla, CA) for 24 h or were exposed to H2O2 (400 μm) for 2 h before RNA isolation. A control cell line for experiments included the murine monocytic cell line RAW264.7 (ATCC TIB-71; American Type Culture Collection, Manassas, VA) that was maintained in α-MEM supplemented with 10% FCS and incubated at 37 C in 5% CO2. The osteoblast cell lines including MC3T3-E1, MC3T3-G2/PA6, and 2T3 were cultured in their appropriate medium.
Primary bone marrow cultures for osteoclast formation
Bone marrow cells were isolated from 3- to 5-wk-old wt C57/BL mice. Briefly, femurs were excised, and the marrow cavity was flushed with α-MEM supplemented with 10% FCS. Cells were washed and plated in 1 ml of the same medium at a density of 2.5 × 106 cells/cm2. Cultures were grown either in complete medium alone (control) or in differentiation medium [dexamethasone (DEX) and VD3, 10−8 m each; Sigma-Aldrich, St. Louis, MO) and incubated in the presence and absence of rmCSF-1 (10–100 ng/ml; R&D Systems). In some experiments, rmRANKL (50 ng/ml) (R&D Systems) was added to cultures. Every 2 d, half the medium was removed and replaced with fresh medium with twice the final concentration of the treatment agent. After 13 d, cultures were harvested for tartrate-resistant acid phosphatase (TRACP) activity or total RNA.
Cell transfection and siRNA knockdown
Osteoblasts were transfected in complete medium with 100 nm siRNAs including Nox1, Nox4, or scrambled control (Dharmacon, Lafayette, CO) for 12 h using X-treme Gene transfection reagent (Roche, Indianapolis, IN). Then cells were placed in SF medium for 24 or 48 h before harvesting to assess siRNA knockdown efficiency or the effect of each siRNA on RANKL mRNA expression, respectively. In parallel experiments, cells transfected with siNox1+siNox4 were placed in SF medium with or without CSF-1 (100 ng/ml) to determine the effect of CSF-1 on RANKL expression under conditions of combined Nox1 and Nox4 knockdown.
Additional experiments were performed to improve Nox4 mRNA knockdown and analyze Nox protein levels. Transfection efficiency was tested using Alexa Fluor 546-labeled negative, nonsilencing control siRNA that enters RNA-induced silencing complex to produce bright red fluorescence (QIAGEN Inc., Valencia, CA). Osteoblasts under SF conditions were transfected with this control siRNA (10 nm) for 24 h at 37 C using Lipofectamine Plus (Invitrogen, Carlsbad, CA) and then examined for red fluorescence using an Olympus FV-500 confocal microscope (556 nm excitation, 573 nm emission). Similar conditions were used to transfect cells with 100 nm siNox1, 200 nm siNox4, or scrambled control. At 24 or 48 h after transfection, cells were analyzed for Nox protein levels or RANKL expression, respectively.
TRACP assays
TRACP activity was assayed according to standard methods using p-nitrophenyl phosphate as a substrate (22). Medium was aspirated from marrow cultures, and cell lysates were prepared. Samples were incubated in 100 μl buffer solution [125 mm sodium acetate buffer (pH 5.2), 100 mm p-nitrophenyl phosphate (Sigma-Aldrich), and 1 mm L(+) sodium tartrate]. The production of p-nitrophenol was determined in 96-well plates by measuring the absorbance at 405 nm at 37 C. Protein amounts were also quantified, and data are expressed as the mean OD per minute per milligram protein. In parallel experiments, cellular TRACP activity was analyzed using the leukocyte acid phosphatase kit (Sigma-Aldrich). Briefly, the cells were washed twice with PBS, fixed for 5 min with citrate/paraformaldehyde/acetone solution, and stained according to the manufacturer’s instructions.
NADPH oxidase assay
NADPH-dependent superoxide production was measured by the lucigenin-enhanced chemiluminescence method as described (23,24). Cells were washed and pelleted in ice-cold PBS and then prepared in 300 μl lysis buffer [20 mm KH2PO4 (pH 7.0), 1 mm EGTA, 1 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 0.5 μg/ml leupeptin] by using a Dounce homogenizer (100 strokes on ice). Homogenates were centrifuged at 800 × g at 4 C for 10 min to remove the unbroken cells and debris, and aliquots were used immediately. To start the assay, 100 μl homogenates were added to 900 μl 50 mm phosphate buffer (pH 7.0) containing 1 mm EGTA, 150 mm sucrose, 5 μm lucigenin, and 100 μm NADPH. Photon emission was measured in a luminometer every 30 sec for 10 min. There was no measurable activity in the absence of NADPH. For each sample, superoxide anion production was expressed as relative chemiluminescence (light) units per milligram protein.
Immunoprecipitation and CSF-1R activity
Primary osteoblasts grown to 80% confluency were placed in SF medium and incubated with rmCSF-1 (0–100 ng/ml) for the indicated time points before harvesting at 48 h. Cell lysates were prepared in ice-cold RIPA buffer containing 1 mm NaVO4 (phosphatase inhibitor). For immunoprecipitation, an equal amount of protein (100 μg) was incubated overnight with rabbit anti-CSF-1R (Upstate Biotechnology, Lake Placid, NY). The immunoprecipitates were bound to protein A- or G-Sepharose (Amersham Biosciences, Piscataway, NJ), rotated at 4 C for 2 h, and washed with ice-cold lysis buffer and PBS. The kinase reaction was performed by incubating the immunobeads in kinase assay buffer [20 mm HEPES (pH 7.4), 20 mm MgCl2, 25 mm β-glycerophosphate, 2 mm dithiothreitol, 1 mm Na3VO4] in the presence of 1 μCi [γ-32P]ATP for 30 min at 37 C. The reaction was stopped by the addition of reducing loading buffer. Samples were boiled for 5 min and analyzed by 10% SDS-PAGE, and the phosphorylated receptor was visualized by autoradiography.
Western blot analysis
Cultured cells as well as bone from sCSF-1 transgenic mice that we have previously reported were analyzed by Western blot. In the transgenic mice, sCSF-1 is selectively expressed in osteoblasts using the osteocalcin promoter, and these mice express high levels of sCSF-1 protein in bone (25). Briefly, hind limbs from 5-wk-old sCSF-1 transgenic mice and wt littermates were removed, and marrow cells were isolated by flushing marrow cavities with media. The remaining bone tissue was placed in liquid nitrogen and crushed. Cultured cells, marrow cells, and bone samples were lysed in ice-cold lysis buffer. Proteins were loaded and resolved on an 8% (CSF-1R) or 12% (RANKL, Nox1, and Nox4) SDS-PAGE and transferred to a polyvinylidene difluoide membrane (PerkinElmer, Norwalk, CT). CSF-1R, RANKL, Nox1, and Nox4 proteins were detected using specific antibodies from Upstate Biotechnology (06-174), Abcam (Cambridge, MA; ab13582), Santa Cruz Biotechnology (Santa Cruz, CA; sc-25545), and Abcam (ab41886), respectively. After exposure with the indicated primary antibody, the immunoblots were washed and incubated with goat antirabbit/mouse-coupled horseradish peroxidase (Santa Cruz) followed by chemiluminescence using ECL reagent (Amersham).
RNA extraction and RT-PCR
Alterations in mRNA expression were examined by RT-PCR. Total RNA was isolated from primary osteoblast or bone marrow cultures using TRIzol reagent (Invitrogen) and treated with deoxyribonuclease I (1 U/μg) to remove any contaminating genomic DNA. First-strand cDNA was reverse-transcribed from 2 μg total RNA using murine Moloney leukemia virus reverse transcriptase, RNAsine, oligo-dT (Invitrogen), and dNTP mix (Promega, Madison, WI) according to the manufacturer’s protocol. Two microliters of RT reaction mixture were subjected to PCR using specific primers (30 pmol each, Table 1) and TaqPlatinum Supermix (Invitrogen) in a final volume of 50 μl. Primers for 18S were used to ascertain that an equivalent amount of cDNA was synthesized. PCR amplification was performed over a range of cycle numbers to ensure that amplification was in the linear range of the curve. PCR products were analyzed in 1% agarose gels, stained with ethidium bromide, and photographed. The density of each band was measured using ImageJ software (National Institutes of Health, Bethesda, MD). The densities of marker mRNA bands were normalized relative to those of 18S mRNA bands. Data are expressed as relative mRNA expression where the ratio of the RANKL and CTR band density in control cultures to the corresponding 18S signal was arbitrarily set at 1. Quantitative real-time PCR for RANKL was performed by TaqMan gene expression assay (Applied Biosystems) (assay ID RANKL: Mm01313943_m1) using an ABI PRISM 7900 HT sequence detection system according to the manufacturer’s protocol. Reaction conditions were as follows: 2 min at 50 C (one cycle), 10 min at 95 C (one cycle), and 15 sec at 95 C and 1 min at 60 C (40 cycles). The relative expression level of RANKL was calculated using the comparative ΔΔCt method after normalization against the expression of 18S rRNA.
Table 1.
Primers sequences and PCR conditions
| Genes | Sequences (5′–3′) | Size (bp) | Cycles | Temperature (°C) |
|---|---|---|---|---|
| CSF-1 | ||||
| Forward | catccaggcagagactgaca | 381 | 30 | 55 |
| Reverse | cttgctgatcctccttccag | |||
| CSF-1R | ||||
| Forward | gacctgctccacttctccag | 304 | 30 | 55 |
| Reverse | ggttcagaccaagcgagaag | |||
| CTR | ||||
| Forward | tgctatgaccggattcatca | 383 | 30 | 55 |
| Reverse | gtcaccctctggcagctaag | |||
| Nox1 | ||||
| Forward | acagaggagagcttgggtga | 320 | 30 | 60 |
| Reverse | cactccaggaaggaaatgga | |||
| Nox4 | ||||
| Forward | ccagaatgaggatcccagaa | 329 | 30 | 60 |
| Reverse | aaaaccctcgaggcaaagat | |||
| Osterix | ||||
| Forward | cttaacccagctccctaccc | 492 | 30 | 60 |
| Reverse | gtggtcgcttctggtaaagc | |||
| Osteocalcin | ||||
| Forward | gcgctctgtctctctgacct | 106 | 30 | 60 |
| Reverse | gccggagtctgttcactacc | |||
| Procollagen 1 | ||||
| Forward | acgtcctggtgaagttggtc | 170 | 30 | 60 |
| Reverse | cagggaagcctctttctcct | |||
| RANKL | ||||
| Forward | agccgagactacggcaagta | 495 | 30 | 55 |
| Reverse | gatggtgaggtgtgcaaatg | |||
| OPG | ||||
| Forward | accaaagtgaatgccgagag | 442 | 30 | 55 |
| Reverse | ttgtgaagctgtgcaggaac | |||
| 18S | ||||
| Forward | gaccataaacgatgccgact | 389 | 20 | 60 |
| Reverse | gaacgccacttgtccctcta |
Statistical analysis
Results were analyzed by one-way ANOVA Newman-Keuls’ test using Prism4 software (GraphPad, San Diego, CA). Data represent the mean ± se of three separate experiments. Significance was determined as P < 0.05.
Results
Primary murine osteoblasts express a functional CSF-1R
Primary cultures expressed the osteoblast-specific transcription factor osterix as well as type I collagen and osteocalcin demonstrating their osteoblast lineage (Fig. 1A). To assess whether cultures contained CD45+ hematopoietic cells, especially monocytes or macrophages (CD11b+ or F4/80+ cells, respectively), flow cytometry was performed (Fig. 1B, upper panel). The histograms of CD45-, CD11b-, and F4/80-stained osteoblasts resembled that of the unstained cells, indicating that monocytes, macrophages, or any other types of hematopoietic cells were not detectable. Results for control bone marrow cells demonstrate the validity of the antibodies used and that surface marker integrity was maintained in the presence of trypsin (Fig. 1B, lower panel). Previous studies suggest that CSF-1 may have a direct effect on osteoblasts. We, therefore, screened primary calvarial osteoblasts for the presence of the CSF-1R using RT-PCR. As shown in Fig. 1C, osteoblasts express the CSF-1R transcript observed in control RAW264.7 cells, and both cell types express CSF-1 ligand. Western blot analysis (Fig. 1D, upper panel) confirmed that osteoblasts express CSF-1R at the protein level, and immunocomplex kinase assay (Fig. 1D, lower panel) demonstrated that the receptor is biologically active. Addition of CSF-1 (100 ng/ml) to cultures induced rapid phosphorylation of the receptor compared with untreated control, and this effect persisted up to 60 min. Receptor phosphorylation was also observed when lower doses of CSF-1 were added to cultures. (Fig. 1D). Similar to primary osteoblast cultures, osteoblast cell lines including MC3T3-E1, MC3T3-G2/PA6, and 2T3 expressed CSF-1R mRNA and protein (Fig. 1E).
Figure 1.
Expression of osteoblast lineage markers and CSF-1R transcripts and effect of CSF-1 on tyrosine phosphorylation of CSF-1R in murine primary osteoblasts. A, Expression of osteoblast lineage markers. Total RNA was isolated from primary cultures in complete medium, and mRNA expression of the indicated genes was determined using RT-PCR. B, FACS analysis of CD45, CD11b, and F4/80 expression in osteoblasts (upper panel) and bone marrow control (lower panel). In histograms, a dotted line represents unstained cells, and solid and dashed lines represent antibody staining in trypsinized and untrypsinized cells, respectively. C, RT-PCR analysis of CSF-1 and CSF-1R mRNAs in osteoblasts (WT OB.) and RAW264.7 cells (control). Total RNA was isolated from cells, and RT-PCR was carried out using murine CSF-1, CSF-1R, or 18S (control) primer sets. D, CSF-1-induced tyrosine phosphorylation. Cells were placed in SF medium and incubated with or without rmCSF-1 (1–100 ng/ml) at the indicated time points before harvesting at 48 h. Western blot analysis of CSF-1R protein (upper panels, 100 μg/lane) and immunocomplex kinase assay (lower panels) are shown. E, CSF-1R mRNA (upper panel) and protein (lower panel) expression in osteoblast cell lines.
CSF-1 inhibits RANKL mRNA expression in primary osteoblasts
Because high concentrations of CSF-1 have been reported to inhibit osteoclast formation, dose and time-course studies were performed for the effect of CSF-1 on RANKL expression in primary osteoblasts. Figure 2A shows that CSF-1 inhibited RANKL expression in a dose-dependent manner. Using an optimal concentration of CSF-1 (100 ng/ml), RANKL mRNA levels progressively declined over time, with a decrease of approximately 75% observed at 24 h (Fig. 2B). Analysis of other osteoblast-associated genes including osteoprotegerin, type I collagen, or osteocalcin were not altered by CSF-1 (Fig. 2C). Because sCSF-1 transgenic mice are a model where osteoblasts are exposed to high levels of CSF-1, bones from these mice were analyzed for RANKL expression. By Western blot analysis (Fig. 2D), abundant RANKL protein was detected in wt, but not in sCSF-1 transgenic, bone, suggesting that CSF-1 may inhibit RANKL in vivo. RANKL was not identified in the bone marrow fraction isolated from wt or transgenic mice.
Figure 2.
Effect of CSF-1 on RANKL mRNA expression in murine primary osteoblasts. A, Dose-dependent effect of CSF-1 on RANKL (upper panel). Cells were placed in SF medium for a total period of 48 h including a 24-h incubation time with or without increasing concentrations of rmCSF-1 before harvesting for total RNA. RT-PCR was performed using murine RANKL or 18S primer sets. B, Time course for the effect of rmCSF-1 on RANKL (upper panel). Cells in SF medium were incubated with or without rmCSF-1 (100 ng/ml) for the indicated time points before harvesting at 48 h. Bar graphs (lower panels) show semiquantitative analysis of RANKL expression shown in upper panels. C, Effect of CSF-1 on osteoprotegerin (OPG) mRNA expression. Experiments were carried out as described in A, and RT-PCR was performed using a murine OPG primer set. D, RANKL protein expression in vivo. Western blot analysis of RANKL expression in bone marrow (left panel) and bone (right panel) lysates from wt and sCSF-1-transgenic (TG) mice are shown. a, P < 0.001; b, P < 0.01; c, P < 0.05; d, P > 0.05 vs. untreated cultures.
High concentrations of CSF-1 decrease osteoclastogenesis
To examine the biological effect of CSF-1-mediated inhibition of RANKL on osteoclastogenesis, primary bone marrow cultures were placed in differentiation medium (VD3 and DEX) and incubated in the presence and absence of 100 ng/ml CSF-1. Figure 3A shows that RANKL as well as CTR mRNA, a marker of mature osteoclasts, were up-regulated in cultures containing differentiation medium compared with control. Semiquantitative analysis of mRNA transcripts showed that incubation with rmCSF-1 partially inhibited RANKL and CTR expression approximately 50 and 30%, respectively (Fig. 3, B and C). In contrast, CTR expression decreased by CSF-1 was restored and slightly enhanced by the addition of exogenous RANKL (Fig. 3C). The pattern of CTR expression correlated with TRACP activity and staining analyzed under the same culture conditions. rmCSF-1 decreased TRACP activity approximately 40% compared with untreated cultures, whereas addition of exogenous rmRANKL abrogated the inhibitory effect of rmCSF-1 on TRACP activity (Fig. 3D). Multinucleated TRACP-positive cells were observed in VD3/DEX-treated but not in untreated cultures. The VD3/DEX-induced TRACP staining was inhibited in presence of rmCSF-1 and restored by rmRANKL addition (Fig. 3E). In separate experiments, the effect of lower concentrations of CSF-1 was assessed using the same culture conditions. RANKL and CTR expression decreased in response to 10 and 25 ng/ml rmCSF-1 compared with cultures in differentiation medium alone (Fig. 3F). A decrease in VD3/DEX-induced TRACP-positive osteoclast formation was also detected in cultures treated with 25 and 50 ng/ml rmCSF-1, with maximal inhibition observed at 100 ng/ml (Fig. 3F). These data suggest that down-regulation of RANKL by high as well as low levels of CSF-1, perhaps via the CSF-1R on osteoblasts, may contribute to decreased osteoclastogenesis.
Figure 3.
Effect of CSF-1 on RANKL and osteoclast marker expression in primary bone marrow cultures. Femurs were flushed, and bone marrow cells were plated at a density of 2.5 × 106 cells/cm2. Cells were incubated in complete medium alone (control) or in differentiation medium (10−8 m VD3/10−8 m DEX) and incubated in the presence and absence of rmCSF-1 (100 ng/ml). In some experiments, rmRANKL (50 ng/ml) was added to cultures. After 13 d, cultures were harvested for total RNA or TRACP assays. A, RT-PCR for RANKL and CTR mRNA expression. B and C, Semiquantitative analysis of RANKL and CTR expression shown in A. D and E, Effect of CSF-1 on TRACP activity. TRACP activity was assayed using p-nitrophenyl phosphate as a substrate, and the OD at 405 nm was determined by spectrophotometry. Osteoclasts (arrows) were identified by positive TRACP staining according to manufacturer’s protocol. F, Effect of CSF-1 concentrations on RANKL and osteoclastogenesis. Experiments were carried out as described in A using indicated doses of rmCSF-1. RT-PCR for RANKL and CTR expression was performed (left panel), and the number of TRACP-positive multinucleated cells (MNCs, defined as cells with at least three nuclei) per well was determined (right panel). a, P < 0.001; b, P < 0.01; c, P < 0.05 vs. 10−8 m VD3/10−8 m DEX-treated cultures.
Involvement of ROS in mediating CSF-1 effects
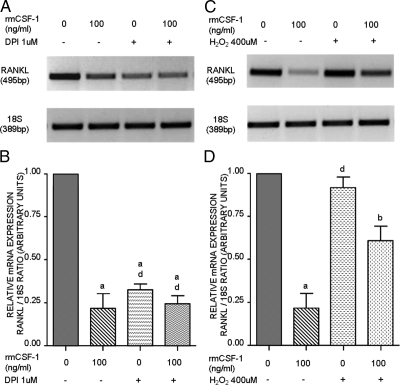
Because redox-regulated signaling is involved in stimulating RANKL expression in primary osteoblasts, we analyzed the effect of CSF-1 on RANKL in cultures treated with DPI or H2O2. Osteoblasts were incubated in presence and absence of 1 μm DPI, an inhibitor of flavoprotein-containing oxidases, in combination with rmCSF-1. At this concentration, DPI has been shown to inhibit Nox activity (26). RT-PCR (Fig. 4A) and semiquantitative analysis of transcripts (Fig. 4B) show that DPI alone inhibits RANKL expression in osteoblasts compared with control, suggesting that ROS maintains RANKL expression. The inhibitory effect of DPI on RANKL was similar to rmCSF-1. Coincubation of DPI with rmCSF-1 had neither a synergistic nor additive effect on RANKL expression, suggesting that they may use the same pathway and that CSF-1 may mediate its effect on RANKL through inhibition of ROS production. In parallel experiments, H2O2 was added to rmCSF-1-treated cultures and was observed to partially reverse the inhibitory effect of CSF-1 on RANKL expression (Fig. 4, C and D). Collectively, these findings suggest that CSF-1-mediated inhibition of RANKL expression is dependent upon a redox mechanism. Because superoxide production stimulates RANKL, we hypothesized that NADPH oxidase activity could participate in mediating the effect of CSF-1.
Figure 4.
Effect of DPI and H2O2 on RANKL expression in murine primary osteoblasts. A, Cells were placed in SF medium for 24 h and then incubated with or without rmCSF-1 (100 ng/ml) in combination or not with the flavoprotein inhibitor DPI (1 μm) before harvesting at 48 h. Total RNA was extracted and subjected to RT-PCR for RANKL. B, Semiquantitative analysis of RANKL expression shown in A. C, Cells incubated with or without rmCSF-1 as described in A were treated or not treated with H2O2 (400 μm) for 2 h before RNA isolation at 48 h. D, Semiquantitative analysis of RANKL expression shown in C. a, P < 0.001; b, P < 0.01; d, P > 0.05 vs. untreated cells.
CSF-1 decreases NADPH oxidase activity and Nox1 and 4 proteins
To further examine the potential role of NADPH-dependent superoxide generation as an intermediary pathway involved in mediating the effect of CSF-1 on RANKL, we analyzed the effect of CSF-1 on NADPH-dependent superoxide production. NADPH-dependent superoxide anion (O2−) production was measured using lucigenin-enhanced chemiluminescence assay. Figure 5A demonstrates that CSF-1 inhibits superoxide production in osteoblasts in a dose-dependent manner, with 50% reduction in activity observed at 50 and 100 ng/ml rmCSF-1. To determine which NAD(P)H oxidase isoforms are expressed in osteoblasts, Nox proteins were analyzed by Western blot. Both Nox1 and Nox4 proteins were expressed by osteoblasts with a predominance of Nox4 (Fig. 5B). Nox2 was also expressed, whereas Nox3 protein was not detected (data not shown). The inhibitory effect of rmCSF-1 on superoxide production was associated with decreased protein levels of Nox1 and Nox4 (Fig. 5B). Semiquantitative analysis of Western blots showed a significant reduction in Nox1 and, to a lesser extent, Nox4 proteins in cultures treated with rmCSF-1 (100 ng/ml) compared with untreated cultures (Fig. 5, C and D). These results suggest that CSF-1 regulates NADPH-dependent superoxide production by regulating the protein expression of Nox1 and Nox4. Taken together with studies on RANKL, these data indicate that CSF-1 may down-regulate RANKL, at least in part, by inhibiting NAD(P)H oxidases.
Figure 5.
Effect of CSF-1 on NADPH-dependent superoxide production and Nox proteins in murine primary osteoblasts. Lysates were prepared from cells incubated under the same conditions described in Fig. 2A. A, NADPH-dependent superoxide generation was measured by lucigenin-enhanced chemiluminescence and expressed as relative chemiluminescent light units per minute per milligram protein. B, Nox1 and Nox4 are expressed by osteoblasts. Equivalent amounts of cell lysates (30 μg protein/lane) were analyzed by Western blot with Nox1 or Nox4 antibodies. C and D, The densities of Nox1 and Nox4 bands shown in B were normalized relative to those of actin (control) bands. Data are expressed as relative protein expression where the ratio of the Nox1 and Nox4 band density in control cultures to the corresponding actin signal was arbitrarily set at 1. The data in A, C, and D were quantified, and results are expressed as the means ± se. a, P < 0.001; b, P < 0.01; c, P < 0.05; d, P > 0.05 vs. untreated cells.
Effect of Nox1 siRNA on RANKL expression in primary osteoblasts: Nox1 knockdown mimics CSF-1 effect
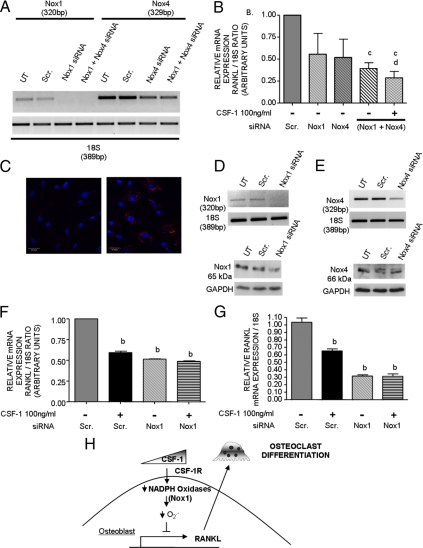
To examine the contribution of Nox oxidase components to RANKL expression, we employed a knockdown strategy. Primary osteoblasts were transfected with siRNAs to mNox1 (100 nm) and mNox4 (100 nm) or scrambled control. Successful knockdown of Nox1 and Nox4 was confirmed by RT-PCR (Fig. 6A). Consistent with Western blot results, untransfected osteoblasts and cells with scrambled control express Nox1 and Nox4 mRNA transcripts, with Nox4 being more abundant. siRNA treatment abolished and moderately decreased Nox1 and Nox4 expression, respectively. Analysis of RANKL expression in siNox1 and siNox4 knockdown cells showed a downward trend but was not significantly different from that observed in cells with scrambled control; however, in cells transfected with both siNox1 and siNox4, RANKL expression decreased. Interestingly, addition of CSF-1 on double-transfected cells did not significantly further decrease RANKL expression (Fig. 6B).
Figure 6.
Effect of Nox1 and Nox4 siRNAs on RANKL expression in murine primary osteoblasts. A, Analysis of siRNA knockdown efficiency. Osteoblasts were not transfected (UT) or transfected in complete medium with 100 nm siRNAs including Nox1, Nox4, or scrambled control (Dharmacon) for 12 h. Then cells were serum starved for 24 h before harvesting total RNA. RT-PCR was performed using specific murine Nox PCR primers or 18S as control. B, Effect of siRNAs on RANKL. Transfected cells were placed in SF medium for a total period of 48 h including a 24-h incubation time with or without CSF-1 (100 ng/ml) before determining the effect of each siRNA on RANKL mRNA expression. Bar graph shows semiquantitative analysis of siRNA treatment on RANKL mRNA expression by RT-PCR. c, P < 0.05 vs. scrambled control; d, P > 0.05 vs. siNox1+siNox4 alone. C, Analysis of siRNA transfection efficiency. Cells were not transfected (left panel) or incubated with Alexa Fluor 546-labeled negative control siRNA (right panel) as described in Materials and Methods, and nuclei were stained with DAPI. Composite overlay of DAPI and red fluorescence visualized by confocal microscopy is shown. D and E, Effect of siRNA knockdown on Nox proteins. Osteoblasts were placed in SF medium and not transfected (UT) or incubated with siNox1 (100 nm), siNox4 (200 nm) or scrambled control. After 24 h, cells were harvested and analyzed by RT-PCR (upper panels) and Western blot (lower panels). F, Effect of siNox1 on RANKL. Transfected cells in SF medium were incubated with or without CSF-1 (100 ng/ml) as described in B. Bar graph shows semiquantitative analysis of siRNA treatment on RANKL mRNA expression by RT-PCR. G, Quantitative real-time PCR for RANKL. Samples from the same experiment shown in F were analyzed by real-time PCR. Relative RANKL mRNA expression levels, normalized against 18S rRNA, were calculated using the 2−ΔΔCt method, and values were graphed as the mean expression level ± se. b, P < 0.01 vs. scrambled control without CSF-1. H, CSF-1 directly inhibits RANKL expression by osteoblasts. Scr., Scrambled control.
Separate experiments were carried out to improve Nox4 knockdown and assess Nox1 and Nox4 at the protein level. Cells were transfected with an Alexa Fluor 546-labeled negative control siRNA, and greater than 90% showed red fluorescence, indicating high transfection efficiency (Fig. 6C). Using similar transfection conditions, Nox1 siRNA (100 nm) abolished Nox1 mRNA and resulted in a marked decline in Nox1 protein by Western blot analysis (Fig. 6D). Nox4 siRNA at 200 nm dramatically decreased Nox4 mRNA levels; however, a substantial decrease in Nox4 protein was not observed when compared with cells transfected with scrambled control (Fig. 6E).
RANKL expression in siNox1 knockdown cells was significantly decreased from that observed in cells with scrambled control (Fig. 6F) and resembled the inhibitory effect of CSF-1 on RANKL in control cells. A further decline in RANKL was not observed when CSF-1 was added to siNox1-transfected cells, providing further evidence that Nox1 mediates the effect of CSF-1. Similar results were observed when the same samples were analyzed by real-time PCR (Fig. 6G). In view of insufficient knockdown of Nox4 protein, decreased RANKL expression observed in double-transfected cells (Fig. 6B) likely reflects primarily knockdown of Nox1 protein. Our results support the hypothesis that Nox1 and, to a lesser extent, Nox4 may be downstream targets for mediating CSF-1 effect on osteoblasts (Fig. 6H). These data suggest that Nox proteins are important mediators of RANKL expression in osteoblasts.
Discussion
Our results demonstrate the first evidence of CSF-1R mRNA and protein expression by primary calvarial osteoblasts and its phosphorylation in response to CSF-1 ligand. CSF-1 inhibited RANKL expression in osteoblasts in a dose- and time-dependent manner, and in vivo, expression of sCSF-1 in transgenic mice was associated with down-regulation of RANKL protein in bone extracts. In primary bone marrow cultures, high as well as lower levels of CSF-1 decreased RANKL expression and led to decreased osteoclast marker expression. Our findings show, for the first time, that CSF-1 regulates NADPH-dependent superoxide generation and the protein expression of Nox1 and Nox4 oxidases. RNA interference studies also suggest that Nox1 and, to a lesser extent, Nox4 are involved in mediating the effect of CSF-1 on RANKL.
Identification of the CSF-1R on osteoblasts is novel and may provide an explanation for results obtained in previous studies related to the effect of CSF-1 on osteoblasts and osteoclasts. In work by Dai et al. (27), CSF-1R was not detected in calvarial cells by Western blotting; however, the osteoblast phenotype of the cells was not assessed, immunoprecipitation was not performed, and technical differences in the methodology could account, in part, for the differences in results between laboratories. Although CSF-1 is necessary for entry of precursors into the preosteoclast pathway, CSF-1 has been reported to differentially regulate osteoclast formation depending on the culture system (28,29,30,31,32,33). Treatment of human colony-forming unit-granulocyte monocyte osteoclast progenitor cultures that lack osteoblast/stromal cells with 10–50 ng/ml CSF-1 in the presence of RANKL increased osteoclast formation, whereas this effect was almost abolished by 100 ng/ml rmCSF-1 (28). In mouse cultures, addition of exogenous CSF-1 to primary bone marrow cultures that contain osteoblast/stromal cells or to cocultures of spleen cells and osteoblast cells causes concentration-dependent inhibition of osteoclastogenesis (29,30,31,33). In both primary marrow and coculture systems, CSF-1 levels much above 8.35 ng/ml were inhibitory to osteoclastogenesis (29,33). Our results showing CSF-1-mediated inhibition of VD3/DEX-induced TRACP-positive osteoclast formation are similar to reports using primary marrow cultures. In a coculture system of spleen cells and ST-2 stromal cells, Perkins and Kling (33) showed that high CSF-1 levels decreased osteoclast numbers and promoted the development of macrophages. Incubation with CSF-1 down-regulated CSF-1R expression and was associated with proliferation of mononuclear cells with decreased CSF-1R binding. In these studies, decreased osteoclastogenesis in response to high CSF-1 was attributed to a shift in progenitor cell differentiation that favors macrophage/monocytic, rather than osteoclastic, lineage. Our findings indicate that high levels of CSF-1 directly down-regulate RANKL expression in osteoblasts and, in primary bone marrow cultures, leads to decreased osteoclast formation as determined by decreased CTR expression and TRACP activity. Taken together, we propose that high levels of CSF-1 may decrease osteoclastogenesis not only by altering the CSF-1R on progenitors but also by down-regulating RANKL in osteoblasts. This is supported by our data in sCSF-1 transgenic mice. These mice have high sCSF-1 levels, and it is tempting to speculate that decreased RANKL expression in osteoblasts contributed to the preferential increase in bone formation and increased cortical bone thickness.
ROS contribute to bone remodeling because they promote osteoclast formation and activity (18,19). In our study using DPI, a flavoprotein inhibitor, we hypothesized that ROS could be an intermediary for CSF-1 effects on osteoblasts. Interestingly, DPI alone down-regulated RANKL and thereby mimicked CSF-1-mediated inhibition of RANKL. When DPI was added to CSF-1-treated cultures, inhibition of RANKL by CSF-1 was comparable to that observed with each agent alone, suggesting that CSF-1 shares a similar pathway as DPI in regulating RANKL. Results showing that H2O2 is capable of partially reversing the inhibitory effect of CSF-1 on RANKL provide further evidence that CSF-1 may mediate its effect on RANKL, at least in part, through inhibition of ROS production.
Because RANKL is an essential factor for osteoclast development and function, it is important to identify the pathways involved its regulation. DPI inhibits electron transport by NADPH oxidases as well as other flavin-containing enzymes (26). Major contributors to ROS generation that are flavoprotein-containing oxidases inhibited by DPI include respiratory complexes of the mitochondria, nitric oxide synthase, and the Nox family members. Production of superoxide anion in osteoblasts has been shown to up-regulate RANKL (20). Taken with the results observed with DPI, our data indicate that the activity of NADPH oxidases may be important for maintaining RANKL expression in osteoblasts.
Nox1, Nox2, Nox3, and Nox4 are members of the Nox family of NADPH oxidases. These enzymes transport electrons from NADPH across cell membranes to generate superoxide and downstream ROS. Nox1, Nox2, and 4 are expressed in multiple cell types including osteoclasts (34). Nox enzymes play an important role in the differentiation and function of osteoclasts and Nox-derived ROS are implicated in the pathogenesis of age-related osteoporosis (35). To date, no study examined Nox expression in osteoblasts. Our findings indicate that osteoblasts express Nox1, Nox2, and Nox4 mRNAs and proteins with Nox4 being the predominant isoform.
High concentrations of CSF-1 decreased NADPH-dependent superoxide production in osteoblasts. This observation is associated with down-regulation of Nox1 and Nox4 proteins and strongly suggests a role for these NADPH oxidases in CSF-1-mediated events. This hypothesis is supported by RNA interference experiments. In osteoblasts transfected with siRNA to Nox1, knockdown of Nox1 protein inhibited RANKL expression. Although we cannot exclude involvement of complexes of the mitochondrial respiratory chain or nitric oxide synthase, our data suggest that Nox1 NADPH oxidase is likely an important intermediary enzyme involved in mediating downstream effects of CSF-1. Whether Nox4 has a role in mediating CSF-1 effects remains to be confirmed.
Clearly, CSF-1 has complex actions that are likely dependent on its local concentration and the cell-to-cell interaction between osteoblasts and osteoclast progenitors. The dose of 100 ng/ml is high compared with circulating levels in normal adult mice (10–19 ng/ml) and closer to levels in fetal mice (40–45 ng/ml) (11,36). Significantly higher circulating levels have been reported in humans with malignancies and may exceed 100 ng/ml in advanced disease (37). Such high circulating levels could dramatically increase local levels. Interestingly, little is known about the physiological local levels of CSF-1 in bone marrow. Membrane- and matrix-associated as well as the soluble form of the cytokine are active. Local levels of CSF-1 may be significantly elevated even in the absence of high circulating levels as the turnover of circulating CSF-1 is relatively high (38). In vitro and in vivo studies suggest that high local levels are feasible and could be achieved via CSF-1 binding to cell membranes and/or extracellular matrix proteins (39,40).
In summary, our data provide the first evidence of a functional CSF-1R in osteoblasts and broadens the potential biological effect of CSF-1 on bone cells in vitro and in vivo. Thus, CSF-1 may regulate osteoclastogenesis not only directly but also indirectly through its effect on osteoblasts. High levels of CSF-1 down-regulate RANKL expression in osteoblasts and, in primary bone marrow cultures, lead to decreased osteoclast formation. Our data suggest a novel association between CSF-1, NADPH-dependent superoxide generation, and RANKL expression. We have identified specific NADPH oxidases, Nox1 and Nox4, as downstream targets of CSF-1 and Nox1 as an important mediator that contributes to RANKL expression in osteoblasts. Nox oxidases may represent targets for treatment of bone disease associated with high bone turnover including osteoporosis. In the context of bone physiology, CSF-1-mediated inhibition of RANKL expression in osteoblasts may provide an important mechanism for preventing excessive osteoclastogenesis during normal skeletal growth. Moreover, high levels of CSF-1, reported at fracture sites (41) and in several tumors (42) may be a key determinant of bone remodeling in fracture repair and metastatic bone disease.
Supplementary Material
Acknowledgments
We thank Dr. Karen Block, Dr. Denis Feliers, Andrea Barrentine, and Bridget Fagg for helpful advice and Dr. Vivienne Rebel for performing FACS analysis. We also thank the Core Optical Imaging Facility at University of Texas Health Science Center, San Antonio, for confocal work.
Footnotes
This work was supported by funding from the National Institutes of Health (AR-42306 to S.L.A.-W.) and Veteran’s Administration Merit Award (S.L.A.-W.).
Disclosure Summary: The authors of this manuscript have nothing to declare.
First Published Online October 9, 2009
Abbreviations: CSF-1, Macrophage colony-stimulating factor; CSF-1R, c-fms receptor; DEX, dexamethasone; DPI, diphenyleneiodonium chloride; FCS, fetal calf serum; NAD(P)H, reduced nicotinamide adenine dinucleotide (phosphate); RANKL, receptor activator of nuclear factor-κB ligand; rmCSF-1, recombinant murine CSF-1; ROS, reactive oxygen species; sCSF-1, soluble CSF-1; SF, serum free; siRNA, small interfering RNA; TRACP, tartrate-resistant acid phosphatase; VD3, 1,25-dihydroxyvitamin D3; wt, wild type.
References
- Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D 2004 The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 15:457–475 [DOI] [PubMed] [Google Scholar]
- Bartocci A, Pollard JW, Stanley ER 1986 Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med 164:956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JW, Bartocci A, Arceci R, Orlofsky A, Ladner MB, Stanley ER 1987 Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature 330:484–486 [DOI] [PubMed] [Google Scholar]
- Inaba T, Yamada N, Gotoda T, Shimano H, Shimada M, Momomura K, Kadowaki T, Motoyoshi K, Tsukada T, Morisaki N 1992 Expression of M-CSF receptor encoded by c-fms on smooth muscle cells derived from arteriosclerotic lesion. J Biol Chem 267:5693–5699 [PubMed] [Google Scholar]
- Pollard JW 1997 Role of colony-stimulating factor-1 in reproduction and development. Mol Reprod Dev 46:54–60; discussion 60–61 [DOI] [PubMed] [Google Scholar]
- Mori T, Bartocci A, Satriano J, Zuckerman A, Stanley R, Santiago A, Schlondorff D 1990 Mouse mesangial cells produce colony-stimulating factor-1 (CSF-1) and express the CSF-1 receptor. J Immunol 144:4697–4702 [PubMed] [Google Scholar]
- Hofstetter W, Wetterwald A, Cecchini MC, Felix R, Fleisch H, Mueller C 1992 Detection of transcripts for the receptor for macrophage colony-stimulating factor, c-fms, in murine osteoclasts. Proc Natl Acad Sci USA 89:9637–9641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EC, Horowitz MC, Baron R, Centrella M, Kacinski BM, Insogna KL 1993 Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res 8:1507–1518 [DOI] [PubMed] [Google Scholar]
- Gyda M, Corisdeo S, Zaidi M, Troen BR 2001 Macrophage colony-stimulating factor suppresses osteoblast formation. Biochem Biophys Res Commun 285:328–334 [DOI] [PubMed] [Google Scholar]
- Wisner-Lynch LA, Shalhoub V, Marks Jr SC 1995 Administration of colony stimulating factor-1 to toothless osteopetrotic rats normalizes osteoblast, but not osteoclast, gene expression. Bone 16:611–618 [DOI] [PubMed] [Google Scholar]
- Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER 2002 Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 99:111–120 [DOI] [PubMed] [Google Scholar]
- Yeung YG, Stanley ER 2003 Proteomic approaches to the analysis of early events in colony-stimulating factor-1 signal transduction. Mol Cell Proteomics 2:1143–1455 [DOI] [PubMed] [Google Scholar]
- Dröge W 2002 Free radicals in the physiological control of cell function. Physiol Rev 82:47–95 [DOI] [PubMed] [Google Scholar]
- Nathan C 2003 Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest 111:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR 1990 Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS 2006 Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res 21:1003–1011 [DOI] [PubMed] [Google Scholar]
- Suda N, Morita I, Kuroda T, Murota S 1993 Participation of oxidative stress in the process of osteoclast differentiation. Biochim Biophys Acta 1157:318–323 [DOI] [PubMed] [Google Scholar]
- Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH 2004 Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127 [DOI] [PubMed] [Google Scholar]
- Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY 2005 A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859 [DOI] [PubMed] [Google Scholar]
- Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ 2005 Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J Biol Chem 280:17497–17506 [DOI] [PubMed] [Google Scholar]
- Harris SE, Bonewald LF, Harris MA, Sabatini M, Dallas S, Feng JQ, Ghosh-Choudhury N, Wozney J, Mundy GR 1994 Effects of transforming growth factor β on bone nodule formation and expression of bone morphogenetic protein 2, osteocalcin, osteopontin, alkaline phosphatase, and type I collagen mRNA in long-term cultures of fetal rat calvarial osteoblasts. J Bone Miner Res 9:855–863 [DOI] [PubMed] [Google Scholar]
- Ek-Rylander B, Barkhem T, Ljusberg J, Ohman L, Andersson KK, Andersson G 1997 Comparative studies of rat recombinant purple acid phosphatase and bone tartrate-resistant acid phosphatase. Biochem J 321(Pt 2):305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin Y, Block K, Hernandez J, Bhandari B, Wagner B, Barnes JL, Abboud HE 2005 Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem 280:39616–39626 [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE 2003 Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol 285:F219–F229 [DOI] [PubMed] [Google Scholar]
- Abboud SL, Ghosh-Choudhury N, Liu LC, Shen V, Woodruff K 2003 Osteoblast-specific targeting of soluble colony-stimulating factor-1 increases cortical bone thickness in mice. J Bone Miner Res 18:1386–1394 [DOI] [PubMed] [Google Scholar]
- Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause KH 2007 NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J 406:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XM, Zong XH, Akhter MP, Stanley ER 2004 Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone. J Bone Miner Res 19:1441–1451 [DOI] [PubMed] [Google Scholar]
- Hodge JM, Kirkland MA, Nicholson GC 2007 Multiple roles of M-CSF in human osteoclastogenesis. J Cell Biochem 102:759–768 [DOI] [PubMed] [Google Scholar]
- Fan X, Biskobing DM, Fan D, Hofstetter W, Rubin J 1997 Macrophage colony stimulating factor down-regulates MCSF-receptor expression and entry of progenitors into the osteoclast lineage. J Bone Miner Res 12:1387–1395 [DOI] [PubMed] [Google Scholar]
- Shinar DM, Sato M, Rodan GA 1990 The effect of hemopoietic growth factors on the generation of osteoclast-like cells in mouse bone marrow cultures. Endocrinology 126:1728–1735 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Udagawa N, Akatsu T, Tanaka H, Isogai Y, Suda T 1991 Deficiency of osteoclasts in osteopetrotic mice is due to a defect in the local microenvironment provided by osteoblastic cells. Endocrinology 128:1792–1796 [DOI] [PubMed] [Google Scholar]
- van de Wijngaert FP, Tas MC, van der Meer JW, Burger EH 1987 Growth of osteoclast precursor-like cells from whole mouse bone marrow: inhibitory effect of CSF-1. Bone Miner 3:97–110 [PubMed] [Google Scholar]
- Perkins SL, Kling SJ 1995 Local concentrations of macrophage colony-stimulating factor mediate osteoclastic differentiation. Am J Physiol 269:E1024–E1030 [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH 2007 The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- Krause KH 2007 Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 42:256–262 [DOI] [PubMed] [Google Scholar]
- Roth P, Stanley ER 1996 Colony stimulating factor-1 expression is developmentally regulated in the mouse. J Leukocyte Biol 59:817–823 [DOI] [PubMed] [Google Scholar]
- Kacinski BM 1997 CSF-1 and its receptor in breast carcinomas and neoplasms of the female reproductive tract. Mol Reprod Dev 46:71–74 [DOI] [PubMed] [Google Scholar]
- Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER 1987 Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci USA 84:6179–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot Y, Fixe P, Trimoreau F, Praloran V 1996 Macrophage colony-stimulating factor levels in the plasma of bone marrow aspirate in several hematological malignancies. Stem cells 14:363–365 [DOI] [PubMed] [Google Scholar]
- Ohtsuki T, Suzu S, Hatake K, Nagata N, Miura Y, Motoyoshi K 1993 A proteoglycan form of macrophage colony-stimulating factor that binds to bone-derived collagens and can be extracted from bone matrix. Biochem Biophys Res Commun 190:215–222 [DOI] [PubMed] [Google Scholar]
- Kon T, Cho TJ, Aizawa T, Yamazaki M, Nooh N, Graves D, Gerstenfeld LC, Einhorn TA 2001 Expression of osteoprotegerin, receptor activator of NF-κB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 16:1004–1014 [DOI] [PubMed] [Google Scholar]
- Lo AS, Taylor JR, Farzaneh F, Kemeny DM, Dibb NJ, Maher J 2008 Harnessing the tumor-derived cytokine, CSF-1, to co-stimulate T-cell growth and activation. Mol Immunol 45:1276–1287 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.