Abstract
In many health care settings, it is uneconomical, impractical, or unaffordable to maintain and access a fully equipped diagnostics laboratory. Examples include home health care, developing-country health care, and emergency situations in which first responders are dealing with pandemics or biowarfare agent release. In those settings, fully disposable diagnostic devices that require no instrument support, reagent, or significant training are well suited. Although the only such technology to have found widespread adoption so far is the immunochromatographic rapid assay strip test, microfluidics holds promise to expand the range of assay technologies that can be performed in formats similar to that of a strip test. In this paper, we review progress toward development of disposable, low-cost, easy-to-use microfluidics-based diagnostics that require no instrument at all. We also present examples of microfluidic functional elements—including mixers, separators, and detectors—as well as complete microfluidic devices that function entirely without any moving parts and external power sources.
Introduction
Over the past 15 years, a variety of microfluidics-based diagnostic devices have been developed by various research groups. These devices hold promise for miniaturization of analysis equipment, improvement in response times, and simplification of analysis procedures. With a few having entered commercial use these systems typically consist of a small microfluidic chip surrounded by a desktop-sized analysis instrument.1–3 Further integration of electronic and fluidic components will allow additional miniaturization of the analysis instrument. Another means to further miniaturize instruments and integrate elements of micro total analysis systems (μTAS) is to eliminate as many power-consuming and otherwise complex elements as possible. These elements can be replaced with passive components that operate without external power by manipulating fluids using gravity, air pressure, or simple manual actions.
In this paper, we review progress toward development of disposable low-cost, easy-to-use TAS microfluidics-based diagnostics that require no instrument at all. We present examples of microfluidic functional elements—including mixers, separators, and detectors—as well as complete microfluidic devices that function entirely without any moving parts and external power sources.
We define “non-instrumented, microfluidics-based diagnostics” as follows:
The device requires only a disposable component and no external reusable instrument for operation. The disposable device can have a number of subcomponents that may include low-cost electronics, as long as the character of the device clearly remains that of a disposable.
The device includes at least one microfluidic feature—such as a microfluidic mixer, aliquoter, separator, concentrator, or reactor—that contributes to its functionality.
We also discuss “minimally instrumented, microfluidics-based diagnostics” that use a very simple external reusable device, such as a battery-operated LED for visualizing a detection line or a reusable plastic lens for viewing results. However, we do not consider microfluidic devices that require external detectors with, for example, electronic output or pump systems to be “minimally instrumented.”
Both non- and minimally instrumented devices lend themselves to applications such as ultra-low-cost disposable qualitative and semiquantitative medical and environmental assays for home, office, and field use, and for sample or reagent preparation tools that provide processed liquids for downstream analysis or synthesis. In this paper, we focus on diagnostic assay applications.
The case for a disposable-only diagnostic
Although most medical diagnostic tests in developed countries are performed in centralized, well-equipped laboratories, significant niches exist for diagnostics that require little or no instrumentation (and are likely to grow). We and others see those niches primarily in three areas: (1) developing-country health care, (2) home testing in developed countries, and (3) diagnostic and bioanalytical disposables for use in natural or man-made bioemergencies by first responders.4–12 In developing countries, central diagnostic laboratories are uncommon and typically serve only portions of major metropolitan areas or the wealthier segments of society.
Although use of portable instruments is feasible in a number of settings, there are many challenges associated with having specialized equipment for point-of-care (POC) diagnostic tests. The up front cost of the instrument and the cost of service and maintenance increase the cost per test and add logistical challenges to testing systems. Cost-effective use requires that a sufficient number of tests be performed over the useful life of the device to justify the expenses. Thus, decisions to procure equipment are largely based on the projected volume of tests that will be performed and the reimbursement rate for testing. In settings with high patient volumes and insurance reimbursement rates, such as hospitals and large medical clinics in the developed world, the cost of equipment can be spread over large volumes of insured patients. But in settings with low testing volume or with little or no insurance reimbursement for testing, diagnostic testing is often not feasible. There are a number of settings in both developed and developing countries that are subject to these constraints and would thus benefit from POC tests that do not require instrumentation.
Non-instrumented, single-use, disposable POC tests would be particularly beneficial in health care settings in low-resource settings. These settings are encumbered by many infrastructure challenges, including lack of access to sterile water or electricity. Furthermore, health settings in the developing world are perennially underbudgeted, and the cost of testing is often passed to the patient, making the cost per test a very important factor.
Even in low-resource settings, cost-effective tests are being adopted and implemented. One of the most dramatic examples of successful POC testing is the widespread implementation of voluntary counseling and testing centers for human immunodeficiency virus (HIV) in developing countries.13 These testing centers have been a key front-line resource in the fight against HIV, and they rely on the availability of inexpensive, POC lateral flow tests. Lateral flow tests for malaria are also replacing smear microscopy in many malaria-endemic regions.14
The value of POC testing in developing countries was characterized in great detail in a supplement to Nature supported by the Bill & Melinda Gates Foundation.15 The supplement was the culmination of the Global Health Diagnostics Forum, a collaborative effort involving the Foundation, RAND, experts in relevant diseases, representatives from diagnostics and technology development arenas, and experts in the modeling of disease impact and the introduction and adoption of diagnostic technologies. The supplement presents a series of models that show an inverse correlation between lives saved and the level of infrastructure required to perform a diagnostic test. Thus, the maximum number of lives saved is always for tests that can be performed with no infrastructure. This provides compelling evidence for development of non-instrumented, single-use POC tests for use in the developing world and should justify further expenditures on research and development in this area.
There are a number of applications for non-instrumented, single-use POC tests in high-resource settings as well. These include applications that require highly distributed testing capacity or are subject to very unpredictable demand. One example of a highly distributed, low-volume testing application is home pregnancy testing. The economics of the market for home pregnancy testing would not support requiring an expensive instrument to perform the test. Contrast this with another widely distributed application, glucose testing, in which multiple tests are performed daily by each home user. Even for a single user, the cost of the instrument can be amortized over many tests.
Single-use POC tests could also be valuable for applications in disaster relief, biodefense, or disease outbreaks. These scenarios are characterized by extremely unpredictable demand, and risk mitigation planning in these fields would benefit from the ability to rapidly deploy a highly distributed testing network. Tests could be stored in tactical locations and deployed rapidly by first responders without incurring costs associated with outfitting each team with a piece of equipment.
A model for a non-instrumented, microfluidics-based μTAS—the ICS assay
For the past decade, immunochromatographic strip (ICS) tests have been one of the very few diagnostic technologies successfully used in the developing world. Although ICS tests are not microfluidic devices, they can be considered μTAS technologies, and they provide a great model for what a microfluidics-based μTAS should strive for in terms of user characteristics, simplicity, cost, integration, and in some cases, even performance.
ICS tests provide POC diagnosis in areas without access to well-equipped and -staffed clinical laboratories. Because they rely on inexpensive, off-the-shelf components and reagents, they are affordable, in many cases costing less than US$2 to the end user. They can be formatted for detection of antigens or antibodies (more recently also nucleic acids) and are usable with a wide range of specimens, making them useful for a wide range of applications.16,17 ICS strips require relatively little and sometimes no sample processing, and they do not require an external instrument. Fig. 1 depicts the key features of an example ICS test, a test for gonorrhoea that was developed specifically for low-resource settings.18
Fig. 1.

Schematic of immunochromatographic strip (ICS) test for gonorrhoea specifically developed for low-resource settings.
The strips are stable for many months at ambient temperatures—a critical feature for settings in which the electrical supply is inconsistent and temperature control is difficult. Because of their low cost and stability, the tests may also be used by epidemiological surveillance teams in the field to gather baseline data or to assess the effect of public health interventions.
Despite their many very positive attributes, lateral flow strips also frequently suffer from limited sensitivity and sometimes specificity. Although some lateral flow assays can achieve sensitivities and specificities in the range of 90% or higher, others, even ones that are in clinical use, can have sensitivity as low as 70%. Those performance values, unacceptable in most high-resource settings, are a huge improvement over the purely syndromic diagnosis common in developing countries. Nevertheless, better tests are needed.
To date, the vast majority of non-instrumented, single-use POC tests are immunoassays in one of three formats: lateral flow, flow through, or solid phase. These tests are relatively simple to perform, inexpensive to produce, and very suitable for many testing applications. Due to inherent limitations in the limit of detection of these platforms, their use is confined to testing for biomarkers that are abundant in the specimen, such as hormones (e.g., pregnancy tests), immunoglobulins (e.g., HIV tests) or abundant pathogen antigens (e.g., malaria tests). A number of infections cannot be diagnosed by monitoring relevant biomarkers with currently available rapid tests (e.g., tuberculosis). Microfluidic immunoassay platforms under development might improve limits of detection and thus extend POC testing to pathogens that are currently not feasible for testing by current rapid test platforms. Microfluidic immunoassay platforms also hold promise for developing simple-to-use tests for more than one pathogen at a time (multiplexed tests) which will enable entirely new diagnostic algorithms that are based on identifying the specific etiological agent (from a panel of possible agents) that causes a particular syndrome, as opposed to determining the presence or absence of a particular pathogen (or the presence or absence of an immune response to that pathogen). Despite the technological potential for developing POC immunoassays with greater sensitivity and specificity, there is an inherent protein-based biomarker cap on the levels of sensitivity and specificity that can be attained with immunoassays depending on the pathogenesis and biology of the infectious agent.
Another exciting advantage of microfluidics is that it enables development of POC platforms that detect nucleic acids (DNA or RNA). Nucleic acid amplification tests are often more sensitive and specific than their immunoassay counterparts. Making this technology comparable in complexity to that of a strip-based rapid diagnostic test by removing the need for an instrument entirely would be nothing less than a quantum leap in POC testing technology.
Designing non-instrumented, microfluidics-based disposables
Mapping diagnostic processes on non-instrumented, microfluidics-based disposables
Requirements of non-instrumented diagnostic systems
Any medical diagnostic device must provide a result, usually the presence (or absence) or concentration of a chemical compound contained (usually) in a tissue or bodily fluid. For the purposes of simple, point-of-care diagnostics, that sample is most likely blood, interstitial fluid, saliva, vaginal fluid or cellular material, or nasal fluid. A diagnostic will either have to be capable of measuring a parameter directly in one of those fluids with relatively minimal interference from other components of that sample, or perform a variety of preparative steps prior to measuring that parameter. Direct measurement is usually impossible, even devices such as electrochemical glucose meters require at least a membrane, and an enzyme layer, to convert a physiological parameter (glucose in whole blood) into a parameter that the electrode can measure (hydrogen peroxide).
In general, steps for performing any diagnostic test include, besides specimen collection and metering (for quantitative analysis), processing steps such as removal of unwanted sample compounds, analyte enrichment, analyte labeling and/or signal amplification, and analyte signal detection.
For all these steps, microfluidic solutions may enable what otherwise requires fairly complex technologies (ranging from a flow cytometer to a micropipette) for POC testing. Microfluidic solutions can be particularly effective when applied to take advantage of unique biomechanical or biophysical properties of the target analyte. Such an approach can elegantly combine many of the steps described above to reduce the complexity of a diagnostic test. We describe below the diversity of requirements for the steps mentioned for diagnosis or management of infectious diseases and present some non- or minimally instrumented microfluidic solutions that are currently under development.
Specimen metering
For some diagnostic applications, quantification is highly desirable (e.g., CD4 counts for HIV case management). Accurate delivery of known specimen volumes is crucial to the reproducibility and accuracy of the test. Simple, commercially available, off-the-shelf solutions are available to perform specimen metering prior to introduction of a specimen onto the POC device. Compelling microfluidic solutions to on-device metering include the use of capillaries.19 A major challenge to diagnostic platforms which has not yet been successfully addressed is developing a robust, user-independent interface between the specimen metering device and the diagnostic device.
Specimen processing and analyte enrichment
Specimen processing can include cell sorting (e.g., for CD4 testing or for malaria diagnostics), separation of sera from cells for immunoassays (IgG and/or IgM detection), and cell lysis for immunoassays (antigen detection) or for nucleic acid amplification tests (NAATs) with subsequent nucleic acid extraction. The use of microfluidics to sort cells and separate cells from whole blood has recently been reviewed.20 There are several approaches currently developed to separate cells from plasma and to separate white blood cells from red blood cells and sort cells.20–23 Several teams are currently developing microfluidic approaches to extracting nucleic acid for NAATs. A major challenge is the need to extract nucleic acid to meet the levels of purity required for more traditional NAAT enzyme-based amplification technologies such as polymerase chain reaction (PCR) and isothermal techniques. This is a multistep process requiring multiple reagents with a wide volume disparity ranging from more than 1 ml of specimen and lysis buffer to less than 50 μl elution buffer. Several groups are working on developing nucleic acid extraction microfluidic devices, including some disposable technologies.24–27 Alternately, nucleic acid-based molecular diagnostics that do not require enzymatic target nucleic acid amplification prior to detection may be more amenable to POC devices by removing the complex nucleic acid extraction process and enzymatic nucleic acid amplification step; however, direct NA assays usually require a more sensitive detection platform than what appears feasible in a non-instrumented device.
Signal amplification and detection
For many diagnostic modalities, signal amplification is intimately associated with detection such as through fluorescence labeling or enzymatic signal amplification through colorimetric fluorescent or chemiluminescent products. Many of these form the basis of diagnostic immunoassays, including lateral flow tests. These approaches have also been applied towards the development of disposable microfluidic devices.19,28 In general, immunoassays can detect protein quantities down to pictogram levels, which is, for example, sufficient for the detection of antibodies to many pathogens; for direct detection of the presence of pathogens, however, NAATs such as PCR-based nucleic acid amplification can provide much better sensitivity (down to the single copu level). NAATs required distinct signal amplification and detection steps until the advent of real-time PCR using fluorescence concomitantly with the amplification cycles, and isothermal amplification technologies that can generate agglutination-like signals, as well as fluorescence and chemiluminescence. Attempts to minimize the resources required for NAATs have included developing lateral flow strip technologies for amplicon detection.16,29,30 Isothermal NAATs are more amenable to simple signal detection technologies but have lagged behind PCR in the microfluidic realm.
There is significant effort to develop “label-free” diagnostic platforms that rely on physical signals such as impedance and vibration in cantilever technologies.28,31,32 These approaches have the potential to reduce reagent costs and test complexity; however, it is difficult to see how those methods could be implemented on a non-instrumented platform.
Integrated POC microfluidic devices
Microfluidic integration of diagnostic processes
There are increasing successes in developing integrated, microfluidic, disposable diagnostic cards that still require instrumentation.11,33–38 Also, reports of disposable microfluidic devices with little or no instrumentation for POC diagnostics are appearing.19,24,39–42 One such report utilizes pressurized gas in a micro-reservoir as an energy source to control sequencing on a disposable card.42 Developing disposable microfluidic NAATs will require either use of nucleic acid signal amplification technology alternatives to PCR and current isothermal NAATs or identification of novel sources of energy to carry out the NAAT. Examples of such concepts are shown in Fig. 4–6 and 14.
Fig. 4.

Configuration of HydroLogic technology. A pressure signal received in the thrust inlet pressurizes the pressure capsule, which in turn causes the acidic solution to pressurize. The pressurized acidic solution ruptures the barrier to the alkaline cell. Microencapsulation of the alkaline controls the delay of a gas-generating reaction and its rate, providing means for controlling the subsequent events in the HydroLogic system.
Fig. 6.

Front (a) and back (b) of exothermal circulation polymerase chain reaction card developed by Wheeler et al.72 The authors have designed a version of this device that would rely on exothermic heating materials instead of electric heaters, thus making the device non-instrumented.
Fig. 14.

Prototype filter dial device for an integrated sample processing device with on-board reagents. Reverse transcription mix stored in two detachable vials: (1) POC, for emerging point-of-care NAT technologies and (2) CF, for central facility surveillance testing.
Components suitable for non-instrumented, microfluidics-based disposables
Pumps
Moving sample fluids and reagents on a diagnostic device requires developing a pressure differential in the flow path to direct fluid in one direction or another. Miniaturized versions of positive-displacement pump designs such as gear or peristaltic pumps have been proposed for microfluidic applications, but these all require some external power source or repetitive motion to control. It is desirable for fluidic motion in a non-instrumented design to be driven by a readily available force such as gravity, capillary action, absorption in porous materials, chemically induced pressures or vacuums (e.g., by a reaction of water with a drying agent), or by vacuum and pressure generated by simple manual action.43 Wicking and capillary action have been widely used to motivate fluids for POC diagnostics. For example, low-cost lateral flow tests demonstrate the elegant and inexpensive use of wicking to drive multiple sample types through all steps of an assay. Pumpless microfluidics have also been described by Weigl et al. (patent WO/2001/026813).43
In addition, flexible finger pumps have been described in patent WO/2003/008102.44 This passive system employs a microchannel to drive fluid at a substantially constant rate, and it can be employed through use of a one-way vent valve or duckbilled valve or finger-blocked vent. Fig. 2 shows a finger-based bellows fabricated using inexpensive materials such as polydimethylsiloxane (PDMS).45 This example has a flexible diaphragm and requires simultaneous blocking of a vent hole during pumping and uncovering this vent during repriming.
Fig. 2.

Example of a finger bellows that primes a wicking pad that then takes over the pumping action.
Patent WO/2003/008102 also describes a microfluidic gravity pump with constant flow rate. This passive system employs a microchannel and a gravity-driven pump consisting of horizontally oriented reservoirs that supply fluid to the microchannel at a substantially constant rate.44 The device may be useful for numerous microfluidic applications such as cell-size sorting.
One promising pumping method is the spring-loaded pump. As an example, a technology called Springfusor (Fig. 3) is marketed by Go Medical Industries Pty Ltd of Australia (http://www.gomedical.com.au/index.php) as a simple, portable, infusion pump to slowly deliver drugs.46 The output from the syringe is controlled to ± 10% flow rate by a short length of narrow-bore flow-control tubing. Not yet commercialized for a microfluidic platform and currently available in only mesofluidic volumes (30 and 100 mL), this technology has several advantages worth mentioning: it is reusable, low cost, compact, and easy to activate, and it requires no external power. Despite these advantages, it is unclear whether such a pump could be a low-cost part of a disposable assay.
Fig. 3.

Springfusor, an example of a simple, portable, spring-loaded infusion pump.
Another fluid-motivating method that may lend itself to non-instrumented diagnostic technologies is called HydroLogic. This technology is currently under development by Plan A Solutions from Boulder, CO. HydroLogic is a self-contained, disposable fluid management system that can handle complex dispensing procedures for various applications, including diagnostics. In this system, internal-controlled chemical reactions provide the pressure source that propels fluid transport. In the configuration depicted in Fig. 4, the propellant is carbon dioxide generated from a reaction between a citric acid solution and baking soda powder. The baking soda powder can be microencapsulated for a controlled delay of reaction, thus forming a timing mechanism. In this manner a timely sequenced chain of dispensing and mixing events can be accomplished. This technology has yet to be tested on microfluidic platforms.
Typical PCR applications involve keeping a sample stationary while using a heat pump or combination of heat sources and heat sinks to cycle the temperature over the necessary three-temperature profile to conduct PCR. Unfortunately, the large thermal momentum and energy required to achieve these temperature profiles creates a barrier to non-instrumented designs using this approach. To address this problem, scientists have demonstrated moving the fluid instead over a series of appropriate temperature zones separated by insulators.47 Although this reduces the heat energy requirements, it introduces a need to move the fluid. Again, this fluid motivation component creates a barrier to non-instrumented design.
One approach considered by the authors to motivate fluid for PCR is the spiral channel design, depicted in Fig. 5, which avoids the requirement for moving parts. Movement of the sample is through capillary and wicking forces only. After reconstitution and mixing, a sacrificial flow strip is inserted into the flow strip well. As the strip wets with wetting buffer, the sample is drawn through the spiral reaction channel while continuously cycling through the three PCR zones. The sample undergoes one PCR cycle per revolution. Once the sample is completely contained in the spiral channel, the user removes the saturated sacrificial flow strip and replaces it with a test strip with colloidal gold control and indicator stripes. The resultant wicking forces from the dry test strip in contact with the wet channel draw the sample through the spiral, completing the 30 cycles and initiating take-up in the test strip. An additional, user-added 100 μL (50 μL each to the buffer and sample wells) washes the channel and acts as a running buffer as it is also drawn into the test strip.
Fig. 5.

Spiral polymerase chain reaction channel designed by PATH.
Another approach, demonstrated on a microlevel by researchers at Lawrence Livermore National Laboratory44,48 is known as thermosyphon, in which only the fluid, not the reaction chamber, is subjected to hot and cold cycling. This approach operates on the principle that the buoyancy of a hot column of water is greater than that of a cooler column. This buoyancy difference sets up a driving head to move the fluid. When applied to PCR, thermal momentum is minimized, allowing for more rapid cycling at a fraction of the power consumption (Fig. 6).
Although these examples rely upon external power, the authors have proposed using exothermic and endothermic reactions thermally coupled to phase change materials (PCM) to replace the micro heating units to establish the thermal gradients to drive fluid flow.
Mixers
In general, mixing small amounts of reagents and samples in microfluidic structures is challenging due to the low Reynolds numbers. In typical microfluidic structures used for diagnostic purposes, the Reynolds number is significantly less than 2100, which is recognized as the transition between laminar (Re < 2100) and turbulent flow (Re > 2100). In laminar flow, mixing mechanisms are limited to diffusion and convection between fluid layers.
Many mixing methods have been proposed for microfluidic structures, including electrohydrodynamic, magnetohydrodynamic, pressure perturbations, centrifugal, electrophoretic effects, pulsed flow (chopping), and an on-card stir bar stator with oscillating field on a card reader. However, these methods all require external power sources.
Thus, we are left with a small group of passive fluid-mixing methods. Some passive methods are designed to reduce the diffusion lengths required for adequate mixing by decreasing layer thicknesses through layering, folding, and stretching. Other methods increase the surface area between fluid layers. Another strategy for passive mixing is to induce eddy currents and small, localized perturbations to encourage turbulent-like flow.
Design considerations for choosing an appropriate mixing method should include: the degree of mixing required, fluid volumes involved, level of sophistication required to manufacture, and the anticipated Reynolds number. Table 1 outlines a variety of passive mixing methods, their mechanism of mixing, advantages, and disadvantages, and Fig. 7 illustrates each method.
Table 1.
Features of various passive mixing methods
| Passive mixing method | Mechanism | Reference | Advantages | Disadvantages |
|---|---|---|---|---|
| Transverse mixing wells | Channels (45°) or biased herringbone structures are cut into layers adjacent to main channel and force cross-over, or transverse component (bas relief) | Kung et al, 199494 | Simple design and good at low Re (0–100) and can induce mixing over a relatively short distance | Multilevel fabrication |
| Spiral micro-channels | Centrifugal effects predicted by Dean number (ratio of inertial and centrifugal forces to viscous forces) | Sudarsan et al, 200695 | Single-level fabrication | Relies on higher Re to be effective |
| Expansion vortex | Abrupt change in cross-sectional area cause jet-like (nozzle) motion causing expansion vortices | Sudarsan et al, 200695 and Alleborn et al. 199796 | Single-level fabrication, effective over a wide range of Re | Relies on higher Re to be effective, relies heavily on correct introduction angle and velocity |
| Channel obstacles | Eddy currents and low pressure redirects – much like an airplane wing | Wang et al. 200197 | Single-level fabrication | Limited to specific manufacturing methods |
| Lamination split and recombine | Increase surface area, continuous diffusion length reduction through channel geometry | Munson et al 200398 | Can be easily combined with other methods | Multilevel fabrication |
| Tesla split and recombine | Increase surface area, continuous diffusion length reduction through channel geometry, makes use of the Coanda effect to split part of the fluid stream and direct it so that it recombines with the opposing flow of the other part of the stream | Schafer et al, 200399 | Single-level fabrication | Relies on higher Re to be effective |
| Hybrid (obstacle nozzle) | Eddy currents and low pressure redirects – much like an airplane wing | Lin et al., 2003100 | Single-level fabrication, effective over a wide range of Re | Limited to specific manufacturing methods |
| Square wave mixer | Eddy currents and low pressure redirects | Mengeaud et al., 2002101 | Single-level fabrication, effective over a wide range of Re | Less effective than chaotic advection methods |
Fig. 7.
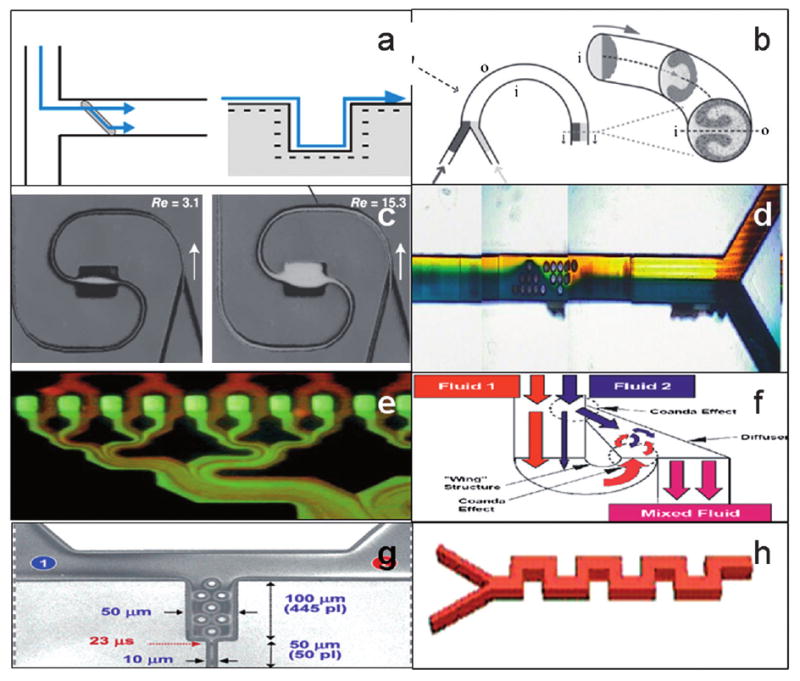
Diagrams of various passive mixing methods: (a) transverse mixing wells, (b) spiral microchannels, (c) expansion vortex, (d) channel obstacles (e) lamination split and recombine, (f) Tesla split and recombine, (g) hybrid (obstacle nozzle), and (h) square wave mixer.
Valves
Non-instrumented valve solutions are limited. Most macro-scale valves have two or more physical positions that block or redirect fluid flow. Typical valve components include a valve stem, bushing, disc, and seat and require activation through hydraulic, pneumatic, manual, or solenoid actuation. In theory, non-instrumented valves for microfluidic applications could be miniaturized versions of their macroscale counterparts. Micro-valves have been developed that utilize magnetic, electrostatic, heat, mechanical, pneumatic, and peizo-electric actuators.48–54 However, the complexity and incompatibility of manufacturing methods has largely limited the success of this approach.
A number of check valves have been demonstrated (Fig. 8). For example, Hasselbrink developed an on-chip check valve and a diverter valve fabricated using wet etching techniques and a nonstick polymer formulation.55 This free-floating check valve, constructed from a mobile concentric cylinder with bypass, is laser-polymerised in situ from a teflon-like polymer. Deshmukh described another free-floating check that demonstrated a flow resistance ratio between closed and open states of more than 100 to 1.56 Deshmukh further characterized bubble expansion and contraction as a means of providing fluid motivation.
Fig. 8.
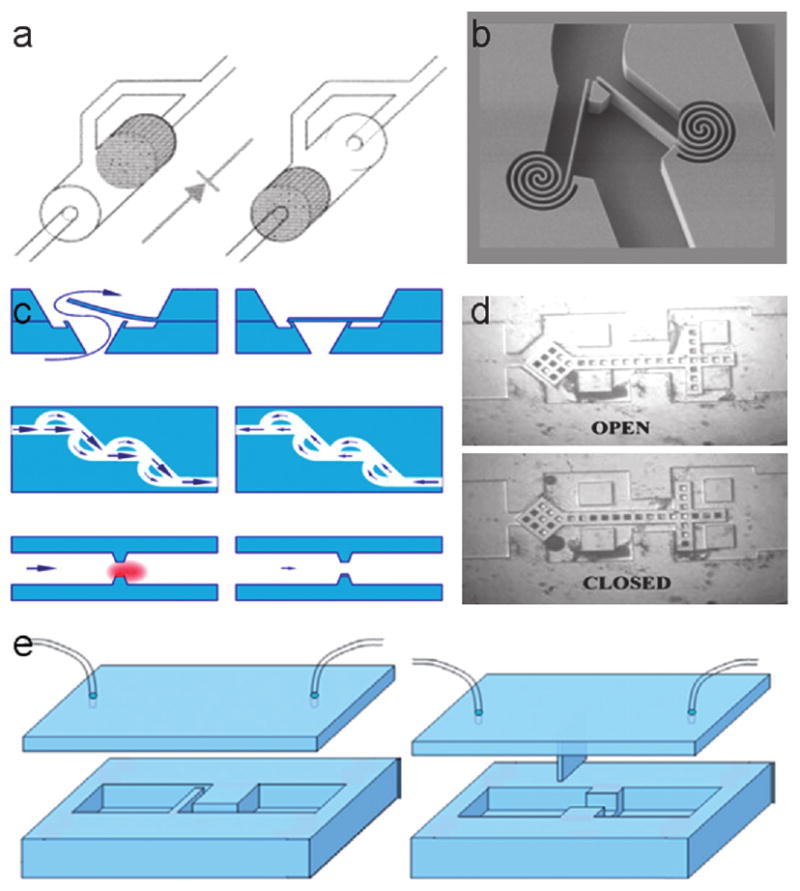
Examples of check valves include: (a) an on-chip check valve and diverter valve described by Hasselbrink, (b) a spring check valve design, (c) a type of PDMS “flap and block” described by Yang et al., (d) a free-floating check valve characterized by Deshmukh, and (e) a design for a flap check valve.93
Yang et al. described two styles of PDMS “flap and block.” The simple design consists of a thin flap that lies perpendicular to fluid flow and a restriction block.57 A two-dimensional model of the design demonstrated saturated leakage at high pressures, but a three-dimensional model demonstrated no leakage at high pressures.
Other innovative valve types potentially suitable for non-instrumented diagnostics include:
Hydraulically actuated (Thorsen et al. 2002)58
Parafin phase-change operated (Pal et al., 2004)59
Ice phase-change operated (Chen et al., 2005)60
Temperature responsive, viscosity controlled (Liu et al., 2002)61
Magnetic field responsive, viscosity controlled (Hartshorne et al 2004)62
Salt-responsive hydrogel volumetric change (Beebe et al., 2003)63
Thermally responsive hydrogel volumetric change (Wang et al., 2005)64
One benefit of hydrovalves is that their responsiveness increases as size decreases. Scaling down hydrovalves to micrometer size greatly improves their response time because this technology relies upon short diffusion lengths.
Of particular interest is the valve described by Wang and Chen, a thermally actuated, self-regulated hydrogel valve for flow control in pneumatically driven microfluidic systems. This valve has many advantages, including ease of fabrication, tight sealing, and ability to withstand relatively high pressures.
Separators
There are several examples of separation techniques that might be multiplexed and integrated into non-instrumented microfluidic platforms.20–27,63,63
The H-filter is based upon the parallel laminar flow of two or more miscible streams in contact with each other. The streams do not mix, but chemicals can diffuse from one stream to the other with smaller molecules diffusing faster than larger ones. This principle can be used to salt from a solution containing DNA or to extract smaller molecules from whole blood. The H-Filter® platform has two input flows and two outputs. With this platform, diffusion can be used to filter unwanted components or to extract desired components from one of several fluids being simultaneously processed. Diffusion along the horizontal section serves as an extractor—pulling certain elements out of the sample and into the diluent. Another, similar separation method relies upon microfluidic/micromagnetic forces to selectively remove living cells from flowing biological fluids without any wash steps (Fig. 9).65
Fig. 9.

Schematic of a separation method relying upon microfluidic/micromagnetic forces to selectively remove living cells from flowing biological fluids without any wash steps.
Concentrators
Diagnostic device concentrators increase concentration of dissolved or dispersed substances or microorganisms (e.g., bacteria, viruses, proteins, toxins, enzymes, antibodies, etc.) under conditions gentle enough to preserve their specific activity or viability. These components are useful to diagnostic applications in that they can increase the signal strength of any diluted substance of interest. The most common method to concentrate or remove bioparticles is centrifugation. However, this method has not been used successfully in microfluidic devices.
Other methods such as chromatography, dielectrophoresis, transverse isoelectric focusing, and ultrasonic trapping have taken advantage of microscale phenomena.66–69 However, due to their reliance on instruments, they are not appropriate for non-instrumented POC devices.
MicroPlumbers Microsciences LLC, of Seattle, WA, has developed an inexpensive concentrator based on isothermal evaporation. One advantage of this innovation is its generic nature, as it is able to concentrate practically any dissolved or dispersed substance or particles at temperatures low enough to retain the viability of any biological component of interest. While the initial tested configuration of this device requires fluid motivation and heat controls, we envision an adaptation of this concentrator that employs the non-instrumented fluid motivation and exothermic heating with PCM temperature modulation described elsewhere in this article. Such an enhancement of this component would eliminate dependence on instrumentation and increase its utility in a POC disposable diagnostic device.
Precise fluid handling
In addition to standard pipette techniques, there are two inexpensive, disposable methods for metering sample and reagent volumes that are worth mentioning here. First, is the Microsafe collection and dispensing tube by Safe-Tec Clinical Products Inc, Ivyland, PA, which is commercially available in 5 μL to 100 μL volumes. These are designed to collect and dispense blood samples at a preset volume using capillary action and a volume controlling air vent.70
Second is the Exact Volume Pipette manufactured by Poly-Pipets, Inc.71 These single-dose disposable pipets are available in a range of sizes from 20 μL to 250 μL. They are designed to dispense the exact volume held in the stem. Any excess fluid from the draw remains in the bottom bulb.
In addition, PATH has developed a single-use, calibrated blood collection and transfer device. This device depicted in Fig. 10 is designed to collect 100 μL of blood from a fingerstick and transfer diluted blood to a microfluidic diagnostic disposable.
Fig. 10.

A prototype device designed to collect 100 μL of blood from a fingerstick and transfer diluted blood to a microfluidic diagnostic disposable.
Heating and cooling mechanisms
Heating and cooling in microfluidics is often accomplished through traditional means— heating via resistive or I2R losses on an element that can be etched onto the microfluidic platform or by using thin film strip heaters. Also, heating and cooling can be accomplished through a Peltier thermoelectric heat pump. As both of these methods require a form of electrical energy and often bulky components, we will not include them in further discussion of non-instrumented microfluidic components.
Passive cooling on a microfluidic platform as described by Wheeler et al. does not require any complex electrical components such as a thermocouple or microelectronics and thus lends itself to use on non-instrumented platforms.72 However, this method has open-loop control, and the absence of closed-loop temperature feedback means that performance varies significantly with ambient temperatures.
Guijt et al. describe a temperature control channel that uses chemical and physical processes to achieve integrated heating and cooling in microfluidic devices.73 Localized heating and cooling are controlled by positioning the endothermic (evaporation of acetone) and exothermic (dissolution of concentrated sulfuric acid in water) processes in microreaction chambers near the reactant flow interface.
Detectors and viewers
The T-sensor is based upon the same laminar diffusion principle described previously for the H-filter but combines sample preparation with self-calibration and detection. Controlled continuous diffusion between sample and reagent/detection streams creates a region of varying saturation of a detection chemistry. The combined reagent/analyte stream can be probed by optical absorption or fluorescence. The T-sensor can be used either downstream from an H-filter or in the presence of nondiffusing particulates. It can be used to detect most chemicals present in a fluid sample, and because it operates only on small sample volumes, it has a very fast response time. Over the past five years, T-sensor assay feasibility has been demonstrated for a variety of clinical parameters, such as blood pH and oxygen, electrolytes, proteins, enzymes, and drugs, by using detection methods ranging from fluorescence and absorption to voltammetry. T-sensor measurements have been demonstrated for analytes directly in whole blood without prior separation of blood cells. In addition, monitoring signal intensities along the T-sensor detection channel (in flow direction) provides a means of measuring the kinetics of a reaction, thus allowing kinetic diagnostic reactions to be measured not as a function of time but of distance from the starting point of interdiffusion.74
Value of integrating components and future progress in battery technology
Although the stand-alone components for hydrogel valving and exothermic heating or cooling do not in themselves constitute a closed-loop feedback system, these elements could be combined for this effect. For example, consider the hydrogel valve described in patent 6523599.75 This valve is responsive to the temperature of an adjacent fluid volume such that the valve is open at low temperatures and constricts to close over a narrow or broad temperature range depending on the desired level of control. Using this valve technology to control the on-chip mixing of exothermic or endothermic reactants could result in a closed-loop temperature control system. This self-regulating system could be further enhanced through use of a phase-change, heat-storing element.
Up to this point, we have constrained our discussion of non-instrumented devices to those that do not rely upon external power. However, new developments in battery technology are worth mentioning. Enfucell is one of several companies that have developed novel battery manufacturing methods based on traditional paper printing and lamination technology. Such developments could allow for inexpensive, disposable batteries integrated into laminar flow and other microfluidic devices.76 Because these devices would not rely upon external power, they would qualify as non-instrumented assays. This might open up additional opportunities for fully integrated disposable optical sensor systems, such as a fluorescent detection system described by which is constructed by self-assembling micron-scale excitation sources and photosensors onto plastic substrates.77
Examples of non-instrumented, microfluidics-based devices and diagnostics
There are currently very few products in the marketplace that fit the definition of a non-instrumented, microfluidics-based disposable diagnostic. One such device is the Micronics ABO bloodtyping card.78 There are, however, several such devices in various stages of development, as well as several devices that could be used as diagnostic devices but are currently commercialized as training and research tools only. We provide examples in the following.
ABO card
Mis-transfusion of blood—the failure to give “the right blood to the right patient at the right time for the right reason”—is the second highest risk among major transfusion hazards. Most errors result from administration of properly labeled blood to the wrong recipient. As little as 30 mL of ABO-incompatible blood can be fatal.
One of the authors (while at Micronics, Inc., Redmond, WA) developed a microfluidics-based disposable device for ABO blood typing that does not require an instrument (Fig. 11).78 The device relies on the diffusion-based interaction of whole blood and antisera in microfluidic channels. All reagents are stored on the card. The typing operation involves placing a droplet of whole blood into an opening on the card and manually activating a bellows to aspirate the fluids and initiate the process. In approximately 10 seconds, the card produces a readable result that remains visible for hours. Images and a legend printed onto each card make interpretation straightforward for untrained personnel.
Fig. 11.

Easy-to-use, fast, and easy-to-interpret disposable device for ABO blood typing developed by Micronics, Inc. All fluids are moved and aliquoted through capillary force and manual on-card bellows pumping. Reagents and sample are mixed passively along laminar flow diffusion interfaces in microchannels. The result visible in the viewing window indicates blood type A, Rh positive.
Non-instrumented, diffusion-based detection devices and separators
While not strictly speaking diagnostics, passive separation and detection devices based on laminar flow diffusion interfaces have the potential to be used as non-instrumented diagnostics.79–90
Most fluids show laminar behavior in miniature flow structures with channel cross sections below 1 mm. This allows the movement of different layers of fluid and particles next to each other in a channel without any mixing other than by diffusion. A sample solution (e.g., whole blood), a receptor solution (e.g., an indicator solution), and a reference solution (a known analyte standard) are introduced in a common channel (T-Sensor) and flow next to each other until they exit the structure. Smaller particles such as ions or small proteins diffuse rapidly across the fluid boundaries, whereas larger molecules diffuse more slowly. Large particles (e.g., blood cells) show no significant diffusion within the time the two flow streams are in contact. Two interface zones are formed between the fluid layers. The ratio of a property (e.g., fluorescence intensity) of the two interface zones is a function of the concentration of the analyte and is largely free of cross-sensitivities to other sample components and instrument parameters. This device allows, for example, one-time or continuous monitoring of the concentration of analytes in microliters of whole blood without the use of membranes or prior removal of blood cells. The principle is demonstrated by the determination of human albumin and ionized calcium in whole blood and serum.
As an example, Fig. 12 shows a hydrostatic pressure-driven, disposable microfluidic detector card based on the T-sensor (diffusion-based detection).88 This device combines the ease of use of a paper test strip with the versatility of a microfluidic system.
Fig. 12.

Hydrostatically driven, integrated T-sensor design (a). A sample is put into the top left reservoir, a reagent (e.g., an indicator dye) is put into the top middle reservoir, and a reference solution with a known concentration of analyte is put into the right reservoir. The comparison of the intensity and position of the two diffusion interaction zones allows a semiquantitative analyte determination. The photograph in the center (b) shows such a T-sensor in operation, standing vertical, as it determines the pH of a buffer solution.
The same laminar fluid diffusion interface behavior can also be used for passive separation, or enrichment, of fast diffusing particles from a mixture of fast (generally small) and more slowly diffusing (generally large) particles. Fig. 13 shows a passive device that can separate chemical compounds by their diffusion coefficients (based on the H-filter method) and produce several microliters of a cleaned-up sample in one minute for further processing. Such a device, for example, can be used as a simple and cheap replacement for a centrifuge that requires no power or moving parts. As an example, this device has been used to separate drug molecules from whole blood as a preprocessing step in a chromatographic application.91
Fig. 13.

Hydrostatic-pressure-driven, integrated H-filter design. The figure shows an H-filter experiment in progress (illuminated with a UV lamp). A sample (e.g., blood) is put into the top left reservoir, and an acceptor reagent (e.g., water or saline) is put into the top right reservoir. Two parallel laminar streams will flow. Smaller components of the sample stream will diffuse into the acceptor stream. The two parallel flows are then split into two separate reservoirs (at the bottom of the drawing) that are connected only through a common vent. The left reservoir then contains the sample solution with a reduced concentration of the extracted component, and the right reservoir contains the acceptor reagent containing the extracted reagent. Both reservoirs can then be harvested from the cartridge for further use or be processed through further integrated microfluidic structures.
Exothermically heated non-instrumented nucleic acid amplification assays
The authors are currently developing a non-instrumented sample preparation device specifically for HIV viral load testing in low-resource settings, although the device will likely have other applications as well (Fig. 14). This device will extract HIV viral RNA and remove the requirement of a functioning cold chain for specimen handling and transportation. The center piece of the device is a filter-dial component that incorporates a silica-based absorption matrix. This filter dial component allows manual switching of the membrane through sample and various reagents, which eliminates the need for centrifugation.
Non-instrumented, nucleic ace amplification tests (NINAAT)
Although PCR has established the standard for nucleic acid amplification tests for more than 30 years, this technique requires a complex thermal profile that typically involves ramping and then dwelling at 54 °C, 74 °C, and 94 °C for annealing, extension, and denaturization. Furthermore, this profile is repeated approximately 30 times for amplification to 109. Typical cycle times are around 1 minute but can be significantly reduced by minimizing the volume of sample and reagents. The complexity of this thermal profile and the energy required to heat both the sample and the associated container, housing, and heating element all add to the energy requirements and create barriers to development of an appropriate non-instrumented device.
A number of methods for detecting clinically relevant nucleic acids do not involve cycling the sample temperature as is necessary for PCR. The obvious advantage of these methods over PCR is that they are isothermal (do not require thermal cycling). This may make them more amenable to non-instrumented POC diagnostics. Many of the methods identified in Table 2 are currently being utilized in USFDA-cleared or -approved diagnostic tests. Other promising methods are at earlier stages of development. Most of these methods use DNA polymerases or reverse transcriptases to amplify nucleic acids, which are then detected directly. Another approach (the bio-barcode method) does not amplify the target sequence at all. Rather, it utilizes the target sequence to capture and purify large quantities of bio-barcode DNA. The purified bio-barcode DNA is then used for detection. The methods vary in their amplification target and product (DNA or RNA). Thus, some methods can amplify RNA directly without a reverse transcription step, making them particularly well suited to detecting RNA viruses such as influenza. Bio-barcode amplification has the potential to detect target RNA and DNA equally well.
Table 2.
Methods for detecting nucleic acids that do not require temperature cycling
| Assay type | Patent holder | Detection limits | Reaction temperature (°C) | Reaction duration | Target template | Amplification product |
|---|---|---|---|---|---|---|
| NASBA | Biomeriex, Organon Tecknika | Comparable to PCR | 65 °C - 5 min; 41 °C -100 min | 105 min | RNA | RNA and DNA |
| TMA | Gen-Probe | > 95% sensitivity for chlamydia test | 95 °C - 10 min; 42 °C - 65 min; 60 °C - 25 min | 140 min | RNA | RNA and DNA |
| SDA | Nanogen (ligation based) / genprobe / BD ProbeTec | 46 copies/reaction | 37 °C (has gotten more complicated with ProbeTec) | 120 min | DNA | DNA |
| LCR | Abbot | Unknown | Unknown | Unknown | DNA | DNA |
| LAMP | Eiken Chemical, Japan | 6 copies/reaction | 95 °C - 5 min; 6 °C - 30 min | 35 min | DNA | DNA |
| HDA | New England Biolabs | 10 copies (NEB website) | 5 °C - 2 min (optional); 64 °C -75–90 minutes | 75–90 min | DNA | DNA |
| LIDA | Pacific Biometrics | 10,000 copies | Unknown | “Minutes” (sighted by company website) | DNA | RNA |
The authors are currently developing a non-instrumented nucleic acid platform that requires absolutely no instrument, power, or external reagents.92 This can be achieved by combining two unrelated technologies. The first technology is exothermic chemical heating, a mature technology currently in use in “ready-to-eat” meals and camping hand-warmers. When thermally coupled with the temperature moderating characteristics of PCMs, a recent innovation developed by the applicants, the resultant heating unit can produce consistent, constant-temperature heat largely independent of external conditions. The second technology is loop-mediated isothermal amplification (LAMP), a novel technology.
Successful amplification of the target genetic sequence with LAMP results in a visible output—turbidity—and thus no further detection device is needed. This disposable device does not require any additional instrumentation and will be almost as rapid and simple to use as a lateral flow strip test—yet will offer the sensitivity and specificity of nucleic acid amplification tests. The proposed assay platform also includes preservation of nucleic acid amplification reagents in dry form inside the LAMP reaction chamber, resulting in a self-contained, point-of-care device. This diagnostic is targeted for use in low-resource settings by minimally trained health workers.
Although an exothermic reaction such as that of quicklime (calcium oxide) and water can generate the necessary heat, this isothermal device will also require a temperature-moderating medium such as a custom-designed paraffin with a specific melting range to keep the temperature constant at the desired level for the desired time. Fig. 15 demonstrates the concept of using heat of fusion as a thermal battery to store heat during a two-phase transition.
Fig. 15.

Diagram of how a PCM can be used to control temperature.
We have also developed components of a diagnostic disposable platform that have the dual purpose of providing molecular diagnosis at the point of care as well as stabilizing specimens for further analysis via a centralized surveillance system. Although not demonstrated specifically for the reagents required for LAMP, preservation of nucleic acid amplification reagents in dry form inside a reaction chamber has also been demonstrated by PATH.
When coupled thermally to an exothermic reaction, these materials can moderate heat from an unacceptable thermal profile, such as that generated by most exothermic reactions, into a temperature band suitable for LAMP. Some materials have performed better than others. We have found that high-melting-temperature paraffin materials tend to have a wide (±5 °C) melting temperature range, due to the variable length of polymer chains, as compared to a precise melting temperature of alloys or ice. So far, the most promising PCM for high temperatures is Cerroshield, an inexpensive alloy with a high melting enthalpy per unit volume and a phase transition temperature of 95 °C. A paraffin product called RT 65 produced by Rubitherm® Technologies GmbH is our leading candidate for the LAMP amplification temperatures. Fig. 16 demonstrates the temperature stability profile of a prototype device that generates heat with a calcium oxide reaction and moderates temperature with a paraffin. Although the device has not been optimized and the paraffin is not customized for a melting temperature to maintain 62.5 ± 2.5 °C, the graphic clearly demonstrates that appropriate temperatures can be achieved and maintained for sufficient time to amplify the DNA of a target pathogen.
Fig. 16.

Thermal profile measured from a modified commercially available self-heating beverage product. Time is in minutes. Although the correct temperature range was not achieved for LAMP, the data demonstrates the capacity of paraffin to moderate peak temperature using a CaO exothermic reaction. Additional insulation will further reduce temperature drop from the peak.
Fig. 17 shows a form factor model and describes the operation of a concept for a non-instrumented LAMP device.
Fig. 17.

Preliminary design of a POC NAAT for malaria. (Left—outside view. Right—cross-section). Length approx. 40 mm. (1) Top screw cap containing exothermic material and PCM for generating 99 °C for several minutes, activated by the screwing action. (2) Color temperature indicator showing when the heating step is complete. (3) Reagent tube containing sample (top), buffer (bottom), and LAMP reagents and primers in dry form (this can be in the sample compartment, or, optionally, in a separate compartment). (4) Bottom screw cap, containing exothermic material and PCM for generating 65 °C for 30–60 minutes, also activated by the screwing action. (5) Color temperature indicator showing when the 65 °C heating step is complete. (6) Viewing window and (7) daylight inlet to observe presence of turbidity, indicating positive reaction. (8) Open sample compartment inside reagent tube, to receive the blood drop. (9) Optional LAMP reagent compartment in reagent tube. (10) Buffer compartment in reagent tube. (11) Spike for dislocating/fracturing membranes that separate reagent tube compartments.
Conclusions
Within the microfluidics and lab-on-a-chip universe, the development of non-instrumented devices, and especially diagnostics, has not been a priority. However, another μTAS, the rapid ICS assay (here the assay actually predates the term μTAS), has been used very successfully in settings that require highly distributed testing capacity, are subject to very unpredictable demand, or demonstrate lack of resources to purchase and maintain instruments. Such settings would include developing-country health care, home testing in developed countries, and diagnostics for use by first responders in natural or man-made bioemergencies.
Some published work is promising—many microfluidic components are fundamentally passive in nature and can be implemented in disposables. Pumps and valves can be replaced by hydrostatic pressure, wicking pads, capillary forces, evaporation pumps, etc.; detection can be done visually in many cases; and heating can be performed using exothermic chemicals. For example, we believe that recent progress with isothermal amplification and exothermic heating will lead to completely disposable NAAT assays.
Significant obstacles to successful development of non-instrumented microfluidic assays remain. While it should be possible to perform sample preparation and even, if needed, target amplification, without the use of instrumentation, it is difficult to envisage how very low signal intensities could be visualized without electronic signal amplification. This may limit the applicability of these assays where extremely high sensitivity is required. Another drawback is that disposables may become quite complex, and possibly bulky and costly, if too many functions that normally would be performed by an instrument are integrated into the disposable.
Nevertheless, non-instrumented microfluidic assays can retain many of the attributes of ICS assays, yet greatly expand their capabilities (for example, to assays other than immunoassays such as nucleic acid amplification-based assays). Given that such assays are greatly needed, especially in low-resource settings, as well as likely commercially feasible in settings with unpredictable demand, we believe that microfluidics researchers should attempt to develop diagnostic devices that require little or no instrumentation and use characteristics similar to those of an ICS assay.
Acknowledgments
The authors acknowledge funding for a portion of this work from the National Institutes of Health (NIAID, as well as funding from NIBIB to establish the Center for Point-of-Care Diagnostics for Global Health at PATH and the University of Washington), and the Bill & Melinda Gates Foundation. The authors would also like to thank PATH employees Jared Wilmoth, Justin Roth, and Darrel Harvey, as well as interns Dennis Tang, Nick Talwar, and Alex Fichtenholz for valuable contributions.
Footnotes
Part of a special issue on Point-of-care Microfluidic Diagnostics; Guest Editors - Professor Kricka and Professor Sia.
References
- 1.Guia A, Xu J. In: Ion Channels in the Pulmonary Vasculature. Yuan JXJ, editor. Taylor and Francis Group; Boca Raton, FL: 2005. pp. 635–649. [Google Scholar]
- 2.Gulliksen A, Solli LA, Drese KS, Sorensen O, Karlsen F, Rogne H, Hovig E, Sirevag R. Lab on a Chip. 2005;5:416. doi: 10.1039/b415525d. [DOI] [PubMed] [Google Scholar]
- 3.Mcmillan WA. Proceedings of the 8th International Symposium on Microbial Detection; 20021. [Google Scholar]
- 4.Chin CD, Linder V, Sia SK. Lab on a Chip. 2007;7:41. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 5.Cirino NM, Musser KA, Egan C. Expert Rev Mol Diagn. 2004;4:841. doi: 10.1586/14737159.4.6.841. [DOI] [PubMed] [Google Scholar]
- 6.Mabey D, Peeling RW, Ustianowski A, Perkins MD. Nat Rev Microbiol. 2004;2:231. doi: 10.1038/nrmicro841. [DOI] [PubMed] [Google Scholar]
- 7.Pipper J, Inoue M, Ng LF, Neuzil P, Zhang Y, Novak L. Nat Med. 2007 doi: 10.1038/nm1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson BH, Nicholson JK. Annu Rev Public Health. 2005;26:281. doi: 10.1146/annurev.publhealth.26.021304.144522. [DOI] [PubMed] [Google Scholar]
- 9.Schulte TH, Bardell RL, Weigl BH. Clinica Chimica Acta. 2002;321:1. doi: 10.1016/s0009-8981(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 10.Tudos AJ, Besselink GJ, Schasfoort RB. Lab on a Chip. 2001;1:83. doi: 10.1039/b106958f. [DOI] [PubMed] [Google Scholar]
- 11.Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Nature. 2006;442:412. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- 12.Yager P, Domingo GJ, Gerdes J. Annu Rev Biomed Eng. 2008;10 doi: 10.1146/annurev.bioeng.10.061807.160524. [DOI] [PubMed] [Google Scholar]
- 13.Respess RA, Rayfield MA, Dondero TJ. AIDS. 2001;15(Suppl 3):S49. doi: 10.1097/00002030-200104003-00007. [DOI] [PubMed] [Google Scholar]
- 14.Craig MH, Bredenkamp BL, Williams CH, Rossouw EJ, Kelly VJ, Kleinschmidt I, Martineau A, Henry GF. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:258. doi: 10.1016/s0035-9203(02)90092-1. [DOI] [PubMed] [Google Scholar]
- 15.Burgess DCH, Wasserman J, Dahl CA. Improved Diagnostic Technologies for the Developing World. Nature Publishing Group; Washington, DC: 2006. [Google Scholar]
- 16.Glynou K, Ioannou PC, Christopoulos TK, Syriopoulou V. Analytical Chemistry. 2003;75:4155. doi: 10.1021/ac034256+. [DOI] [PubMed] [Google Scholar]
- 17.Seal J, Braven H, Wallace P. IVD Technology Magazine. 2006:41. [Google Scholar]
- 18.Burgess D, Odugwu S, Matsyshen D, Radebe F, Wright J, Steele M, Koornhof H. Evaluation of a new rapid test for Gonorrhea in high risk populations in Johannesburg, Republic of South Africa. 2008 [Google Scholar]
- 19.Pugia MJ, Blankenstein G, Peters RP, Profitt JA, Kadel K, Willms T, Sommer R, Kuo HH, Schulman LS. Clinical Chemistry. 2005;51:1923. doi: 10.1373/clinchem.2005.052498. [DOI] [PubMed] [Google Scholar]
- 20.Toner M, Irimia D. Annu Rev Biomed Eng. 2005;7:77. doi: 10.1146/annurev.bioeng.7.011205.135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huh D, Bahng JH, Ling Y, Wei HH, Kripfgans OD, Fowlkes JB, Grotberg JB, Takayama S. Analytical Chemistry. 2007;79:1369. doi: 10.1021/ac061542n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji HM, Samper V, Chen Y, Heng CK, Lim TM, Yobas L. Biomed Microdevices. 2008;10:251. doi: 10.1007/s10544-007-9131-x. [DOI] [PubMed] [Google Scholar]
- 23.VanDelinder V, Groisman A. Analytical Chemistry. 2007;79:2023. doi: 10.1021/ac061659b. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya A, Klapperich CM. Analytical Chemistry. 2006;78:788. doi: 10.1021/ac051449j. [DOI] [PubMed] [Google Scholar]
- 25.Breadmore MC, Wolfe KA, Arcibal IG, Leung WK, Dickson D, Giordano BC, Power ME, Ferrance JP, Feldman SH, Norris PM, Landers JP. Analytical Chemistry. 2003;75:1880. doi: 10.1021/ac0204855. [DOI] [PubMed] [Google Scholar]
- 26.Poeckh T, Lopez S, Fuller AO, Solomon MJ, Larson RG. Anal Biochem. 2008;373:253. doi: 10.1016/j.ab.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian H, Huhmer AF, Landers JP. Anal Biochem. 2000;283:175. doi: 10.1006/abio.2000.4577. [DOI] [PubMed] [Google Scholar]
- 28.Bhattacharya S, Jang JS, Yang LJ, Akin D, Bashir R. Journal of Rapid Methods and Automation in Microbiology. 2007;15:1. [Google Scholar]
- 29.Baeumner AJ, Pretz J, Fang S. Analytical Chemistry. 2004;76:888. doi: 10.1021/ac034945l. [DOI] [PubMed] [Google Scholar]
- 30.Dineva MA, Candotti D, Fletcher-Brown F, Allain JP, Lee H. Journal of Clinical Microbiology. 2005;43:4015. doi: 10.1128/JCM.43.8.4015-4021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maraldo D, Rijal K, Campbell G, Mutharasan R. Analytical Chemistry. 2007;79:2762. doi: 10.1021/ac0621726. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Xu J, Ma W, Zheng W. Biotechnol Adv. 2006;24:243. doi: 10.1016/j.biotechadv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Manz A, Day PJ. Lab on a Chip. 2007;7:1413. doi: 10.1039/b708362a. [DOI] [PubMed] [Google Scholar]
- 34.Easley CJ, Karlinsey JM, Bienvenue JM, Legendre LA, Roper MG, Feldman SH, Hughes MA, Hewlett EL, Merkel TJ, Ferrance JP, Landers JP. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19272. doi: 10.1073/pnas.0604663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu RH, Sharp KV, Olsen MG, Strembler JG, Santiago RJ. Journal of Microelectromechanical Systems. 2000;9:190. [Google Scholar]
- 36.Weigl BH, Gerdes J, Tarr P, Yager P, Dillman L, Peck R, Ramachandran S, Lemba M, Kokoris M, Nabavi M, Battrell F, Hoekstra D, Klein EJ, Denno DM. Proceedings of SPIE International Society for Optical Engineering. 2006;6112:611202. [Google Scholar]
- 37.Yeung SH, Medintz IL, Greenspoon SA, Mathies RA. Clinical Chemistry. 2008;54:1080. doi: 10.1373/clinchem.2007.102319. [DOI] [PubMed] [Google Scholar]
- 38.Yeung SW, Lee TM, Cai H, Hsing IM. Nucleic Acids Research. 2006;34:e118. doi: 10.1093/nar/gkl702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn CH, Choi JW, Beaucage G, Nevin JH, Lee JB, Puntambekar A, Lee JY. Proceedings of the IEEE. 2004;92:154. [Google Scholar]
- 40.Bhattacharyya A, Klapperich CM. Biomedical Microdevices. 2007;9:245. doi: 10.1007/s10544-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 41.Kim DS, Lee SH, Ahn CH, Lee JY, Kwon TH. Lab on a Chip. 2006;6:794. doi: 10.1039/b516495h. [DOI] [PubMed] [Google Scholar]
- 42.Ahn CH, Choi JW, Beaucage G, Nevin JH, Lee JB, Puntambekar A, Lee JY. Proceedings of the IEEE. 2004;92:154. [Google Scholar]
- 43.Weigl BH, Williams WC, Hayenga J, Bardell R, Schulte T. International patent WO/2001/026813. Microfluidics without electrically or mechanically operated pumps. 2001 Apr 19;
- 44.Takayama S, Chang J, Huh D, Zhu X, Cho B, Smith G. International patent WO/2003/008102. Microfluidic gravity pump with constant flow rate. 2003 Jan 30;
- 45.Weigl BH. Microfluidics for low cost medical diagnostics. 2008 [Google Scholar]
- 46.Millar Forbes A, Capes DF, Tang E, Smith N, McKenzie TA, Wang LP, Bett KF, Australian J. Hospital Pharm. 1992;6:22. [Google Scholar]
- 47.Yao L, Liu B, Chen T, Liu S, Zuo T. Biomed Microdevices. 2005;7:253. doi: 10.1007/s10544-005-3999-0. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty I, Tang WC, Bame DP, Tang TK. Sensors and Actuators A (Physical) 2000;83:188. [Google Scholar]
- 49.Evans JD, Liepmann D. The bubble spring and channel (bsac) valve: an actuated, bi-stable mechanical valve for in-plane fluid control. 1999 [Google Scholar]
- 50.Fu C, Rummler Z, Schomburg W. Journal of Micromechanics and Microengineering. 2003;13:S96. [Google Scholar]
- 51.Huff MA, Mettner MS, Lober TA, Schmidt MA. Solid-State Sensor and Actuator Workshop 4th Technical Digest. Institute of Electrical and Electronics Engineers; Hilton Head Island, SC: 1990. pp. 123–127. [Google Scholar]
- 52.Kluge S, Klink G, Woias P. American laboratory. 1998;30:17. [Google Scholar]
- 53.Papavasiliou AP, Pisano AP, Liepmann D. High-speed and bi-stable electrolysis-bubble actuated gate valves. 2001 [Google Scholar]
- 54.Shikida M, Sato K, Tanaka S, Kawamura Y, Fujisaki Y. Journal of Microelectromechanical Systems. 1994;3:76. [Google Scholar]
- 55.Hasselbrink EF, Jr, Shepodd TJ, Rehm JE. Analytical Chemistry. 2002;74:4913. doi: 10.1021/ac025761u. [DOI] [PubMed] [Google Scholar]
- 56.Deshmukh AA, Liepmann D, Pisano AP. Characterization of a Micro-mixing, Pumping, and Valving System. University of California; Berkeley, CA: 2001. [Google Scholar]
- 57.Yang B, Lopez GC, Lin Q, Rosenbloom AJ. Proceedings of 2003 ASME International Mechanical Engineering Congress; November 16–21, 2003; Washington, DC: American Society of Mechanical Engineers (ASME); 2003. pp. 369–372. [Google Scholar]
- 58.Thorsen T, Maerkl SJ, Quake SR. Science. 2002;298:580. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- 59.Pal R, Yang M, Johnson BN, Burke DT, Burns MA. Analytical Chemistry. 2004;76:3740. doi: 10.1021/ac0352934. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z, Wang J, Qian S, Bau HH. Lab on a Chip. 2005;5:1277. doi: 10.1039/b508275g. [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Enzelberger M, Quake S. Electrophoresis. 2002;23:1531. doi: 10.1002/1522-2683(200205)23:10<1531::AID-ELPS1531>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 62.Hartshorne H, Backhouse CJ, Lee WE. Sensors and Actuators B Chemical. 2004 [Google Scholar]
- 63.O'Connor SD, Karp CD. 6 676 835. Microfluidic separators Nanostream Incorporated. 2004 Jan 13;
- 64.Wang J, Chen Z, Mauk M, Hong KS, Li M, Yang S, Bau HH. Biomedical Microdevices. 2005;7:313. doi: 10.1007/s10544-005-6073-z. [DOI] [PubMed] [Google Scholar]
- 65.Xia N, Hunt TP, Mayers BT, Alsberg E, Whitesides GM, Westervelt RM, Ingber DE. Biomedical Microdevices. 2006;8:1387. doi: 10.1007/s10544-006-0033-0. [DOI] [PubMed] [Google Scholar]
- 66.Durr M, Kentsch J, Muller T, Schnelle T, Stelzle M. Electrophoresis. 2003;24:722. doi: 10.1002/elps.200390087. [DOI] [PubMed] [Google Scholar]
- 67.Macounova K, Cabrera CR, Yager P. Analytical Chemistry. 2001;73:1627. doi: 10.1021/ac001013y. [DOI] [PubMed] [Google Scholar]
- 68.Wang XB, Yang J, Huang Y, Vykoukal J, Becker FF, Gascoyne PR. Analytical Chemistry. 2000;72:832. doi: 10.1021/ac990922o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiklund M, Nilsson S, Hertz HM. Journal of Applied Physics. 2001;90:421. [Google Scholar]
- 70.Safe-Tec Clinical Products website. Microsafe page. 2008 Jul 1; http://www.safe-tecinc.com/microsafe.htm.
- 71.Poly-Pipets Incorporated. 2008 Jul 1; website http://www.polypipets.com/pagetwo.html.
- 72.Wheeler EK, Benett K, Ness P, Straton J, Richards T, Weisgraber A, Papavasiliou A. Disposable polymerase chain reaction device. University of California; Livermore, CA: 2001. [Google Scholar]
- 73.Guijt RM, Dodge A, van Dedem GW, de Rooij NF, Verpoorte E. Lab on a Chip. 2003;3:1. doi: 10.1039/b210629a. [DOI] [PubMed] [Google Scholar]
- 74.Yager P. Paul Yager page. 2008 Jun 30; http://faculty.washington.edu/yagerp/6-30-2008.
- 75.Balliel M. Casting furnace with centrally located heating element for producing directionally solidified castings. 6 523 599. Alstom. 2003 Feb 25;
- 76.Home page. Enfucell. 2008 Jun 9; website http://www.enfucell.com/index.htm 2008.
- 77.Kim SS, Saeedi E, Meldrum DR, Parviz BA. 2nd International Conference on Nano/Micro Engineered and Molecular Systems (NEMS)'07; Bankok, Thailand: Institute of Electrical and Electronics Engineers (IEEE); 2007. pp. 927–931. [Google Scholar]
- 78.Weigl BH. Microfluidic platform for complex sample analysis starting from raw samples, mainstreaming microfluidics: diffusing microfluidics technology in the marketplace. 2008 [Google Scholar]
- 79.Garcia E, Yager P. In: Micro Total Analysis Systems 2005. Jensen KF, Han J, arrison DJ, oldman J, editors. Transducer Research Foundation; San Diego, CA: 2005. pp. 1443–1445. [Google Scholar]
- 80.Hatch A, Yager P. Micro Total Analysis Systems 2001. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 571–572. [Google Scholar]
- 81.Hatch E, Garcia E, Yager P. In: Micro Total Analysis Systems 2003. Northrup MA, Jensen KF, Harrison DJ, editors. Springer Publishing; New York, NY: 2003. pp. 1215–1218. [Google Scholar]
- 82.Hawkins K, Hatch A, Chang H, Yager P. Proceedings of Microtechnologies in Medicine and Biology. IEEE Press; Madison, WI: 2002. pp. 535–540. [Google Scholar]
- 83.Kamholz AE, Yager P. Biophysical Journal. 2001;80:155. doi: 10.1016/S0006-3495(01)76003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kamholz AE. PhD. University of Washington, Bioengineering; 2002. [Google Scholar]
- 85.Munson MS, Hawkins KR, Hasenbank MS, Yager P. Lab on a Chip. 2005;5:856. doi: 10.1039/b501035g. [DOI] [PubMed] [Google Scholar]
- 86.Nelson KE, Foley JO, Mashadi-Hossein A, Yager P. In: Micro Total Analysis Systems 2005. Jensen KF, Han J, Harrison DJ, Voldman J, editors. Transducer Research Foundation; San Diego, CA: 2005. pp. 1000–1002. [Google Scholar]
- 87.Weigl BH. Medical Design Online [serial online] 1999 [Google Scholar]
- 88.Weigl BH, Yager P. Science. 1999;283:346. [Google Scholar]
- 89.Yager P. In: Lab-on-a-chip: Chemistry in Miniaturized Synthesis and Analysis Systems. Oosterbroek RE, van den Berg A, editors. Lavoisier; Paris: 2003. [Google Scholar]
- 90.Yager P, Cabrera C, Kamholz A. In: Separation Methods in Microanalytical Systems. Kutter JP, Fintschenko Y, editors. CRC Press; Boca Raton, FL: 2005. [Google Scholar]
- 91.Jandik P, Weigl BH, Kessler N, Cheng J, Morris CJ, Schulte T, Avdalovic N. J Chromatogr A. 2002;954:33. doi: 10.1016/s0021-9673(02)00160-7. [DOI] [PubMed] [Google Scholar]
- 92.Weigl BH, Domingo G, Gerlach J, Tang D, Harvey D, Talwar N, Fichtenholz A, van Lew B, LaBarre P. Proceedings of SPIE. 2008;6886:688604. [Google Scholar]
- 93.Liepmann D, Pisano AP. MEMS and Microfluidic Research at UC Berkeley Micro Valves and Pumps. Berkeley Sensor and Actuator Center; Berkeley, CA: 2005. [Google Scholar]
- 94.Kung CF, Chen CF, Chu CC, Tseng FG. Nanotech 2004. 2004 [Google Scholar]
- 95.Sudarsan AP, Ugaz VM. Lab Chip. 2006;6:74–82. doi: 10.1039/b511524h. [DOI] [PubMed] [Google Scholar]
- 96.Alleborn N, Nandakumar K, Raszillier H, Durst F. J Fluid Mech. 1997;330:169. [Google Scholar]
- 97.Wang H, Iovenitti P, Harvey E, Masood S. Proceedings SPIE, BioMEMS and Smart Nanostructures. 2001;4590:204–212. [Google Scholar]
- 98.Munson MS, Yager P. Mesa Monographs. In: Micro Total Analysis Systems 2003. Northrup MA, Jensen KF, Harrison DJ, editors. 2003. pp. 495–498. [Google Scholar]
- 99.Schafer H, Chemnitz S, Seibel K. Nano-Micro-Interface Conference; Berlin, Germany. 2003. [Google Scholar]
- 100.Lin Y, Gerfen GJ, Rousseau DL, Yeh S. Anal Chem. 2003;75:5381–5386. doi: 10.1021/ac0346205. [DOI] [PubMed] [Google Scholar]
- 101.Mengeaud V, Josserand J, Girault HH. Anal Chem. 2002;74:4279–4286. doi: 10.1021/ac025642e. [DOI] [PubMed] [Google Scholar]


