Abstract
Increased matrix metalloproteinase (MMP) proteolytic activity contributes to the pathogenesis of many neuroinflammatory and neurodegenerative conditions in the CNS. To fully understand this process, it is important to define the MMP expression profile of specific cell types, including the CNS-resident cells astrocytes and microglia. While previous studies have characterized astrocyte MMP expression by using mixed glial cultures, these results are likely complicated by the presence of contaminating microglia within these cultures. In the current study, we sought to clarify this complexity, by taking a novel approach to prepare pure astrocyte cultures entirely devoid of microglia, by promoting neural stem cell (NSC) differentiation into astrocytes. The MMP expression profile of mixed glial cultures, neurosphere-derived astrocytes, and pure microglia was characterized by RNase protection assay. This revealed that MMP gene expression is largely cell-type specific. Astrocytes constitutively expressed MMP-11, MMP-14, and MMP-2 and showed induction of MMP-3 in response to IL-1β but did not respond to lipopolysaccharide (LPS). In contrast, microglia constitutively expressed high levels of MMP-12 and showed strong induction of MMP-9 and MMP-14 in response to LPS. Gelatin zymography confirmed that LPS and TNF-α induced strong expression of MMP-9 in microglia but not astrocytes. In summary, these studies demonstrate that neurosphere-derived astrocytes represent an attractive alternative system in which to study astrocyte behavior in vitro. Using this system, we have shown that astrocytes and microglia express distinct sets of MMP genes and that microglia, not astrocytes, are the major source of MMP-9 in response to LPS or TNF-α.
Keywords: microglia, astrocytes, MMP
INTRODUCTION
In the CNS, glial cells play active roles in regulating the homeostatic balance. As an integral part of the blood-brain barrier (BBB), astrocytes help to limit the influx of potentially toxic factors into the CNS (Ballabh et al., 2004; del Zoppo and Milner, 2006; Huber et al., 2001; Janzer and Raff, 1987). They also absorb excess levels of potassium ions and excitotoxic neurotransmitters such as glutamate (Nedergaard et al., 2003; Ransom et al., 2003; Ridet et al., 1997). As the principal immune effector cells in the CNS, microglia also play a vital function by maintaining immune surveillance (Carson, 2002). Following stimulation by invading microorganisms, trauma, or as part of autoimmune disease, both astrocytes and microglia undergo transformation into activated phenotypes (Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Raivich et al., 1999). It is now widely accepted that this cellular activation can be both beneficial and detrimental to the overall health of the CNS. When astrocytes undergo reactive gliosis, they provide a useful function by attempting to limit the damage within the CNS, but this also leads to formation of a reactive glial scar (Ridet et al., 1997), which prevents neurite regeneration (Fawcett, 1997; Fawcett and Asher, 1999) and remyelination (Franklin and ffrench-Constant, 1996). In a similar vein, microglia respond very quickly to stimuli and become highly migratory aggressive cells that phagocytose foreign microorganisms and tissue debris, thus paving the way for tissue regeneration (Hanisch and Kettenmann, 2007; Kreutzberg, 1996; Raivich et al., 1999). However, chronically activated microglia can also lead to unwanted and excessive tissue damage, such as that which occurs during autoimmune breakdown of myelin in multiple sclerosis (MS) (Gonzalez-Scarano and Baltuch, 1999; Ransohoff, 1999; Trapp et al., 1999).
The matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidase enzymes that in the CNS are responsible for remodeling of the extracellular matrix (ECM) (Rosenberg, 2002; Yong et al., 2001) and cleavage of proteins present in myelin (Chandler et al. 1995; Proost et al., 1993) and tight junction complexes (Gurney et al., 2006; Lohmann et al., 2004; Yang et al., 2007). Together with the tissue inhibitors of metalloproteinases (TIMPs), the MMPs form a dynamic system that maintains the homeostatic balance of ECM breakdown or regeneration (Crocker et al., 2004; Rosenberg, 2002). In many disease states, this balance is lost, and excessive MMP proteolytic activity is thought to contribute to the pathogenesis of a number of CNS diseases, including MS, cerebral ischemia, Parkinson's disease, Alzheimer's disease, and traumatic injury (Crocker et al., 2004; Rosenberg, 2002).
Recently, we analyzed the MMP expression profile in purified cell cultures of microglia and enriched astrocytes (Crocker et al., 2006). In these studies, we used the traditional method of mixed glial cultures to represent astrocytes, which consist predominantly of astrocytes with variable numbers of contaminating microglia. This revealed that microglia and astrocytes appear to show an overlapping MMP expression profile. However, as we used the mixed glial culture system to represent astrocytes, we could not exclude the possibility that some of the MMPs within the mixed glial cultures were originating from contaminating microglia within this culture. This is an extremely important point, especially in light of the fact that the vast majority of previous studies of astrocyte MMP expression employed the same mixed glial culture system (Arai et al., 2003; Lee et al., 2003; Liu et al., 2006; Liuzzi et al., 2004; Shin et al., 2007; Tejima et al., 2006; Wang et al., 2002). For this reason, in the current study, we took an alternative approach to obtain pure astrocyte cultures entirely devoid of microglia, by adapting a neurosphere culture system.
Neural stem cells (NSC) are tripotential, giving rise to neurons, astrocytes, or oligodendrocytes (Reynolds et al., 1992; Reynolds and Weiss, 1992; Reynolds and Weiss, 1996), but importantly not microglia (Levison et al., 2003), which are derived from the hematopoietic lineage (Hess et al., 2004; Rezaie and Male, 1999; Simard and Rivest, 2004). In the current study, we obtained pure astrocyte cultures by growing NSC in the presence of media containing 10% serum, which promotes NSC differentiation into astrocytes (Brunet et al., 2004; Loo et al., 1995; Sakai et al., 1990). After confirming astrocyte purity and a total absence of microglia in these cultures, we then employed the RNase protection assay (RPA) to characterize the MMP expression profile in the three different types of culture: traditional mixed glial cultures, pure neurosphere-derived astrocyte cultures, and pure microglial cultures. Gelatin zymography was then used to specifically examine astrocyte and microglial expression of MMP-2 and MMP-9 at the protein level, following stimulation by LPS, IL-1β, or TNF-α.
MATERIALS AND METHODS
Cell Culture
The use of animals in this protocol was approved by the Committee on Animal Protocols, Department of Animal Resources, The Scripps Research Institute. Mixed glial cultures were prepared from postnatal (day 0–2) C57Bl/6 mouse pups, as previously described (Milner and Campbell, 2002; Milner and Campbell, 2003). Cultures were maintained in poly-d-lysine coated T75 flasks in DMEM (Sigma-Aldrich, St. Louis, MO) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich) for 7–10 days, before being mechanically shaken to yield microglia. Cells that maintained adherence to the flask, consisting predominantly of astrocytes, were then plated out into six-well plates (Nunc, Naperville, IL) coated with poly-d-lysine. Purified microglia were also plated into poly-d-lysine coated six-well plates. The purity of these microglial cultures was >99% as determined by Mac-1 positivity in flow cytometry.
Primary cultures of neurospheres were obtained from postnatal (P0–P2) C57BL6 mouse brains as described previously (Jacques et al., 1998; Milner, 2007). Neurospheres were subsequently passaged every 5–7 days into fresh flasks with fresh media. Neurospheres were differentiated into astrocytes by culturing them on poly-d-lysine coated T75 flasks in DMEM containing 10% FBS and cultured to confluence. After 2–3 weeks, these cultures were passaged into six-well plates and maintained in DMEM containing 10% FBS. The astrocyte purity of these cultures was >99% as determined by GFAP positivity by immunocytochemistry. Flow cytometry and immunocytochemistry demonstrated the total absence of Mac-1 positive cells in these cultures.
Immunocytochemistry
Mixed glial cultures and NSC-derived astrocytes were cultured on poly-d-lysine coated glass coverslips in DMEM containing 10% FBS. All incubations were performed at room temperature with washes in between. Cells were blocked in 5% normal goat serum (NGS) in PBS for 30 min then live-labeled with a Mac-1 monoclonal antibody for 1 h, then incubated with anti-rat-Cy3 for 30 min, fixed in acid/alcohol (95:5) at –20°C for 30 min, then incubated with anti-GFAP for 1 h, followed by FITC-anti-rabbit for 30 min before being mounted in aquamount (Polysciences, Warrington, PA). The MMP localization studies were performed the same way except that cultures were first fixed in acetone/methanol (50:50) at –20°C for 30 min. Monoclonal antibodies against MMP-3 or MMP-9 were obtained from R&D Systems. Monoclonal antibodies specific for the integrin subunits α1 (Ha1/29), α5 (5H10-27), α6 (GoH3) and Mac-1 (M1/70) and isotype control monoclonal antibodies were obtained from BD Pharmingen (La Jolla, CA). The TLR4 rat monoclonal antibody (MTS510) was obtained from eBioscience (La Jolla, CA). The mouse monoclonal antibody against β-dystroglycan (43DAG/8D5) was obtained from Novocastra, Newcastle-upon-Tyne, United Kingdom). The rabbit polyclonal anti-GFAP antibody was obtained from Sigma.
RNase Protection Assay
Multiprobe RNase Protection Assays (RPA) were used to quantitatively assess changes in the expression of MMP genes, as previously described (Crocker et al., 2006). For the RPA analysis, mixed glial cultures, astrocytes, and microglia were maintained under serum-free conditions for 24 h either under control conditions (no cytokines) or in the presence of LPS (20 ng/ml; Sigma-Aldrich) or IL-1β (10 ng/ml; Peprotech, Rocky Hill, NJ). RPA analyses were performed on total RNA samples (5–10 μg) prepared using TriReagent (Sigma-Aldrich). For analysis, each lane represented an RNA sample derived from individually treated wells. RPA gels were visualized using Kodak Biomax MR autoradiography film, and densitometry was performed on scanned images of the developed autoradiographs using NIH Image J (version 1.37) software. Expression is presented as arbitrary units that represent the ratio of the signal intensity for each gene relative to the internal loading control, the ribosomal protein L32. It was determined that longer exposure times (2–7 days) were required to resolve the expression of MMP genes, which resulted in saturation of L32 signals. To correct for this, autorad signal intensities for the MMP genes were corrected to the L32 band intensities from shorter exposure times (8–12 h). Analyses of MMP mRNA expression by RPA were done in triplicate. Identification of significant treatment differences was determined using a two-way analysis of variance followed by Newman-Keuls tests for pair-wise comparison. The null hypothesis was rejected when p < 0.05.
Flow Cytometry
Flow cytometry was used to detect cell surface expression levels of integrins, β-dystroglycan, and TLR4 as described previously (Milner and Campbell, 2002). Mixed glial cultures, neurosphere-derived astrocytes, or microglia were cultured in serum-free medium for two days before analysis. In the TLR4 experiments, cells were incubated with no cytokine, IL-1β (10 ng/ml), or LPS (20 ng/ml) for two days before TLR4 expression was analyzed. Cells were removed from culture plates nonenzymatically using a rubber policeman. Nonspecific antibody binding was evaluated using isotype control antibodies. The fluorescent intensity of labeled cells was analyzed with a Becton Dickinson FACScan machine (San Diego, CA), with 10,000 events recorded for each condition.
Gel Zymography
Gelatin zymography was used to detect the activity of MMP-9, as previously described (Heo et al., 1999). Microglial cells were plated at a density of 2 × 105 cells/well in six-well plates. Mixed glial cultures and pure astrocytes were cultured to confluence. Mixed glial cultures, astrocytes, or microglia were switched to serum-free conditions for two days either under control conditions (no cytokines) or in the presence of LPS (1–10,000 ng/ml), IL-1β (1–100 ng/ml), or TNF-α (1–100 ng/ml; R&D Systems, Minneapolis, MN). After two days, cell culture supernatants were collected and analyzed for MMP-2 and MMP-9 activity by gel zymography.
RESULTS
Establishment of Astrocyte Cultures Devoid of Microglia
The overall goal of this study was to define the MMP expression profile of astrocytes and microglia. To do this, we first had to obtain pure astrocyte cultures entirely devoid of microglia. While astrocyte MMP expression has been examined in previous studies, all of these used the mixed glial culture system (Arai et al., 2003; Lee et al., 2003; Liu et al. 2006; Liuzzi et al., 2004; Shin et al., 2007; Tejima et al., 2006; Wang et al., 2002). As mixed glial cultures contain variable amounts of microglial cells (Liu et al., 2006; Milner and Campbell, 2002; Saura et al., 2003), which express high levels of MMPs (Crocker et al., 2006; Rosenberg et al., 2001), it seems highly likely that contaminating microglia will contribute to the MMP expression pattern of astrocyte cultures obtained via the mixed glial culture method, thus complicating the analysis. To overcome this problem, we took a different approach to obtain pure astrocyte cultures entirely devoid of microglia, by promoting NSC differentiation into astrocytes. Neurospheres were prepared as previously described (Milner, 2007), by culturing postnatal neural cells under nonadherent conditions in the presence of EGF and FGF2. Cells within neurospheres are a mixture of NSC and neural precursor cells (NPC) that have tripotential capacity, giving rise to neurons, astrocytes, or oligodendrocytes (Reynolds et al., 1992; Reynolds and Weiss, 1992; Reynolds and Weiss, 1996). Neurosphere differentiation into astrocytes was promoted, as previously described, by culturing neurospheres on adherent poly-d-lysine coated plates in media containing high serum (10% FBS) (Sakai et al., 1990; Loo et al., 1995; Brunet et al., 2004).
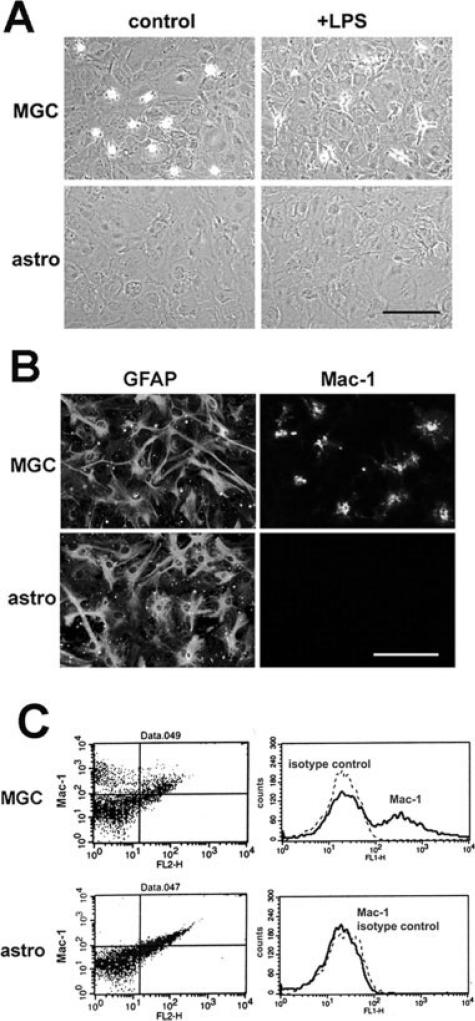
As shown in Fig. 1A, a typical mixed glial culture contains a basal layer of well-spread phase-dark astrocytes and a superficial layer of loosely attached phase-bright microglia. Neurospheres that were differentiated in the presence of 10% FBS also formed a flat layer of well-spread phase-dark astrocytes, but in contrast to the mixed glial culture, these cultures contained no loosely attached phase-bright cells. When both types of culture were treated with lipopolysaccharide (LPS), microglia within the mixed glial culture changed morphology from loosely attached rounded-up cells to well attached spread cells (Fig. 1A). However, consistent with the notion that NSCs do not generate microglia, no morphologically detectable microglia were seen in the neurosphere-derived astrocyte cultures. To confirm the lack of microglia in these cultures, immunocytochemistry for the microglia-specific marker Mac-1was also performed on mixed glial cultures and neurosphere-derived astrocyte cultures. As shown in Fig. 1B, although mixed glial cultures contained a majority of GFAP-positive astrocytes, and a small number of Mac-1-positive microglial cells, the neurosphere-derived astrocyte cultures contained only GFAP-positive cells, with no Mac-1-positive cells present. These two types of culture were also analyzed by flow cytometry for expression of Mac-1. Consistent with the phase and immunocytochemical analysis, many Mac-1 positive microglia were detected in the mixed glial cultures (varying between 5 and 20% of the cell population); however, none were observed in the neurosphere-derived pure astrocyte cultures (Fig. 1C). Thus, the neurosphere-derived astrocyte cultures represent a pure GFAP-positive cell population that contains no microglial cells.
Fig. 1.
Neurosphere-derived pure astrocyte cultures contain no contaminating microglia. Mixed glial cultures (MGC) and pure astrocyte cultures (astro) were prepared as described in the Materials and Methods section, and then analyzed for the appearance of microglia, by phase (A), immunocytochemistry (B) or flow cytometry (C). Scale bar = 50 μm. Note that under phase microscopy (A), phase-bright microglia are seen in the MGC, both under control conditions and in the presence of LPS (20 ng/ml), but none are present in the pure astrocyte cultures. Immunocytochemistry (B) demonstrated the presence of many GFAP-positive astrocytes in both the MGC and pure astrocyte cultures, but Mac-1 positive microglia were seen only in the MGC. Flow cytometry (C) confirmed the presence and absence of Mac-1 positive microglia in the MGC and pure astrocyte cultures, respectively.
Astrocytes Derived from Neurospheres or Mixed Glial Cultures Show Similar Expression Patterns of the Cell Adhesion Molecules, Integrins and Dystroglycan
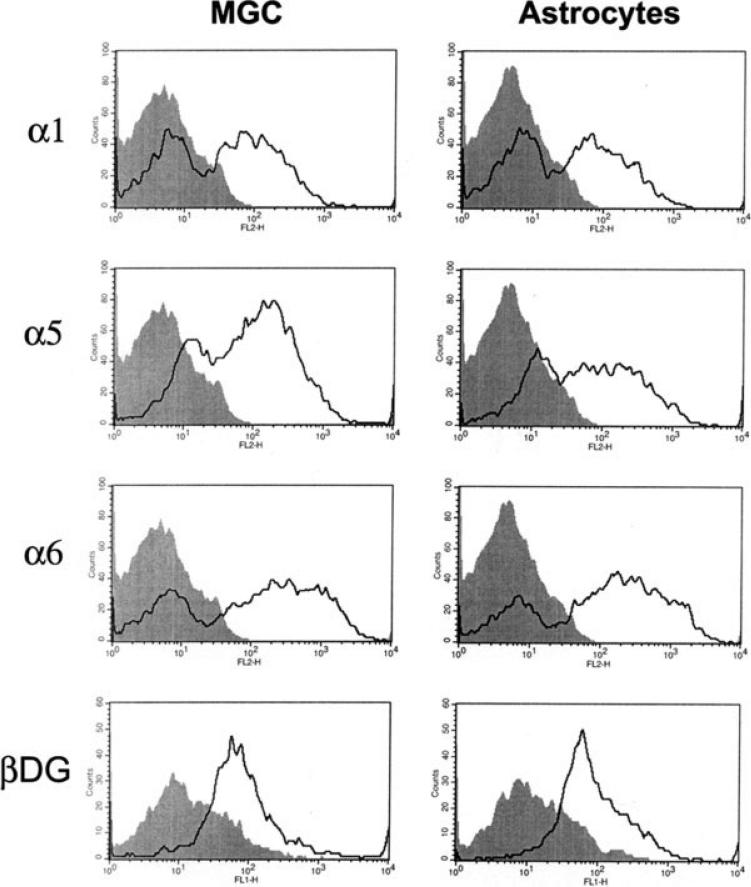
To investigate whether astrocytes derived from neurospheres are essentially the same cells as astrocytes within mixed glial cultures, we also compared the expression of a number of different cell adhesion molecules between the two different cultures. We have shown previously that astrocytes within a mixed glial culture express a specific repertoire of β1 class integrins including, the α1, α5, and α6 subunits (Milner and ffrench-Constant, 1994; Shaw et al., 1996) and also express an alternative class of laminin receptor, dystroglycan (Milner et al., 2008). Confluent cultures of mixed glial cultures or pure astrocytes were switched to serum-free conditions for two days, and then cell surface expression of adhesion molecules were analyzed by flow cytometry. As shown in Fig. 2, mixed glial cultures and pure neurosphere-derived astrocytes expressed similar levels of the α1, α5, and α6 integrin subunits and also of the β-dystroglycan subunit, thus revealing a very similar expression pattern of astrocytes obtained from the two different cell culture systems.
Fig. 2.
Comparison of cell adhesion molecule expression on astrocytes derived from neurospheres or mixed glial cultures. Mixed glial cultures (MGC) and pure astrocyte cultures were prepared as described in the Materials and Methods section, switched to serum-free conditions for two days, and then analysed for cell surface expression of the integrin subunits α1, α5, and α6, and the β-dystroglycan subunit (isotype control antibody: shaded filled line; integrin or dystroglycan antibodies: open line). These expression profiles are representative of three different experiments performed. Note that pure astrocytes and mixed glial astrocytes expressed a very similar profile of integrin subunits and dystroglycan.
Expression of the LPS Receptor, TLR4 Is High on Microglia but Low on Astrocytes Derived from Neurospheres or Mixed Glial Cultures
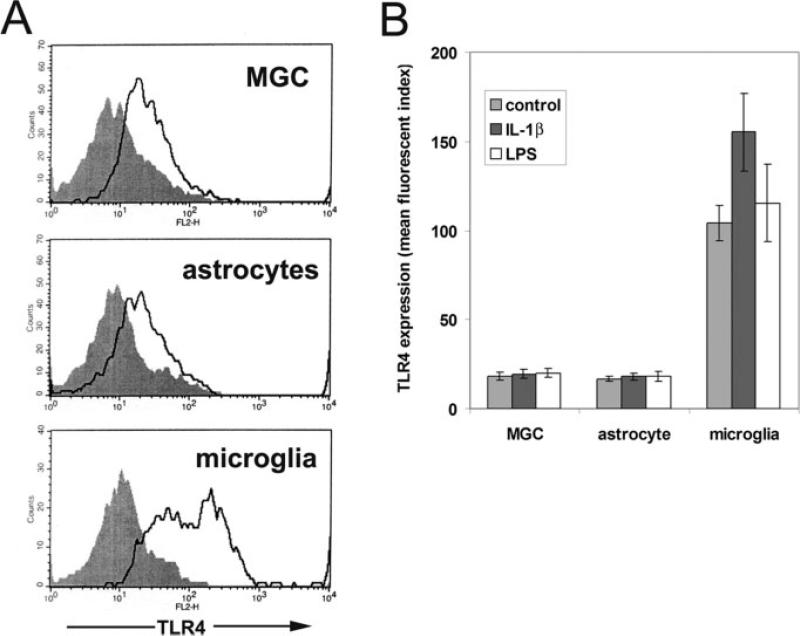
As previous studies have shown that TLR4 expression is much higher on microglia than on astrocytes (Jack et al., 2005), we also examined expression of TLR4 on astrocytes from the two different types of culture and compared this to microglia. The three different types of culture were switched to serum-free conditions for two days, and cell surface expression of TLR4 analyzed by flow cytometry. As shown in Fig. 3A, TLR4 was expressed by both types of astrocyte culture but at only a relatively low level compared with microglia. Quantification of the mean fluorescent index of the different cell types revealed that neurosphere-derived astrocytes expressed 16.7 ± 1.5 U, mixed glial cultures expressed 18.3 ± 2.1 U, and microglia expressed 104.3 ± 9.9 U (n = 3 experiments). Thus, TLR4 expression by the two different astrocyte cell populations was very similar, with microglial expression approximately six-fold that of astrocytes. Next, we investigated whether proinflammatory stimuli such as IL-1β or LPS would modulate TLR4 expression on astrocytes and microglia. The three different types of culture were incubated for two days under serum-free conditions in the presence of IL-1β or LPS, and then cell surface expression of TLR4 was examined by flow cytometry. As shown in the graph in Fig. 3B, neither IL-1β nor LPS significantly affected TLR4 expression by astrocytes from either source. In contrast, microglial expression of TLR4 was significantly increased by IL-1β (from 104.3 ± 9.9 U under control conditions to 155.3 ± 21.7 U in the presence of IL-1β, p < 0.05) but unaffected by LPS. Thus, we have compared neurosphere-derived astrocytes with mixed glial culture astrocytes and found that on the basis of morphology, GFAP expression, and expression repertoire of integrins, dystroglycan, and TLR4, these cells are essentially identical in all parameters tested. The notable exception is that mixed glial cultures contain significant amounts of microglial cells, while the neurosphere-derived astrocytes are totally microglial-free. This establishes neurosphere-derived astrocytes as a good in vitro model in which to study the behavior of astrocytes, free of the complicating effects of contaminating microglia.
Fig. 3.
TLR4 expression by mixed glial cultures, pure astrocytes, and pure microglia. Mixed glial cultures (MGC) and pure cultures of astrocytes or microglia were prepared as described in the Materials and Methods section, switched to serum-free conditions for two days, and then analysed for cell surface expression of TLR4 (isotype control antibody: shaded filled line; TLR4 antibody: open line). These expression profiles are representative of three different experiments performed. Note that TLR4 was expressed at a similar low level by mixed glial cultures and pure astrocytes, but at a much higher level by microglia (A). The influence of IL-1β or LPS on TLR4 expression in astrocytes and microglia was investigated by incubating the three different types of culture with IL-1β (10 ng/ml) or LPS (20 ng/ml) for 2 days, before analyzing TLR4 expression by flow cytometry (B). The points represent the mean ± SD of three experiments. Note that mixed glial cultures and pure astrocytes showed the same low level of TLR4 expression, which was not affected by IL-1β or LPS. In contrast, microglia express markedly higher levels of TLR4 and this was significantly increased by IL-1β.
Astrocytes and Microglia Express Distinct Sets of MMP Genes
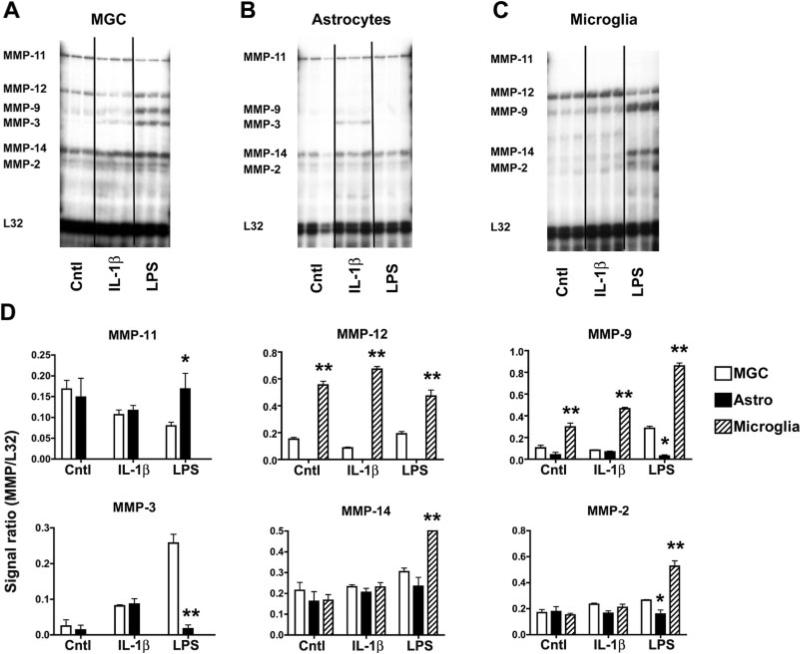
Having established a reliable method to obtain microglia-free astrocyte cultures, we next characterized MMP gene expression in the three different types of glial culture: mixed glial culture, neurosphere-derived astrocytes, and pure microglia. After 24 h in serum-free culture, the expression pattern of different MMP genes was examined by RNase protection assay (RPA) using a well-characterized multiprobe set (Crocker et al., 2006; Pagenstecher et al., 1997). This analysis revealed that under basal conditions, mixed glial cultures expressed detectable levels of mRNA for MMP-11, MMP-12, MMP-14, and MMP-2 (Fig. 4A). Under basal conditions, the pure astrocyte cultures expressed detectable levels of mRNA for the MMP-11, MMP-14, and MMP-2 genes but no MMP-12 (Fig. 4B), while in contrast, microglia expressed high levels of mRNA for the MMP-12 gene and lower levels of mRNA for MMP-9, MMP-14, and MMP-2 genes (Fig. 4C). The MMP expression profiles of the mixed glial cultures and microglia confirm our previous findings (Crocker et al., 2006). The interesting observation from the current study is that pure astrocyte cultures expressed all the MMPs found in mixed glial cultures (MMP-11, MMP-14, and MMP-2), with the exception of MMP-12. As pure microglial cultures constitutively express high levels of MMP-12, this strongly suggests that the MMP-12 gene expression detected in mixed glial cultures originates specifically from microglia. Furthermore, by inference, it demonstrates that neurosphere-derived astrocytes and mixed glial culture astrocytes have an identical expression pattern of MMPs.
Fig. 4.
Analysis of the MMP expression profile in: (A) mixed glial cultures (MGC), (B) pure astrocytes, or (C) pure microglia. The three different types of culture were prepared, as described in the Materials and Methods section, and then cultured in serum-free medium on poly-d-lysine coated plates. MMP expression was examined by RNase protection assay (RPA) during control conditions (Cntl) or after 24-h stimulation by IL-1β (10 ng/ml) or LPS (20 ng/ml) (left to right). MMP expression is presented as arbitrary units that represents the ratio of the signal intensity for each gene relative to the internal loading control, the ribosomal protein L32. This revealed that under basal conditions, mixed glial cultures expressed the MMP-11, MMP-12, MMP-14, and MMP-2, while pure astrocytes expressed MMPs-11, MMP-14, and MMP-2, but no MMP-12. Microglia expressed high levels of the MMP-12 gene and lower levels of the MMP-9, MMP-14, and MMP-2. Quantification of the RPAs (D) revealed that LPS stimulated induction of MMP-3 and MMP-9 in the mixed glial cultures (open bars) but had no effect on pure astrocytes (black bars). In contrast, LPS stimulated strong expression of MMP-9 and MMP-14 in microglia (hatched bars). IL-1β promoted MMP-3 expression in pure astrocytes and mixed glial cultures. Asterisks indicate significance by ANOVA: *P < 0.05, **P < 0.001 vs. mixed glial culture expression of each MMP for the same treatment.
We next examined the influence of proinflammatory mediators on glial MMP expression. Cultures were treated with IL-1β (10 ng/ml) or lipopolysaccharide (LPS; 20 ng/ml) for 24 h, and MMP gene expression was analyzed by RPA. In mixed glial cultures (Fig. 4A) and pure neurosphere-derived astrocyte cultures (Fig. 4B), IL-1β induced low levels of MMP-3 gene expression. IL-1β had no detectable effect on MMP gene expression in microglia. Interestingly, the influence of LPS on the three types of culture was markedly different. Pure astrocyte cultures were totally unresponsive to LPS (Fig. 4B). In contrast, LPS treatment of pure microglia caused a strong increase in the mRNA levels for the MMP-9 and MMP-14 (Fig. 4C). These two genes were also upregulated following LPS treatment of the mixed glial cultures, in which MMP-3 also showed a marked increase (Fig. 4A). This LPS-induced MMP-3 upregulation was observed only in the mixed glial culture and not in the pure astrocyte or pure microglial cultures, suggesting that it results from interplay between microglia and astrocytes.
In summary, these findings demonstrate that expression of MMP genes in CNS primary glial cells are largely cell-type specific. Astrocytes constitutively express MMP-11, MMP-14, and MMP-2 and show small induction of MMP-3 in response to IL-1β. Microglia constitutively express high levels of MMP-12, lower levels of MMP-9, and very low levels of MMP-14 and MMP-2, but then show strong induction of MMP-9 and MMP-14 in response to LPS. Importantly, this study demonstrates that the LPS-induction of MMP-9 in mixed glial cultures originates from microglial cells not astrocytes.
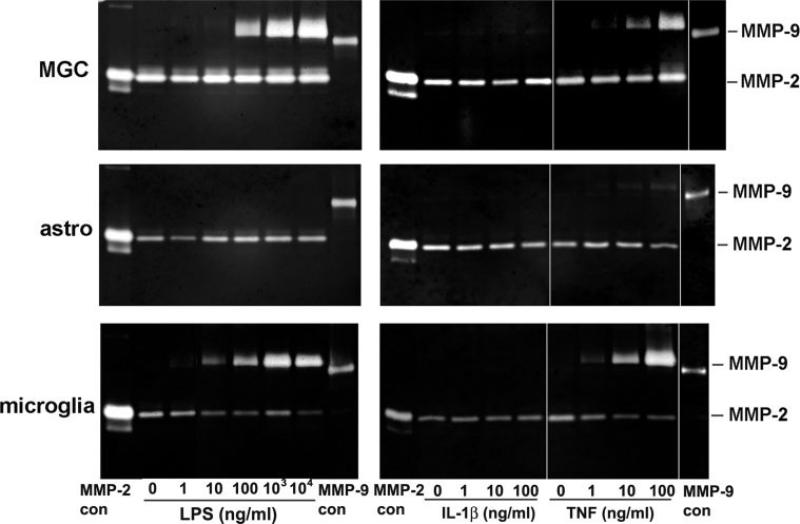
Microglia but not Astrocytes, Express High Levels of Pro-MMP-9 Protein Following Activation by LPS or TNF-α
As the RPA analysis indicated that microglia, not astrocytes are the source of MMP-9 following stimulation by LPS, we next investigated whether this was also true at the level of protein expression, by using gelatin zymography. The three different types of culture were maintained under serum-free conditions for two days, either under control conditions (no cytokines) or in the presence of LPS (10–1000 ng/ml), IL-1β (1–100 ng/ml), or TNF-α (1–100 ng/ml). Previous studies have shown that these factors induce MMP-9 expression in mixed glial cultures (Arai et al., 2003; Crocker et al., 2006; Lee et al., 2003; Liu et al., 2006; Liuzzi et al., 2004) and have often been interpreted as showing that LPS, IL-1β, or TNF-α promote astrocyte expression of MMP-9. After two days incubation, cell culture supernatants were collected and analyzed for MMP-2 and MMP-9 activity by gel zymography (see Fig. 5). This revealed that under basal conditions, mixed glial cultures, pure neurosphere-derived astrocytes and pure microglia all expressed MMP-2 but no detectable MMP-9. MMP-2 expression in mixed glial cultures or pure astrocyte cultures was not significantly altered by LPS, IL-1β, or TNF-α. Following stimulation by LPS, both mixed glial cultures and microglia showed strong induction of MMP-9. Consistent with previous observations (Milner et al., 2007), microglia expressed the higher molecular mass (92 kD) pro-MMP-9 form but none of the lower molecular mass (82 kD) activated form. In contrast, and consistent with our RPA data, pure neurosphere-derived astrocyte cultures showed no induction of MMP-9 in response to LPS, even at the highest dose tested (10 μg/ml). IL-1β-induction of MMP-9 was not detected in any of the cultures. In a similar manner to LPS, TNF-α strongly induced pro-MMP-9 in the mixed glial and pure microglial cultures. By contrast, TNF-α promoted only very low levels of pro-MMP-9 expression in pure astrocyte cultures. In summary, these gel zymography experiments support the RPA data, in showing that expression of pro-MMP-9 was strongly induced in microglia by LPS or TNF-α, whereas pure neurosphere-derived astrocytes did not express pro-MMP-9 in response to LPS and showed only minimal pro-MMP-9 expression when stimulated by TNF-α.
Fig. 5.
The influence of LPS, IL-1β, or TNF-α on MMP-9 expression in mixed glial cultures (MGC), pure astrocytes (astro) or pure microglia. The three different types of culture were prepared, as described in the Materials and Methods section, and then cultured in serum-free medium on poly-d-lysine coated plates. After 2-days culture, cell supernatants were analyzed for MMP-9 activity by gel zymography. Note that LPS and TNF-α stimulated strong expression of MMP-9 in both the MGC and pure microglial cultures. In contrast, pure cultures of astrocytes did not express MMP-9 in response to LPS and expressed only minimal levels in response to TNF-α. IL-1β evoked no detectable MMP-9 in any of the cultures.
We also performed immunocytochemical studies on mixed glial cultures to determine which glial cell type expresses MMP-3 or MMP-9 (see Fig. 6). In mixed glial cultures that were treated with LPS for two days, the MMP-9 signal always colocalized to Mac-1 positive microglial cells (Fig. 6, panels A–C). In contrast, the MMP-3 positive signal was diffusely distributed throughout the basal layer of astrocytes and not localized to microglia (Fig. 6, panels D–F). This supports the conclusion from the RPA and gel zymography analysis, confirming that microglia are the dominant source of MMP-9, while astrocytes express MMP-3.
Fig. 6.
Cell-type localization of MMP-3 and MMP-9 in mixed glial cultures. Mixed glial cultures were prepared as described in the Materials and Methods section, and then subcultured on poly-d-lysine coated glass coverslips. Cultures were treated with LPS (20 ng/ml) for two days before being analyzed by dual immunofluorescence with antibodies against Mac-1 (A), MMP-9 (B), Mac-1/MMP-9 merge (C), Mac-1 (D), MMP-3 (E) and Mac-1/MMP-3 merge (F). Scale bar = 50 μm. Note that MMP-9 showed a strong colocalization with Mac-1 positive microglia, while in contrast, MMP-3 showed a diffuse distribution in the basal layer of astrocytes and was not colocalized to microglia.
DISCUSSION
Until now, all studies of astrocyte MMP expression have employed the traditional mixed glial culture system (Arai et al., 2003; Lee et al., 2003; Liu et al. 2006; Liuzzi et al., 2004; Shin et al., 2007; Tejima et al., 2006; Wang et al., 2002), which contains microglia in addition to astrocytes (Milner and Campbell, 2002; Saura et al., 2003; Liu et al., 2006). As microglia also strongly express their own MMPs, this has complicated the interpretations of studies using this culture system. In the current work, we used an alternative approach of differentiating NSC into astrocytes, as a strategy to obtain pure astrocyte cultures devoid of microglia, and then compared the MMP expression profiles of mixed glial cultures with pure cultures of astrocytes or microglia. Three main conclusions arose from this study. First, neurosphere-derived astrocytes represent a valid and reliable system to obtain pure astrocyte cultures, free of microglia. Second, microglia and astrocytes show distinct MMP expression profiles. Third, microglia, but not astrocytes, are the major source of MMP-9 in response to the proinflammatory mediators LPS or TNF-α.
Derivation of Pure Astrocyte Cultures from NSC
The vast majority of cell culture studies of astrocytes have used the traditional mixed glial culture system (Arai et al., 2003; Lee et al., 2003; Liu et al., 2006; Liuzzi et al., 2004; Shin et al., 2007; Tejima et al., 2006; Wang et al., 2002). However, it is very difficult to totally remove all microglia from mixed glial cultures as microglia are located not just on top of the astrocytes but also within the astrocyte monolayer (Saura et al., 2003). Consequently, after removal of the microglial top layer by mechanical shaking, there is a fairly rapid reconstitution of this layer due to microglial migration from within the astrocyte layer. Most studies estimate that the astrocyte purity of these cultures never exceeds 95%, with the remaining 5% cells being microglia (Liu et al., 2006; Milner and Campbell, 2002; Saura et al., 2003). Recently, a new method was described to more effectively remove microglia from mixed glial cultures, which involves treating the cultures with Ara C for 5–6 days followed by a short exposure (60 min) to l-leucine methyl ester (LME) (Hamby et al., 2006).
In this study, we took a novel approach to obtain pure astrocyte cultures entirely devoid of microglia. NSCs are tripotential, giving rise to neurons, astrocytes, and oligodendrocytes (Reynolds et al., 1992; Reynolds and Weiss, 1992; Reynolds and Weiss, 1996). Importantly, microglia are not derived from NSCs (Levison et al., 2003); rather they are derived from hematopoietic origin and colonize the CNS during development (Hess et al., 2004; Rezaie and Male, 1999; Simard and Rivest, 2004). In this study, NSC differentiation into astrocytes was promoted by culturing neurospheres in 10% FBS, as previously described (Brunet et al., 2004; Loo et al., 1995; Sakai et al., 1990). Using this approach, microglia were never observed in these cultures, and both immunocytochemistry and flow cytometry confirmed that Mac-1-positive microglial cells were entirely absent from these cultures.
An important issue regarding these cultures is to what degree neurosphere-derived astrocytes resemble those from a mixed glial culture. We observed that both sources of astrocyte displayed similar morphology by phase microscopy and all cells in culture expressed high levels of GFAP (see Fig. 1). Cell surface expression of the α1, α5, and α6 integrin subunits, the β-dystroglycan subunit, and TLR4 was near identical for astrocytes from the two different sources. Previous studies have shown that the two sources of astrocyte show similar expression profiles of αv integrins (Milner et al., 2001), and as astrocyte αv integrins show developmental regulation, this implies that neurosphere-derived astrocytes and mixed glial astrocytes are at an equivalent stage of differentiation. In addition, the RPA analysis showed that in the basal state, neurosphere-derived astrocytes expressed an almost-identical pattern of MMP genes as the astrocyte-enriched population of the mixed glial culture. The only exception was that the mixed glial culture also expressed MMP-12, but as we have shown that MMP-12 (macrophage metalloelastase) is expressed at very high levels by microglia (Crocker et al., 2006), this strongly implies that all the MMP-12 present in mixed glial cultures emanates from microglia. Finally, we have found that neurosphere-derived astrocytes and mixed glial cultures express an identical pattern of tissue inhibitor of metalloproteinases (TIMP) genes (Crocker and Milner, manuscript in preparation). When taken together, these combined analyses indicate that the two different sources of astrocyte are largely equivalent. As neurosphere-derived astrocyte cultures are pure and contain no contaminating microglia, this system represents a preferable alternative for preparing astrocyte cultures, when compared with traditional mixed glial cultures. This has significant implications, not just for the study of MMP/TIMP expression, but also for examining many other facets of basic cell biology in astrocytes.
Glial Cell-Specific MMP Expression
A clear message to emerge from these studies is that astrocytes and microglia express distinct sets of MMP genes. Astrocytes were found to constitutively express MMP-11, MMP-14, and MMP-2 and showed small induction of MMP-3 in response to IL-1β. In comparison, microglia constitutively expressed high levels of MMP-12, lower levels of MMP-9 and even low levels of MMP-14 and MMP-2 but then showed strong induction of MMP-9 and MMP-14 in response to LPS. The high level of MMP-12 expression by microglia is consistent with previous observations showing MMP-12 expression in macrophage-like cells in demyelinated lesions of both MS patients and in EAE (Vos et al., 2003; Toft-Hansen et al., 2004).
Of all MMPs, MMP-9 has probably attracted the most interest. MMP-9 has been implicated in the pathogenesis of many different CNS disorders, including multiple sclerosis (MS) (Anthony et al., 1997; Maeda and Sobel, 1996), ischemic stroke (Cunningham et al., 2005; Lee et al., 2004; Rosenberg et al., 2001), and neoplasia (Rao et al., 1996; Yamamoto, et al. 1996). In MS, MMP-9 levels are markedly upregulated during demyelination (Anthony et al. 1997; Cossins et al., 1997; Maeda and Sobel, 1996), and in an animal model of demyelination, MMP-9 knockout mice are resistant, at least early in life (Dubois et al., 1999). From the wealth of studies, it appears that several different cell types within the CNS can produce MMP-9, including microglia (Kauppinen and Swanson, 2005; Liuzzi et al., 1999), astrocytes (Arai et al., 2003; Muir et al., 2002), and oligodendrocytes (Oh et al., 1999; Larsen et al., 2003; Larsen et al., 2006).
Several in vitro studies have suggested that astrocytes express MMP-9 in response to LPS (Lee et al., 2003; Liu et al., 2006; Liuzzi et al., 2004; Shin et al., 2007) and other proinflammatory mediators, including TNF-α (Arai et al., 2003). However, as these studies employed the traditional mixed glial culture system, which contain significant amounts of microglia, we reasoned that the MMP-9 expression in these cultures may be a product of microglia rather than astrocytes. In our current study, both the RPA and gel zymography clearly demonstrated that microglia, but not astrocytes, are the major source of MMP-9 following stimulation by LPS or TNF-α. LPS induced strong MMP-9 expression both in pure microglial and mixed glial cultures but not in pure astrocyte cultures. In a similar manner, TNF-α promoted strong MMP-9 expression in pure microglial and mixed glial cultures but not in pure astrocyte cultures. These findings contradict the notion that astrocytes are a significant source of MMP-9 (Arai et al., 2003; Lee et al., 2003; Liu et al., 2006; Shin et al., 2007) and, instead, strongly suggest that in previous studies of LPS-treated mixed glial cultures, it is most likely that the MMP-9 activity came from contaminating microglia, not from astrocytes.
Cell culture studies do not always totally reflect the situation in vivo; specifically, it has been suggested that glial cells in vitro show a partially activated phenotype, perhaps as a consequence of contact with factors present in tissue culture serum. Importantly, the main observation of this study, that microglia are the main source of MMP-9 in vitro, is consistent with our previous in vivo demonstration that in the chronic inflammatory CNS of EAE affected mice, MMP-9 is expressed specifically by microglia but not by astrocytes or oligodendrocytes.
In this study, pure astrocytes were totally unresponsive to LPS, as indicated by a lack of change in MMP expression. Consistent with this, LPS also had no effect on astrocyte expression of TLR4 or on the expression of TIMPs (Crocker and Milner, unpublished observations). This lack of response to LPS is consistent with the demonstration that astrocytes express only low levels of the LPS receptor TLR4 (Jack et al., 2005; Lehnardt et al., 2003; Lehnart et al., 2002). It also supports our notion (Crocker et al., 2006) that in mixed glial cultures, LPS acts on astrocytes via an indirect mechanism, whereby LPS stimulates microglia to produce IL-1β (Chauvet et al., 2001), which then stimulates astrocytes. This explains the LPS-induction of astrocyte TIMP-1 in mixed glial cultures (Crocker et al., 2006) and also accounts for our observation that IL-1β triggers astrocyte MMP-3 expression both in mixed glial and pure astrocyte cultures, but LPS itself triggers MMP-3 expression only in mixed glial cultures.
Our analysis showed that MMP-3 is induced specifically in astrocytes following stimulation by IL-1β. This result is consistent with previous findings (Crocker et al., 2006; Deb et al., 2003; Falo et al., 2006; Muir et al., 2002), although it does contradict an earlier study describing microglial MMP-3 expression following reperfusion injury in an animal model of stroke (Rosenberg et al., 2001). The reason for this difference is presently unclear, although it may reflect the more complex in vivo environment. In vivo, MMP-3 promotes activation of pro-MMP-9 (Ramos-DeSimone et al., 1999), and it was recently shown that MMP-3 released from dying neurons stimulates microglial activation (Kim et al., 2005). Taken together, this suggests that in vivo, MMP-3 released by IL-1β-stimulated astrocytes or dying neurons may play a dual role both to stimulate microglial activation and to activate microglial pro-MMP-9.
In summary, we have successfully generated pure astrocyte cultures entirely devoid of microglia and demonstrated that these cells are largely equivalent to astrocytes from mixed glial cultures. We have used this system to characterize MMP expression profiles of astrocytes and microglia by RPA and gel zymography. This analysis revealed that astrocytes and microglia express distinct sets of MMP genes and that microglia, not astrocytes, are the major source of MMP-9 in response to the proinflammatory stimuli LPS or TNF-α. These findings contradict the notion that astrocytes are a significant source of MMP-9 and, instead, strongly suggest that in previous studies of LPS-treated mixed glial cultures (Arai et al., 2003; Lee et al., 2003; Liu et al., 2006; Shin et al., 2007), it was most likely that MMP-9 activity came from microglia, not astrocytes. In light of these findings, some of the interpretations drawn from previous studies describing astrocyte expression of MMP-9 in mixed glial cultures can now be revisited using pure cell populations.
ACKNOWLEDGMENT
This is manuscript number 19043 from The Scripps Research Institute.
Grant sponsor: National Multiple Sclerosis Society; Grant numbers: JF 2125A1/1, TA 3021A1/1, R-01 AI042314.
REFERENCES
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri M, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. [PubMed] [Google Scholar]
- Arai K, Lee SR, Lo EH. Essential role for ERK mitogen-activated protein kinase in matrix metalloproteinase-9 regulation in rat cortical astrocytes. Glia. 2003;43:254–264. doi: 10.1002/glia.10255. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: An overview. Structure, regulation and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Grollimund L, Chatton JY, Lengacher S, Magistretti PJ, Villemure JG, Pellerin L. Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia. 2004;46:8–17. doi: 10.1002/glia.10348. [DOI] [PubMed] [Google Scholar]
- Carson MJ. Microglia as liasons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Coats R, Gearing AJ, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- Chauvet N, Palin K, Verrier D, Poole S, Dantzer R, Lestage J. Rat microglial cells secrete predominantly the precursor of interleukin-1beta in response to lipopolysaccharide. Eur J Neurosci. 2001;14:609–617. doi: 10.1046/j.0953-816x.2001.01686.x. [DOI] [PubMed] [Google Scholar]
- Cossins JA, Clements JM, Ford J, Miller KM, Pigott R, Vos W, Van der Valk P, de Groot CJ. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol. 1997;94:590–598. doi: 10.1007/s004010050754. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Milner R, Pham-Mitchell N, Campbell IL. Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. J Neurochem. 2006;98:812–823. doi: 10.1111/j.1471-4159.2006.03927.x. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Pagenstecher A, Campbell IL. The TIMPs tango with MMPs and more in the central nervous system. J Neurosci Res. 2004;75:1–11. doi: 10.1002/jnr.10836. [DOI] [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003;970:205–213. doi: 10.1016/s0006-8993(03)02344-8. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Milner R. Integrin-matrix interactions in the cerebral microvasculature. Arterioscler Thromb Vasc Biol. 2006;26:1966–1975. doi: 10.1161/01.ATV.0000232525.65682.a2. [DOI] [PubMed] [Google Scholar]
- Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B. Resistance of young gelatinaseB-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest. 1999;104:1507–1515. doi: 10.1172/JCI6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falo MC, Fillmore HL, Reeves TM, Phillips LL. Matrix metalloproteinase-3 expression profile differentiates adaptive and maladaptive synaptic plasticity induced by traumatic brain injury. J Neurosci Res. 2006;84:768–781. doi: 10.1002/jnr.20986. [DOI] [PubMed] [Google Scholar]
- Fawcett J. Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell tissue Res. 1997;290:371–377. doi: 10.1007/s004410050943. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Asher RA. The glial scar and CNS repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Franklin RJM, ffrench-Constant C. Transplantation and repair in multiple sclerosis. In: Russell W, editor. The molecular biology of multiple sclerosis. John Wiley and Sons; New York, NY: 1996. pp. 231–242. [Google Scholar]
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neourosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Jack CS, Arbour N, Manusow J, Montgrain V, Blain M, McCrea E, Shapiro A, Antel JP. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol. 2005;175:4320–4330. doi: 10.4049/jimmunol.175.7.4320. [DOI] [PubMed] [Google Scholar]
- Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, ffrench-Constant C. Neural precursor chain migration and division are regulated through different β1 integrins. Development. 1998;125:3167–3177. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kauppinen TM, Swanson RA. Poly (ADP-ribose) polymerase-1 promotes microglial activation, proliferation and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–2296. doi: 10.4049/jimmunol.174.4.2288. [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O, Shin DH, Chun HS, Beal MF, Joh TH. Matrix metalloproteinase-3: A novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Larsen PH, DaSilva AG, Conant K, Yong VW. Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J Neurosci. 2006;26:2207–2214. doi: 10.1523/JNEUROSCI.1880-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SR, Tsuiji K, Lo EH. Role of matrix metalloproteinases in delayed neuronal damage after transient global cerebral ischemia. J Neurosci. 2004;24:671–678. doi: 10.1523/JNEUROSCI.4243-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Shin CY, Yoo BK, Ryu JR, Choi EY, Cheong JH, Ryu JH, Ko KH. Induction of matrix metalloproteinase-9 (MMP-9) in lipopolysaccharide-stimulated primary astrocytes is mediated by extracellular signal-regulated protein kinase 1/2 (Erk1/2). Glia. 2003;41:15–24. doi: 10.1002/glia.10131. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Druckman SK, Young GM, Basu A. Neural stem cells in the subventricular zone are a source of astrocytes and oligodendrocytes, but not microglia. Dev Neurosci. 2003;25:184–196. doi: 10.1159/000072267. [DOI] [PubMed] [Google Scholar]
- Liu W, Rosenberg GA, Liu KJ. AUF-1 mediates inhibition by nitric oxide of lipolysaccharide-induced matrix metalloproteinase-9 expression in cultured astrocytes. J Neurosci Res. 2006;84:360–369. doi: 10.1002/jnr.20895. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Mastroianni CM, Latronico T, Mengoni F, Fasano A, Lichtner M, Vullo V, Riccio P. Anti-HIV drugs decrease the expression of matrix metalloproteinases in astrocytes and microglia. Brain. 2004;127:398–407. doi: 10.1093/brain/awh049. [DOI] [PubMed] [Google Scholar]
- Liuzzi GM, Santacroce MP, Peumans WJ, Van Damme EJ, Dubois B, Opdenakker G, Riccio P. Regulation of gelatinases in microglia and astrocyte cell cultures by plant lectins. Glia. 1999;27:53–61. doi: 10.1002/(sici)1098-1136(199907)27:1<53::aid-glia6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Lohmann C, Krischke M, Wegener J, Galla HJ. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004;995:184–196. doi: 10.1016/j.brainres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Loo DT, Althoen MC, Cotman CW. Differentiation of serum-free mouse embryo cells into astrocytes is accompanied by induction of glutamine synthetase activity. J Neurosci Res. 1995;42:184–191. doi: 10.1002/jnr.490420205. [DOI] [PubMed] [Google Scholar]
- Maeda A, Sobel RA. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropath and Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- Milner R. A novel three-dimensional system to study interactions between endothelial cells and neural cells of the developing central nervous system. BMC Neurosci. 2007;8:3. doi: 10.1186/1471-2202-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Cytokines regulate microglial adhesion to laminin and astrocyte extracellular matrix via protein kinase C-dependent activation of the α6β1 integrin. J Neurosci. 2002;22:1562–1572. doi: 10.1523/JNEUROSCI.22-05-01562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- Milner R, Crocker SJ, Hung S, Wang X, Frausto RF, Del Zoppo GJ. Fibronectin- and vitronectin-induced microglial activation and matrix metalloproteinase-9 expression is mediated by integrins α5β1 and αvβ5. J Immunol. 2007;178:8158–8167. doi: 10.4049/jimmunol.178.12.8158. [DOI] [PubMed] [Google Scholar]
- Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: Changes in αv-associated β subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo G. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Relvas JB, Fawcett J, ffrench-Constant C. Developmental regulation of alpha v integrins produces functional changes in astrocyte behavior. Mol Cell Neurosci. 2001;18:108–118. doi: 10.1006/mcne.2001.1003. [DOI] [PubMed] [Google Scholar]
- Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, ellis C, Fawcett JW, Rogers JH. Matrix metallproteinases and their inhbitors are produced by overlapping populations of activated astrocytes. Brain Res Mol Brain Res. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Oh LY, Larsen PH, Krekowski CA, Edwards DR, Donovan F, Werb Z, Yong VW. Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes. J Neurosci. 1999;19:8464–8475. doi: 10.1523/JNEUROSCI.19-19-08464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Campbell IL. RNase protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J Immunol Methods. 1997;206:1–9. doi: 10.1016/s0022-1759(97)00077-x. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A, Stalder AK, Kincaid CL, Shapiro L, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens form human myelin basic protein. Biochem Biophys Res Comm. 1993;192:1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Ramos-DeSimone N, Hahn-Dantona E, Sipley J, Nagase H, French DL, Quigley JP. Activation of matrix metalloproteinase-9 (MMP-9) via a converging plasmin/stromelysin-1 cascade enhances tumor cell invasion. J Biol Chem. 1999;274:13066–13076. doi: 10.1074/jbc.274.19.13066. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Mechanisms of inflammation in MS tissue: Adhesion molecules and chemokines. J Neuroimmunol. 1999;98:57–68. doi: 10.1016/s0165-5728(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last). Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Rao JS, Yamamoto M, Mohaman S, Gokaslan ZL, Fuller GN, Stetler-Stevenson WG, Rao VH, Liotta LA, Nicolson GL, Sawaya RE. Expression and localization of 92 kDa type IV collagenase/gelatinase B (MMP-9) in human gliomas. Clin Exp Metastasis. 1996;14:12–18. doi: 10.1007/BF00157681. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Tetzlaff W, Weiss S. A multipotent, EGF-responsive striatal embryonic progenitor cell produces neurones and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1708–1709. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: A review. Microsc Res and Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ridet J, Malhotra S, Privat A, Gage F. Reactive astrocytes: Cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: Activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Rawson C, Lindburg K, Barnes D. Serum and transforming growth factor beta regulate glial fibrillary acidic protein in serum-free-derived mouse embryo cells. Proc Natl Acad Sci U S A. 1990;87:8378–8382. doi: 10.1073/pnas.87.21.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44:183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]
- Shaw CE, Milner R, Compston DAS, ffrench-Constant C. Analysis of integrin expression on oligodendrocytes during axoglial interaction by using rat-mouse xenocultures. J Neurosci. 1996;16:1163–1172. doi: 10.1523/JNEUROSCI.16-03-01163.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CY, Lee WJ, Choi JW, Choi MS, Ryu JR, Oh SJ, Cheong JH, Choi EY, Ko KH. Down-regulation of matrix metalloproteinase-9 expression by nitrix oxide in lipopolysaccharide-stimulated rat primary astrocytes. Nitric Oxide. 2007;16:425–432. doi: 10.1016/j.niox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J. 2004;18:998–1000. doi: 10.1096/fj.04-1517fje. [DOI] [PubMed] [Google Scholar]
- Tejima E, Zhao B-Q, Tsuji K, Rosell A, van Leyen K, Gonzalez RG, Montaner J, Wang X, Lo E. Astrocytic induction of matrix metalloproteinase-9 and edema after brain hemorrhage. J Cereb Blood Flow Metab. 2006;27:460–468. doi: 10.1038/sj.jcbfm.9600354. [DOI] [PubMed] [Google Scholar]
- Toft-Hansen H, Nuttall RK, Edwards DR, Owens T. Key metalloproteinases are expressed by specific cell types in experimental autoimmune encephalomyelitis. J Immunol. 2004;173:5209–5218. doi: 10.4049/jimmunol.173.8.5209. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Vos CM, van Haastert ES, de Groot CJ, van der Valk P, de Vries HE. Matrix metalloproteinase-12 is expressed in phagoctyic macrophages in active muliple sclerosis lesions. J Neuroimmunol. 2003;138:106–114. doi: 10.1016/s0165-5728(03)00036-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Jung J-C, Fini ME, Lo E. Secretion of matrix metalloproteinase-2 and -9 after mechanical trauma injury in rat cortical cultures and involvement of MAP kinase. J Neurotrauma. 2002;19:615–625. doi: 10.1089/089771502753754082. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. 1996;56:384–392. [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]