Abstract
CD4+ T cells respond to peripheral endogenous superantigen stimulation by undergoing deletion or TCR revision. The latter involves RAG re-expression, TCR gene rearrangement, and expression of a novel TCR. TCR-revised T cells are functional and express a diverse TCR repertoire. Because TCR revision harbors the potential to create self-reactivity, it is important to explore whether T cells known to be self-reactive (regulatory T cells) or those involved in autoimmunity (Th17 cells) arise from TCR revision. Interestingly, we observed that Foxp3+ cells are excluded from revising their TCR and that only a small fraction of postrevision cells expresses Foxp3. In contrast, Th17 cells are 20 times more frequent among revised than among C57BL/6 CD4+ T cells, indicating that postrevision cells are biased toward the Th17 lineage. The link between Th17 differentiation and TCR revision might be highly relevant to the role of Th17 cells in promoting autoimmunity.
Recognition of Ag by peripheral CD4+ T cells in the absence of inflammation can lead to T cell deletion, the persistence of T cells in a state of anergy, or conversion into Foxp3-expressing regulatory T cells (Treg)3 (1, 2). A fourth pathway traveled by CD4+ T cells responding to chronic stimulation results in the rescue of cells upon the expression of a revised TCR (3–15). This pathway, labeled TCR revision, occurs in Vβ5 TCR β-chain (Vβ5)-only transgenic (Tg) mice due to chronic TCR triggering mediated by a superantigen encoded by the defective endogenous mammary tumor virus (Mtv)-8 (3, 5–8, 13, 14).
The Mtv-8-encoded superantigen interacts with Vβ5+ TCRs and, although it fails to eliminate Vβ5-expressing T cells in the thymus of H-2b mice, it drives the deletion of peripheral Vβ5+CD4+ T cells in wild-type and Vβ5 Tg C57BL/6 (B6) mice (3, 7, 8). In Vβ5 Tg mice, the decline of Vβ5+CD4+ T cells is paralleled by the appearance of CD4+ T cells that express TCR β-chains other than Vβ5 (3, 14). The generation of these Vβ5− TCRβ+ cells does not require the thymus (5, 8) and has been linked to peripheral RAG expression and Vβ to DJβ rearrangements, indicating that these novel surface TCR β-chains result from TCR gene rearrangement in the lymphoid periphery (14). For unknown reasons, postrevision T cells in Vβ5 Tg mice do not express Vβ5 on the surface despite expressing Vβ5 mRNA (our unpublished observation). Revised T cells display a diverse repertoire of TCR β-chains with N regions that are shorter than thymically generated sequences (13, 14). Postrevision T cells accumulate over time in unmanipulated and thymectomized Vβ5 Tg mice and can comprise up to 40% of peripheral CD4+ T cells, indicating that revision gives rise to functional, long lived T cells that are maintained in the periphery. Nonetheless, it remains largely unknown into which CD4+ T cell subsets revised T cells are capable of differentiating.
The random nature of TCR gene rearrangement inherently has the potential to create T cells carrying autoreactive TCR. TCR revision takes place in germinal centers (6) that could provide an environment for selecting against self-reactivity, but it is not known to what extent self-tolerance is enforced among T cells undergoing TCR revision. The potential for generating self-reactive TCR led us to hypothesize that revised T cells might be prone to convert into Treg (1, 16). However, we now report that Treg do not undergo Mtv-8 mediated peripheral deletion, are excluded from TCR revision, and are underrepresented among revised T cells. In contrast, revised T cells contain a 20-fold higher frequency of IL-17-producing Th17 T cells than is normally found in B6 mice. Thus, our data indicate that TCR revision gives cells a strong bias toward differentiating into Th17 T cells. The link between Th17 differentiation and the generation of autoreactive TCR upon peripheral revision (15, 17) might be vital for understanding the mechanisms underlying the promotion of autoimmunity by Th17 T cells (18).
Materials and Methods
Mice
Vβ5 Tg B6, non-Tg littermate control B6, C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II TCR Tg B6), Mtv-8+ Vβ5 Tg, and Mtv− Vβ5 Tg mice were all bred and maintained in specific pathogen-free facilities at the University of Washington (Seattle, WA) and used at 4–45 wk of age. Vβ5 Tg B6 mice carry Mtv 8, 9, 17, and 30, Mtv-8+ mice carry only Mtv-8, and Mtv− mice are negative for all known Mtv. The latter two strains are both H-2b and were derived by crossing and intercrossing Vβ5 Tg to wild-derived Mtv− mice (3). All experiments were performed in compliance with University of Washington Institutional Animal Care and Use Committee regulations.
Detecting and phenotyping Foxp3+ and Th17 T cells
RBC-lysed single cell suspensions from spleens were first incubated with an FcR-blocking 2G24 Ab and then surface stained with Abs specific for CD4, CD8, CD25, CD44, CD69, CD62L, CD103, GITR, ICOS, TCRβ, or Vβ5, all from BD Biosciences or eBioscience. Subsequent intracellular staining for Foxp3-expressing cells was performed using a Foxp3 staining kit (eBioscience) in accordance with the provided manual.
To detect IL-17 producing T cells, 4 × 106 cells were transferred into 96-well plates in DMEM containing 10% FCS, antibiotics, and 50 μM 2-ME. PMA (0.3 μg/ml; Sigma-Aldrich) and Ionomycin (0.3 μg/ml; Calbiochem) were added and the samples were incubated for 40 min at 37°C. Cultures were supplemented with 7 μg/ml brefeldin A (Sigma-Aldrich) and incubated for an additional 5.5 h. Cells were washed, surface stained, fixed, permeabilized with the BD Cytofix/Cytoperm kit (BD Biosciences), and stained intracellularly with anti-IL-17A (BD Biosciences or eBioscience).
Suppression assays
Splenocytes from 18- to 23-wk-old B6 or Vβ5 Tg mice and 8- to 10-wk-old B6 mice were stained for CD4, CD8, and CD25 and sorted as CD4+CD25+. Cells from young B6 mice were also sorted into CD4+CD25− populations. CD25− cells (2 × 104) were plated and 2-fold dilutions of CD25+ cells (starting with 2×104 per well) were added along with 8×104 T-depleted irradiated splenocytes from B6 mice as APC. The cultures were stimulated with 2 μg/ml Con A for 72 h. [3H]Thymidine (1 μCi) was added 12 h before cell harvest.
Results and Discussion
Vβ5+CD4+ T cells are enriched for Foxp3-expressing T cells
Vβ5 Tg B6 mice express a rearranged TCR β-chain derived from a Kb/OVA-specific T cell clone. Because these mice exhibit diverse TCR α-chain gene rearrangements (3), their T cell repertoire is polyclonal and contains both CD4+ and CD8+ T cells. However, splenocytes isolated from all but very young Vβ5 Tg mice show an inverted CD4 to CD8 ratio due to a gradual decline in the number of peripheral CD4+ T cells (Fig. 1A). Peripheral CD8+ T cell numbers remain stable at ∼10 × 106 cells per spleen.
FIGURE 1.
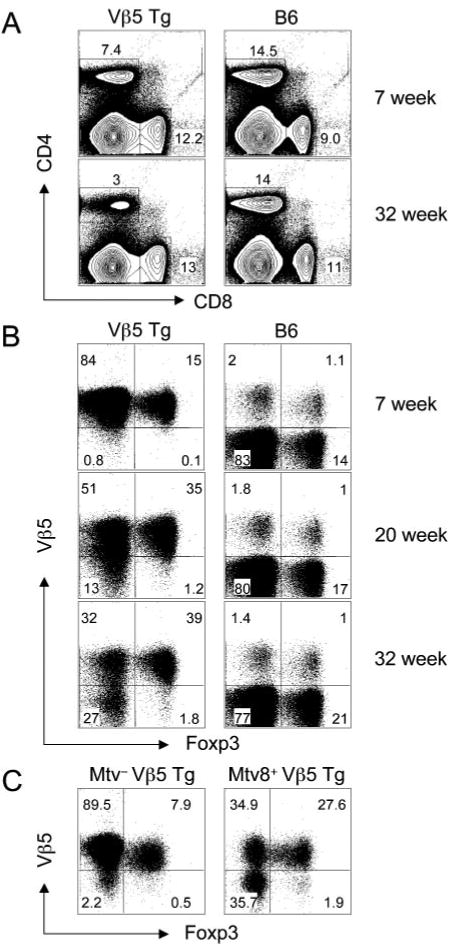
The presence of an Mtv-8-encoded superantigen skews CD4+Vβ5+ T cells toward the Foxp3+ T cell lineage. A, Splenocytes from 7- and 32-wk-old Vβ5 Tg or B6 mice were stained with anti-CD4 and anti-CD8. B, CD4+ splenocytes from 7-, 20-, and 32-wk-old Vβ5 Tg and B6 mice were stained for surface Vβ5 and intracellular Foxp3. C, CD4+ splenocytes from 40-wk-old Vβ5 Tg Mtv− or Vβ5 Tg Mtv-8+ mice were stained with anti-Vβ5 and anti-Foxp3.
We noted that in parallel to the decline of total CD4+ T cells, the frequency of CD4+ Foxp3-expressing T cells increases from 15% in 7-wk-old to 41% in 32-wk-old Vβ5 Tg mice (Fig. 1B). Interestingly, CD4+ T cells expressing endogenously rearranged Vβ5 TCR β-chains in B6 mice also include a large fraction of Treg such that ∼40% of Vβ5+CD4+ T cells in the 32-wk-old B6 mouse express Foxp3, whereas only ∼21% of Vβ5−CD4+ T cells express Foxp3.
To determine whether the increased frequency of Foxp3+ T cells depends on Mtv-8, we analyzed Vβ5 Tg mice that are negative for all known Mtv or positive solely for Mtv-8. Interestingly, although 30% of CD4+ T cells in the Mtv-8+ mouse express Foxp3, only 8% did so in the age-matched Mtv− mouse (Fig. 1C). Thus, the increased frequency of Foxp3+ cells among Vβ5+CD4+ T cells depends on Mtv-8.
Revised T cells do not convert into Treg and Foxp3 expression protects cells from deletion and TCR revision
As reported previously (3), Mtv-8 mediates not only the decline of Vβ5+CD4+ T cells but also the appearance of Vβ5− revised T cells that are pan-TCRβ+. Interestingly, for Vβ5 Tg mice of all ages, Foxp3 expression is almost exclusively restricted to Vβ5+ cells, with only a few Vβ5− cells that are Foxp3+ (Fig. 1B).
To further investigate this skewed representation of Foxp3+ T cells, we determined the frequency of Foxp3-expressing T cells among CD4+Vβ5+ and CD4+Vβ5− T cells in Vβ5 Tg and B6 mice (Fig. 2A). In both, Vβ5+ T cells are skewed toward expressing Foxp3, such that 40–50% are Foxp3+ whereas only ∼8% of revised Vβ5− cells in Vβ5 Tg mice are Foxp3+. The latter frequency is 2.5 times lower than in Vβ5− CD4+ T cells from age-matched B6 mice (Fig. 2A). Thus, revised cells are not prone to differentiate into Treg. Furthermore, because <10% of Foxp3+ T cells in 18- to 45-wk-old Vβ5 Tg mice are Vβ5− while up to 50% of Foxp3− cells are Vβ5−, the data also indicate that cells expressing Foxp3 are precluded from revising their TCR (Fig. 2B). Whether the few Vβ5−Foxp3+ T cells originate from Foxp3+ cells that have undergone TCR revision or from Foxp3− cells that have revised their TCR and subsequently differentiated into Foxp3-expressing cells is not known.
FIGURE 2.
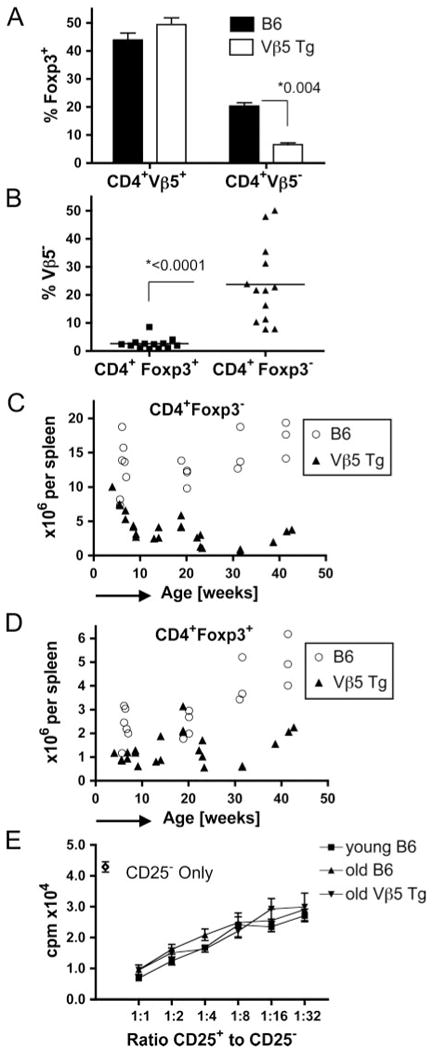
Treg are spared from superantigen-driven deletion and TCR revision. A and B, Splenic CD4+ T cells isolated from Vβ5 Tg or B6 mice at 18–42 wk of age (n > 10) were analyzed for Foxp3 and Vβ5 expression. In A, the fraction of CD4+Vβ5+ and CD4+Vβ5− T cells expressing Foxp3 is shown for both types of mice. In B, the fraction of Vβ5− cells within CD4+Foxp3+ and CD4+Foxp3− cells in 18- to 42-wk-old Vβ5 Tg mice is plotted. Bars represent the mean values. C and D, Splenocytes from B6 or Vβ5 Tg mice of the indicated ages were analyzed for CD4, Vβ5, and Foxp3 expression. Absolute numbers of splenic CD4+Foxp3− (C) and CD4+Foxp3+ cells (D) are plotted against age. E, Con A-activated CD4+CD25− T cells from 8- to 10-wk-old B6 mice were incubated for 72 h alone or with 2-fold dilutions of CD4+CD25+ T cells from B6 mice aged 8–10 wk (young B6), 18- to 23-wk-old B6 (old B6), or 18- to 23-wk-old Vβ5 Tg (old Vβ5 Tg), starting at a ratio of 1:1. The ratio of CD25+ to CD25− cells is plotted against the measured activity in cpm of incorporated [3H]thymidine. Error bars indicate SD in A and E and p values determined by Student's t test are given in A and B.
To determine whether the increased frequency of Foxp3+ T cells among Vβ5+ T cells is due to the expansion of Treg or to differential sensitivity to Mtv-8-mediated deletion, we analyzed Vβ5 Tg and B6 mice aged 4–42 wk and calculated the absolute numbers of splenic CD4+Foxp3+ and CD4+Foxp3− cells. In contrast to B6 mice, the number of CD4+Foxp3− cells decreases in Vβ5 Tg mice with age (Fig. 2C), whereas the number of CD4+Foxp3+ cells in Vβ5 Tg mice remains relatively stable over the same period (Fig. 2D). This suggests that Foxp3+ and Foxp3− cells respond differently to chronic peripheral stimulation, such that cells lacking Foxp3 are deleted or forced to revise their TCR while, in contrast, CD4+Vβ5+ cells expressing Foxp3 do not undergo either of these fates because their number and TCRβ remain unchanged. Moreover, our data indicate that TCR revision does not generate Treg. The fraction of Treg among peripheral CD4+ T cells increases with age (19). The reasons for this increase are unknown but could involve processes similar to those described above, such that some Foxp3− T cells are deleted from the repertoire due to reactivity to self-Ag, whereas Foxp3+ T cells are spared from deletion to increase in prevalence over time.
Chronic superantigen stimulation does not alter the phenotype or function of Treg
Having shown that Treg are spared from superantigen-mediated deletion and TCR revision, we analyzed whether chronic recognition of superantigen alters Treg phenotype or function. Sorted CD25+CD4+ T cells from old Vβ5 Tg and from young and old B6 mice exhibited a comparable degree of suppression; thus, chronic superantigen recognition does not influence Treg suppressive capacity (Fig. 2E). Moreover, phenotyping Vβ5+ or Vβ5− CD4+Foxp3+ T cells isolated from spleens of 45-wk-old B6 mice for expression levels of ICOS, GITR, CD25, CD44, CD62L, and CD69 did not reveal significant differences, although a slight increase in the fraction of CD103+ cells among Vβ5+Foxp3+CD4+ T cells was noted (data not shown).
Vβ5 Tg mice have an elevated frequency of Th17 T cells that carry revised TCR
We observed that PMA/Ionomycin-stimulated (or anti-CD3 plus anti-CD28-stimulated) CD4+ splenocytes from aged Vβ5 Tg mice contain a much higher frequency of IL-17 producers than wild-type B6 mice (Fig. 3A and data not shown). Both young and aged Vβ5 Tg mice contain IL-17-producing T cells at 10-fold higher frequencies than do age-matched B6 mice, and these Th17 lineage cells express normal levels of IL-17 but no IFN-γ (data not shown). Thus, ∼1% of total CD4+ T cells in young Vβ5 Tg mice and ∼3.5% of CD4+ T cells in old Vβ5 Tg mice make IL-17 (Fig. 3B). Interestingly, although CD4+ IL-17+ T cells from young and old Vβ5 Tg express similar surface levels of pan-TCRβ (Fig. 3C, left panel), they differ in their levels of surface Vβ5. Thus, IL-17+ T cells in young Vβ5 Tg mice express Vβ5, whereas in old Vβ5 Tg mice, most IL-17-producing cells are Vβ5− (Fig. 3C, right panel). These data show that while young Vβ5 Tg mice already contain an elevated frequency of Vβ5+ Th17 T cells, these are outnumbered in older mice by cells that have a revised Vβ5− TCR. Considering that, on average, 25% of total CD4+ T cells in 18-to 42-wk-old Vβ5 Tg mice are Vβ5− (Fig. 2B) and that ∼3% Th17 cells exist among total CD4+ T cells (Fig. 3B), this means that 12% of revised T cells are Th17 lineage cells. Compared with the average 0.4–0.8% IL-17+ of total CD4+ T cells in naive B6 mice (Fig. 3B), this is about a 20-fold bias toward the Th17 lineage.
FIGURE 3.
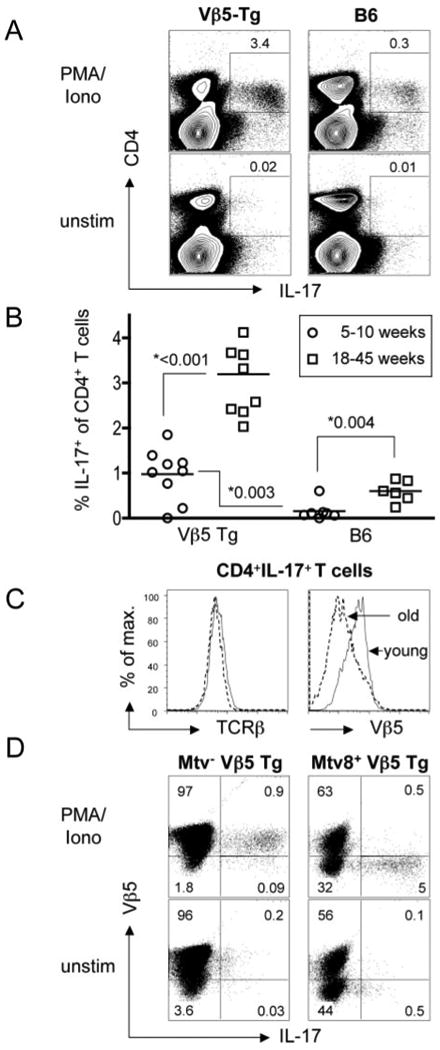
Mtv-8+ Vβ5 Tg mice have elevated frequencies of Th17 T cells that display revised TCR. Splenocytes from 5- to 10-wk-old and 18- to 45-wk-old B6 and Vβ5 Tg mice were stimulated for 6 h with PMA/Ionomycin (PMA/Iono) or left untreated (unstim). A, Representative flow data are depicted for total splenocytes from 23-wk-old mice. Percentages refer to IL-17+ T cells among total CD4+ T cells. B, For all analyzed mice, the fraction of CD4+ T cells producing IL-17 in response to PMA/ionomycin stimulation is plotted and the p values determined by Student's t test are shown. C, The levels of pan-TCRβ (left panel) or Vβ5 staining (right panel) are depicted for gated CD4+IL-17+ cells from representative young and old Vβ5 Tg mice. D, Splenocytes from a 40-wk-old Mtv− Vβ5 Tg (left panels) and a Mtv-8+ Vβ5 Tg mouse (right panels) were stimulated for 6 h with PMA/Ionomycin (upper row) or left untreated (lower row). Shown are CD4+ gated T cells stained for surface Vβ5 and intracellular IL-17.
We used Mtv− and Mtv-8+ mice to test whether chronic stimulation via Mtv-8 contributes to the generation of Th17 T cells or whether the Vβ5 repertoire is intrinsically prone to give rise to Th17 T cells. The 40-wk-old Vβ5 Tg Mtv− mouse has a frequency of IL-17+ T cells similar to that observed in age-matched B6 mice (Fig. 3, compare B with D). In contrast, ∼14% of Vβ5−CD4+ T cells in the Mtv-8+ animal produce IL-17 (Fig. 3D). Thus, the expression of Vβ5 by itself does not intrinsically bias cells toward the Th17 lineage but, instead, Mtv-8 promotes TCR revision and differentiation into IL-17+ CD4+ T cells.
In our model, it is possible that chronic superantigen stimulation expands an already existing population of Th17 T cells and renders them highly susceptible to TCR revision. Alternatively, superantigen-driven TCR revision may bias cells toward Th17 differentiation, perhaps through the generation of autoreactive TCR. In fact, previous work suggests that self-Ag recognition can support the differentiation of CD4+ T cells into Th17 cells (20, 21). For unknown reasons, T cells in OT-II TCRαβ (Vα2Vβ5) Tg B6 mice do not undergo TCR revision despite the fact that this mouse line expresses both Vβ5 and Mtv8. Interestingly, the frequency of CD4+IL-17+ T cells in these mice is comparable to that in B6 mice (data not shown), suggesting a correlation between elevated numbers of Th17 cells and TCR revision, not solely the expression of both Vβ5 and Mtv-8.
It is of interest that while Th17 cells play an important role in conferring protection from Citrobacter rodentium (18), Vβ5 Tg mice are unusually sensitive to this pathogen (22), possibly due to an overzealous Th17 response. In addition to conferring protection from some pathogens, the Th17 population has also been shown to be an important component of autoimmunity. Our work establishes a relationship between TCR revision and the generation of Th17 cells, whereas other reports suggest a connection between TCR revision and autoimmunity (15, 17).
Footnotes
This work was supported by the Howard Hughes Medical Institute, National Institutes of Health Grants AI19335 (to M.J.B.) and AG13078 (to P.J.F.), and a fellowship from the German Academic Exchange Service (DAAD) (to D.Z.).
Abbreviations used in this paper: Treg, regulatory T cell; B6, C57BL/6; Mtv, mammary tumor virus; Tg, transgenic; Vβ5, Vβ5 TCR β-chain.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 3.Blish CA, Gallay BJ, Turk GL, Kline KM, Wheat W, Fink PJ. Chronic modulation of the TCR repertoire in the lymphoid periphery. J Immunol. 1999;162:3131–3140. [PubMed] [Google Scholar]
- 4.Bynoe MS, Viret C, Flavell RA, Janeway CA., Jr T cells from epicutaneously immunized mice are prone to T cell receptor revision. Proc Natl Acad Sci USA. 2005;102:2898–2903. doi: 10.1073/pnas.0409880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper CJ, Orr MT, McMahan CJ, Fink PJ. T cell receptor revision does not solely target recent thymic emigrants. J Immunol. 2003;171:226–233. doi: 10.4049/jimmunol.171.1.226. [DOI] [PubMed] [Google Scholar]
- 6.Cooper CJ, Turk GL, Sun M, Farr AG, Fink PJ. Cutting edge: TCR revision occurs in germinal centers. J Immunol. 2004;173:6532–6536. doi: 10.4049/jimmunol.173.11.6532. [DOI] [PubMed] [Google Scholar]
- 7.Fink PJ, Fang CA, Turk GL. The induction of peripheral tolerance by the chronic activation and deletion of CD4+Vβ5+ cells. J Immunol. 1994;152:4270–4281. [PubMed] [Google Scholar]
- 8.Fink PJ, Swan K, Turk G, Moore MW, Carbone FR. Both intrathymic and peripheral selection modulate the differential expression of Vβ5 among CD4+ and CD8+ T cells. J Exp Med. 1992;176:1733–1738. doi: 10.1084/jem.176.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang CY, Golub R, Wu GE, Kanagawa O. Superantigen-induced TCR α locus secondary rearrangement: role in tolerance induction. J Immunol. 2002;168:3259–3265. doi: 10.4049/jimmunol.168.7.3259. [DOI] [PubMed] [Google Scholar]
- 10.Kondo E, Wakao H, Koseki H, Takemori T, Kojo S, Harada M, Takahashi M, Sakata S, Shimizu C, Ito T, et al. Expression of recombination-activating gene in mature peripheral T cells in Peyer's patch. Int Immunol. 2003;15:393–402. doi: 10.1093/intimm/dxg040. [DOI] [PubMed] [Google Scholar]
- 11.Lantelme E, Palermo B, Granziero L, Mantovani S, Campanelli R, Monafo V, Lanzavecchia A, Giachino C. Cutting edge: recombinase-activating gene expression and V(D)J recombination in CD4+CD3low mature T lymphocytes. J Immunol. 2000;164:3455–3459. doi: 10.4049/jimmunol.164.7.3455. [DOI] [PubMed] [Google Scholar]
- 12.Li TT, Han S, Cubbage M, Zheng B. Continued expression of recombination-activating genes and TCR gene recombination in human peripheral T cells. Eur J Immunol. 2002;32:2792–2799. doi: 10.1002/1521-4141(2002010)32:10<2792::AID-IMMU2792>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.McMahan CJ, Fink PJ. RAG reexpression and DNA recombination at T cell receptor loci in peripheral CD4+ T cells. Immunity. 1998;9:637–647. doi: 10.1016/s1074-7613(00)80661-5. [DOI] [PubMed] [Google Scholar]
- 14.McMahan CJ, Fink PJ. Receptor revision in peripheral T cells creates a diverse Vβ repertoire. J Immunol. 2000;165:6902–6907. doi: 10.4049/jimmunol.165.12.6902. [DOI] [PubMed] [Google Scholar]
- 15.Vaitaitis GM, Poulin M, Sanderson RJ, Haskins K, Wagner DH., Jr Cutting edge: CD40-induced expression of recombination activating gene (RAG) 1 and RAG2: a mechanism for the generation of autoaggressive T cells in the periphery. J Immunol. 2003;170:3455–3459. doi: 10.4049/jimmunol.170.7.3455. [DOI] [PubMed] [Google Scholar]
- 16.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagner DH, Jr, Vaitaitis G, Sanderson R, Poulin M, Dobbs C, Haskins K. Expression of CD40 identifies a unique pathogenic T cell population in type 1 diabetes. Proc Natl Acad Sci USA. 2002;99:3782–3787. doi: 10.1073/pnas.052247099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25− Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 20.Hirota K, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J Exp Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maggio-Price L, Nicholson KL, Kline KM, Birkebak T, Suzuki I, Wilson DL, Schauer D, Fink PJ. Diminished reproduction, failure to thrive, and altered immunologic function in a colony of T-cell receptor transgenic mice: possible role of Citrobacter rodentium. Lab Anim Sci. 1998;48:145–155. [PubMed] [Google Scholar]


