Abstract
Background:
The prevalence of pneumothorax associated with travel in patients with interstitial lung diseases is unknown. In patients with lymphangioleiomyomatosis (LAM), in whom pneumothorax is common, patients are often concerned about the occurrence of a life-threatening event during air travel. The aim of this study was to determine the prevalence of pneumothorax associated with air travel in patients with LAM, idiopathic pulmonary fibrosis (IPF), and sarcoidosis.
Methods:
Records and imaging studies of 449 patients traveling to the National Institutes of Health were reviewed.
Results:
A total of 449 patients traveled 1,232 times; 299 by airplane (816 trips) and 150 by land (416 trips). Sixteen of 281 LAM patients arrived at their destination with a pneumothorax. In 5 patients, the diagnosis was made by chest roentgenogram, and in 11 patients by CT scans only. Of the 16 patients, 14 traveled by airplane and 2 by land. Seven of the 16 patients, 1 of whom traveled by train, had a new pneumothorax; 9 patients had chronic pneumothoraces. A new pneumothorax was more likely in patients with large cysts and more severe disease. The frequency of a new pneumothorax for LAM patients who traveled by airplane was 2.9% (1.1 per 100 flights) and by ground transportation, 1.3% (0.5 per 100 trips). No IPF (n = 76) or sarcoidosis (n = 92) patients presented with a pneumothorax.
Conclusions:
In interstitial lung diseases with a high prevalence of spontaneous pneumothorax, there is a relatively low risk of pneumothorax following air travel. In LAM, the presence of a pneumothorax associated with air travel may be related to the high incidence of pneumothorax and not to travel itself.
A concern of patients with cystic or bullous lung diseases, especially those associated with spontaneous pneumothorax, is its occurrence during air travel, which could be life threatening.1 Frequently, because of fear of pneumothorax, patients are reluctant to travel, especially by airplane, which may have a substantial impact on quality of life. Because cabins of commercial aircraft are pressurized only to a barometric pressure equivalent to an altitude of 1,524 to 2,438 m, during the airplane's ascent the cabin pressure falls, and according to the Boyle law, gas expands within body cavities including lung cysts or nonfunctioning bullae.1 This change in volume poses a potential risk of pneumothorax for patients with interstitial lung diseases.
Few data are available regarding the prevalence of pneumothorax associated with traveling in patients with interstitial lung diseases. In the case of lymphangioleiomyomatosis (LAM), a disorder affecting women that is characterized by cystic lung destruction, lymphatic abnormalities, and abdominal tumors (eg, angiomyolipomas),2–6 registry data6 indicate that 4.8% of patients with a history of pneumothorax experienced one episode related to air travel. A mail survey of 276 LAM patients who traveled by plane 454 times revealed 10 events of pneumothorax, 8 of which were confirmed by chest roentgenogram.7 Five of the ten patients had symptoms suggestive of pneumothorax prior to the flights; overall, the incidence of pneumothorax was 2.2 per 100 flights and 4 episodes per 100 patients.
Since 1995, patients with interstitial lung diseases, including LAM, idiopathic pulmonary fibrosis (IPF), and sarcoidosis, traveled to the National Institutes of Health (NIH) to participate in research studies. The majority of patients traveled by airplane, from within the United States, Canada, and other parts of the world. Patients underwent chest roentgenograms on hospital admission, and most underwent a CT scan of the thorax on the following day. The purpose of our study was to determine the prevalence of pneumothorax associated with travel in patients with LAM, IPF, and sarcoidosis.
Materials and Methods
Study Population
The population comprised 281 subjects with LAM (National Heart, Lung, and Blood Institute [NHLBI] protocol 95-H-0186), 76 patients with IPF (NHLBI protocols 99-H-0056, 99-H-0068, and 04-H-0211), and 92 patients with sarcoidosis (NHLBI protocols 82-H-0032, 96-H-0100, and 06-H-0072) who were admitted to the NIH Clinical Research Center, a research hospital where patients are enrolled in research protocols. Protocols allowed us to perform CT scans and chest roentgenograms, and to publish findings. The chair of the institutional review board indicated that additional consent was not required because patients were aware that imaging data would be used for research purposes. Patients traveled to the NIH Clinical Research Center between 1999 and 2008. Patient participation was approved by the institutional review board of the NHLBI; written consent was obtained from all subjects.
Study Design
Records were reviewed to determine whether there was evidence (chest roentgenogram and/or CT scan) of a pneumothorax. Information was collected regarding mode of travel, grade of disease severity, lung cyst size (CT scan), lung function, and history of pneumothorax and pleurodesis.
Chest Roentgenograms and CT Scans
Radiologic studies were reviewed by a radiologist and a pulmonologist, who were both blinded to subject travel status. For LAM and IPF patients, the severity of lung disease and cyst size were graded semiquantitatively by CT scans, as previously described.8,9 Patients who had 1 to 10 cysts were placed in the category of minimal disease (grade 0). If > 10 cysts were identified, the lungs were divided in three zones (upper, middle, and lower). The extent of involvement in each of the zones was graded according to the percentage of the volume judged abnormal using the following scale: grade I, < 30% abnormal lung; grade II, 30 to 60% abnormal; and grade III, > 60% abnormal. CT scans were also assigned scores according to the average cyst size, as follows: size I < 0.5 cm; size II, 0.5 to 1.0 cm, and size III, > 1.0 cm. For sarcoidosis, the roentgenologic severity of disease was graded, as is common, from 0 to IV.10
Pulmonary Function Tests
Lung volumes, flow rates, and single-breath diffusing capacity of the lung for carbon monoxide (Dlco) were measured (V̇max 229; SensorMedics; Yorba Linda, CA), according to the American Thoracic Society recommendations.11–13
Statistical Analysis
The rate of lung function declines over time for patients with LAM, in whom serial lung function data obtained throughout several years were available, was calculated as previously reported.14 Because in LAM patients the most useful pulmonary function tests in assessing disease severity and progression are for FEV1 and Dlco,14 data analysis is restricted to these two parameters. The Student t test was employed to compare data sets. Analysis of variance (ANOVA) was employed to evaluate lung function data for groups of study subjects. All p values reported are two sided, and the data are shown as the mean ± SEM.
Results
Demographics
Table 1 shows the clinical and physiologic characteristics of the 449 patients. All LAM patients were female; 240 were white, 18 were Asian American, 17 were African American, 4 were Hispanic, and 2 were from Pacific islands. The diagnosis of LAM was established by tissue biopsy in 169 patients and CT scan findings in 112 patients. One hundred forty patients had a history of pneumothorax, and 122 had undergone pleurodesis.
Table 1.
Clinical and Physiologic Data of 449 Patients With Interstitial Lung Diseases
| Variables | LAM | IPF | Sarcoidosis |
|---|---|---|---|
| Patients, No. | 281 | 76 | 92 |
| Age, yr | 47.7 ± 0.6 | 62.7 ± 1.1 | 47.9 ± 0.9 |
| Gender, No. | |||
| Female | 281 | 23 | 36 |
| Male | 0 | 53 | 56 |
| TLC, % predicted | 96.1 ± 0.9 | 71.5 ± 2.1 | 86.0 ± 1.5 |
| FRC, % predicted | 98.8 ± 1.4 | 69.5 ± 2.2 | 79.8 ± 1.8 |
| RV, % predicted | 114.9 ± 2.1 | 70.1 ± 2.4 | 86.3 ± 2.4 |
| RV/TLC, % | 50.0 ± 1.0 | 33.9 ± 0.8 | 31.7 ± 0.7 |
| FVC, % predicted | 86.3 ± 1.1 | 73.9 ± 2.3 | 89.0 ± 1.8 |
| FEV1, % predicted | 70.5 ± 1.6 | 85.2 ± 2.4 | 91.9 ± 2.2 |
| FEV1/FVC, % | 60.0 ± 1.0 | 82.6 ± 0.8 | 77.2 ± 0.9 |
| Dlco, % predicted | 66.6 ± 1.5 | 58.2 ± 2.4 | 73.7 ± 2.2 |
| Ground trips, No. | 206 | 89 | 121 |
| Air trips, No. | 536 | 159 | 121 |
| Pneumothorax, No. | 16 | 0 | 0 |
Data are presented as the means ± SEM, unless otherwise indicated. Pulmonary function data, except RV/TLC and FEV1/FVC ratios, are shown as percent predicted values. FRC = functional residual capacity; RV = residual volume; TLC = total lung capacity.
Twenty-three of the IPF patients were female and 53 were male. Sixty-seven were white, four were Hispanic, four were Asian, and two were African American. The diagnosis of IPF was made by biopsy in 62 patients and in 14 by clinical and radiographic data. No patient had a history of pneumothorax.
Fifty-six of the 92 patients with sarcoidosis were male, and 36 were female. Thirty-seven patients were white, 1 was Hispanic, and 54 were African American. The diagnosis of sarcoidosis was made by lung, lymph node, or skin biopsy in 88 patients, and by clinical and radiologic data in 4 patients. No patient had a history of pneumothorax.
Mode of Travel
The 449 patients traveled to the NIH 1,232 times; 299 patients traveled by airplane for a total of 816 trips, and 200 traveled by ground for a total of 416 trips. Of the 816 flights, 9 were from Europe, 11 were from Central and South America, 4 were from the Middle East, 6 were from Pacific islands, 6 were from Alaska, 62 were from Canada, and 718 were from within the United States.
The 281 LAM patients traveled to the NIH 742 times; 77 patients by ground for a total of 206 trips, and 204 patients by airplane for a total of 536 flights. For the patients with IPF, there were a total of 248 trips; 47 of the 76 patients traveled by airplane (159 trips), and 29 traveled by ground (89 trips). For the sarcoidosis cohort, there were a total of 242 visits; 48 traveled by airplane (121 trips), and 44 chose ground travel (121 trips). There was no significant difference in lung function between those who traveled by ground and those who chose air transportation.
Severity of Lung Disease
LAM:
Pulmonary function test results are shown in Table 1. Severe disease (CT scan grade III) was present in 80 patients; 61 patients had moderate disease (CT scan grade II), 125 patients had mild disease (CT scan grade I), and 15 patients had minimal disease (CT scan grade 0); 43 patients had large cysts, 126 patients had moderate cyst-size lesions, and 112 patients had small cysts.
IPF:
Lung function data are shown on Table 1. CT scans showed that 15 patients had CT scan grade III disease, 25 had grade II disease, 33 had grade I disease, and 3 had grade 0 disease. Fifteen of the 76 patients had lung cysts > 1 cm in diameter.
Sarcoidosis:
Lung function data are shown in Table 1. Radiologic data showed that 14 patients had stage IV sarcoidosis, 24 patients had stage III sarcoidosis, 28 patients had stage II sarcoidosis, 17 patients had stage I sarcoidosis, and 9 patients had stage 0 sarcoidosis.10
Prevalence of Pneumothorax
LAM:
Pneumothorax was diagnosed in 16 patients, 5 by chest roentgenograms and 11 by CT scans only. Of the 16 patients in whom pneumothorax was diagnosed, 14 had traveled by airplane and 2 by ground transportation. In 9 of the 16 patients, the pneumothorax preceded the study visits. Seven patients, one of whom traveled by car, had evidence of a pneumothorax that was not present at the previous visit. The six patients who traveled by airplane had flights with a mean distance of 3,092 ± 845 miles from Pakistan, Ecuador, Arkansas, California, Texas, and Idaho. In three of the seven patients, the diagnosis was made by chest roentgenogram and in the remaining four by CT scan. In no patient could we ascertain whether the pneumothorax occurred during the trip; patients did not relate respiratory symptoms that suggested the presence of a pneumothorax. Based on these data, we estimated that the frequency of a new pneumothorax, potentially related to flying, in a LAM population of 204 patients was 2.9% (6 of 204 patients). Pneumothorax was observed in 1.1% of 536 flights. For the 77 patients who traveled by car or train, the corresponding figure was 1.3% (1 of 77 patients). Pneumothorax was observed in 0.5% of 206 ground trips. Three of the seven patients with a new pneumothorax required subsequent pleurodesis. In three of the remaining patients, the pneumothorax resolved spontaneously; in the other patient, it was not treated and became chronic.
Of the nine patients with chronic pneumothorax, eight traveled by airplane. We reviewed their CT scans (mean, 3.2 ± 0.5 CT scans per patient) before and after enrollment in the current study. We found that the pneumothoraces had been present for a mean duration of 2.2 ± 0.3 years prior to study enrollment (Table 2). We estimated the volume of the pneumothoraces, assuming they were ellipsoidal in shape and found that during mean duration of 4.4 ± 0.4 years of follow-up, the pneumothoraces did not increase significantly (82 ± 40 to 107 ± 52 mL; p = 0.702).
Table 2.
Characteristics of 16 LAM Patients With Pneumothorax
| Patient No. | CT Scan Score | Cyst Size | Prior Pneumothorax /Pleurodesis | Type of Pneumothorax* | Mode of Travel | Oxygen Use |
|---|---|---|---|---|---|---|
| 1 | 3 | III | Yes/Yes | Chronic (3) | Train | Yes |
| 2 | 1 | II | Yes/Yes | Chronic (3.5) | Airplane | No |
| 3 | 1 | III | Yes/Yes | New | Airplane | No |
| 4 | 2 | III | Yes/Yes | Chronic (2) | Airplane | Yes |
| 5 | 0 | III | Yes/Yes | New | Airplane | No |
| 6 | 1 | III | Yes/Yes | Chronic (4) | Airplane | No |
| 7 | 1 | III | Yes/Yes | Chronic (2) | Airplane | No |
| 8 | 2 | III | Yes/Yes | Chronic (2) | Airplane | Yes |
| 9 | 0 | III | Yes/Yes | New | Airplane | No |
| 10 | 1 | III | Yes/Yes | New | Car | No |
| 11 | 2 | III | Yes/Yes | New | Airplane | Yes |
| 12 | 1 | III | Yes/Yes | New | Airplane | Yes |
| 13 | 1 | III | Yes/Yes | Chronic (2.5) | Airplane | No |
| 14 | 3 | III | Yes/Yes | New | Airplane | Yes |
| 15 | 1 | III | Yes/No | Chronic (1) | Airplane | No |
| 16 | 1 | II | No/No | Chronic (1) | Airplane | No |
CT scan: grade 0, 1 to 10 lung cysts; grade I, < 30% abnormal lung; grade II, 30 to 60% abnormal; grade III, > 60% abnormal. Average cyst size: I, < 0.5 cm; II, 0.5 to 1.0 cm; III, > 1.0 cm.
*The duration of chronic pneumothoraces (in years) before patients traveled to the NIH is shown in parentheses.
IPF and Sarcoidosis:
No patient with IPF or sarcoidosis had a pneumothorax at the time of admission to the NIH Clinical Research Center.
Characteristics of the LAM patients With Pneumothorax:
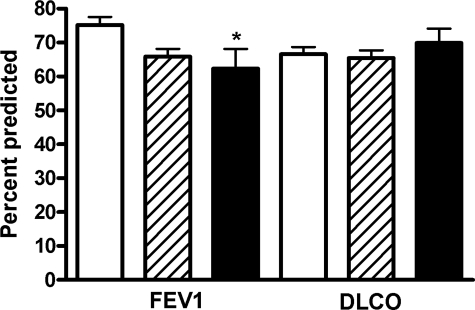
A history of pneumothorax was present in 15 of the 16 patients; 14 had previously undergone pleurodesis (Table 2). There was a trend for FEV1 to be lower in patients who presented with a pneumothorax (acute and chronic) [62 ± 6%] and in patients with a history of pneumothorax (66 ± 2%) than in those without a history of pneumothorax (75 ± 2%). However, as shown in Figure 1, only the difference in FEV1 between those who had a pneumothorax and those who never had one was statistically significant (p < 0.01 [by ANOVA]). There were no differences in Dlco among the three groups of patients (Fig 1). The rate of FEV1 decline tended to be greater (121 ± 21 mL/yr) in patients with pneumothorax than that in patients without pneumothorax (80 ± 7 mL/yr), but the difference was not statistically significant. Fourteen of the 16 patients with pneumothorax had predominantly size III cysts (Table 2).
Figure 1.
Percent predicted FEV1 and Dlco in LAM patients without a history of pneumothorax (white columns), patients who had prior pneumothorax (crosshatched columns), and patients who presented with pneumothorax (black columns). Patients presenting with a pneumothorax had significantly lower FEV1 (* = p < 0.01 by ANOVA) than patients who never had experienced a pneumothorax.
Discussion
In this retrospective study of 449 patients with interstitial lung diseases who traveled to the NIH from within the United States, Canada, and other parts of the world, we found evidence of recent pneumothorax potentially associated with travel only in patients with LAM. Among 281 LAM patients, 7 had evidence of a new pneumothorax. However, because pretravel radiologic imaging was not available and none of the seven patients with a pneumothorax experienced symptoms of dyspnea or chest pain during or after travel, it was not possible to determine whether or not the pneumothorax occurred prior to or during traveling. That is, a relationship between the development of the pneumothorax and travel could not be established. A second limitation of our study is that a selection bias may have occurred because patients with more severe disease, potentially at a greater risk for the development of a pneumothorax, may have avoided enrollment into our protocols. However, the patients whom we identified with pneumothorax had degrees of lung disease severity ranging from minimal to severe (Table 2).
Assuming the pneumothoraces were caused by the air travel, the frequency of such an event was low (1.1 per 100 flights and 2.9 per 100 patients). A common denominator among these patients was a history of pneumothorax; 15 patients had a history of pneumothorax, confirming that such a history appears to be a risk for subsequent pneumothorax.15
The fact that many of our patients had prior pleurodesis may have lowered the risk for pneumothorax and influenced our results. However, Chu et al3 found that 19 of 24 patients with pneumothorax had previously undergone pleurodesis. Also, Almoosa et al15 found that chemical and surgical pleurodesis, although reducing the recurrence of pneumothorax, were still associated with a recurrence rate of 27% and 32%, respectively.
In contrast with the patients with LAM, none of the patients with IPF or sarcoidosis presented with pneumothorax. This may reflect the fact that compared to LAM, spontaneous pneumothorax is uncommon in IPF and sarcoidosis. Indeed, McLoud et al16 reported a 7.4% prevalence of pneumothorax in 95 patients with IPF, whereas in patients with sarcoidosis, pneumothorax occurred in only 2.5 to 2.7% of the patients.17–19
Our study is important because it is the first one conducted in patients with interstitial lung diseases in which the frequency of pneumothorax associated with traveling, especially flying, included the review of chest radiographs and CT scans. We are not aware of prior publications where the prevalence of pneumothorax during air travel has been radiologically evaluated in patients with either sarcoidosis or IPF. Further, a prior publication7 addressing this issue in LAM patients was a mail survey, not an actual review of chest radiographs and CT scans.
Our data may be helpful to patients who are considering air travel and assist physicians in counseling such patients. Indeed, we believe that patients with LAM may safely travel by airplane provided they have no clinical or radiologic evidence of pneumothorax or a history of recent pneumothorax. The relatively low risk of pneumothorax associated with air travel may be even lower in those patients with small lung cysts, mild disease, and no history of pneumothorax. In addition, the possibility that the association between flying and the development of a pneumothorax may be coincidental cannot be discounted. That is, patients with a history of recurrent pneumothorax are more likely to have them, regardless of traveling by any route or not traveling at all.
In agreement with prior observations,9 we found that LAM patients who had pneumothoraces tend to have lower FEV1, and a CT scan pattern consisting of larger cysts scattered throughout relatively normal lung parenchyma. This suggests that the presence of larger cysts predisposes patients both to pneumothorax and greater rates of FEV1 decline. However, the possibility that a lower FEV1 is directly related to the pneumothorax cannot be excluded.
Finally, an interesting finding of our study was the observation that the size of chronic loculated pneumothoraces did not seem to increase progressively with air travel. This is consistent with the report by Currie et al20 of two patients with chronic pneumothoraces who were stable for at least 1 year. Although these are limited data, they do suggest that the presence of a chronic stable pneumothorax in patients with LAM may not pose additional risks for air travel. Further follow-up with additional patients is warranted. A different situation would be that of a patient with a recent pneumothorax. The complete resolution of a pneumothorax for at least a week prior to flying is recommended.1 In patients with LAM, however, our study shows that the presence, or the complete absence, of a pneumothorax cannot be ascertained by a chest roentgenogram. It is imperative, as illustrated by the report of Pollock-BarZiv et al,7 that patients with symptoms suspicious for pneumothorax undergo appropriate radiologic testing before being approved for air travel. This may require a CT scan of the thorax.
Acknowledgments
Author contributions: Drs. Taveira-DaSilva and Moss were responsible for writing the manuscript. Dr. Burstein and Ms. Hathaway collected and organized the demographic data for the LAM patients. Drs. Fontana and Gochuico performed the studies and organized the data for the sarcoidosis and IPF patients, respectively. Dr. Avila read the CT scans of the patients.
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: The authors thank Dr. Martha Vaughan for discussions and review of the manuscript. The authors also thank the LAM Foundation and the Tuberous Sclerosis Alliance for their assistance in recruiting patients. This study would not have been possible without the cooperation of patients with IPF, sarcoidosis, or LAM, who in many cases traveled great distances to participate in our clinical research protocols.
Abbreviations:
- ANOVA
analysis of variance
- Dlco
diffusing capacity of the lung for carbon monoxide
- IPF
idiopathic pulmonary fibrosis
- LAM
lymphangioleiomyomatosis
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
Footnotes
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute and the National Human Genome Research Institute, National Institutes of Health.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
For editorial comment see page 655
References
- 1.British Thoracic Society Standards of Care Committee. Managing passengers with respiratory disease planning air travel: British Thoracic Society recommendations. Thorax. 2002;57:289–304. doi: 10.1136/thorax.57.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitaichi M, Nishimura K, Itoh H, et al. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am J Respir Crit Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 3.Chu SC, Horiba K, Usuki J, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 4.Urban TJ, Lazor R, Lacronique J, et al. Pulmonary lymphangioleiomyomatosis: a study of 69 patients. Medicine. 1999;78:321–337. doi: 10.1097/00005792-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Johnson S. Lymphangioleiomyomatosis. Eur Respir J. 2006;27:1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 6.Ryu JH, Moss J, Beck GJ, et al. The NHLBI Lymphangioleiomyomatosis Registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollock-BarZiv S, Cohen MM, Downey GP, et al. Air travel in women with lymphangioleiomyomatosis. Thorax. 2007;62:176–180. doi: 10.1136/thx.2006.058537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avila NA, Chen CC, Chu SC, et al. Pulmonary lymphangioleiomyomatosis: correlation of ventilation-perfusion scintigraphy, chest radiography, and CT with pulmonary function tests. Radiology. 2000;214:441–446. doi: 10.1148/radiology.214.2.r00fe41441. [DOI] [PubMed] [Google Scholar]
- 9.Steagall WK, Glasgow CG, Hathaway OM, et al. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Cell Mol Physiol. 2007;93:L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 10.Mihailovic-Vucinic V, Jovanovic D. Pulmonary sarcoidosis. Clin Chest Med. 2008;29:459–473. doi: 10.1016/j.ccm.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique; 1995 update. Am Rev Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 14.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, et al. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 15.Almoosa KF, Ryu JH, Mendez J, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 16.McLoud TC, Carrington CB, Gaensler EA. Diffuse infiltrative lung disease: a new scheme for description. Radiology. 1983;149:353–363. doi: 10.1148/radiology.149.2.6622676. [DOI] [PubMed] [Google Scholar]
- 17.Sharma OP. Sarcoidosis: unusual manifestations. Postgrad Med. 1977;61:67–73. doi: 10.1080/00325481.1977.11712149. [DOI] [PubMed] [Google Scholar]
- 18.Rockoff SD, Rohatgi PK. Unusual manifestations of sarcoidosis. AJR Am J Roentgenol. 1985;144:513–528. doi: 10.2214/ajr.144.3.513. [DOI] [PubMed] [Google Scholar]
- 19.Frouarakis ME, Bouros D, Voulodaki A, et al. Pneumothorax as a first manifestation of sarcoidosis. Chest. 1997;112:278–280. doi: 10.1378/chest.112.1.278. [DOI] [PubMed] [Google Scholar]
- 20.Currie GP, Kennedy A-M, Paterson E, et al. A chronic pneumothorax and fitness to fly. Thorax. 2007;62:187–189. doi: 10.1136/thx.2004.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]