Abstract
Background
The voluntary, maximum inclined posture reflects the self-perceived limits of stability. Parkinson’s disease is associated with small, bradykinetic postural weight shifts while standing but it is unclear whether this is due to reduced limits of stability and/or to the selection of abnormal strategies for leaning. The aim of this study was to investigate the effects of Parkinson’s disease and levodopa medication on voluntary limits of stability and strategies used to reach these limits.
Methods
Fourteen subjects with Parkinson’s disease (OFF and ON levodopa) and 10 age-matched controls participated in the study. Functional limits of stability were quantified as the maximum center of pressure excursion during voluntary forward and backward leaning. Postural strategies to achieve functional limits of stability were assessed by (i) body segments alignment, (ii) the difference between center of pressure and center of mass in preparation for a lean, (iii) the timing and the velocity of the preparation phase.
Findings
Functional limits of stability were significantly smaller in subjects with Parkinson’s disease compared to control subjects. Subjects with Parkinson’s disease maintained their stooped posture while leaning, initiated leaning with a smaller difference between center of pressure and center of mass and had a slower leaning velocity compared to control subjects. Levodopa enlarged the limits of stability in subjects with Parkinson’s disease because of an increase in maximum forward, but not backward leans, but did not significantly improve postural alignment, preparation for a leaning movement, or velocity of leaning.
Interpretation
Parkinson’s disease reduces functional limits of stability as well as the magnitude and velocity of postural preparation during voluntary, forward and backward leaning while standing. Levodopa improves the limits of stability but not the postural strategies used to achieve the leaning.
Keywords: Parkinson’s disease, Postural control, Limits of stability, Levodopa, Voluntary body leaning
1. Introduction
Postural stability is the ability to maintain equilibrium under both static and dynamic conditions, such as during quiet stance (Corriveau et al., 2004; van Wegen et al., 2002; Winter et al., 1998), in response to postural perturbations (Horak et al., 2005; Jacobs et al., 2005; Patton et al., 1999), or during the postural preparation for movements (Hass et al., 2005; Rocchi et al., 2006). One way to quantify postural stability involves measuring the limits of stability. The limits of stability can be defined, under dynamic conditions, as the maximum displacement of the center of body mass during a feet-in-place response to external postural perturbations that can be controlled without a fall or a step (Horak et al., 2005). To investigate limits of stability in the absence of external perturbations, the maximum, voluntary, inclined posture can be used (Schieppati et al., 1994; van Wegen et al., 2001). Statically holding the center of body mass near the forward or backward limits of foot support simulates functional positions that occur in motor tasks such as in the transition from stance to gait and from sit to stand (Newton, 2001). Limits of stability, quantified by the maximum, voluntary inclined posture may be considered “functional” limits of stability, since they are influenced by subjective perception, internal postural control abilities, and environmental factors, and not only by body biomechanics or segment properties (Holbein and Redfern, 1997). One way to measure functional limits of stability involves quantification of the maximum center of pressure (COP) displacement with respect to the base of support (Binda et al., 2003).
Postural instability is a frequent problem in subjects with Parkinson’s disease (PD) (Dibble and Lange, 2006; Nardone and Schieppati, 2006; Rocchi et al., 2002) and has a great impact on their quality of life, often resulting in falls, subsequent injury, and increased fear of falling. Previous studies reported reduced antero-posterior COP excursions in PD subjects in their ON dopaminergic medication state compared with age-matched control subjects while voluntarily leaning (Bartolic et al., 2005; Schieppati et al., 1994). Another study (van Wegen et al., 2001) did not detect any differences in COP position at maximum leans between healthy and PD subjects. However, the previous studies investigated postural stability while statically maintaining the maximum inclined posture, and did not consider the anticipatory and executive phases used to reach the maximal inclinations or the influence of levodopa on the limits of stability (i.e., OFF vs. ON state).
The purpose of the present study was to investigate how PD subjects manage their forward and backward functional limits of stability, and how this is affected by levodopa. Since COP displacements reflect not only displacement of the body center of mass (COM) (Blaszczyk and Klonowski, 2001), but also anticipatory postural control (Corriveau et al., 2004; Hass et al., 2005), we used (i) the relationship between COP and COM, (ii) leaning velocity and duration, and (iii) body segments alignment, to investigate the postural strategies used to achieve the forward and backward stability limits.
2. Methods
2.1. Participants
Fourteen patients with idiopathic PD (mean age 65.6 years, SD 8.7), see Table 1, and 10 age-matched control subjects (mean age 64.9 years, SD 8) free of any neurological or musculoskeletal disorders, participated in this study. All subjects gave informed consent in accordance with the OHSU Institutional Review Board.
Table 1.
Characteristics of subjects with Parkinson’s disease
| SubjID | Age (yrs) |
Disease duration (yrs) |
UPDRSa |
Rigidityb |
Posturec |
|||
|---|---|---|---|---|---|---|---|---|
| OFF | ON | OFF | ON | OFF | ON | |||
| 1 | 67 | 9 | 59 | 34 | 15 | 8 | 5 | 1 |
| 2 | 73 | 24 | 64 | 55 | 14 | 8 | 5 | 6 |
| 3 | 76 | 14 | 63 | 42 | 12 | 10 | 3 | 3 |
| 4 | 74 | 17 | 57 | 23 | 11 | 6 | 5 | 2 |
| 5 | 75 | 13 | 32.5 | 21 | 10 | 5 | 4 | 4 |
| 6 | 56 | 15 | 29.5 | 19 | 3.5 | 2 | 3 | 3 |
| 7 | 73 | 10 | 70 | 53 | 17 | 13 | 6 | 5 |
| 8 | 57 | 3 | 26 | 13 | 10 | 7 | 3 | 0 |
| 9 | 55 | 13 | 43 | 13 | 6 | 0 | 4 | 1 |
| 10 | 55 | 10 | 39.5 | 23 | 6 | 6 | 3.5 | 2.5 |
| 11 | 52 | 5 | 42 | 16 | 10 | 0 | 2 | 2 |
| 12 | 67 | 13 | 43 | 13 | 7 | 0 | 1 | 0 |
| 13 | 67 | 15 | 59 | 34.5 | 14 | 9 | 3 | 1 |
| 14 | 71 | 14 | 48 | 39 | 5 | 4 | 3 | 2 |
| Mean | 65.6 | 12.5 | 48.3 | 28.5 | 10.0 | 5.6 | 3.6 | 2.3 |
| SD | 8.7 | 5.1 | 13.9 | 14.5 | 4.1 | 4.0 | 1.3 | 1.8 |
| P = 0.001 | P = 0.007 | P = 0.03 | ||||||
UPDRS Motor Subscale, /108.
Item #22 of UPDRS, /20.
Item #28 and 30 of UPDRS, /8.
All subjects with PD were sensitive to levodopa as noted by the Motor Subscale (Part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS), (Fahn et al., 1987), reported in Table 1. PD subjects were tested in their practical OFF state after at least 12 h of medication wash-out, and again on the same day in their ON state, at least 1 h after taking their usual dose of medication. All subjects with PD had gait difficulties, impaired balance, and moderate to severe PD (from III to IV on the Hoehn and Yahr scale). These subjects were approved for deep brain stimulation surgery, attesting to homogeneity of the PD group, consistent with surgery inclusion criteria (Broggi et al., 2003). A summary of PD subjects’ characteristics is reported in Table 1.
2.2. Procedure
At the beginning of a trial, the subjects stood with each foot on a separate, side by side, force plate with feet parallel at their comfortable stance width. Initial stance position was consistent from trial-to-trial by tracing foot outlines on the force plates and by coaching subjects to maintain their initial COP position prior to each trial based on oscilloscope COP traces. Subjects were asked to maintain an upright standing position with arms crossed on the chest, eyes open and gaze straight ahead at an art poster 3-m ahead of them. To allow for subsequent parameters normalization, foot length was measured, from the heel to the tip of the hallux, with an electronic calliper.
Starting from an upright, natural position, subjects performed 3 tasks sequentially: (1) maximum forward lean (1 repetition acquired for 15 s), (2) maximum backward lean (1 repetition acquired for 15 s), and (3) quiet stance (3 repetitions of 60 s each). Subjects were asked to lean as far as they could at their comfortable speed, without lifting their toes or heels or flexing their hips, and to hold their maximum position for at least 5 s.
2.3. Measurements
2.3.1. Force platform data
Four vertical forces were recorded from each strain-gauge, custom-made force plate at 480 Hz, low-pass filtered at 8 Hz, and down-sampled at 20 Hz. The excursion of the total body COP (i.e., the application point of the total ground-reaction force) was computed from the vertical forces (Henry et al., 2001), both in the antero-posterior (AP) and medio-lateral (ML) direction.
2.3.2. Body kinematics
A movement analysis system (Motion Analysis, Santa Rosa, CA) with six video cameras and sampling frequency of 60 Hz recorded the kinematics of body segments. Reflective markers were placed on both feet and on the right side of the body on the following bony landmarks: fifth metatarsal head, lateral malleolus, lateral femoral condyle, greater trochanter, anterior superior iliac spine, clavicular acromion, elbow, temple of head, and mastoid process. Body segment kinematics, and appropriate anthropometric tables (Winter et al., 1998), were used to estimate the position of the total body COM in the sagittal plane. In addition, we reconstructed the shank, thigh, and trunk segment angles with respect to vertical to characterize postural alignment.
2.4. Data analysis and extracted parameters
The leaning tasks consisted of a motion phase followed by a maximal leaning phase. The 3 quiet stance trials were considered to characterize the natural standing of subjects, through the estimation of the average COP position.
2.4.1. Functional limits of stability
The steady-state positions of AP COP during backward and forward maximal lean were used to quantify the functional limits of stability (fLOS). Their extension was estimated as
where maxFW and maxBW represented the average AP COP over the first 5 s of stabilized, forward and backward leaning, respectively (see Fig. 1A). fLOS, maxFW, and maxBW were normalized to foot length, and are, in the following, expressed as a percent of foot length. The 5 s window of stabilized, maximal leaning was manually identified analyzing AP COP time-series.
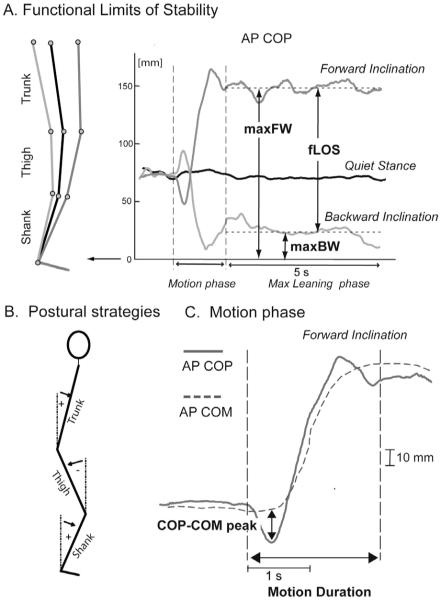
Fig. 1.
Signals collected from a representative control subject and main parameters considered in the data analysis. (A) Functional limits of stability and parameters that quantify the maximal leaning phase. (B) Parameters that characterize the motion phase (example for forward leaning).
The steady-state positions of ML COP were also computed during maximal leans, to check for potential, lateral asymmetries.
To express the COP coordinates in an anatomically-based reference frame, the position of the AP COP was referenced to the lateral malleolus marker, and the position of the ML COP was referenced to the mid-point between right and left malleolus markers.
2.4.2. Postural strategies
Postural strategies were characterized by means of average segmental kinematics. Average inclination of the trunk, thigh, and shank segments with respect to vertical were used to describe the body segments alignment (postural attitude) during the 3 tasks (see Fig. 1B for details).
2.4.3. Motion phase of the leaning tasks
The onset of the motion phase was detected by a threshold-based algorithm, with threshold set as twice the standard deviation (SD) of AP COP during the initial, standing position of each trial (Fig. 1). The motion phase was considered completed when AP COP ended its rapid migration to a new steady-state position, coincident with the start of the leaning phases (see Fig. 1C). The size of the anticipatory postural adjustments to initiate the motion phase of the lean was quantified by the peak of the COP-COM time series (see Fig. 1C) (Massion, 1992). The motion phase of leaning was characterized by its duration (motion duration, Fig. 1C), and by the ratio between the AP COP path and the motion duration (motion velocity).
2.4.4. Statistical analyses
Group means and SD of the means are summarized in the text. For each parameter, a separate one-way ANOVA was used to detect differences between the control versus PD OFF and between the control versus PD ON groups. A repeated measures ANOVA was used to compare PD subjects OFF and ON. Correlations between functional limits of stability parameters and the UPDRS Motor Subscale and the UPDRS items characterizing rigidity and posture (Items 22 and 28 and 30, respectively) were investigated using Pearson’s correlation analysis. For the entire set of statistical analyses the level of significance was set at P < 0.05. All the analyses were performed with NCSS Software, Kaysville, Utah.
3. Results
3.1. Functional limits of stability
The mean position of AP COP in quiet stance and during maximal backward leaning was not significantly different between control and PD subjects, both in the OFF and ON states, as shown in Fig. 2A. Similarly, the mean position of ML COP during the 3 tasks was not different between control and PD subjects, or between PD subjects in the OFF and ON states.
Fig. 2.
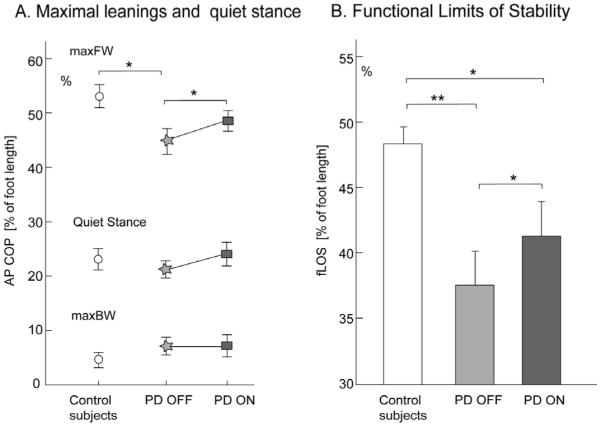
Functional limits of stability in control and parkinsonian subjects. (A) Position of antero-posterior center of pressure (mean and SD) during the maximal leaning tasks and in quiet stance. (B) Functional limits of stability (mean and SD) quantified as the difference between maximal forward and maximal backward lean position. * P < 0.05, ** P < 0.01.
Maximal forward leaning was significantly smaller in PD subjects in the OFF state compared to control subjects (P < 0.05), and was increased by levodopa, although remained smaller than normal (Fig. 2A). MaxFW reached a mean of 53.1% (SD 2.1) of foot length ahead of the lateral malleoli in control subjects versus 44.7% (SD 2.4) in PD OFF and 48.5% (SD 1.9) in PD ON.
The magnitude of the functional limits of stability, as measured by fLOS (Fig. 2B), and expressed as percent of foot length, was significantly smaller in PD OFF, compared to control subjects (37.6% (SD 2.6) and 48.5% (SD 1.2), respectively, with P < 0.01). Levodopa significantly increased fLOS in PD subjects, (41.4% (SD 2.6)), however, fLOS remained significantly smaller than normal values (P < 0.05). All 14 PD subjects increased their fLOS when ON except one subject, who was the least responsive to levodopa (see the Motor UPDRS Motor subscale and rigidity score in Table 1, Subject #14). All correlations between fLOS and UPDRS Motor subscale were not significant (ranging from -0.56 to -0.42 with 0.09 < P < 0.17) even after we removed two-outliers (Subjects #2 and #7 in Table 1) who had been unable to maintain backward lean for 5 s.
3.2. Postural strategy
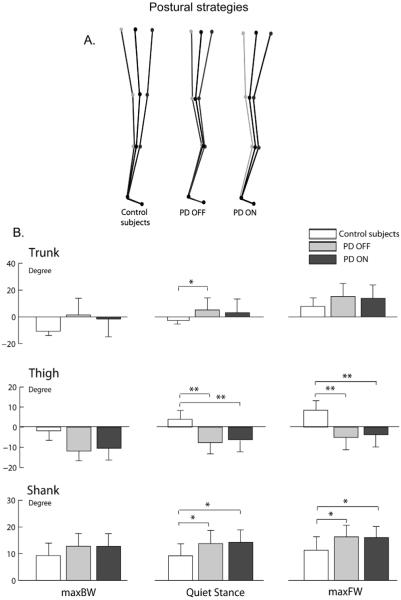
During quiet stance, the kinematic analysis of body segment alignment with respect to vertical confirmed the typical stooped posture in PD subjects. Fig. 3A shows the group average, sagittal body alignment as stick diagrams for the three subject groups. Compared to control subjects, PD subjects OFF showed larger forward inclination of the trunk (P < 0.05), larger backward inclination of the thigh (P < 0.01), and larger forward inclination of the shank (P < 0.05), reflecting their increased hip, knee, and ankle joint flexion. Levodopa decreased forward trunk inclination to some extent, although not significantly, but did not change thigh or shank inclinations, which remained significantly different from control subjects’ values (P < 0.01 and P < 0.05, respectively).
Fig. 3.
Postural strategies during maximal leaning tasks and quiet stance in control and parkinsonian subjects, represented by: (A) average stick diagrams. (B) Trunk, thigh, and shank inclinations (mean and SD). *P < 0.05, **P < 0.01.
During forward lean, all subjects significantly increased their forward trunk inclination compared to quiet stance (P < 0.05; Fig. 3B upper panel). However, unlike control subjects, PD subjects, both OFF and ON, maintained similar leg alignment as during quiet stance, with a smaller forward thigh inclination and a smaller forward shank inclination than control subjects (P < 0.05). In addition, PD subjects, both OFF and ON, maintained the knees flexed during backward leaning, as highlighted by corresponding shank and thigh inclination values and by stick diagrams.
3.3. Motion phase of the leaning tasks
The algorithm chosen for detecting the onset of movement proved valid, after comparing between groups the SDs of COP coordinates at the baseline prior to leaning. As expected, we found that such SDs did not differ between PD, both OFF and ON, and control subjects in the short time they spent in natural standing before leaning.
The AP COP-COM time-series is shown in Fig. 4A during the backward and forward leaning tasks for a representative control subject.
Fig. 4.
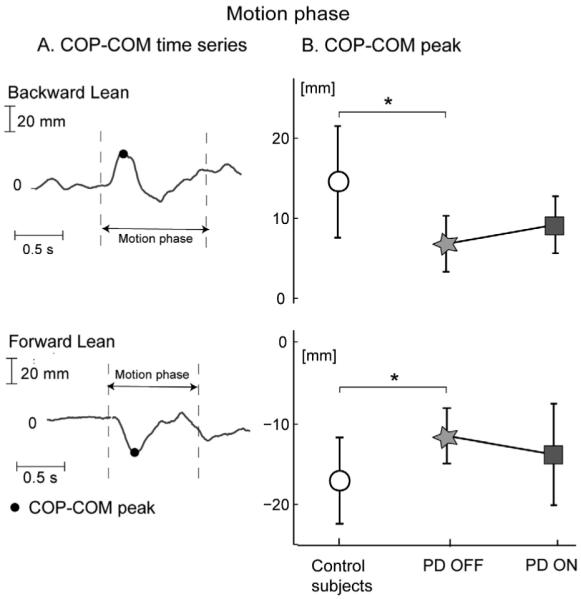
Peak of COP-COM time series during backward and forward leaning. (A) Example of COP-COM time-series for a representative control subject. (B) COP-COM peaks for control and parkinsonian subjects (mean and SD). *P < 0.05.
Fig. 4B summarizes the group means and SD of the COP-COM peak. The COP-COM peak was significantly smaller in PD subjects OFF compared to control subjects (P < 0.05), both for the forward and backward leaning. Levodopa did not significantly change the COP-COM peak.
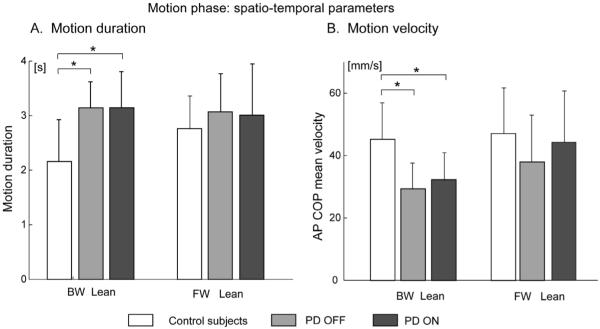
The spatio-temporal parameters of the motion phase are shown in Fig. 5. During backward leaning, PD subjects, both OFF and ON, showed significantly longer and slower movements compared to control subjects (P < 0.05). In contrast, during forward leaning, movement duration and velocity did not differ significantly between control subjects and PD subjects OFF. Levodopa did not change significantly movement duration and velocity.
Fig. 5.
Spatio-temporal characterization of the motion phase (mean and SD) in control and parkinsonian subjects. (A) Motion duration. (B) Motion velocity quantified by the AP COP mean velocity.
4. Discussion
The present study showed that subjects with PD have smaller functional limits of stability in the sagittal plane compared to age-matched control subjects. The small stability limits in PD subjects was primarily due to a reduction of maximum forward body leaning. The small maximum forward lean in PD subjects may be related to their impaired postural preparation for gait initiation (Burleigh-Jacobs et al., 1997; Ferrarin et al., 2002; Rocchi et al., 2006) that similarly requires a preparatory forward lean. In contrast to the forward direction, stability limits in the backward direction were not significantly different between control and PD subjects. This result could be due to an age-effect or “floor”-effect on maximum backward inclination common to both PD and control subjects due to biomechanical constraints for backward leaning (Schieppati et al., 1994).
We did not find any left-right asymmetry during the leaning tasks. However, future studies aimed at a better characterization of postural stability should more extensively evaluate COP position in both the AP and ML directions, during longer leans. Indeed, previous studies found differences in medio-lateral sway between PD and control subjects during body sagittal inclinations (Adkin et al., 2005; van Wegen et al., 2001).
Unlike a previous study (Schieppati et al., 1994), we did not see a significant difference in average COP position during quiet stance between PD and control subjects. Such differences might be explained by different inclusion criteria for PD subjects (our subjects where candidates for DBS surgery) and by the specific instructions for subjects to gaze forward and to maintain consistent initial COP position prior to each trial.
Postural kinematic strategies (Horak et al., 1997) in the steady-state upright and leaning positions confirmed the typical, stooped posture of PD subjects (Jacobs et al., 2005). PD subjects also maintained their stooped posture during the voluntary leaning tasks (Bloem et al., 1999). The stooped posture probably contributed to the reduced forward limits of stability, because the flexed ankle, knee and hip joints resulted in longer ankle plantarflexor muscles and larger antigravity forces required to maintain equilibrium. This unchanged body posture is consistent with previous studies showing that PD subjects have difficulty in changing postural strategies with changes in initial conditions (Burleigh-Jacobs et al., 1997; Chong et al., 2000; Jacobs et al., 2005; Rocchi et al., 2006). Although our subjects were instructed to move without flexion/extension of knee or hip, both control and PD subjects were not able to use a pure, inverted pendulum-like behavior but flexed the hips for forward leans and flexed the knees for backward leans.
PD subjects participating in our study were highly sensitive to levodopa, as shown by changes in their UPDRS Motor subscale (see Table 1). Interestingly, the medication increased their limits of stability but did not change postural strategies used to reach such limits. It is possible that reduced rigidity played a role in allowing larger stability limits with levodopa, even if we did not find significant correlations between the parameter fLOS and the UPDRS measures of rigidity. Indeed, previous studies showed that PD subjects’ background EMG is quieter and COM moves farther and faster in response to external perturbations and during quiet stance when ON levodopa, consistent with reduced rigidity, (Horak et al., 1996). Increased functional stability limits in the ON state may be related to reduction of leg, and not axial, rigidity, because a previous study showed no reduction of axial rigidity with levodopa (Wright et al., 2007).
Postural preparation for the voluntary leaning movement, characterized by the peak of the COP-COM time series, was impaired in subjects with PD, particularly in the OFF state, consistently with other tasks requiring anticipatory postural adjustments (Burleigh-Jacobs et al., 1997; Crenna et al., 2006; Rocchi et al., 2006). The COP-COM variable has been shown to detect stability during preparation for a voluntary rise from a chair (Hass et al., 2005). Our results showed reduced COP-COM peak in preparation for a lean as well as reduced functional stability limits in PD subjects, suggesting that PD affect both preparation and achievement of limits of stability. Subjects with PD reached their functional stability limits slowly compared to control subjects, during backward, but not during forward, leaning. The slowness of backward leaning may reflect weakness in the ankle extensors or a perceived difficulty of the backward leaning motor task. In fact, slowness of movement may reveal cautiousness or fear of falling and a higher perceived difficulty of the backward leaning task (Franchignoni et al., 2005). In this case, rehabilitation programs focused on increasing postural limits of stability and/or reducing fear of falling may be useful for PD.
The present study highlights the importance of a quantitative approach for postural evaluation in PD. In fact, the lack of correlation between the UPDRS Motor subscale and limits of stability parameters is consistent with poor specificity of the UPDRS Motor subscale for the postural requirements associated with a voluntary lean. Forward voluntary leaning may be a good clinical measure of postural ability in PD by reflecting composite effects of segment orientation, perceived postural stability, fear of falling, whole body kinaesthesia and leg rigidity.
Our results showed that levodopa improves the static, functional limits of stability, but did not ameliorate postural preparation for a leaning movement or postural kinematic strategies for leaning. These findings suggest separate central mechanisms and different constraints on perceived postural limits of stability, multisegmental postural alignment, and postural preparation for whole body movement.
References
- Adkin AL, Bloem BR, Allum JH. Trunk sway measurements during stance and gait tasks in Parkinson’s disease. Gait Posture. 2005;22:240–249. doi: 10.1016/j.gaitpost.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bartolic A, Pirtosek Z, Rozman J, Ribaric S. Postural stability of Parkinson’s disease patients is improved by decreasing rigidity. Eur. J. Neurol. 2005;12:156–159. doi: 10.1111/j.1468-1331.2004.00942.x. [DOI] [PubMed] [Google Scholar]
- Binda SM, Culham EG, Brouwer B. Balance, muscle strength, and fear of falling in older adults. Exp. Aging Res. 2003;29:205–219. doi: 10.1080/03610730303711. [DOI] [PubMed] [Google Scholar]
- Blaszczyk JW, Klonowski W. Postural stability and fractal dynamics. Acta Neurobiol. Exp. (Wars) 2001;61:105–112. doi: 10.55782/ane-2001-1390. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Beckley DJ, van Dijk JG. Are automatic postural responses in patients with Parkinson’s disease abnormal due to their stooped posture? Exp. Brain Res. 1999;124:481–488. doi: 10.1007/s002210050644. [DOI] [PubMed] [Google Scholar]
- Broggi G, Franzini A, Marras C, Romito L, Albanese A. Surgery of Parkinson’s disease: inclusion criteria and follow-up. Neurol. Sci. 2003;24(Suppl 1):S38–S40. doi: 10.1007/s100720300037. [DOI] [PubMed] [Google Scholar]
- Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Movement Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- Chong RK, Horak FB, Woollacott MH. Parkinson’s disease impairs the ability to change set quickly. J. Neurol. Sci. 2000;175:57–70. doi: 10.1016/s0022-510x(00)00277-x. [DOI] [PubMed] [Google Scholar]
- Corriveau H, Hebert R, Raiche M, Dubois MF, Prince F. Postural stability in the elderly: empirical confirmation of a theoretical model. Arch. Gerontol. Geriatr. 2004;39:163–177. doi: 10.1016/j.archger.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Crenna P, Carpinella I, Rabuffetti M, Rizzone M, Lopiano L, Lanotte M, Ferrarin M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson’s disease. Exp. Brain Res. 2006;172:519–532. doi: 10.1007/s00221-006-0360-7. [DOI] [PubMed] [Google Scholar]
- Dibble LE, Lange M. Predicting falls in individuals with Parkinson disease: a reconsideration of clinical balance measures. J. Neurol. Phys. Ther. 2006;30:60–67. doi: 10.1097/01.npt.0000282569.70920.dc. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, The UPDRS Development Committee . Unified Parkinson’s disease rating scale. In: Fahn S, et al., editors. Recent Developments in Parkinson’s Disease. Macmillan Healthcare Information; Florham Park, New Jersey: 1987. pp. 153–163. [Google Scholar]
- Ferrarin M, Lopiano L, Rizzone M, Lanotte M, Bergamasco B, Recalcati M, Pedotti A. Quantitative analysis of gait in Parkinson’s disease: a pilot study on the effects of bilateral subthalamic stimulation. Gait Posture. 2002;16:135–148. doi: 10.1016/s0966-6362(01)00204-1. [DOI] [PubMed] [Google Scholar]
- Franchignoni F, Martignoni E, Ferriero G, Pasetti C. Balance and fear of falling in Parkinson’s disease. Parkinsonism Relat. Disord. 2005;11:427–433. doi: 10.1016/j.parkreldis.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson’s disease. Arch. Phys. Med. Rehabil. 2005;86:2172–2176. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J. Neurophysiol. 2001;85:559–570. doi: 10.1152/jn.2001.85.2.559. [DOI] [PubMed] [Google Scholar]
- Holbein MA, Redfern MS. Functional stability limits while holding loads in various positions. Int. J. Ind. Ergon. 1997;19:387–395. doi: 10.1016/s0169-8141(96)00023-6. [DOI] [PubMed] [Google Scholar]
- Horak FB, Dimitrova D, Nutt JD. Direction-specific postural instability in subjects with Parkinson’s disease. Exp. Neurol. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Horak FB, Frank J, Nutt J. Effects of dopamine on postural control in parkinsonian subjects: scaling, set, and tone. J. Neurophysiol. 1996;75:2380–2396. doi: 10.1152/jn.1996.75.6.2380. [DOI] [PubMed] [Google Scholar]
- Horak FB, Henry SM, Shumway-Cook A. Postural perturbations: new insights for treatment of balance disorders. Phys. Ther. 1997;77:517–533. doi: 10.1093/ptj/77.5.517. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Dimitrova DM, Nutt JG, Horak FB. Can stooped posture explain multidirectional postural instability in patients with Parkinson’s disease? Exp. Brain Res. 2005;166:78–88. doi: 10.1007/s00221-005-2346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Nardone A, Schieppati M. Balance in Parkinson’s disease under static and dynamic conditions. Movement Disord. 2006;21:1515–1520. doi: 10.1002/mds.21015. [DOI] [PubMed] [Google Scholar]
- Newton RA. Validity of the multi-directional reach test: a practical measure for limits of stability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M248–M252. doi: 10.1093/gerona/56.4.m248. [DOI] [PubMed] [Google Scholar]
- Patton JL, Pai Y, Lee WA. Evaluation of a model that determines the stability limits of dynamic balance. Gait Posture. 1999;9:38–49. doi: 10.1016/s0966-6362(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Horak FB. Effects of deep brain stimulation and levodopa on postural sway in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2002;73:267–274. doi: 10.1136/jnnp.73.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson’s disease: influence of initial stance conditions. Neurosci. Lett. 2006;406:128–132. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Hugon M, Grasso M, Nardone A, Galante M. The limits of equilibrium in young and elderly normal subjects and in Parkinsonians. Electroencephalogr. Clin. Neurophysiol. 1994;93:286–298. doi: 10.1016/0168-5597(94)90031-0. [DOI] [PubMed] [Google Scholar]
- van Wegen EE, van Emmerik RE, Riccio GE. Postural orientation: age-related changes in variability and time-to-boundary. Hum. Movement Sci. 2002;21:61–84. doi: 10.1016/s0167-9457(02)00077-5. [DOI] [PubMed] [Google Scholar]
- van Wegen EE, van Emmerik RE, Wagenaar RC, Ellis T. Stability boundaries and lateral postural control in Parkinson’s disease. Motor Control. 2001;5:254–269. doi: 10.1123/mcj.5.3.254. [DOI] [PubMed] [Google Scholar]
- Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J. Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- Wright GW, Gurfinkel VS, Nutt JD, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson’s disease: direct measurements of trunk and hip torque. Exp. Neurol. 2007;208(1):38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]