Abstract
Psychological stressors precipitate and maintain stress-induced psychopathology, and it is likely that altered amygdala function underlies some of the deleterious effects of psychological stress. To understand the mechanisms underlying the linkage between the response to psychological stressors and maladaptive or psychopathological responses, we have focused on amygdala responsivity in animal models employing species-specific psychological stressors. In the present study, we characterized the effects of a 15-min exposure to a natural predator, the ferret, on rat behavior and the expression of the somatostatin family of genes in the amygdala. We examined the somatostatin family of genes because substantial evidence shows that central somatostatin systems are altered in various neuropsychiatric illnesses. We report that rats respond to acute ferret exposure with a significant increase in fearful and anxious behaviors that is accompanied by robust amygdala activation and an increase in somatostatin receptor 2 (sst2) messenger RNA expression within the amygdala and anterior cingulate cortex. These studies are the first to show stress-induced changes in amygdala sst2 expression and may represent one mechanism by which psychological stress is linked to adaptive and maladaptive behavioral responses.
Keywords: Amygdala, anxiety, behavior, cingulate, stress, gene expression
It is widely recognized that stress can have profound effects on brain and behavior and that stressful life events are important factors in the development of psychopathologies such as depression and anxiety disorders [see Heim & Nemeroff (1999) for review]. Among the brain regions involved in the stress response, the amygdala is of interest because it is also involved in emotional processing and its function is altered in relation to psychopathology (Amaral 2002; Davis & Whalen 2001; LeDoux 2003). Numerous studies suggest that the amygdala mediates both conditioned and unconditioned fear responses (Amaral 2003; Blanchard & Blanchard 1972; Kalin et al. 2004; LeDoux 2000) and human functional imaging studies show that some patients with anxiety and depressive disorders have increased amygdala activation (Drevets 2003; Rauch et al. 2003).
The neuropeptide somatostatin (SST) was first described (Krulich et al. 1968) based on its ability to potently inhibit growth hormone and thyroid-stimulating hormone secretions (Reichlin 1983). Substantial evidence demonstrates that central SST systems are altered in various neuropsychiatric illnesses. For example, SST cerebrospinal fluid concentrations are decreased in patients with depression, dementia and Parkinson's disease [see (Rubinow et al. 1988) for review]. Furthermore, somatostatin receptor 2 (sst2) knockout mice display increased anxiety-related behaviors in a number of paradigms (Viollet et al. 2000). Lastly, SST containing cells and fibers occur throughout the brain, including the amygdala (Brownstein et al. 1975; Dierickx & Vandesande 1979; Finley et al. 1981; Krisch 1978). Inhibitory interneurons within the basolateral nucleus of the amygdala (BLA) and gamma amino butyric acid (GABA)-containing projections from the central nucleus of the amygdala (CeA) co-express SST (Batten et al. 2002; McDonald & Mascagni 2002; Muller et al. 2007).
The actions of SST and the highly related peptide cortistatin (de Lecea 2005; de Lecea et al. 1996) are mediated by five different seven transmembrane domain receptor-subtypes (sst1–5) (Bruno et al. 1992; Hoyer et al. 1995; Kluxen et al. 1992; Li et al. 1992; Meyerhof et al. 1992; O'Carroll et al. 1992). The SST receptors couple to G-proteins (Raynor & Reisine 1992) and trigger multiple intracellular signaling cascades (Buscail et al. 1994). Using nonselective radio-ligands, autoradiographic receptor-binding studies have shown a widespread central nervous system (CNS) distribution for the SST-binding sites (Martin et al. 1991; Moyse et al. 1989). Immunohistochemistry using receptor-specific antibodies confirmed the widespread brain distribution of sst2 (Dournaud et al. 1996; Schindler et al. 1997) but not of sst1 (Helboe et al. 1998), sst3 (Handel et al. 1999) or sst5 (Stroh et al. 1999). Taken together, these studies suggest that sst2 receptors represent the majority of SST-binding sites in the rat CNS. Cells and processes immunopositive for sst2 have been observed in a number of brain regions, including the amygdala, cerebral cortex, locus ceruleus and hippocampus (Dournaud et al. 1996; Schindler et al. 1997).
Given the SST alterations observed in neuropsychiatric illness and that this gene family is robustly expressed within the amygdala, we focused our efforts on characterizing the extent to which expression of the SST family of genes within the amygdala is modulated by acute predator stress exposure.
Methods
Animals
Male Sprague–Dawley rats (Charles River, Wilmington, MA, USA) weighing 275–300 g were housed singly with lights on from 0700 to 1900 h. To control for possible diurnal variability in behavioral or biochemical indices, all testing and sacrificing occurred between 1000 and 1300 h. All rats were handled for 6 days prior to testing to minimize subsequent handling-related stress and the novelty-related stress associated with changing housing cages. Six ferrets (Marshall Farms, North Rose, NY, USA) were used in these studies and were housed in pairs. Food and water were available ad libitum for both the rats and ferrets.
Ferret predator paradigm
We previously reported increased stress-like behaviors in rats following a direct ferret exposure (Roseboom et al. 2007). In the previous paradigm, rats in small protective wire cages (length 8.5 by width 6 by height 5.5 inches) were placed directly into the ferret housing cages. In an effort to improve our ability to quantify the behavioral data, the ferret exposure paradigm was modified in the present studies by placing the rats in standard rat housing cages (length 18 by width 10 by height 8 inches) one foot from the ferret housing cages. The standard rat housing cages allowed the rats to more easily engage in a wider variety of behaviors, and although the rats could still hear, see and smell the ferrets, no direct physical contact between ferret and rat test cage was possible with the current paradigm. Each rat was exposed to the ferret colony using a housing cage that had been cleaned and sanitized before use.
The ferret predator paradigm consists of the following steps: rats are handled for six consecutive days for 1–2 min to habituate them to the experimenter removing them from and placing them into rat housing cages. In addition, during these 6 days, rats are placed into new standard rat housing cages three times to habituate them to the novelty of exposure to a clean cage. In experiment 1, in which rat behavior was assessed, the rats are placed in an empty rat housing cage for 15 min in a room outside of the rat housing colony that is free of ferret odors once daily for four additional days. These exposures were to habituate the rats to the novelty of the room. The fourth exposure is videotaped to assess rat baseline behavior. On the predator exposure day, rats are placed in a standard rat housing cage without bedding inside the ferret colony room directly in front of the ferret housing cage for 15 min. All ferret exposure sessions in experiment 1 were videotaped and the rat behavior assessed by a trained reviewer. The reviewer is not blind to condition because of differences between the ferret colony room and the room in which baseline behaviors were observed.
Experimental design
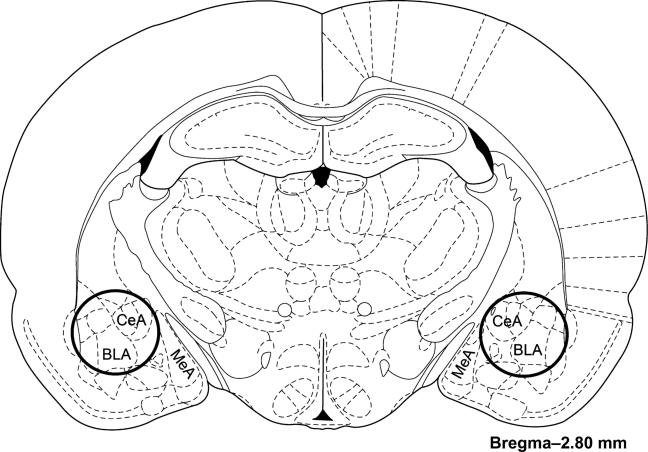
In experiment 1 (n = 48), the behavior of all the rats was observed at baseline and following 15 min of ferret exposure. In experiments 2 and 3, because behavior was not assessed, rats were not habituated to the behavioral testing procedure. In experiment 2 (n = 36), 18 rats were exposed to the ferret predator paradigm and 18 rats remained in their home cage to serve as controls. All rats were killed by decapitation 3 h following cessation of the ferret exposure and the brains were rapidly removed. The whole brain was placed in a block and it was cut into 2-mm-thick coronal sections. The majority of the amygdala (including the CeA and BLA) was removed with a 2-mm-diameter punch tool from a tissue slice that was located from –1.6 to –3.6 mm posterior of bregma. An atlas image (Paxinos & Watson 1998) was adapted to show the location of the punches (Fig. 1). The tissue punch was then frozen on dry ice and stored at –80°C until used for gene expression analysis. In experiment 3 (n = 16), eight rats were exposed to the ferret predator paradigm and eight rats served as home cage controls. All rats were killed by decapitation 3 h following cessation of the ferret exposure and their brains were rapidly removed and placed in isopentane at –30°C. The whole brains were stored at –80°C until used for in situ hybridization analysis.
Figure 1. Schematic image depicting the location of the amygdala tissue punches in experiment 2.
The whole rat brain was placed in a block and it was cut into 2-mm-thick coronal sections. The majority of the amygdala was removed with a 2 mm diameter punch tool from the tissue slice that was located from –1.6 to –3.6 mm posterior of bregma. The punches are depicted as dark solid line circles on the image adapted from an atlas (Paxinos & Watson 1998).
Behavioral testing
In experiment 1, the durations of the following behaviors were scored: rearing (raising both front paws off the floor of the test cage), grooming (licking and rubbing with paws of any accessible area of skin and fur), and locomotion and behavioral inhibition (BI). Behavioral inhibition is defined as the sum of freezing (a period of at least 3 seconds during which there is an absence of all movement except those required for respiration) and hypervigilance. Hypervigilance is characterized by an absence of locomotion with accompanying minor head movements associated with sensory acquisition (sniffing and vibrissa movement) (Roseboom et al. 2007). While freezing may be considered a form of hypervigilance, the hypervigilance and freezing behaviors were scored separately. Therefore, BI (the sum of freezing and hypervigilance) does not measure the same behavior twice.
Affymetrix gene chip analysis
In experiment 2, amygdala were combined into separate pools (each with three animals) from the 18 control rats and 18 stress rats, resulting in six pools for each condition. Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. The same RNA samples were used for the gene chip analysis and the quantitative real-time polymerase chain reaction (qRT-PCR) studies. Total RNA was quantified spectrophotometrically. The complementary RNA (cRNA) synthesis, labeling, hybridization and expression analysis were performed according to the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix Inc., Santa Clara, CA, USA). The quality of the cRNA synthesis was determined from the 3′/5′ ratio of rat housekeeping genes within the array. Each pool of RNA from the two conditions was used to prepare cRNA and each of these was hybridized to an independent Affymetrix Rat Genome 230 2.0 Array resulting in six arrays per condition. Expression levels for each set of probes were determined using the robust multigene average (gcRMA) and were imported into GeneSpring GX software (Version 7.3.1; Agilent Technologies, Palo Alto, CA, USA). Expression levels in the stressed condition were compared with the home cage control condition with each member of the SST gene family (SST, cortistatin, sst1, sst2, sst3, sst4 and sst5).
Quantitative real time-polymerase chain reaction analysis
Those gene expression changes that were statistically significant on the array were confirmed with qRT-PCR analysis. For each of the RNA pools from the home cage control and stressed conditions, a single reverse transcription reaction was performed to generate cDNA. Four qRT-PCR runs for each gene of interest were performed on each cDNA reaction. The PCR primers were designed using Primer Express 2.0 software, and each combination of gene-specific primers were optimized using rat whole brain cDNA. The following primers were used: for sst2, sense primer 5′-CCCGGTGGAAAGCAGCTA-3′ from nucleotides (NT) 1721 to 1738 of GenBank accession number NM_019348 and antisense primer 5′-CGCGCGGAACTTTGATTT-3′ from NTs 1768 to 1785 resulting in a 65 base pair amplicon; c-fos, sense primer 5′-AGAAATAAATCGCTATATCCACGTACTG-3′ from NT 1814 to 1841 of X06769 and antisense primer 5′-CAATGAACATGGACGCTGAAGA-3′ from NT 1846 to 1867 resulting in a 54 base pair amplicon; for ribosomal protein S12 (rps12), sense primer 5′-GCATCCAACTGTGATGAGCC-3′ from NT 275 to 294 of NM_031709 and antisense primer 5′-ACAACGCAACTGCAACCAAC-3′ from NTs 428 to 447 resulting in a 173 base pair amplicon. The Sequence Detection System 5700 (Applied Biosystems, Foster City, CA, USA) was used for qRT-PCR analysis.
Each PCR reaction contained a gene-specific forward and reverse primer (900 nm final concentration) and SYBR green PCR master mix (Applied Biosystems). The PCR temperature profile began with 10 min at 95°C for template denaturation and AmpliTaq Gold activation. Fluorescence generated by the SYBR green dye was measured during 40 cycles that alternated between 15 seconds at 95°C for denaturation, 60 seconds for annealing and extension at 56°C. To ensure that a single PCR product was being amplified, a product dissociation curve was then generated by cycling from 95 to 56°C and back to 95°C. The Ct value, the number of cycles required to reach a preset threshold level of fluorescence, was compared with a standard curve generated by performing qRT-PCR analysis on serial dilutions of rat whole brain cDNA. Relative quantities of PCR product were derived from the equation of the line determined by linear regression of the log of the cDNA amount in the standard curve vs. the corresponding Ct value. The rps12 gene was used as an endogenous internal control gene. The rps12 RNA was amplified in different wells on the same plate as the gene of interest using rps12 specific primers (500 nm final concentration) and relative levels of rps12 were determined in a manner identical to the genes of interest as described above. To normalize the data for an individual experimental sample, the mean of the replicate qRT-PCR signals for the gene of interest was divided by the mean of the replicate rps12 qRT-PCR signals. The normalized signal for the six stressed samples were then averaged and compared with the averaged normalized signal for the six home cage control samples.
In situ hybridization histochemistry
The double-stranded sst2 in situ hybridization probe was obtained by PCR amplification of rat whole brain cDNA and corresponded to bases 1386–2023 of GenBank accession number NM_019348. This PCR product was subcloned into pCRII-Topo (Invitrogen, Carlsbad, CA, USA). The plasmid was linearized with the restriction enzyme SpeI (Promega, Madison, WI, USA) and antisense [α-35S] uracyl tri-phosphate (UTP)-labeled riboprobe was generated by in vitro transcription using T7 Riboprobe System (Promega) as previously described (Herringa et al. 2004). Coronal sections (20 μm) were fixed in 4% paraformaldehyde and subsequently processed for in situ hybridization as previously described (Hsu et al. 2001).
Probed sections were exposed to phosphor screens for 4 days and scanned with a Typhoon phosphorimager (GE Healthcare, Piscataway, NJ, USA) at 50 μm resolution. All exposures were within the linear range of the phosphor screens. ImageQuant 5.0 software (GE Healthcare) was used to analyze positively hybridized probe signals. During the quantification process, the experimenter was blind to the experimental condition associated with each image. We analyzed 20 sections per animal for the BLA, 16 for the medial habenula (MHb) and 10 each for the medial nucleus of the amygdala (MeA), CeA, paraventricular nucleus of the hypothalamus (PVN) and anterior cingulate cortex (AC). Regions of interest (ROI) with fixed dimensions were utilized to assess signal intensity along the rostral–caudal extent of the BLA (1.2 by 1.35 mm ellipse), CeA (1.1 mm diameter circle) and MeA (1.65 by 2.0 mm ellipse), PVN (1.25 by 0.9 mm ellipse), AC (0.45 by 1.1 mm triangle) and MHb (0.6 mm diameter circle) in each hemisphere. Signal background was calculated by using an identically shaped ROI that was placed over an adjacent region that showed only very low hybridization signal. For the BLA, the object was placed over the thalamus, for the AC it was placed over the caudate putamen and for the CeA, MeA, MHb and PVN it was placed over the internal capsule. The sections that were used for signal quantification corresponded to the following anterior/posterior co-ordinates relative to Bregma – BLA (–1.3 to –4.1 mm), CeA (–1.5 to –3.3 mm), MeA (–2.0 to –3.5 mm), MHb (–1.5 to –4.1 mm), PVN (–1.5 to –1.9 mm) and the AC (0.2 to –1.3 mm). With the exception of the PVN, the highest average signal volume from consecutive sections was determined for each hemisphere in each region using a rolling average. For AC, PVN, MeA and CeA, the rolling average consisted of three sections and for BLA and MHb, the rolling average consisted of five sections. In each rat, the highest average signal volume from the left and right hemispheres were averaged together. For PVN, because the signals for the left and right hemispheres were in close proximity, a single ellipse was drawn that contained signal from both hemispheres. In addition, because there were only 3–4 sections per animal that contained a PVN signal, a rolling average method was not used. Instead, the PVN data represent the highest signal volume obtained from a single section for each animal.
Histological analysis
Rats were deeply anesthetized with sodium pentobarbital and perfused transcardially with isotonic saline followed by 10% formalin. Brains were stored in 10% sucrose and 10% formalin mixture before sectioning. Sections were stained with cresyl violet and examined under light microscopy to show the anatomy of the nuclei of interest.
Statistical analysis
Because the behavioral data were not normally distributed, it was analyzed with nonparametric Mann–Whitney U-tests using GraphPad Prism 4.03 software (GraphPad Software, San Diego, CA, USA). Initial analysis of the Affymetrix array data was performed with GeneSpring GX software such that lists of genes in which there was a significant difference between the control and ferret stress groups were obtained using nonparametric Mann–Whitney U-tests. For the specific analysis of the c-fos gene and SST family of genes, unpaired t-tests were performed using GraphPad Prism on both the Affymetrix expression array and qRT-PCR data. The data from the in situ hybridization studies was analyzed with unpaired Student's t-tests using GraphPad Prism.
Results
Experiment 1: ferret exposure increases stress-like behaviors in rats
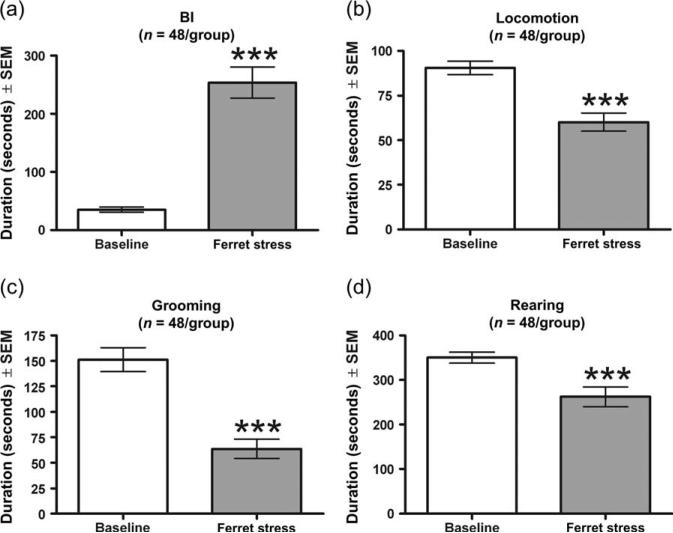
To assess the impact of psychological stress on behavior in this paradigm, rats were exposed in a separate cage to ferrets for a period of 15 min. Compared with control rats, ferret-exposed rats exhibited significantly increased levels of BI (U = 185.5, P < 0.001; Fig. 2a). In addition, ferret exposure significantly decreased total duration of locomotion (U = 547, P < 0.001; Fig. 2b), grooming (U = 419, P < 0.001; Fig. 2c) and rearing (U = 719, P = 0.002; Fig. 2d).
Figure 2. Acute ferret exposure increased levels of anxiety-related behaviors.
Ferret exposure significantly increased the duration of BI (a) and significantly decreased the duration of locomotion (b), grooming (c) and rearing (d). Bars represent the mean ± SEM for 48 independent determinations at baseline and during ferret exposure. ***P < 0.001 compared with control.
Experiment 2a: ferret exposure increases c-fos messenger RNA expression in the amygdala
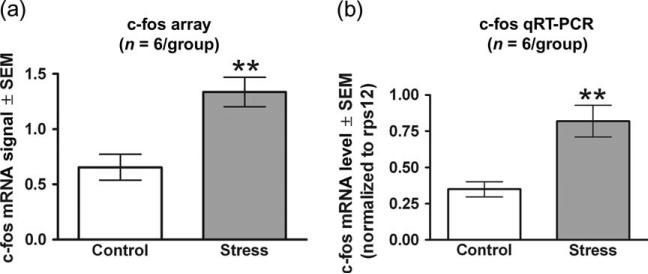
To determine if the amygdala was activated by ferret exposure, c-fos mRNA levels were assessed on Affymetrix expression arrays performed on samples obtained 3 h post-ferret exposure. The results were confirmed by qRT-PCR performed on the same samples. Compared with control rats, ferret exposure significantly increased c-fos mRNA expression as measured with Affymetrix expression arrays by 104% in the amygdala (t10 = 3.861, P = 0.003; Fig. 3a). The qRT-PCR studies confirmed this result, showing a 134% increase in ferret-induced c-fos mRNA expression in the amygdala (t10 = 3.893, P = 0.003; Fig. 3b).
Figure 3. Ferret exposure increases c-fos mRNA within the amygdala as observed from Affymetrix expression array data and validated by qRT-PCR analysis.
Gene expression changes were first obtained using the Rat Genome 230 2.0 array and subsequently validated by qRT-PCR. Each of the two techniques used the same six pools of RNA (each pool comprised of three animals) for both the experimental and control conditions. (a) Expression data obtained from the arrays (one array for every pool) were gcRMA processed and the resulting normalized values were averaged for each condition. (b) The qRT-PCR data were obtained by averaging the results from four separate qPCR runs on a single cDNA synthesis for each RNA pool. The numbers represent mRNA levels expressed as a ratio following normalization to the rps12 housekeeping gene mRNA levels. Ferret stress produced an increase in c-fos mRNA as measured by both array and qRT-PCR. Bars represent the mean ± SEM for six independent determinations at baseline and following ferret exposure. **P < 0.01 compared with control.
Experiment 2b: analysis of the stress-induced changes in mRNA expression for the SST gene family
In examining changes in mRNA levels 3 h post-ferret exposure, Affymetrix gene chip analysis showed that of 31 099 probe sets representing over 30 000 transcripts and variants derived from over 28 000 different genes, on average 62.0% (19 268) of the probe sets were detected as present on each chip. Of these genes, there were a total of 1360 (7% of the detected probe sets) that showed a statistically significant stress-induced change in expression, with 951 genes increasing in expression and 409 genes decreasing in expression.
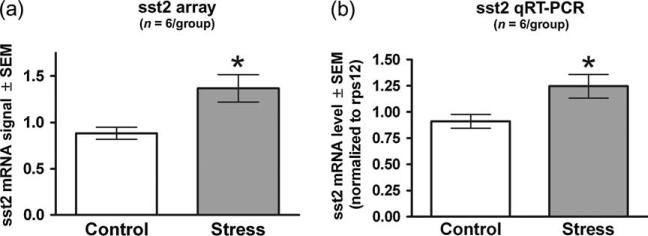
When examining the SST gene family with Affymetrix expression array analysis (Table 1), the sst2 gene was the only member of the family that showed a significant change in expression. Specifically, amygdala levels of sst2 mRNA were 55% higher following ferret stress (t10 = 3.009, P = 0.0131; Fig. 4a) compared with home cage control animals. The qRT-PCR studies confirmed this result, showing a 37% increase in ferret stress-induced sst2 mRNA expression in the amygdala (t10 = 2.565, P = 0.028; Fig. 4b). It should be noted that although it did not reach statistical significance on the Affymetrix expression arrays (t10 = 1.923; P = 0.083; Table 1), there was a trend for sst4 mRNA expression to be increased (by 28%) in the ferret stress group.
Table 1.
SST gene family Affymetrix expression array data
| Gene | Home cage control expression | Ferret-induced expression | % change |
|---|---|---|---|
| SST | 0.917 ± 0.079 | 1.049 ± 0.103 | +14 |
| Cortistatin | 1.010 ± 0.050 | 1.024 ± 0.076 | +1 |
| sst1 | 0.956 ± 0.079 | 1.040 ± 0.070 | +9 |
| sst2 | 0.883 ± 0.065 | 1.367 ± 0.147 | +55* |
| sst3 | 0.943 ± 0.113 | 1.074 ± 0.084 | +14 |
| sst4 | 0.936 ± 0.036 | 1.195 ± 0.130 | +28 |
| sst5 | 1.013 ± 0.032 | 0.985 ± 0.011 | –3 |
Gene expression changes were obtained using Affymetrix Rat Genome 230 2.0 arrays on six pools of RNA (each poolcomprised of three animals) for both the ferret stress and control conditions as described in Materials and methods. Expression data obtained from the arrays (one array for every pool) were gcRMA processed and the resulting normalized values were averaged for both conditions. The normalized data for each member of the SST family (SST, cortistatin and sst1-5) are presented in the table. Ferret stress produced a significant increase in only the sst2 mRNA. Values represent the mean ± SEM for six independent determinations at baseline and during ferret exposure.
P < 0.05 compared with control.
Figure 4. Ferret exposure increases sst2 mRNA within the amygdala as observed from Affymetrix expression array data and validated by qRT-PCR analysis.
Gene expression changes were first obtained using the Rat Genome 230 2.0 array and subsequently validated by qRT-PCR. Each of the two techniques used the same six pools of RNA (each pool comprised of three animals) for both the experimental and control conditions. (a) Expression data obtained from the arrays (one array for every pool) were gcRMA processed and the resulting normalized values were averaged for each condition. (b) The qRT-PCR data were obtained by averaging the results from four separate qPCR runs on a single cDNA synthesis for each RNA pool. The numbers represent mRNA levels expressed as a ratio following normalization to the rps12 housekeeping gene mRNA levels. Ferret stress produced an increase in sst2 mRNA as measured by both array and qRT-PCR. Bars represent the mean ± SEM for six independent determinations at baseline and following ferret exposure. *P < 0.05 compared with control.
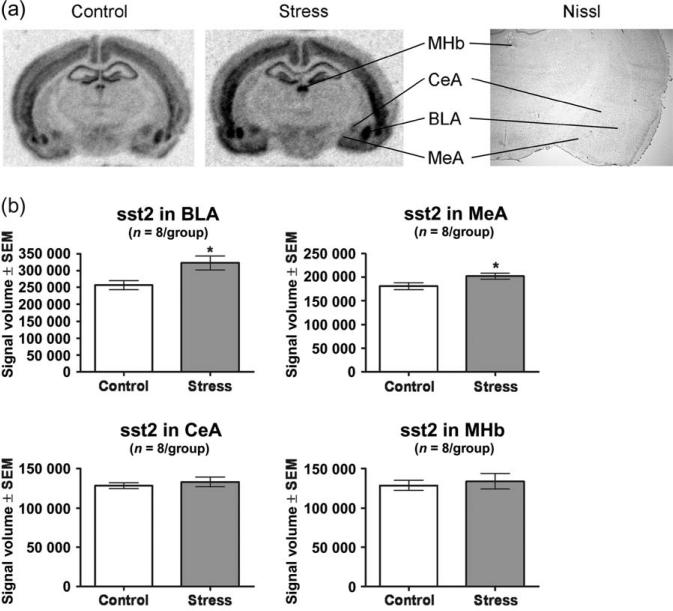
Experiment 3: localization of the sst2 changes in mRNA expression by in situ hybridization
This study sought to localize the changes in sst2 mRNA expression to specific nuclei within the amygdala and to determine whether sst2 mRNA levels changed in other brain regions 3 h post-ferret exposure. Ferret exposure significantly increased expression of sst2 mRNA within the BLA by 26% compared with the control condition (t14 = 2.653, P = 0.019; Fig. 5a,b) and induced a modest (12%) significant increase within the MeA (t14 = 2.144, P = 0.050; Fig. 5a,b). In contrast, ferret exposure did not alter sst2 mRNA levels within the CeA (t14 = 0.6635, P = 0.518; Fig. 5a,b).
Figure 5. Ferret exposure increased sst2 mRNA levels within the BLA and MeA but not the CeA and MHb.
In situ hybridization against sst2 mRNA was performed on the brains from eight ferret-stressed rats and from eight home cage controls. Ferret-stressed rats were killed 3 h following the cessation of exposure; control rats remained in their home cages until the time of killing. (a) Phosphor images showing the MHb, CeA, BLA and MeA regions from a control and ferret-stressed rat along with a Nissl-stained section showing the same regions. (b) Ferret exposure produced an increase in sst2 mRNA signal volume within the BLA and MeA but not the MHb and CeA. Bars represent the mean ± SEM for each group from eight independent determinations. *P < 0.05 compared with control.
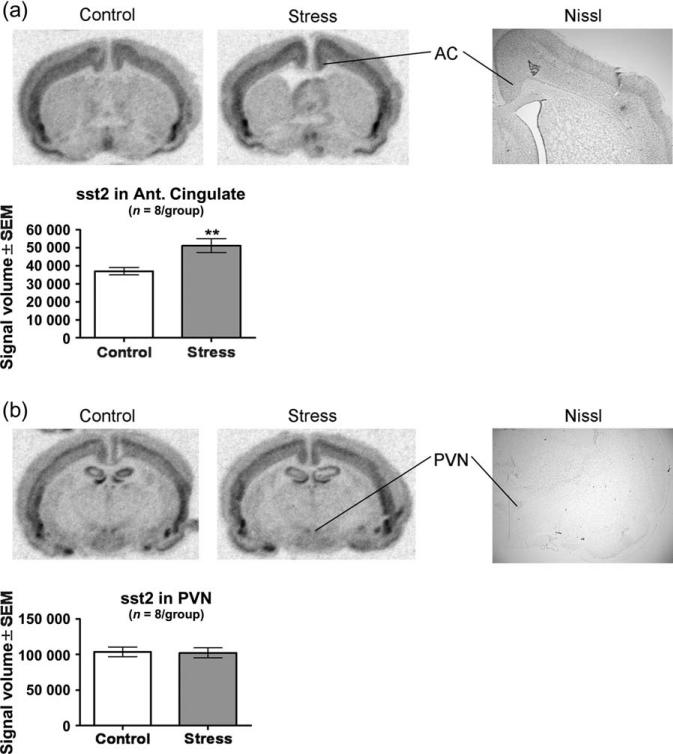
Examination of the in situ hybridization images from the same brain slices showed that in addition to the amygdala, sst2 is widely expressed throughout the brain. We examined expression within the MHb because sst2 levels within this region were among the highest in the CNS. Ferret exposure did not alter levels of sst2 mRNA within the MHb (t14 = 0.4302, P = 0.674; Fig. 5a,b). We also examined sst2 expression within the AC because this cortical region is associated with the contextual regulation of anxiety and fear (Davidson et al. 2000; Kalin et al. 2005) and found that ferret exposure significantly increased expression of sst2 mRNA within the AC by 38% compared with the control condition (t14 = 3.208, P = 0.006; Fig. 6a). The PVN is one of the major regions of SST and sst2 expression within the brain related to the neuroendocrine effects of SST (Crowley & Terry 1980; Dournaud et al. 1996). Ferret exposure did not alter levels of sst2 mRNA within the PVN (t14 = 0.1495, P = 0.883; Fig. 6b).
Figure 6. Ferret exposure increased sst2 mRNA levels within the BLA, MeA and anterior cingulate cortex (AC) but not the CeA, MHb and PVN.
In situ hybridization against sst2 mRNA was performed on the brains from eight ferret-stressed rats and from eight home cage controls. Ferret-stressed rats were killed 3 h following the cessation of exposure; control rats remained in their home cages until time of killing. (a) Phosphor images showing the AC from a control and ferret-stressed rat along with a Nissl-stained section showing the same region. Ferret exposure produced an increase in sst2 mRNA signal volume within the AC. (b) Phosphor images showing the PVN from a control and ferret-stressed rat along with a Nissl-stained section showing the same region. Ferret exposure did not alter sst2 mRNA signal volume within the PVN. Bars represent the mean ± SEM for each group from eight independent determinations. **P < 0.01 compared with control.
Discussion
Understanding the neural and biochemical pathways that underlie the adaptive and maladaptive responses to stress will provide insight into the neurobiology of stress-related psychopathology. The present study, consistent with our earlier work (Roseboom et al. 2007) and that of others (Masini et al. 2005), shows that ferret exposure, an ethologically relevant psychological stressor, elicits anxiety-related behaviors in rats. The current ferret exposure paradigm is a modification from our previous work with the current paradigm having the rat in a larger cage that was placed near the ferret housing cage but not inside it. Although the current ‘indirect ferret exposure paradigm’ qualitatively appears to be a milder stressor than the previous direct ferret paradigm, it nonetheless robustly increased stress-like behaviors. These ferret-evoked behavioral changes are defensive responses and are consistent with those observed following exposure to other natural predators. Previous reports showed that rats exposed to cat or cat odors display an increase in fear-related behavioral responses such as freezing, avoidance and risk assessment (Blanchard et al. 1989; McGregor et al. 2002).
Given the importance of the amygdala in mediating adaptive and maladaptive fear and anxiety responses (Blanchard & Blanchard 1972; Kalin et al. 2004; LeDoux et al. 1988), it is particularly significant that in this study as well as others using predator odor (Dielenberg et al. 2001; Masini et al. 2005), ferret exposure increased c-fos mRNA expression within the amygdala. Using immunohistochemistry, we previously found that direct ferret exposure increased the number of Fos immunoreactive cells within the MeA, CeA and BLA (Roseboom et al. 2007).
A critical step in mediating adaptive or maladaptive responses to stress is the regulation of downstream late-response genes. To identify downstream late-response genes that are altered following ferret exposure, we employed microarray technologies. Of particular interest was the SST family of genes because of their involvement with stress and stress-related disorders (Fendt et al. 1996; Rubinow et al. 1988; Viollet et al. 2000; Zhang et al. 1999). Our microarray studies showed a ferret-induced increase in sst2 expression within the amygdala that was confirmed by qRT-PCR but did not show significant changes in mRNA expression for the other members of the SST family of genes. The lack of positive findings for the other members of the SST family of genes is consistent with sst2 being the SST receptor directly implicated in mediating stress and anxiety-like behaviors (Viollet et al. 2000). Thus, it is likely that sst2 is the member of the SST family that is most relevant to stress-induced psychopathology. However, it is important to acknowledge that stress-induced changes in the other members of the SST gene family may follow a different time–course than what was assessed in the current experiment. Finally, it is possible that the proteins of the other members of the SST family may play a role in response to stress although our results suggest their mRNA levels do not change.
Both the BLA and MeA play a role in the unconditioned response to predator threat. Previous studies using cat or fox odor have shown a stress-induced increase in the number of Fos-positive cells (Funk & Amir 2000; McGregor et al. 2004) and increased c-fos mRNA expression (Masini et al. 2005) within the MeA and BLA. Furthermore, direct ferret exposure increased the number of Fos-positive cells within the MeA and BLA (Roseboom et al. 2007). Using excitotoxic lesions or chemical inactivation, many studies have linked BLA and MeA to increases in fear and anxiety-like behaviors following exposure to predator odors (Blanchard et al. 2005; Li et al. 2004; Muller & Fendt 2006; Takahashi et al. 2005). Our in situ hybridization studies show an increase in sst2 mRNA expression in the MeA and BLA in response to ferret exposure, suggesting the possibility that sst2 within these regions is involved in mediating the response to ferret predator threat.
The role of the CeA in the unconditioned response to predator threat is less clear. Studies have shown that exposure to cat odor (Dielenberg et al. 2001) or ferret odor (Masini et al. 2005) failed to increase expression of c-fos mRNA within the CeA. However, other studies have shown that exposure to fox odor (Day et al. 2004) or ferret exposure (Roseboom et al. 2007) significantly increased the number of CeA Fos-positive cells. In the present study, we show that ferret exposure does not alter expression of sst2 within the CeA suggesting that sst2 within the CeA does not play a role in mediating responses induced by psychological stress. However, the lack of an sst2 mRNA change does not rule out the possibility of a predator stress-induced increase in SST signaling in the CeA.
We examined other brain regions outside the amygdala that showed significant sst2 expression. Although the MHb displays robust sst2 expression, this expression does not appear to be affected by ferret exposure. This is consistent with reports suggesting that the MHb does not have a prominent role in the stress response (Chastrette et al. 1991; Roseboom et al. 2007).
The AC has been implicated in emotion regulation and has reciprocal projections to the basolateral regions of the amygdala (Allman et al. 2001). Human imaging studies show alterations in AC function in relation to depression (Fitzgerald et al. 2007). Preclinical studies suggest that the AC modulates depression-related coping behaviors in rats (Bissiere et al. 2006) and activation of the AC is associated with the regulation of threat-induced freezing in monkeys (Kalin et al. 2005). Previous reports have shown an increased number of Fos-positive neurons within the AC following cat exposure (Canteras et al. 1997). Our studies show a significant increase in sst2 mRNA expression in the AC in response to ferret exposure, suggesting a role for this brain region in response to psychological stress.
The PVN is integral to the stress response and predator exposure can activate the PVN as evidenced by an increase in the level of corticotropin-releasing factor mRNA (Figueiredo et al. 2003). While examination of our in situ hybridization images showed that the PVN is a major site of sst2 expression, we did not observe stress-induced alterations in sst2 mRNA levels in the PVN. As a full time–course study was not performed, one should be cautious in interpreting these findings. Furthermore, the lack of an sst2 mRNA change does not rule out the possibility of a predator stress-induced increase in SST signaling in the PVN.
Numerous associations between alterations in SST levels and human pathology have been described. Decreased levels of cerebral spinal fluid SST have been reported in senile dementia (Oram et al. 1981), Parkinson's disease (Dupont et al. 1982), Alzheimer's disease (Wood et al. 1982) and depression (Rubinow et al. 1983, Sunderland et al. 1987). In addition, an SST analog has been successfully used to treat panic-like attacks (Abelson et al. 1990).
In preclinical studies, long-term (25 days) peripheral administration of SST modifies food intake, body weight, gut motility and taste preference (Scalera & Tarozzi 1998a,b). Acute brain site-specific activation of sst receptors in the neostriatum or ventral pallidum/substantia innominata decreases locomotion (Marazioti et al. 2005; Tashev et al. 2004) and in the pontine reticular nucleus blocks fear-potentiated acoustic startle (Fendt et al. 1996). It is important to note that there are no studies examining anxiety-like or depressive behaviors following SST administration into the rat amygdala. However, whole cell recordings show that SST induces an inwardly rectifying K+ current in amygdala neurons resulting in a dampening of cell excitability (Meis et al. 2005). Taken together, these studies suggest that SST may play an important role in the responses to fear and emotion-related stimuli within the amygdala.
Somatostatin receptor 2 knockout studies have showed that sst2 knockout mice exposed to stress display a behavioral profile that is consistent with increased anxiety (Viollet et al. 2000). This suggests that sst2 activation may be anxiolytic and perhaps also have antidepressant effects. However, the results of knockout studies must be viewed with caution as some of the behavioral alterations may result from compensatory changes in other systems resulting from development in the absence of sst2.
In conclusion, we show that acute ferret exposure elicits an increase in anxiety-like behaviors, activates the amygdala and increases sst2 mRNA levels within the BLA, MeA and AC. Given the importance of these regions in mediating the stress response and emotion regulation, our findings suggest that sst2 may play a role in mediating both the normal and pathological responses to stress exposure. Furthermore, given that increased SST signaling has been shown to be anxiolytic and that sst2 knockout mice display increased stress-related and anxiety-like behaviors, we postulate that the observed stress-induced increases in sst2 expression within the amygdala may serve to mitigate the effects of stress or facilitate adaptation to stress.
Acknowledgments
The authors thank Daniel Mackenrodt from the University of Wisconsin (Madison, WI, USA) for assisting in the performance of the behavioral studies. This work was funded by National Institutes of Health grant MH40855 (N.H.K.), the University of Wisconsin HealthEmotions Research Institute and Meriter Hospital.
References
- Abelson JL, Nesse RM, Vinik A. Treatment of panic-like attacks with a long-acting analogue of somatostatin. J Clin Psychopharmacol. 1990;10:128–132. doi: 10.1097/00004714-199004000-00010. [DOI] [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Batten TF, Gamboa-Esteves FO, Saha S. Evidence for peptide co-transmission in retrograde- and anterograde-labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci. 2002;98:28–32. doi: 10.1016/s1566-0702(02)00026-7. [DOI] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe HR, Cryan JF. The rostral anterior cingulate cortex modulates depression but not anxiety-related behaviour in the rat. Behav Brain Res. 2006;175:195–199. doi: 10.1016/j.bbr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Hori K. Ethoexperimental approaches to the study of defense. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental Approaches to the Study of Behavior. Kluwer Academic Publishers; Boston: 1989. pp. 114–136. [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Brownstein M, Arimura A, Sato H, Schally AV, Kizer JS. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975;96:1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Nat Acad Sci U S A. 1992;89:11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscail L, Delesque N, Esteve JP, Saint-Laurent N, Prats H, Clerc P, Robberecht P, Bell GI, Liebow C, Schally AV, Vaysse N, Susini C. Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Nat Acad Sci U S A. 1994;91:2315–2319. doi: 10.1073/pnas.91.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Chiavegatto S, Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- Chastrette N, Pfaff DW, Gibbs RB. Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Res. 1991;563:339–344. doi: 10.1016/0006-8993(91)91559-j. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Terry LC. Biochemical mapping of somatostatinergic systems in rat brain: effects of periventricular hypothalamic and medial basal amygdaloid lesions on somatostatin-like immunoreactivity in discrete brain nuclei. Brain Res. 1980;200:283–291. doi: 10.1016/0006-8993(80)90920-8. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–151. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Dierickx K, Vandesande F. Immunocytochemical localization of somatostatin-containing neurons in the rat hypothalamus. Cell Tissue Res. 1979;201:349–359. doi: 10.1007/BF00236995. [DOI] [PubMed] [Google Scholar]
- Dournaud P, Gu YZ, Schonbrunn A, Mazella J, Tannenbaum GS, Beaudet A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Dupont E, Christensen SE, Hansen AP, de Fine Olivarius B, Orskov H. Low cerebrospinal fluid somatostatin in Parkinson disease: an irreversible abnormality. Neurology. 1982;32:312–314. doi: 10.1212/wnl.32.3.312. [DOI] [PubMed] [Google Scholar]
- Fendt M, Koch M, Schnitzler HU. Somatostatin in the pontine reticular formation modulates fear potentiation of the acoustic startle response: an anatomical, electrophysiological, and behavioral study. J Neurosci. 1996;16:3097–3103. doi: 10.1523/JNEUROSCI.16-09-03097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–5258. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Finley JC, Maderdrut JL, Roger LJ, Petrusz P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience. 1981;6:2173–2192. doi: 10.1016/0306-4522(81)90006-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20426. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Helboe L, Stidsen CE, Moller M. Immunohistochemical and cytochemical localization of the somatostatin receptor subtype sst1 in the somatostatinergic parvocellular neuronal system of the rat hypothalamus. J Neurosci. 1998;18:4938–4945. doi: 10.1523/JNEUROSCI.18-13-04938.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain Res Mol Brain Res. 2004;131:17–25. doi: 10.1016/j.molbrainres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Lombardo KA, Herringa RJ, Bakshi VP, Roseboom PH, Kalin NH. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neuroscience. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluxen FW, Bruns C, Lubbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Nat Acad Sci U S A. 1992;89:4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch B. Hypothalamic and extrahypothalamic distribution of somatostatin-immunoreactive elements in the rat brain. Cell Tissue Res. 1978;195:499–513. doi: 10.1007/BF00233892. [DOI] [PubMed] [Google Scholar]
- Krulich L, Dhariwal AP, McCann SM. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology. 1968;83:783–790. doi: 10.1210/endo-83-4-783. [DOI] [PubMed] [Google Scholar]
- de Lecea L. Cortistatin: a natural somatostatin analog. J Endocrinol Invest. 2005;28:10–14. [PubMed] [Google Scholar]
- de Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR, Henriksen SJ, Sutcliffe JG. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature. 1996;381:242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Forte M, North RA, Ross CA, Snyder SH. Cloning and expression of a rat somatostatin receptor enriched in brain. J Biol Chem. 1992;267:21307–21312. [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Marazioti A, Kastellakis A, Antoniou K, Papasava D, Thermos K. Somatostatin receptors in the ventral pallidum/substantia innominata modulate rat locomotor activity. Psychopharmacology (Berl) 2005;181:319–326. doi: 10.1007/s00213-005-2237-z. [DOI] [PubMed] [Google Scholar]
- Martin JL, Chesselet MF, Raynor K, Gonzales C, Reisine T. Differential distribution of somatostatin receptor subtypes in rat brain revealed by newly developed somatostatin analogs. Neuroscience. 1991;41:581–593. doi: 10.1016/0306-4522(91)90351-n. [DOI] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meis S, Sosulina L, Schulz S, Hollt V, Pape HC. Mechanisms of somatostatin-evoked responses in neurons of the rat lateral amygdala. Eur J Neurosci. 2005;21:755–762. doi: 10.1111/j.1460-9568.2005.03922.x. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Wulfsen I, Schonrock C, Fehr S, Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Nat Acad Sci U S A. 1992;89:10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyse E, Slama A, Videau C, de Angela P, Kordon C, Epelbaum J. Regional distribution of somatostatin receptor affinity states in rat brain: effects of divalent cations and GTP. Regul Pept. 1989;26:225–234. doi: 10.1016/0167-0115(89)90190-0. [DOI] [PubMed] [Google Scholar]
- Muller M, Fendt M. Temporary inactivation of the medial and basolateral amygdala differentially affects TMT-induced fear behavior in rats. Behav Brain Res. 2006;167:57–62. doi: 10.1016/j.bbr.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- O'Carroll AM, Lolait SJ, Konig M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992;42:939–946. [PubMed] [Google Scholar]
- Oram JJ, Edwardson J, Millard PH. Investigation of cerebrospinal fluid neuropeptides in idiopathic senile dementia. Gerontology. 1981;27:216–223. doi: 10.1159/000212476. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Raynor K, Reisine T. Somatostatin receptors. Crit Rev Neurobiol. 1992;6:273–289. [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Bakshi VP, Trentani A, Newman SM, Kalin NH. Predator threat induces behavioral inhibition, pituitary-adrenal activation and changes in amygdala CRF-binding protein gene expression. Psychoneuroendocrinology. 2007;32:44–55. doi: 10.1016/j.psyneuen.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Gold PW, Post RM, Ballenger JC, Cowdry R, Bollinger J, Reichlin S. CSF somatostatin in affective illness. Arch Gen Psychiatry. 1983;40:409–412. doi: 10.1001/archpsyc.1983.01790040063009. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Davis CL, Post RM. Somatostatin in neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 1988;(Suppl 12):S137–S155. doi: 10.1016/0278-5846(88)90077-2. [DOI] [PubMed] [Google Scholar]
- Scalera G, Tarozzi G. Somatostatin administration alters taste preferences in the rat. Peptides. 1998a;19:1565–1572. doi: 10.1016/s0196-9781(98)00108-9. [DOI] [PubMed] [Google Scholar]
- Scalera G, Tarozzi G. Somatostatin administration modifies food intake, body weight, and gut motility in rat. Peptides. 1998b;19:991–997. doi: 10.1016/s0196-9781(98)00053-9. [DOI] [PubMed] [Google Scholar]
- Schindler M, Sellers LA, Humphrey PP, Emson PC. Immunohistochemical localization of the somatostatin SST2(A) receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- Stroh T, Kreienkamp HJ, Beaudet A. Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain. J Comp Neurol. 1999;412:69–82. doi: 10.1002/(sici)1096-9861(19990913)412:1<69::aid-cne5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Rubinow DR, Tariot PN, Cohen RM, Newhouse PA, Mellow AM, Mueller EA, Murphy DL. CSF somatostatin in patients with Alzheimer's disease, older depressed patients, and age-matched control subjects. Am J Psychiatry. 1987;144:1313–1316. doi: 10.1176/ajp.144.10.1313. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Tashev R, Belcheva S, Belcheva I. Differential effects of somatostatin on exploratory behavior after unilateral injections in to rat neostriatum. Peptides. 2004;25:123–128. doi: 10.1016/j.peptides.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Viollet C, Vaillend C, Videau C, Bluet-Pajot MT, Ungerer A, L'Heritier A, Kopp C, Potier B, Billard J, Schaeffer J, Smith RG, Rohrer SP, Wilkinson H, Zheng H, Epelbaum J. Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur J Neurosci. 2000;12:3761–3770. doi: 10.1046/j.1460-9568.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- Wood PL, Etienne P, Lal S, Gauthier S, Cajal S, Nair NP. Reduced lumbar CSF somatostatin levels in Alzheimer's disease. Life Sci. 1982;31:2073–2079. doi: 10.1016/0024-3205(82)90099-6. [DOI] [PubMed] [Google Scholar]
- Zhang K, Hamanaka K, Kitayama I, Soya H, Yoshizato H, Nakase S, Uesugi Y, Inui K, Nomura J, Okazaki Y. Decreased expression of the mRNA for somatostatin in the periventricular nucleus of depression-model rats. Life Sci. 1999;65:PL87–P94. doi: 10.1016/s0024-3205(99)00326-4. [DOI] [PubMed] [Google Scholar]