Abstract
Background:
In patients with idiopathic pulmonary fibrosis (IPF), our objectives were to identify predictors of abnormal heart rate recovery (HRR) at 1 min after completion of a 6-min walk test (6MWT) [HRR1] and 2 min after completion of a 6MWT (HRR2), and to determine whether abnormal HRR predicts mortality.
Methods:
From 2003 to 2008, we identified IPF patients who had been evaluated at our center (n = 76) with a pulmonary physiologic examination and the 6MWT. We used logistic regression to identify predictors of abnormal HRR, the product-limit method to compare survival in the sample stratified on HRR, and Cox proportional hazards analysis to estimate the prognostic capability of abnormal HRR.
Results:
Cutoff values were 13 beats for abnormal HRR1 and 22 beats for HRR2. In a multivariable model, predictors of abnormal HRR1 were diffusing capacity of the lung for carbon monoxide (odds ratio [OR], 0.4 per 10% predicted; 95% confidence interval [CI], 0.2 to 0.7; p = 0.003), change in heart rate from baseline to maximum (OR, 0.9; 95% CI, 0.8 to 0.97; p = 0.01), and having a right ventricular systolic pressure > 35 mm Hg as determined by transthoracic echocardiogram (OR, 12.7; 95% CI, 2.0 to 79.7; p = 0.01). Subjects with an abnormal HRR had significantly worse survival than subjects with a normal HRR (for HRR1, p = 0.0007 [log-rank test]; for HRR2, p = 0.03 [log-rank test]); these results held for the subgroup of 30 subjects without resting pulmonary hypertension (HRR1, p = 0.04 [log-rank test]). Among several candidate variables, abnormal HRR1 appeared to be the most potent predictor of mortality (hazard ratio, 5.2; 95% CI, 1.8 to 15.2; p = 0.004).
Conclusion:
Abnormal HRR after 6MWT predicts mortality in IPF patients. Research is needed to confirm these findings prospectively and to examine the mechanisms of HRR in IPF patients.
Idiopathic pulmonary fibrosis (IPF) is a severe, progressive, fibrosing interstitial lung disease without effective therapy and a poor prognosis. Median survival times have been observed to be as low as 2.5 years.1 Investigators2–8 have identified several prognostic variables in IPF, including age, gender, disease duration, symptom severity, radiologic features, functional capacity, and both baseline and serial changes in measures of pulmonary physiology and gas exchange. Despite the numerous prognostic variables that inform discussions about prognosis in IPF patients, heterogeneity in the disease course complicates making accurate survival predictions.
Heart rate recovery (HRR), specifically the failure of the heart rate to decline at 1 or 2 min postexercise, is associated with increased mortality.9–11 Heart rates in patients with COPD recover less at 1 min than control subjects (mean [± SD] heart rates, 20 ± 9 vs 11 ± 9, respectively; p < 0.0001), and in COPD patients the failure of heart rate to drop by > 14 beats 1 min after exercise is associated with a fivefold increased risk of death over a mean follow-up duration of 43 months.12 HRR after exertion has not been examined as a prognostic marker in patients with IPF.
The 6-min walk test (6MWT) is a marker of functional exercise capacity that is increasingly used in the initial and longitudinal clinical assessments of patients with IPF. In these patients, the distance walked during the 6MWT is highly reproducible (test-retest reliability, 0.98) over short time intervals (eg, 1 to 2 weeks) and is highly correlated (r = 0.78) with peak oxygen uptake measured during a cardiopulmonary exercise test to volitional fatigue.13 Given the low cost and simplicity of the 6MWT and its apparent validity as an exercise challenge and measure of functional capacity in patients with IPF, we hypothesized that it would provide an ideal setting in which to measure HRR. The main objectives of this study were to define the cutoff values for abnormal HRR, to examine the predictors of an abnormal HRR, and to determine whether an abnormal HRR after a 6MWT carries prognostic value in patients with IPF.
Materials and Methods
Subjects
The study sample consisted of 76 consecutive patients with IPF who were evaluated at our center between January 1, 2003, and January 1, 2008, who completed pulmonary function tests (PFTs) and a 6MWT and were enrolled into our longitudinal database used to examine the natural history of fibrosing interstitial lung disease. The study was approved by the National Jewish Health Institutional Review Board. The diagnosis of IPF was made in accordance with the most recently established consensus guidelines from the American Thoracic Society (ATS)/European Respiratory Society (ERS).14,15 Various treatment regimens were recommended over the course of follow-up; these included no therapy, N-acetyl cysteine (NAC) therapy alone, prednisone therapy alone, therapy with an immunomodulatory agent (eg, azathioprine, cyclophosphamide, or mycophenolate mofetil) with or without NAC and with or without prednisone.
Testing
PFTs were performed according to ATS/ERS criteria.16–19 At our center, 6MWT is performed according to the ATS/ERS criteria (in terms of instructions to patients); however, the test is modified by recording both heart rate and peripheral oxygen pulse oximetric saturation (Spo2) at the end of each minute of the 6MWT, as well as by recording heart rate at 1, 2, and 3 min after completion of the test with the patient seated. The 6MWT test is begun after the patient has been seated for 5 min. Pulse oximeters (Ohmeda Biox 3740 or Ohmeda 3900; Datex-Ohmeda, Inc; Laurel, MD) with finger probes were used. These oximeters display heart rate and Spo2. Supplemental oxygen flow rates were used according to the subjects' current oxygen prescriptions.
Data Collection
Clinical data were collected by chart review and database query. HRR was defined as the difference between a subject's heart rate at the 6th min of the 6MWT and at either 1 min after completion of the 6MWT (HRR1) or 2 min after completion of the 6MWT (HRR2).
Statistical Analysis
Like Cole and colleagues9 and LeBlanc and Crowley,20 we determined the value for abnormal HRR in our sample by finding the maximum value for the log-rank χ2 statistic for all possible cutoff points for abnormal HRR between the 10th and 90th percentiles (at each of 1 and 2 min of recovery) for the study sample. By using this method, we identified the cutoff values to be 13 beats at 1 min and 22 beats at 2 min after the 6MWT.
We present the baseline data as counts and measures of central tendency (means with SD for normally distributed data, and medians with interquartile ranges for nonnormally distributed continuous data). Continuous variables were compared by using t tests or a nonparametric equivalent where appropriate. Categorical variables were compared using the χ2 test or Fisher exact test where appropriate. We used multivariable logistic regression to identify independent predictors of abnormal HRR (ie, heart rate dropping by ≤ 13 beats at 1 min into recovery and by ≤ 22 beats at 2 min into recovery). We first performed bivariate analyses with each predictor. Variables with p values < 0.15 in bivariate analysis were selected as candidates for the final model. We used the “selection = stepwise” feature in the software that was used (SAS PROC LOGISTIC; SAS Institute; Cary, NC) to yield the most parsimonious models for abnormal HRR (dependent variable). We used the product-limit method to derive survival and Kaplan-Meier curves to display survival for the study sample stratified on HRR (abnormal vs normal). Because elevated right ventricular systolic pressure (RVSP) by transthoracic echocardiogram appeared to be a driver of abnormal HRR, we examined whether abnormal HRR carried prognostic value among subjects without elevated RVSP by generating Kaplan-Meier curves for this subset.
For the entire study sample, as well as for the subgroup without elevated RVSP, we used Cox proportional hazards analysis to examine the effect of abnormal HRR and other meaningful predictors of survival. We first performed bivariate analyses with each predictor. Variables with p values < 0.15 in bivariate analysis were selected as candidates for the final model. To yield the most parsimonious survival models and to avoid problems raised by the substantial collinearity among most of the variables, we used the “selection = stepwise” feature in the software used (SAS PROC PHREG; SAS Institute). We confirmed that the proportionality assumption was met for the HRR1 and HRR2 variables by examining log(-log) plots. Survival time was calculated from the time of the 6MWT to death or censoring (subjects were censored if they were alive at last contact). Vital status was ascertained on June 10, 2008, by either records review or query of the Social Security Death Index. For subjects who performed more than one 6MWT at our center, we chose the earliest test to allow for the longest follow-up time. We used a statistical software package (SAS, version 9.1.3; SAS Institute) to perform all statistical analyses, and we considered p values < 0.05 to represent statistical significance.
Results
The majority (n = 70) of subjects were white men in their seventh decade of life. Most subjects had undergone surgical lung biopsy to diagnose IPF. Few subjects (n = 15) had a history of coronary artery disease (CAD), and none had clinically significant or symptomatically apparent CAD at the time of testing. Cardiovascular medication use and therapeutic regimens for IPF were similar between subgroups stratified on HRR1 (Table 1).
Table 1.
Baseline Characteristics of Study Subjects
| Variables | All Subjects (n = 76) | HRR ≤ 13 Beats(n = 29) | HRR > 13 Beats(n = 47) | p Value |
|---|---|---|---|---|
| Age, yr | 68.4 (8.4) | 68.5 (7.5) | 68.4 (9.0) | 0.9 |
| Gender | 0.6 | |||
| Male | 61 | 25 | 37 | |
| Female | 15 | 4 | 10 | |
| Surgical biopsy | 46 | 18 | 29 | 1.0 |
| FVC, % | 68.7 (17.2) | 62.4 (17.3) | 72.5(16.2) | 0.01 |
| Dlco, % | 44.4 (14.4) | 35.0 (9.7) | 49.4 (14.0) | < 0.0001 |
| Time from diagnosis to 6MWT, d | 82 (0–280) | 55 (0–286) | 94 (0–270) | 0.5 |
| 6MWD, feet | 1,277 (263) | 1,135 (212) | 1,364 (255) | < 0.0001 |
| 6MWT nadir Spo2 < 89% | 36 | 17 | 19 | 0.16 |
| Baseline heart rate | 77 (13) | 82 (14) | 74 (11) | 0.01 |
| Maximum heart rate during 6MWT | 106 (11) | 105 (12) | 106 (10) | 0.6 |
| Heart rate 1 min after 6MWT, beats/min | 89 (15) | 99 (14) | 84 (12) | < 0.0001 |
| Heart rate 2 min after 6MWT, beats/min | 84 (13) | 91 (13) | 79 (11) | < 0.0001 |
| Supplemental O2 flow rate | ||||
| None | 43 | 11 | 32 | 0.02* |
| 1–3 L/min | 13 | 6 | 7 | |
| 4–5 L/min | 11 | 6 | 5 | |
| ≥ 6 L/min | 9 | 6 | 3 | |
| IPF medications | ||||
| Prednisone | 15 | 7 | 8 | |
| Azathioprine | 3 | 1 | 2 | |
| NAC | 7 | 2 | 5 | |
| Cyclophosphamide | 4 | 3 | 1 | |
| Mycophenolate mofetil | 1 | 0 | 1 | |
| None | 46 | 16 | 30 | 0.5* |
| History of CAD | 15 | 5 | 10 | 0.8 |
| RVSP | ||||
| > 35 mm Hg by TTE | 17 | 11 | 6 | 0.02 |
| ≤ 35 mm Hg by TTE | 30 | 8 | 22 | 0.2 |
| No echo | 29 | 10 | 19 | 0.6 |
| Cardiac medications | ||||
| β-Blocker | 18 | 6 | 12 | |
| Diltiazem or verapamil | 3 | 0 | 3 | |
| Digoxin | 1 | 1 | 0 | |
| α-Blocker | 3 | 1 | 2 | |
| None | 56 | 21 | 33 | 0.8* |
| Follow-up, d | 487 (240–913) | 365 (235–792) | 561 (245–994) |
Values are given as the mean (SD) or median (interquartile range), unless otherwise indicated. p Values reflect the comparison between groups stratified by HRR. TTE = transthoracic echocardiogram.
*For none vs any (oxygen or drug) comparisons between groups.
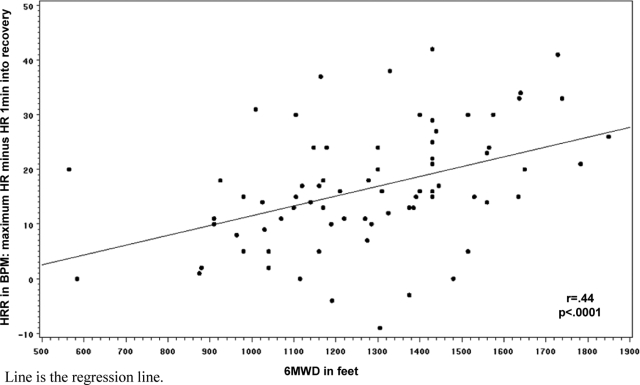
We identified cutoff values for an abnormal HRR1 to be 13 beats and HRR2 to be 22 beats. Predictors of an abnormal HRR1 or HRR2 from bivariate analyses are displayed in Table 2. In the final models, independent predictors of abnormal HRR1 included having an RVSP (estimated by transthoracic echocardiogram) of > 35 mm Hg (odds ratio [OR], 4.8; 95% confidence interval [CI], 1.2 to 19.0; p = 0.03), the change in heart rate from baseline to 6 min (OR, 0.9; 95% CI, 0.8 to 0.97; p = 0.01), and diffusing capacity of the lung for carbon monoxide (Dlco) [OR, 0.4 per 10% predicted; 95% CI, 0.2 to 0.7; p = 0.003]; 6-min walk distance (6MWD) and change in heart rate were predictors of abnormal HRR2 (OR: 0.9 per 10 feet walked [95% CI, 0.94 to 0.99; p = 0.04] vs 0.9 [95% CI, 0.8 to 0.9; p = 0.01], respectively). Figure 1 shows the relationship between HRR1 and 6MWD.
Table 2.
Bivariate Analysis of Predictors of Abnormal HRR
| HRR1 ≤ 13 Beats |
HRR2 ≤ 22 Beats |
|||
|---|---|---|---|---|
| Variables | OR | p Value | OR | p Value |
| Age | 1.0 | 0.96 | 1.0 | 0.5 |
| Male gender | 1.3 | 0.7 | 1.8 | 0.3 |
| FVC, per 10% predicted | 0.7 | 0.02 | 0.8 | 0.1 |
| Dlco, % | 0.4 | 0.0003 | 0.6 | 0.01 |
| 6MWD, per 10 feet | 0.9 | 0.001 | 0.9 | 0.003 |
| Using supplemental O2 | 3.5 | 0.01 | 1.9 | 0.2 |
| Nadir Spo2 | 0.8 | 0.03 | 0.9 | 0.9 |
| Baseline heart rate | 1.1 | 0.02 | 1.0 | 0.03 |
| Maximum heart rate | 0.9 | 0.6 | 0.9 | 0.1 |
| Change in heart rate | 0.9 | 0.001 | 0.8 | 0.03 |
| RVSP > 35 mm Hg by echocardiogram | 4.2 | 0.01 | 1.4 | 0.52 |
Change in heart rate = maximum heart rate minus baseline heart rate.
Figure 1.
Relationship between HRR and 6MWD.
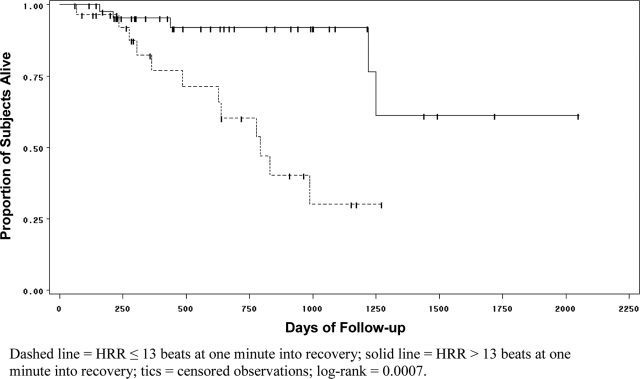
In time-to-event survival analysis, subjects with abnormal HRR1 had significantly worse survival (mean survival time, 715.7 ± 67 vs 1,168.4 ± 48 days) than subjects with normal HRR1 (ie, decline > 13 beats at 1 min) [Fig 2]. At any time during the follow-up period, the hazard of death for subjects with abnormal HRR1 was significantly greater than that for subjects with a normal HRR1 (hazard ratio [HR], 5.2; 95% CI, 1.8 to 15.2; p = 0.002). All results were similar for abnormal HRR2; subjects with HRR2 of ≤ 22 beats had significantly worse survival times than subjects with HRR2 of ≥ 22 beats (data not shown; p = 0.03 [log-rank]). The hazard of death for subjects with abnormal HRR2 was significantly greater than that for subjects with a normal HRR2 (HR, 3.6; 95% CI, 1.1 to 11.6; p = 0.04).
Figure 2.
Kaplan-Meier curve for survival among 76 subjects stratified according to whether heart rate declined by 13 beats from min 6 during the 6MWT to 1 min after the 6MWT.
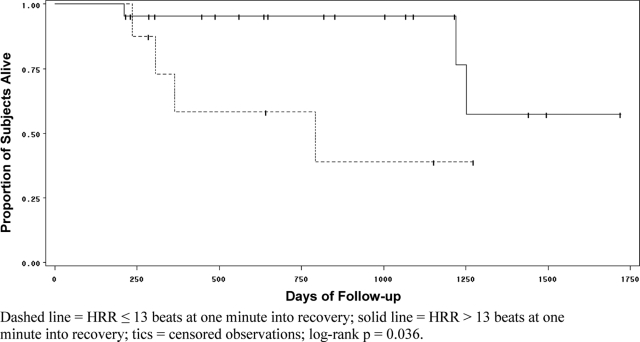
For the 30 subjects with echocardiographically estimated RVSP < 35 mm Hg, the results of bivariate analyses are displayed in Table 3. Significant predictors of abnormal HRR1 included FVC, Dlco, 6MWD, and change in heart rate; for abnormal HRR2, the only significant predictor was a change in heart rate. Among the 30 subjects in this subgroup, in time-to-event survival analysis, abnormal HRR remained a significant predictor of survival (Fig 3). The mean survival time for subjects with abnormal HRR1 was 589.2 ± 104 days vs 1,197.8 ± 57 days for those with a normal HRR1.
Table 3.
Bivariate Analysis of Predictors of Abnormal Heart Rate Recovery Among 30 Subjects With No TEE Evidence of PH
| HRR1 ≤ 13 Beats |
HRR2 ≤ 22 Beats |
|||
|---|---|---|---|---|
| Variables | OR | p Value | OR | p Value |
| Age | 0.9 | 0.9 | 1.0 | 0.9 |
| Male gender | 1.6 | 0.7 | 6.0 | 0.1 |
| FVC, per 10% predicted | 0.5 | 0.04 | 0.9 | 0.6 |
| Dlco, % | 0.2 | 0.02 | 0.7 | 0.2 |
| 6MWD, per 10 feet | 0.9 | 0.04 | 0.9 | 0.6 |
| Using supplemental O2 | 1.1 | 0.9 | 1.1 | 0.9 |
| Nadir Spo2 | 0.8 | 0.2 | 1.0 | 0.8 |
| Baseline heart rate | 1.1 | 0.1 | 1.0 | 0.2 |
| Maximum heart rate | 0.9 | 0.4 | 0.9 | 0.1 |
| Change in heart rate | 0.8 | 0.02 | 0.8 | 0.01 |
See Table 2 for explanation of term.
Figure 3.
Kaplan-Meier curve for survival among the 30 subjects with no transthoracic echocardiographic evidence of PH stratified according to whether heart rate declined by 13 beats from min 6 during the 6MWT to 1 min after 6MWT.
Using data from the entire study sample, abnormal HRR1 was the strongest predictor of survival (Table 4). Abnormal HRR1 was the only variable selected in the final multivariable model (HR, 4.5; 95% CI, 1.5 to 13.7; p = 0.008). To examine the impact of cardiovascular medications on HRR, we performed similar time-to-event, bivariate, and multivariable analyses after excluding subjects who were receiving therapy with β-blockers, chronotropically active calcium channel blockers, digoxin, and α-blocking agents, and the results were no different from those just reported. Likewise, the results were similar for analyses performed on data from the subgroup of 43 subjects not using supplemental oxygen (baseline Spo2, > 88%).
Table 4.
Bivariate Cox Proportional Hazards Survival Analysis
| Variables | HR | 95% CI | p Value |
|---|---|---|---|
| HRR ≤ 13 at 1 min | 5.2 | 1.8–15.2 | 0.002 |
| HRR ≤ 22 at 2 min | 3.6 | 1.1–11.6 | 0.04 |
| Age | 1.0 | 0.9–1.1 | 0.2 |
| Male gender | 5.9 | 0.8–44.6 | 0.1 |
| FVC, per 10% predicted | 0.7 | 0.5–0.9 | 0.004 |
| Dlco, % | 0.5 | 0.3–0.8 | 0.01 |
| 6MWD, per 10 feet | 0.9 | 0.95–0.99 | 0.01 |
| Using supplemental O2 | 2.4 | 0.9–6.2 | 0.1 |
| Nadir Spo2 | 0.8 | 0.6–1.2 | 0.4 |
| Baseline heart rate | 1.0 | 0.9–1.1 | 0.7 |
| Maximum heart rate | 0.9 | 0.9–1.0 | 0.6 |
| Change in heart rate | 1.0 | 0.9–1.1 | 0.3 |
| Has RVSP > 35 mm Hg | 4.7 | 1.6–13.4 | 0.004 |
See Table 2 for explanation of term.
Discussion
The 6MWT is a simple, relatively low-cost procedure that is being used with increasing frequency for the evaluation of patients with IPF. However, one data point that is often neither collected nor considered when reviewing results from this test is heart rate in the recovery period. In this study, we examined 76 subjects with very well-defined IPF and found that the failure of heart rate to decline after exertion (by > 13 beats at 1 min or by > 22 beats at 2 min) is a strong predictor of mortality.
Recognizing that exercise-induced heart rate elevation is due to a combination of sympathetic activation and parasympathetic withdrawal, that the return of postexercise heart rate to normal is due to parasympathetic reactivation (ie, vagal tone),21 and that increased vagal activity is associated with reduced risk of death, Cole and colleagues9,10 hypothesized that HRR would be an important prognostic marker in patients who had been referred for cardiac exercise testing. Indeed, they observed that abnormal HRR1 was an independent predictor of mortality in that patient population. Subsequently, Froelicher and colleagues11,22 identified that abnormal HRR2 was an independent predictor of the presence of CAD by angiography as well as a predictor of mortality in male US veterans.
Lacasse and colleagues12 found that heart rate declines less in patients with COPD than in healthy control subjects at 1 min into recovery after a symptom-limited stepwise exercise test performed on a cycle ergometer. They also found that abnormal HRR1 of ≤ 14 beats was a strong predictor of mortality in patients with COPD. These investigators hypothesized that COPD-related autonomic dysfunction (either cardiac or perhaps due to increased airways resistance) might account for this finding. Seshadri and coinvestigators23 examined the relationship between HRR and PFTs in 627 subjects who had undergone maximum symptom-limited exercise treadmill tests and PFTs within the same year at their center. They found that abnormal HRR (defined as a decline in heart rate of ≤ 12 beats at 1 min into recovery) was associated with FEV1, impaired functional capacity as assessed by metabolic equivalents during the exercise test, male gender, and age. Regarding the few subjects with restrictive pulmonary physiology, these authors commented that there are no data linking restrictive pulmonary physiology and autonomic dysfunction, and they called for further research into this putative association.
It is reassuring to see that the cutoff values we identified for abnormal HRR1 and HRR2 are strikingly similar to those found by these other investigators. In our study sample, we identified different predictors of abnormal HRR from the ones that Seshadri and colleagues23 identified. This is likely due to the differences in underlying disease (the cohort in the study by Seshadri et al23 consisted predominantly of subjects with obstructive pulmonary physiology, whereas, as expected, our IPF subjects all had restrictive pulmonary physiology) as well as differences in the variables assessed in each study. Specifically, we believed it was important to examine the contribution of Dlco because impaired gas transfer is one of the hallmark physiologic abnormalities in IPF patients. Likewise, because so many of our IPF patients have secondary pulmonary hypertension (PH), we investigated whether elevated RVSP might account for some of the variability in abnormal HRR. These variables, noted to be important predictors of abnormal HRR in our study, were not examined by Seshadri et al23 or Lacasse et al.12
So, why might some patients with IPF have abnormal HRR? From our data, it appears that elevated RVSP and impaired diffusing capacity are at least two of the drivers, and that a greater increase in heart rate from baseline to 6 min is protective. In analyses not shown here, we found a significant relationship between 6MWD and change in heart rate (r = 0.3; p = 0.01); thus, as expected, the further a subject walks, the greater the increase in heart rate from baseline (ie, the greater the change in heart rate). Added to that, Figure 1 shows that the greater the 6MWD, the better the heart rate recovers. However, fitness does not fully explain HRR; in analyses that are not shown here, after accounting for 6MWD, HRR remained a significant predictor of mortality with an HR of 3.9 (p = 0.02); in that model, the HR after 6MWD was 0.9 (p = 0.14).
Among subjects without PH (at rest), FVC, Dlco, 6MWD, and change in heart rate were significant predictors of (or protectors from) abnormal HRR1. Thus, more severe IPF (as demonstrated by lower FVC, Dlco, and 6MWD) increased the likelihood for abnormal HRR1. Given that these same variables have been shown to predict survival in IPF patients, it makes sense that HRR is also a predictor. Our data would suggest that this extremely simple-to-collect variable is at least as potent a predictor as many other variables in IPF. When all candidate variables were given equal chances for inclusion in a final survival model, abnormal HRR was selected as the only predictor, and no other variables added significant predictive power.
Our study has limitations. The sample size was small. The retrospective design means that IPF medication regimens and data collection were not systematically implemented. Certain information was not collected and could have aided in elucidating the mechanisms for the results observed. Whether any of our subjects with normal resting RVSP had exercise-induced PH is unknown but possible. In fact, absent right heart catheterization, we cannot be certain of the status of PH in any of our subjects (even those with echocardiograms). Likewise, whether, as it is proposed to occur in COPD patients, IPF induces autonomic dysfunction is unknown but warrants further investigation. Like other investigators,24 we found certain variables (eg, FVC and Dlco) to predict mortality. Lama and colleagues7 observed that among IPF subjects whose resting Spo2 was ≥ 88% without using supplemental oxygen, desaturation to < 89% during a 6MWT was a strong predictor of survival. Differences in entry criteria (nearly half of our subjects used supplemental oxygen during the 6MWT) and altitudes at which the two studies were performed confound any comparisons of their results. At the altitude in Denver (5,280 feet above sea level), on average, resting Spo2 is not as high as that at sea level. Furthermore, the cutpoint for exercise Spo2 that is associated with mortality, as in the article by Lama et al,7 has yet to be defined at altitudes other than that of Ann Arbor, MI. All of these issues make prospective validation of our results at lower (or higher) altitudes all the more important.
Despite these limitations, we believe our study has merit. The study sample had very well-defined IPF, the results of our study are similar to those obtained by other investigators, and the findings are novel for this patient population. The inferences drawn from this study should not be that abnormal HRR is the absolutely most potent prognosticator in IPF, but we do think that it adds to the cadre of prognostic variables for this disease. Moving forward, it will be important to investigate abnormal HRR after 6MWT even more carefully. This can be accomplished only if the 6MWT (and data collection during and after) is standardized. In future studies, the putative relationship between PH (whether at rest or only with exercise) and abnormal HRR can also be better delineated. These studies should be prospective and have survival as the outcome.
In summary, some patients with IPF have abnormal HRR after exertion, and the presence of elevated RVSP and impaired Dlco appear to be two drivers of this abnormality. Even in IPF patients without elevated resting RVSP, abnormal HRR is a significant predictor of mortality. Future investigations should determine whether IPF patients have autonomic dysfunction, as postulated to account for abnormal HRR in patients with COPD, and prospective studies should be performed to confirm our findings and to examine more carefully the relationship between IPF-related PH and abnormal HRR.
Acknowledgments
Author contributions: Dr. Swigris contributed to the study conceptualization and design. Drs. Swigris, Fischer, Cosgrove, Frankel, Brown, and Ms. Kervitsky contributed to the data collection. Dr. Swigris, Mr. Swick, and Mr. Sprunger contributed to the data abstraction. Drs. Swigris and Wamboldt contributed to the statistical analysis. All of the authors contributed to the preparation, revision, and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations:
- ATS
American Thoracic Society
- CAD
coronary artery disease
- CI
confidence interval
- Dlco
diffusing capacity of the lung for carbon monoxide
- ERS
European Respiratory Society
- HR
hazard ratio
- HRR
heart rate recovery
- HRR1
heart rate recovery at 1 min after completion of the 6-min walk test
- HRR2
heart rate recovery at 2 min after completion of the 6-min walk test
- IPF
idiopathic pulmonary fibrosis
- NAC
N-acetyl cysteine
- OR
odds ratio
- PFT
pulmonary function test
- PH
pulmonary hypertension
- RVSP
right ventricular systolic pressure
- 6MWD
6-min walk distance
- 6MWT
6-min walk test
- Spo2
pulse oximetric saturation
Footnotes
This work was supported by the National Institutes of Health [grant No. NHLBI SCOR HL67671].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 2.Collard HR, King TE, Jr, Bartelson BB, et al. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KR, Andrei AC, Murray S, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 8.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 9.Cole CR, Blackstone EH, Pashkow FJ, et al. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 10.Cole CR, Foody JM, Blackstone EH, et al. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med. 2000;132:552–555. doi: 10.7326/0003-4819-132-7-200004040-00007. [DOI] [PubMed] [Google Scholar]
- 11.Shetler K, Marcus R, Froelicher VF, et al. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980–1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 12.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir Med. 2005;99:877–886. doi: 10.1016/j.rmed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Eaton T, Young P, Milne D, et al. Six-minute walk, maximal exercise tests: reproducibility in fibrotic interstitial pneumonia. Am J Respir Crit Care Med. 2005;171:1150–1157. doi: 10.1164/rccm.200405-578OC. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society and European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment; international consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 15.American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias: this joint statement of the American Thoracic Society (ATS) and European Respiratory Society (ERS) was adopted by the ARS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 16.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 17.Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc M, Crowley J. Relative risk trees for censored survival data. Biometrics. 1992;48:411–425. [PubMed] [Google Scholar]
- 21.Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24:1529–1535. doi: 10.1016/0735-1097(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski MJ, Vetrovec GW, Froelicher VF. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. Am J Cardiol. 2004;93:445–449. doi: 10.1016/j.amjcard.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Seshadri N, Gildea TR, McCarthy K, et al. Association of an abnormal exercise heart rate recovery with pulmonary function abnormalities. Chest. 2004;125:1286–1291. doi: 10.1378/chest.125.4.1286. [DOI] [PubMed] [Google Scholar]
- 24.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]