Abstract
Background:
When designing multicenter clinical trials, it is important to understand the characteristics of children who have received ventilation in PICUs.
Methods:
This study involved the secondary analysis of an existing data set of all children intubated and mechanically ventilated from 16 US PICUs who were initially screened for a multicenter clinical trial on pediatric acute lung injury (ALI).
Results:
A total of 12,213 children between 2 weeks and 18 years of age who were intubated and mechanically ventilated were included, representing 30% of PICU admissions (center range, 20 to 64%). Of the children who received ventilation, 22% had cyanotic congenital heart disease; 26% had respiratory failure but not bilateral pulmonary infiltrates on chest radiograph; 8% had chronic respiratory disease; 7% had upper airway obstruction; and 5% had reactive airway disease. At least 1,457 patients (15%) with respiratory failure lacked an arterial line. Of these patients, 97% had a positive end-expiratory pressure ≤ 8 cm H2O, and 80% were supported on an Fio2 of ≤ 0.40. Moreover, 104 of 904 patients (12%) with pulse oximetric saturation (Spo2) and Fio2 measurements available would have met the oxygenation criteria for ALI according to Spo2/Fio2 ratio criteria.
Conclusions:
At least 30% of children in a cross-section of US PICUs are endotracheally intubated, and 25% of those with respiratory failure do not fulfill the radiographic criteria for ALI. Although few patients without an indwelling arterial line require more than modest ventilator support, many may still meet the oxygenation criteria for ALI. These findings will facilitate sample size calculations and help to determine feasibility for future trials on pediatric mechanical ventilation.
Mechanical ventilation is used routinely in PICUs, with 30 to 64% of patients admitted to the PICU requiring ventilator support.1 Although relatively ubiquitous in its use, the reasons for mechanical ventilation and management strategies vary, depending not only on disease state, but also on PICU size, location, time of year, and patient population served.2
Although this heterogeneity represents the diverse genetic, demographic, socioeconomic, and severity of illness makeup of PICUs, it makes designing randomized trials with ventilated patients challenging. In a time when multicenter trials are paramount for the evaluation of the generalizability and efficacy of new strategies or treatment modalities, information about the reality of current practice is needed. Understanding the epidemiology of diagnoses leading to the use of mechanical ventilation as well as the current management practices will facilitate the accurate assessment and feasibility of patient recruitment, and provide data for calculations for multicenter trials on children who are mechanically ventilated.
Designing trials for children with lung injury poses additional challenges. The 1994 American European Consensus Conference on Acute Lung Injury and ARDS3 created uniform guidelines for the diagnosis of both conditions; however, the guidelines require an invasive measure of Pao2 to calculate a Pao2/Fio2 ratio (PF). Prior to the now routine use of pulse oximetry and capnography, frequent arterial blood gas sampling was the norm for patients with respiratory failure. Although arterial sampling is still often used for patients who may need hemodynamic support or who are receiving significant amounts of supplemental oxygen, indwelling arterial lines are being used less often.4–8 As such, we hypothesize that a large subset of patients would otherwise meet the criteria for acute lung injury (ALI) but do not have an arterial line or arterial blood gas measurements available to compute a PF.
The purpose of this study was to describe the characteristics of children receiving mechanical ventilation in a cross-section of 16 US PICUs. We also report the prevalence of different disease states,9 the degree of oxygenation support, and the relationship between arterial catheters and Pao2, which is a necessary diagnostic criteria for studies on ALI or ARDS.4 This information will guide sample size estimates and determine the feasibility for future trials on pediatric mechanical ventilation.
Materials and Methods
We conducted a review of data collected from patients screened for a prospective multicenter trial10 on prone positioning for children with ALI. Screening took place over a 3-year period between May 2001 and April 2004. All patients between 2 weeks and 18 years of age who were intubated and mechanically ventilated in 16 US PICUs were included in the data set. Specific information about the presence or absence of diagnostic criteria for ALI or ARDS was gathered for each patient. For a subset of patients who did not meet the criteria for ALI or ARDS or who had a contraindication to being placed prone, additional diagnostic information was available. In addition, for a subgroup of patients who did not have an arterial line for computation of the PF, information on Fio2, positive end-expiratory pressure (PEEP), and pulse oximetric saturation (Spo2) was available. When Spo2 was ≤ 97% and Fio2 was available, an Spo2/Fio2 ratio (SF) was calculated.
For all 16 PICUs, center type, location, month, and year of PICU admission were available. Eight of the centers provided information regarding the total number of patients admitted to the PICU during the study period, which served as the denominator for estimating the overall incidence of mechanical ventilation (Table 1). The institutional review board at Childrens Hospital Los Angeles (Los Angeles, CA) approved the study.
Table 1.
Subgroups of Data Available
| Variables | Patients, Total No. | Sites Providing Data |
|---|---|---|
| Dates of ICU admission | 12,213 | 16 |
| Met age criteria | 11,536 | 16 |
| ICU admissions, No. | 5,385 | 8 |
| Non-ALI diagnosis | 8,225 | 16 |
| Lack of arterial blood gas measurements | 1,457 | 16 |
| Fio2 | 1,052 | 15 |
| Spo2 | 904 | 15 |
| PEEP | 840 | 12 |
Centers were required to report primary but not all exclusion criteria for the prone-position study. As such, this table displays the total number of patients for which the variables of interest were available and how many centers provided this information.
Statistical analysis was performed using several statistical software packages (Statistica, version 5.5; Statsoft; Tulsa, OK; SAS, version 9.0; SAS Inc; Chicago, IL; and Stata, version 10; StataCorp; College Station, TX). Categorical variables were analyzed with a Yates-corrected Pearson χ2 test. For comparison of categorical variables across multiple institutions, observed vs expected χ2 analysis was performed. Continuous variables were expressed as medians and interquartile ranges and were analyzed using nonparametric one-way analysis of variance with a Kruskal-Wallis test for medians. Multiple comparisons were made using the Scheffé test on mean ranks at a significance level of 0.05.
Results
A total of 12,213 patients who were intubated and mechanically ventilated were included from 16 US PICUs of varying size and acuity. Six PICUs had a relatively high volume (> 1,000 admissions per year), six PICUs had a medium volume (600 to 1,000 admissions per year), and four PICUs had a low volume (< 600 admissions per year). Eleven centers have fellowship training programs. Three were from the northeast and eastern United States, four were from the south and southeastern United States, five were from the Midwest, and four were from the western United States. Table 2 shows center-specific details with regard to screening.
Table 2.
Center-Specific Characteristics
| Center | Start Date | End Date | Patients Screened, Total No. | PICU Admissions, No. | Separate Cardiac Unit? | Fellowship Training Program? |
|---|---|---|---|---|---|---|
| 1 | 5/1/01 | 4/23/04 | 1,386 | NA | Y | Y |
| 2 | 8/15/01 | 3/9/03 | 307 | NA | N | Y |
| 3 | 8/1/01 | 4/30/04 | 1,390 | 4,504 | N | Y |
| 4 | 8/2/01 | 9/22/03 | 748 | 2,938 | N | Y |
| 5 | 9/3/01 | 4/16/04 | 1,675 | 4,958 | N | Y |
| 6 | 8/27/01 | 4/22/04 | 1,246 | NA | Y | Y |
| 7 | 7/30/01 | 3/6/03 | 222 | 959 | N | Y |
| 8 | 8/20/01 | 4/24/04 | 1,237 | NA | N | Y |
| 9 | 5/27/02 | 4/10/04 | 2,112 | NA | Y | Y |
| 10 | 4/29/02 | 4/23/04 | 686 | 3,430 | N | Y |
| 11 | 8/11/03 | 4/25/04 | 196 | NA | N | Y |
| 12 | 7/7/03 | 4/14/04 | 133 | NA | N | N |
| 13 | 7/7/03 | 12/15/03 | 450 | 703 | N | N |
| 14 | 8/25/03 | 2/08/04 | 211 | NA | N | N |
| 15 | 10/6/03 | 4/11/04 | 159 | 385 | N | N |
| 16 | 1/23/04 | 4/17/04 | 55 | 180 | N | N |
NA = not available; Y = yes; N = no.
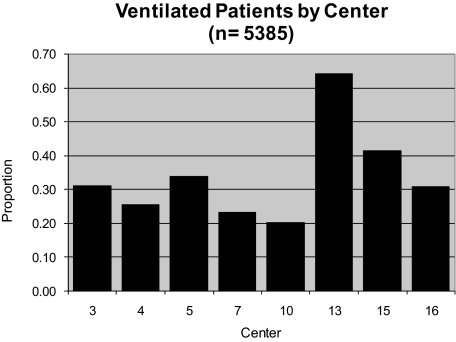
Of the total number of patients, 11,536 were between 2 weeks and 18 years of age, and had been endotracheally intubated and mechanically ventilated. Examining the eight institutions where the number of PICU admissions was available, 5,385 of the 18,057 patients (29.82%) required intubation and mechanical ventilation. However, there was significant intercenter variability, with ranges between 20% and 64% (Fig 1). Given that one cannot be absolutely certain that all patients who were intubated and mechanically ventilated during this period were included in the study, this calculated prevalence likely represents a minimum proportion of intubated patients.
Figure 1.
Overall proportion of patients receiving mechanical ventilation by center. Of note, the proportion was calculated as the number of patients screened per the total number of PICU admissions over the given time period.
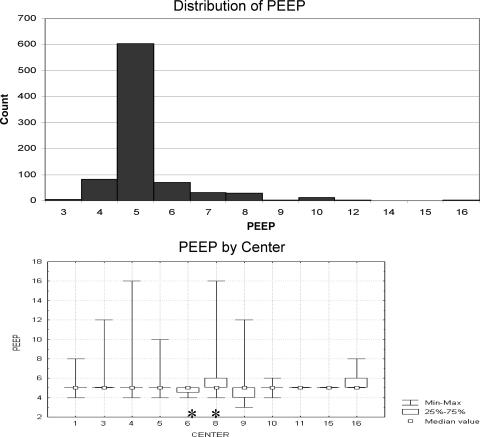
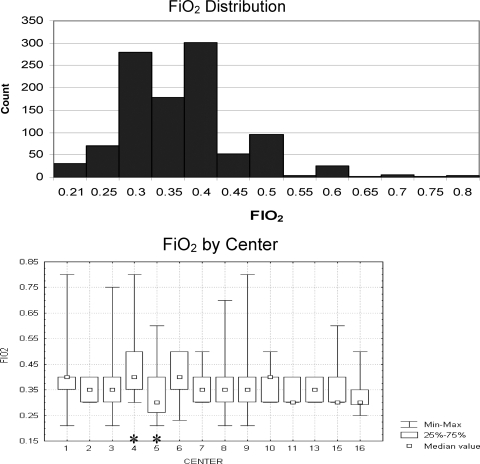
A subset of 1,457 patients with respiratory failure had no arterial blood gas measurements available for computation of the PF. Although there may have been more patients without an arterial line who were not enrolled in the prone-positioning study for other reasons, this finding represents at least 15% of all the patients receiving mechanical ventilation during the period of study. Within this subset, 1,052 patients had information available regarding Fio2, and 840 patients had information on PEEP available. The median PEEP was 5 cm H2O (71%), with 90% having a PEEP ≤ 6 cm H2O and 97% having a PEEP ≤ 8 cm H2O (Fig 2, top). There was little variability with PEEP choice among centers, and although the median PEEP was statistically different among centers (p = 0.012 [Kruskal-Wallis test]), this finding was due largely to differences between only two centers (Scheffé test for multiple comparisons) [Fig 2, bottom]. The median Fio2 was 0.35, with 81% of all patients having an Fio2 ≤ 0.4, 96% having an Fio2 ≤ 0.5, and 99% having an Fio2 ≤ 0.65 (Fig 3, top). Again, there was some variability in Fio2 among centers, although the variation was predominantly due to only two centers (p < 0.001 [Kruskal-Wallis test]; Scheffé test for multiple comparisons) [Fig 3, bottom].
Figure 2.
Top: distribution of PEEP for patients without arterial lines. Note that 97% of patients without arterial lines are managed with PEEP < 8. Bottom: distribution of PEEP by center. Note the similarity in median PEEP among all centers. For centers 1 to 5, 10, 11, and 15, the interquartile range values for PEEP were identical to the median values. Variability from analysis of variance (*) is predominantly due to mean ranks between centers 6 and 8 based on results of a Scheffé test for multiple comparisons.
Figure 3.
Top: distribution of Fio2 for patients without arterial lines. Note that 96% of patients are managed with Fio2 < 0.5. Bottom: Fio2 distribution by center. Note the similarity in median Fio2 among all centers. Variability from analysis of variance (*) is predominantly due to mean ranks between centers 4 and 5 based on the results of a Scheffé test for multiple comparisons.
A subset of 904 patients without an arterial line had information available on both Fio2 and Spo2. For those patients with a documented Spo2 ≤ 97% and a concurrent Fio2, an SF was calculated. In previous work,11–13 we have found that SFs < 270 correspond to PFs < 300. Using these criteria, 104 of these 904 patients would have met the oxygenation criteria for ALI.
A total of 8,225 patients had information on a non-ALI diagnosis available. Within this subset, 22% had cyanotic congenital heart disease, although at least three of the institutions had a dedicated cardiac ICU that did not screen patients. As such, this number likely underrepresents the percentage of patients intubated with cyanotic congenital heart disease. Twenty-six percent of patients had respiratory failure but did not have bilateral pulmonary infiltrates seen on chest radiograph. A heterogeneous group of respiratory diagnoses, including chronic respiratory disease, upper airway obstruction, and reactive airway disease, constituted 20% of the subset. Nonpulmonary conditions, including neuromuscular disease, cerebral hypertension, spinal instability, abdominal wounds, heart failure, and other diagnoses, constituted 26% of patient conditions. Extracorporeal membrane oxygenation and do-not-resuscitate conditions each were responsible for 2% of the patients, and lung or bone marrow transplantation together constituted 2% of patients in PICUs (Table 3).
Table 3.
Breakdown of Patients by Diagnostic Category
| Diagnostic Categories | Count(n = 8,225) | % |
|---|---|---|
| No bilateral lung disease | 2,104 | 25.6 |
| Cyanotic heart disease | 1,784 | 21.7 |
| Congestive heart failure | 655 | 8.0 |
| Chronic lung disease | 673 | 8.2 |
| Upper airway obstruction | 585 | 7.1 |
| Reactive airways disease | 404 | 4.9 |
| Spinal instability | 435 | 5.3 |
| Neuromuscular disease | 318 | 3.9 |
| Abdominal wound | 262 | 3.2 |
| Supported on extracorporeal membrane oxygenation | 195 | 2.4 |
| Bone marrow/lung transplant | 185 | 2.2 |
| Cerebral hypertension | 196 | 2.4 |
| Care considered futile | 170 | 2.0 |
| Other | 259 | 3.1 |
Discussion
Data from this cross-section of 16 US PICUs demonstrate that mechanical ventilation occurs commonly in these settings, although the reasons for ventilation are quite diverse. Given this heterogeneity, this study should be used to gauge the relative frequency of different diagnostic categories for which children require mechanical ventilation and help to determine the feasibility of future trials on different subgroups of ventilated patients.
Moreover, when looking to design future trials on ALI, it is important to consider that at least 25% of patients with respiratory failure do not fulfill the radiographic criteria for ALI and ARDS. From this data set, it is difficult to get an overall estimate of the prevalence of ALI in children receiving mechanical ventilation. There were several patients with a concurrent condition that precluded them from lying prone or for whom information about bilateral pulmonary infiltrates or PF was not available. At least 60% of patients did not have bilateral pulmonary infiltrates, had congenital heart disease, or had congestive heart failure. However, within the remaining 40% of patients, we do not know how many met other criteria for ALI. It is likely that several patients with, for example, abdominal wounds, cerebral hypertension, and neuromuscular disease would meet the criteria for ALI.
The reported prevalence of ALI and ARDS varies greatly. Farias et al1 reported that 2% of all children who receive mechanical ventilation for > 12 h have ARDS, echoing previous findings by Timmons et al.14 However, this percentage is lower than the 7.6% reported by Randolph et al,2 who examined patients who had received ventilation for > 24 h but did not have confirmatory chest radiograph results and instead relied on physician coding of ARDS. As expected, the prevalence of ALI is higher, ranging from 6.2 to 9.9% of children who received mechanical ventilation for a minimum of 12 or 24 h.15,16
It also appears from our data that children with more severe lung injury may have arterial blood gas data available to calculate PFs. In general, most patients who are managed without arterial lines are receiving modest ventilator support, with 90% having a PEEP ≤ 6 cm H2O and 96% having an Fio2 ≤ 0.5. Nonetheless, when attempting to design studies that target patients with less severe lung injury (eg, ALI vs ARDS), the arterial blood gas criteria may be too stringent. Looking at just the time of active enrollment for the prone study, a total of 8,017 patients were screened. Within this group, 7,833 did not meet the study inclusion criteria or met some exclusion criteria. Eight-two patients met the criteria for ALI but were not enrolled because of parent refusal, language barriers, issues of consent, or missed enrollment windows. When combined with the 102 patients who were randomized for the study, overall 184 patients met the diagnostic criteria for ALI. However, an additional 104 patients would have met the oxygenation criteria for ALI if the noninvasive surrogate of SF was used when PF was not available. Therefore, one can estimate that an additional 36% of patients could be captured for studies on ALI if this surrogate measure for arterial PF is substituted.
Finally, these data highlight the difficulty of designing an adequately powered study using mortality as an outcome for children with ALI. Given the relatively low incidence of ALI in children receiving mechanical ventilation, combined with estimated mortality rates between 8% and 22%,5,17,18 a hypothetical study comparing two tidal volume strategies (6 to 8 mL/kg vs 10 to 12 mL/kg) to detect a 5% reduction in mortality from 20 to 15%, assuming a two-tailed hypothesis with an α level of 0.05 and a power of 0.8, would require 975 patients per group. Assuming a 7% incidence of ALI15,16 and a desired enrollment of 1,950 patients with a 50% enrollment rate,17 a total of 55,714 intubated patients would need to be screened. This number could be reduced to 35,657 by using a noninvasive surrogate such as an SF. Given that over a 3-year period approximately 12,000 pediatric patients receiving mechanical ventilation in 16 US PICUs were screened, a study using mortality as an outcome would likely require 48 PICUs over the same 3-year period using a surrogate measure like SF or 74 PICUs using the strict PF criterion. Therefore, an adequately powered randomized controlled trial with mortality as an outcome measure in children with ALI simply may not be feasible.
Using a combined metric, such as ventilator-free days, could reduce these numbers.19 Looking at the results of the prone study, the mean number of ventilator-free days was 15.8 in the supine group, with an SD of 8.5 days. Therefore, to detect a reduction of 2 ventilator-free days (similar to the results of the ARDS Network tidal volume trial20), 284 patients per group would be required, which is one-third fewer patients than would be required to power a trial for a significant mortality reduction.
There are limitations to this study. Centers were required to report primary exclusion criteria but not all exclusion criteria for the prone study. For this reason, not all data elements were available for every patient. However, in the analysis, care was taken to report proportions in reference to the number of patients for which that data element was available. Because of this limitation, an overall estimate of the prevalence of ALI cannot be reliably obtained. Moreover, specific information regarding primary diagnosis was not available, although large diagnostic categories that served as reasons for exclusion from the prone study were analyzed. Finally, screening periods were different across the 16 PICUs, so center-related differences regarding the proportion of patients who are mechanically ventilated may be subject to seasonal variability, depending on the months of the year during which the centers screened patients. Nevertheless, the data from this large number of children receiving mechanical ventilation will be extremely useful to help design future studies.
In conclusion, at least 30% of all children in a heterogeneous cross-section of US PICUs are intubated and mechanically ventilated. The reasons for intubation and mechanical ventilation are quite variable, but close to 50% have primarily respiratory disorders, and 30% have primarily cardiovascular disorders. Although there is remarkable similarity in the patients whom clinicians find acceptable to manage without an indwelling arterial line, the PF requirement to make the diagnosis of ALI can significantly hamper eligibility for multicenter trials on ALI.
Acknowledgments
Author contributions: Dr. Khemani's primary role was data management, analysis, and writing of the manuscript. Dr. Markovitz assisted with data analysis, confirmed methodology, assisted with writing the manuscript, and provided significant editorial assistance. As principal investigator of the original clinical trial, Dr. Curley provided data, assisted with interpretation of data, confirmed methodology, and provided significant editorial assistance.
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: The authors thank Elizabeth A. Mitchell, BSc, and Michelle A. LaBrecque, MSN, RN, for their vigilant attention to the detail necessary to analyze these data.
Abbreviations:
- ALI
acute lung injury
- PEEP
positive end-expiratory pressure
- PF
Pao2/Fio2 ratio
- SF
pulse oximetric saturation/Fio2 ratio
- Spo2
pulse oximetric saturation
Footnotes
Dr. Curley and this work are partially supported by the National Institutes of Health [grant no. RO1 NR05336].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Farias JA, Frutos F, Esteban A, et al. What is the daily practice of mechanical ventilation in pediatric intensive care units? A multicenter study. Intensive Care Med. 2004;30:918–925. doi: 10.1007/s00134-004-2225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randolph AG, Meert KL, O'Neil ME, et al. The feasibility of conducting clinical trials in infants and children with acute respiratory failure. Am J Respir Crit Care Med. 2003;167:1334–1340. doi: 10.1164/rccm.200210-1175OC. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination: the consensus committee. Intensive Care Med. 1994;20:225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 4.Khemani RG, Markovitz B, Curley MAQ. Selection of positive end expiratory pressure (PEEP) and fraction of inspired oxygen for mechanically ventilated children without an arterial line [abstract] Pediatr Crit Care Med. 2007;35(suppl):A232. [Google Scholar]
- 5.Willson DF, Thomas NJ, Markovitz BP, et al. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 6.Merlani P, Garnerin P, Diby M, et al. Quality improvement report: linking guideline to regular feedback to increase appropriate requests for clinical tests: blood gas analysis in intensive care. BMJ. 2001;323:620–624. doi: 10.1136/bmj.323.7313.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilon CS, Leathley M, London R, et al. Practice guideline for arterial blood gas measurement in the intensive care unit decreases numbers and increases appropriateness of tests. Crit Care Med. 1997;25:1308–1313. doi: 10.1097/00003246-199708000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Numa AH, Newth CJ. Assessment of lung function in the intensive care unit. Pediatr Pulmonol. 1995;19:118–128. doi: 10.1002/ppul.1950190207. [DOI] [PubMed] [Google Scholar]
- 9.Khemani RG, Markovitz B, Curley MAQ. Epidemiologic factors of mechanically ventilated PICU patients in the United States [abstract] Pediatr Crit Care Med. 2007;8(suppl):A39. [Google Scholar]
- 10.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khemani RG, Bart RD, Patel N, et al. Comparison of the Spo2/Fio2 ratio and Pao2/Fio2 ratio in children. Chest. 2009;135:662–668. doi: 10.1378/chest.08-2239. [DOI] [PubMed] [Google Scholar]
- 12.Thomas NJ, Willson DF, Shih M, et al. Defining acute lung disease in children with the oxygenation saturation index. Pediatr Crit Care Med. 2009 doi: 10.1097/PCC.0b013e3181b0653d. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the Spo2/Fio2 ratio and the Pao2/Fio2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 14.Timmons OD, Havens PL, Fackler JC. Predicting death in pediatric patients with acute respiratory failure: Pediatric Critical Care Study Group; Extracorporeal Life Support Organization. Chest. 1995;108:789–797. doi: 10.1378/chest.108.3.789. [DOI] [PubMed] [Google Scholar]
- 15.Dahlem P, van Aalderen WMC, Hamaker ME, et al. Incidence and short-term outcome of acute lung injury in mechanically ventilated children. Eur Respir J. 2003;22:980–985. doi: 10.1183/09031936.03.00003303. [DOI] [PubMed] [Google Scholar]
- 16.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 17.Curley MA, Arnold JH, Thompson JE, et al. Clinical trial design: effect of prone positioning on clinical outcomes in infants and children with acute respiratory distress syndrome. J Crit Care. 2006;21:23–32. doi: 10.1016/j.jcrc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld DA, Bernard GR. Acute Respiratory Distress Syndrome Network (ARDSnet). Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Acute Respiratory Distress Syndrome Network (ARDSnet) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]