Abstract
The C. elegans distal tip cell (DTC) provides a niche for germline stem cells in both hermaphrodites and males. The hermaphrodite distal tip cell (hDTC) also provides “leader” function to control gonadal elongation and shape, while in males, leader function is allocated to the linker cell (LC). Therefore, the male distal tip cell (mDTC) serves as a niche but not as a leader. The C. elegans homolog of E/Daughterless, HLH-2, was previously implicated in hDTC specification. Here we report that HLH-2 is also critical for hDTC maintenance, hDTC niche function and hDTC expression of a lag-2/DSL ligand reporter. We also find that HLH-2 functions in males to direct linker cell specification and to promote both mDTC maintenance and the mDTC niche function. We conclude that HLH-2 functions in both sexes to promote leader cell specification and DTC niche function.
Keywords: HLH-2, lag-2, distal tip cell, stem cell niche, morphogenesis, leader function, organogenesis, gonadogenesis
Introduction
Organogenesis relies on the precise temporal and spatial control of growth, morphogenesis and cell specification. Most organs analyzed to date are controlled by a combination of organ-specific regulators and more basic regulators and pathways used to control fundamental cellular processes. Our interest has been how organogenesis programs can be modulated to generate functionally and morphologically distinct organs. The approach we have taken to unveil modulatory mechanisms is analysis of a sexually dimorphic organ, the C. elegans gonad.
The C. elegans hermaphrodite and male gonads derive from two somatic gonadal progenitor (SGP) cells and two germ cell progenitors. The SGPs undergo sex-specific divisions during the first larval stage (L1) to generate three regulatory cells plus progenitors for somatic gonadal structures (Kimble and Hirsh, 1979; Kimble and White, 1981) (Figs. 1A, 1B). This work focuses on controls of the regulatory cells, including both their specification and function. The regulatory cells include two “distal tip cells” (DTCs) in each sex, an “anchor cell” (AC) in hermaphrodites and a “linker cell” (LC) in males. These cells govern gonadal growth and morphogenesis as well as vulval induction, and therefore are crucial regulators of organogenesis.
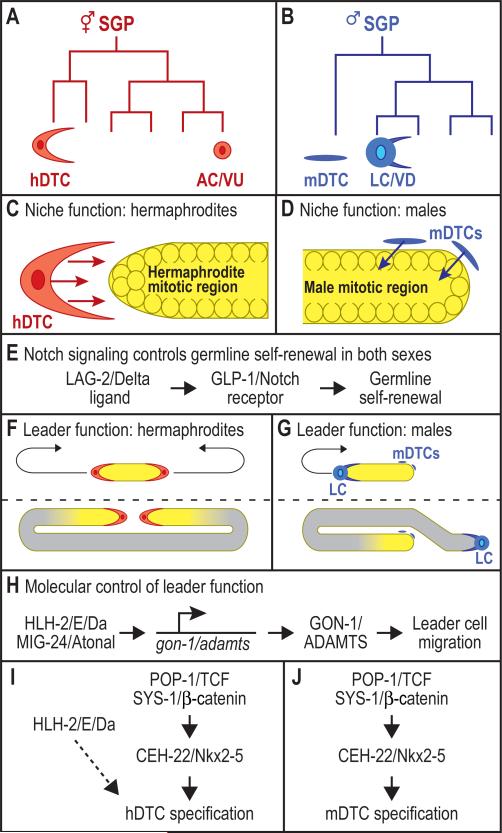
Fig. 1.
Origin, function and regulation of somatic gonadal regulatory cells. (A, B) The somatic gonadal progenitor (SGP) generates the DTC in one or two divisions. Vertical lines, cells; horizontal lines, cell divisions. Distal daughters to left, proximal daughters to right. For complete lineage, see Kimble & Hirsh (1979). (A) Each hermaphrodite SGP generates one hDTC and one regulatory cell with potential to become either an anchor cell (AC) or ventral uterine precursor (VU). (B) Each male SGP generates one mDTC and one regulatory cell with potential to become either a linker cell (LC) or vas deferens precursor (VD). (C, D) DTCs signal to germ cells in the mitotic region (yellow) to promote self-renewal and inhibit differentiation. Germline stem cells are found within the mitotic region. See Kimble and Crittenden (2007) for review. (C) A single hDTC resides at the distal end of the hermaphrodite gonadal arm. (D) Two mDTCs (blue) reside at the distal end of the male gonadal arm. (E) Notch signaling is critical for niche function and germline self-renewal. LAG-2 provides a DSL ligand for Notch signaling in hermaphrodites, but its role in males has not been tested. The GLP-1/Notch receptor is used in both sexes. (F, G) Distinct cells provide leader function in the two sexes. Shown are gonadal shapes in young larvae (above) and late larvae (below). Leader cells migrate in a path (arrows) to guide elongation and shape changes. Mitotically-dividing germ cells, yellow; all other gonadal cells in grey. Anterior, left. Dorsal, up. See Introduction for references. (F) Hermaphrodite leader function resides in the hDTCs, which guide elongation of two gonadal arms in each animal. (G) Male leader function resides in the single linker cell (LC), which guides elongation of the gonadal arm in each male. (H) Molecular control of leader cell migration. HLH-2/E/Da and MIG-24/Atonal regulate transcription of gon-1/adamts, which encodes a secreted metalloprotease required for leader cell migration. See Introduction for references. (I, J) Molecular control of DTC specification in hermaphrodites (I) and males (J). POP-1/TCF and SYS-1/β-catenin regulate transcription of ceh-22/Nkx2.5 to specify both hDTCs and mDTCs (Lam et al., 2006). HLH-2 has been implicated in hDTC specification (Karp and Greenwald, 2004), but its position in the pathway was not explored in previous studies. NHR-25 antagonizes POP-1 and SYS-1 in hermaphrodites (Asahina et al., 2006), but is omitted from this diagram for simplicity.
The hermaphrodite DTC (hDTC) and male DTC (mDTC) have a common function in providing a niche for germline stem cells (Figs. 1C, 1D). That DTC “niche function” relies on GLP-1/Notch signaling, which is required for germline self-renewal in both sexes (reviewed in Kimble and Crittenden, 2007). The GLP-1/Notch receptor is expressed in self-renewing germ cells in both sexes; the LAG-2/Delta ligand is expressed in the hermaphrodite DTC and controls Notch signaling and self-renewal in hermaphrodites (Fig. 1E) (Henderson et al., 1994; Lambie and Kimble, 1991; Tax et al., 1994). Little is known about how lag-2 is regulated in the hDTCs or about its role in males.
The DTCs in the two sexes differ in their control of gonadal shape. In hermaphrodites, each hDTC guides formation of a U-shaped gonadal arm (Fig. 1F). By contrast, the mDTC does not provide leader function in the male gonad. Instead, a different cell, called the linker cell (LC), leads formation of the single J-shaped gonad arm in the male gonad (Fig. 1G). Crucial for leader function in both sexes is GON-1, a secreted ADAMTS metalloprotease (Blelloch and Kimble, 1999). The control of gon-1 expression in hDTCs and male LCs relies on HLH-2 and MIG-24, two basic helix-loop-helix (bHLH) transcription factors that can heterodimerize and bind the gon-1 promoter (Fig. 1H; Tamai and Nishiwaki, 2007). HLH-2 is the sole C. elegans member of the E/Daughterless bHLH protein family; E proteins form heterodimers with other bHLH proteins and bind target gene regulatory elements through the E-box motif, CANNTG (Bertrand et al., 2002). MIG-24 belongs to the Atonal bHLH protein superfamily (Voutev and Hubbard, 2008).
Specification of both hDTCs and mDTCs relies on three key transcription factors: POP-1/TCF, SYS-1/β-catenin and CEH-22/Nkx2.5 (Figs. 1I, 1J). Loss of POP-1, SYS-1 or CEH-22 eliminates DTCs (Kidd et al., 2005; Lam et al., 2006; Miskowski et al., 2001; Siegfried and Kimble, 2002), and ectopic SYS-1 or CEH-22 produces extra hDTCs (Kidd et al., 2005; Lam et al., 2006). The sys-1 and ceh-22 genes are expressed in DTC progenitors and in the DTCs themselves, if only briefly after they are born (Lam et al., 2006; Phillips et al., 2007). Indeed, the ceh-22 gene is a direct target of transcriptional activation by POP-1/TCF and SYS-1/β-catenin (Lam et al., 2006) (Figs. 1I, 1J). By contrast, the nuclear hormone receptor, NHR-25/SF-1/FtzF1, inhibits hDTC specification by antagonizing POP-1/TCF and SYS-1/β-catenin (Asahina et al., 2006).
Specification of the hDTC is also influenced by HLH-2/E/Daughterless (Karp and Greenwald, 2004). Reduction of HLH-2 eliminates some hDTCs, and ectopic HLH-2 can generate extra hDTCs. HLH-2 expression begins after hDTCs are born and continues throughout adulthood (Karp and Greenwald, 2004; Krause et al., 1997). The role of HLH-2 in the male gonad has not been investigated.
In this work, we investigate HLH-2 controls of hDTCs, mDTCs and male linker cells. An important tool for our studies is hlh-2(tm1768), a likely hypomorphic deletion that we used in combination with hlh-2 RNAi to strongly reduce hlh-2 activity. We confirm that HLH-2 affects hDTC specification and hDTC leader function, as previously reported (Karp and Greenwald, 2004), and go on to show that HLH-2 is also critical for hDTC niche function, probably by regulating DTC expression of the LAG-2/DSL ligand. In males, we find that HLH-2 promotes both mDTC maintenance and the mDTC niche function and is also essential for LC specification. Therefore, HLH-2 functions in both sexes to promote both the niche and leader functions.
Materials and methods
Nematode strains and maintenance
All strains were derivative of Bristol strain N2 and were maintained at 20°C unless stated otherwise. The following mutations were used: LGI: hlh-2(tm1768) (this work) (described below); sys-1(q544) (Miskowski et al., 2001); sys-1(os63) (Kidd et al., 2005), pop-1(q624), pop-1(q645) (Siegfried and Kimble, 2002); LGV: ceh-22(q632) (Lam et al., 2006; Siegfried et al., 2004), him-5(e1490) (Hodgkin, 1997). Some of the mutations studied were homozygous sterile and therefore maintained as heterozygotes using dominant GFP balancers. The sys-1 and pop-1 mutations were balanced by hT2[qIs48](I;III) (Miskowski et al., 2001), and ceh-22(q632) was balanced by nT1[qIs51](IV;V) (Siegfried et al., 2004).
We also used the molecular markers qIs57[lag-2::GFP, unc-119(+)] II, qIs56[lag-2::GFP, unc-119(+)] V (Siegfried et al., 2004), qIs95[sys-1::VENUS::SYS-1; ttx-3::dsRed] III (Phillips et al., 2007), and qIs90[ceh-22b::VENUS] (Lam et al., 2006) as well as the heat-inducible transgenes arIs63[hsp16-2::hlh-2; dpy-20(+); ttx-3::GFP] (Karp and Greenwald, 2004) and qIs131[hs::ceh-22b, ttx-3::dsRed] (this work). The qIs131 insertion was made by gamma irradiation-mediated integration of qEx557 (Lam et al., 2006).
The hlh-2(tm1768) strain was obtained from the Japanese National BioResource Project and outcrossed six times to wild-type N2 larvae prior to use. We followed the deletion by nested PCR, using the following primers: Outer set: 5’: TCCTGGTGGCATTCCGGTCA, 3’: GGGATGTCATCCATCCGATA. Inner set: 5’: AGTGTGGGCTCATACCGGCT, 3’: GCTCCAGCCCAATTCCCTAC. The deletion breakpoints and novel insertion were confirmed by sequencing.
The tm1768 allele deletes a 628 bp region of the hlh-2 locus (Fig. 2A) and inserts 17 novel bp. Below, the sequence flanking the deletion/insertion is indicated in CAPs with the novel insertion in lowercase: GATTATCCTTATAATAagctgttgttggctttaACGAATTCTGCAGA. This alteration is predicted to be in frame with the wild-type HLH-2 ORF, producing an in-frame deletion/insertion that deletes 93 amino acids, inclusive of residues 36-128, and introduce the following six novel amino acids: KLLLAL.
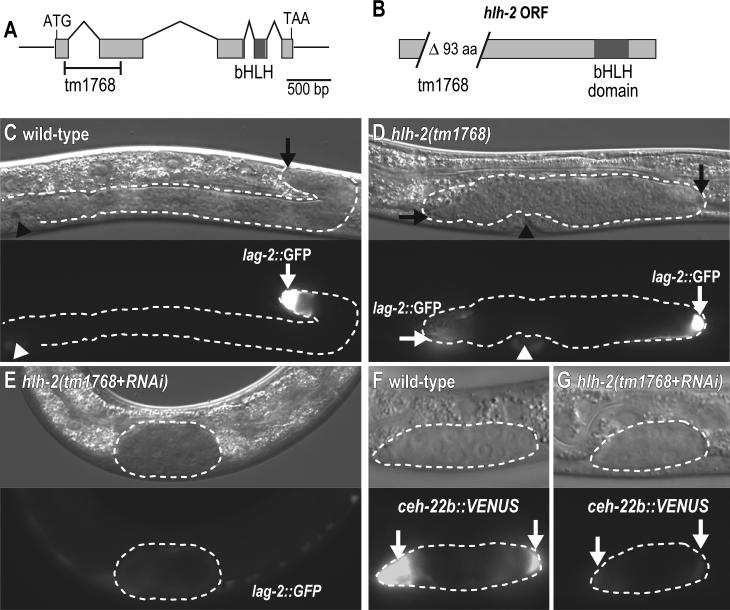
Fig. 2.
Strong HLH-2 reduction in a hlh-2 mutant treated with hlh-2 RNAi. (A) The hlh-2 locus. Boxes, exons; lines, introns; region encoding bHLH DNA-binding domain, dark grey. Bracketed region is deleted in hlh-2(tm1768) deletion/insertion allele. (B) The HLH-2 ORF. The predicted hlh-2(tm1768) protein harbors a 93 aa deletion and six amino acid insertion of novel sequence in the N-terminal region. (C-G) Animals grown at 25°C. Anterior, left. Dorsal, up. Top, Nomarski; bottom, fluorescence; dotted lines, outline of gonad; arrows, hDTCs. (C-E) L4 hermaphrodites carrying lag-2::GFP, qIs57. Arrowhead, vulva. Exposure times: (C) and (D), 4 ms, (E) 20 ms. (C) Wild-type gonad is elongated and reflexed. lag-2::GFP is bright in the hDTC. (D) hlh-2(tm1768) gonad extends slightly but fails to reflex. lag-2::GFP is bright in the hDTCs. Left hDTC is out of the focal plane, but GFP is still visible. (E) hlh-2(tm1768) treated with hlh-2 RNAi. Gonad is not elongated or reflexed; no hDTCs are present. A faint lag-2::GFP signal can be detected in the somatic gonad when exposed for 20 ms; signal was barely detectable when exposed for 4 ms, which was the exposure time used in (C) and (D) (not shown). (F, G) L2 hermaphrodites carrying ceh-22b::VENUS reporter, qIs90. (F) Wild-type larva treated for RNAi but on empty vector. ceh-22b::VENUS is bright in the hDTCs. (G) hlh-2(tm1768) mutant treated with hlh-2 RNAi. ceh-22b::VENUS is barely detectable.
RNA interference (RNAi)
All RNAi experiments used the E. coli strain HT115(DE3) transformed with RNAi clones (below) and maintained under selection with carbenicillin (60 μg/mL) and tetracycline (50 μg/mL). Feeding RNAi plates were prepared as described (Timmons et al., 2001), except for use of 50 μg/mL of tetracycline and 1 mM IPTG. For most experiments, overnight bacterial cultures were grown with selection in 2XYT broth, diluted 1:250 with fresh 2XYT and antibiotics, then grown to an OD600 of ∼0.4. Cultures were then induced for 3-4 h with 0.4 mM IPTG, concentrated 5-10x, transferred to feeding RNAi plates and incubated at room temperature for 24-36 h. Feeding RNAi plates were either used immediately or stored at 4°C for up to three weeks prior to use.
Except where noted, feeding RNAi was performed using L1 larvae, which were synchronized as follows: gravid adults grown at 20°C were treated with a hypochlorite solution for 4-5 minutes. Embryos were then washed five times in M9 solution, stored in M9 with gentle agitation for 16-30 h at either 20°C or 25°C as specified, then concentrated, plated on feeding RNAi plates and maintained at the specified temperature.
For L3 feeding RNAi experiments, synchronized L1 larvae were initially plated on standard plates with OP50, and then transferred to RNAi plates as L3 larvae. The next day, late L4s were moved to new RNAi plates. At 24 h past L4, animals were removed and processed for analysis.
All RNAi clones were variants of pPD129.36 (Timmons and Fire, 1998). For control RNAi experiments, we used the “empty” pPD129.36 vector. For hlh-2 RNAi, we made two different feeding RNAi clones designed to target different regions of the hlh-2 mRNA. The PCR primers used were: Clone 1: 5’: ATGGCGGATCCAAATAGCCAACTTACG, 3’: CTTCTGAAGGTGGAAGGTAACC. Clone 2: 5’: GGTCTTGGTGGAGATACCAACTTG, 3’: AAACCGTGGATGTCCAAACTGCGC. The nhr-25 RNAi clone was constructed using the following PCR primers: 5’: GCTACAATGATGCCAATGACAACT, 3’: TCGGTTGACAGTAGTGCGAATC.
lag-2 and hlh-2 reporter transgenes
Most lag-2::GFP experiments were performed using either qIs56 or qIs57, which contain the 3.2 kb sequence 5’ of the lag-2 translational start codon, fused to GFP (Blelloch et al., 1999; Siegfried et al., 2004). For analysis of E-box sites in the lag-2 promoter, we cloned the expression plasmids pJK1231 (wild-type E-boxes) and pJK1230 (mutated E-boxes), using promoter sequences from pXK52 and pXK61, respectively (kindly provided by Iva Greenwald and described in Karp and Greenwald, 2003). Both pJK1231 and pJK1230 contain the 3.3 kb lag-2 promoter region upstream from the translational start codon, fused to GFP. In pJK1230, the six E-box sites have been mutated from CANNTG to AANNAG (Fig. 4A), as described by Karp & Greenwald (2003).
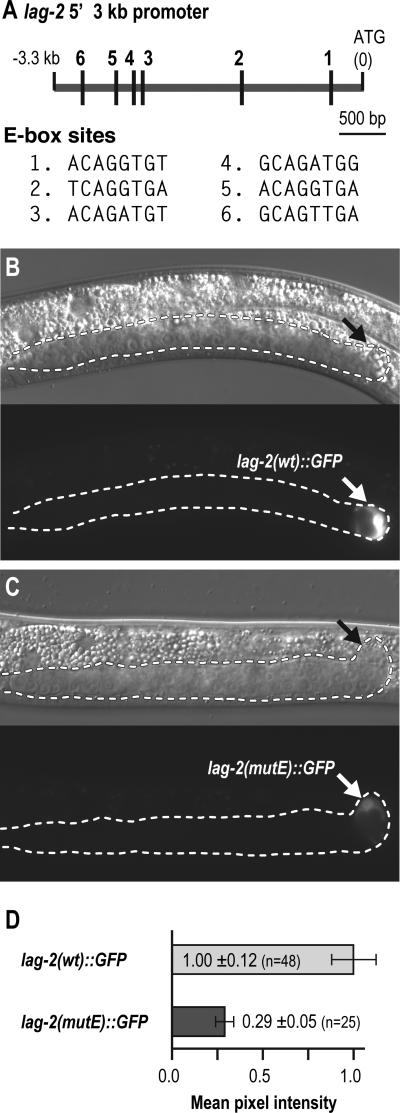
Fig. 4.
E-box sites are required for robust lag-2::GFP expression in hDTCs. (A) Top, the 3.3 kb region upstream of the lag-2 translational start codon. Vertical lines, locations of putative E-box sites. Scale bar, 500 bp. Bottom, E-box sequences with number corresponding to sites in diagram above. For E-box sites 2 and 3, the reverse strand sequence is displayed. (B, C) L3 hermaphrodite larvae carrying a lag-2::GFP transgene. Exposure time, 10 ms. Conventions as in Fig. 2. (B) lag-2 promoter in the reporter transgene carries the wild-type (wt) sequence. GFP expression in the hDTC is robust. (C) lag-2 promoter in the reporter transgene has mutated E-boxes. GFP expression in the hDTC is faint. (D) Quantification of GFP in hDTCs carrying either the wild-type or E-box mutated lag-2 promoter. Normalized mean pixel intensity ± standard error is displayed.
Transgenic lines containing either pJK1230 or pJK1231 were generated by injection into adult hermaphrodites by standard methods (Mello and Fire, 1995). In all cases, injection mixtures contained 10 ng/μl of KP#708 (Pttx-3::dsRed) (a gift from Josh Kaplan), 100 ng/μl of S. cerevisiae genomic DNA (Novagen) that was fragmented by sonication, and 1 ng/μl of either pJK1230 or pJK1231.
For analysis of hlh-2 expression, GFP cDNA was recombineered into the fosmid WRM0610dG01, just after the hlh-2 start codon. A 12 kb fragment was then PCR amplified that spanned 8.3 kb 5’ and 0.6 kb 3’ of the hlh-2 locus. Transgenic lines were generated by injection of this fragment (2 ng/μl) into smg-1(cc546 ts)I; him-5(e1490) animals together with 10 ng/μl of KP#708 (Pttx-3::dsRed) and 30 ng/μl of BamHI digested S. cerevisiae genomic DNA.
Analysis of fluorescent markers
In most cases, Nomarski and fluorescence micrographs were obtained on a Zeiss Imager.D1 microscope. Most imaging used a 63x/1.4 objective lens, while a 40x lens was used for animal images in Figs. 3E-F and Fig. S3. Images were captured in Openlab 5.02 with a Hamamatsu camera. Examination of L4 and adult mDTCs was done using a Zeiss Axioplan 2 microscope with a Hamamatsu camera and a Zeiss LSM Meta confocal attachment. All microscope and camera settings were kept constant between images for strains being compared, except where noted in the figure legends. When imaging for quantification of GFP fluorescence, exposure times and gain were reduced to assure that captured images were at sub-saturating levels. To minimize effects of photobleaching on the analysis, only one DTC was imaged per animal, unless using the confocal.
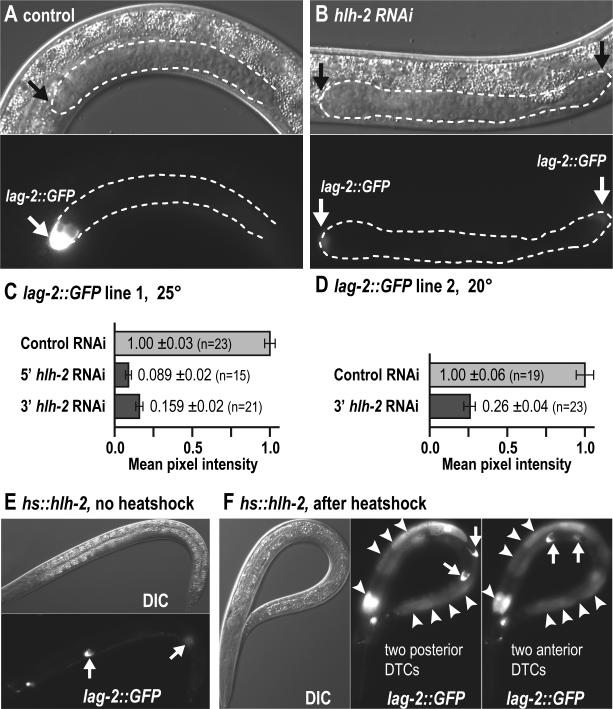
Fig. 3.
hlh-2 is required for robust lag-2::GFP DTC expression. (A, B, E, F) Hermaphrodite L3 larvae, carrying the lag-2::GFP reporter qIs57. Exposure times: (A) and (B), 4 ms, (E) and (F), 5 ms. (A) Control RNAi-treated wild-type animal at 25°C. Anterior gonad arm with bright lag-2::GFP expression in the hDTC. (B) hlh-2 RNAi-treated wild-type animal at 25°C. Gonad arm extension is reduced and both arms are shown; lag-2::GFP is faint in the hDTCs. (C, D) Quantification of lag-2::GFP pixel intensity in hDTCs. Normalized mean pixel intensity +/− standard error quantified from fluorescent hDTC images of control RNAi-treated or hlh-2 RNAi-treated L3 larvae. n=number of hDTCs scored. 5’ hlh-2 RNAi and 3’ hlh-2 RNAi, distinct feeding RNAi clones. (C) Line 1, qIs57, 25°C. (D) Line 2, qIs56, 20°C. (E, F) L3 hermaphrodites carrying arIs63 (hs::hlh-2), a heatshock-inducible hlh-2 transgene. Maintained at 20°C. (E) hs::hlh-2 without heat shock. Two hDTCs are specified and robustly express lag-2::GFP. Posterior hDTC slightly out of the focal plane. No fluorescence in the intestine. (F) hs::hlh-2 after heatshock and then maintained at 20°C. Left, DIC; middle, focal plane with two anterior hDTCs; right panel, focal plane with two posterior hDTCs. All robustly express lag-2::GFP. Bright ectopic fluorescence observed in the intestine (arrowheads).
GFP abundance was quantitated using ImageJ software (http://rsb.info.nih.gov/ij), and only scored when a DTC was specified, as defined below. The cell body of each DTC was outlined and mean pixel intensity measured. Background was subtracted using an identically shaped region adjacent to the DTC. When scoring pixel intensity from extremely dim images, brightness and contrast were initially adjusted to facilitate outlining of the DTC body, but reset prior to measurement.
ceh-22b::VENUS expression was assessed in L2 and L3 larvae. VENUS levels in the hDTCs were qualitatively assessed and scored as dim if the expression level fell below the range observed in controls.
Scoring gonadal defects
hDTCs were scored in L3s, L4s, or early adults. Hermaphrodite gonadal arms were scored in L4s or early adults, or L3s that had undergone at least one round of VPC divisions. A cell was scored as an hDTC by lag-2::GFP expression and either characteristic morphology or proper position along a gonad arm. Male DTCs, LCs, and arms were scored in late L2 or early L3 larvae by lag-2::GFP and their morphology and location within the gonad. In both sexes, gonad arms were scored by elongation from the central gonad, which was invariably coupled with the presence of a leader cell.
Mitotic Region analyses
We extruded and DAPI-stained germ lines as described (Crittenden et al., 2006). Germ cell nuclei in the Mitotic Region nuclei stain throughout the nucleus, whereas nuclei of germ cells in meiotic prophase adopt other patterns. Germ cell positions are designated by row along the distal to proximal axis, with germ cells next to the DTC in row 1. The proximal boundary of the Mitotic Region occurs roughly between rows 20 and 21 in hermaphrodites and between rows 26 and 27 in males (Crittenden et al., 1994; D. Morgan and J. Kimble, manuscript in preparation). To count germ cell number, we marked Mitotic Region boundaries on a computer screen with OpenLab software and counted nuclei in each focal plane.
Heat-shock experiments
For all heat-shock experiments, synchronized L1 larvae were staged using a compound microscope, and heat-shocked when most animals had four germ cells and two somatic gonadal cells. With arIs63, the heat-shock regimen was: 33°C for 1 h, 20°C or 25°C (as specified) for 90-120 minutes, 33°C for 30 minutes. Animals were then maintained at the specified temperature until scoring. For qIs131, the regimen was similar but the second 33°C treatment was omitted.
Sequence analysis
Genomic sequences for C. elegans lag-2 and putative C. briggsae and C. remanei lag-2 orthologs were obtained from WormBase (WS180 17 Sept. 2007 http://ws180.wormbase.org/). A C. brenneri lag-2 ortholog was identified using the Washington University Genome Sequence Center's BLAST server (http://genome.wustl.edu/). Sequence alignments were done using DNA Strider (version1.4f2) and by inspection. E-box sites were identified as described (Karp and Greenwald, 2003).
Results
Strong hlh-2 reduction with a mutant plus RNAi
Previous analyses used RNAi or weak hlh-2 mutants to reduce hlh-2 function (e.g. Karp and Greenwald, 2003; Karp and Greenwald, 2004; Krause et al., 1997; Portman and Emmons, 2000). To obtain stronger reduction, we used hlh-2 RNAi together with an hlh-2 deletion allele. For RNAi, we fed newly hatched L1s to circumvent embryonic lethality, as done previously (Karp and Greenwald, 2004). To control for possible off target RNAi effects, we used two distinct RNAi vectors that target different regions of the hlh-2 locus, 5’ hlh-2 RNAi and 3’ hlh-2 RNAi (see Materials and methods); these two vectors caused the same defects in all of our assays (below). The hlh-2(tm1768) allele contains an in-frame deletion/insertion within the first two exons (Fig. 2A); it is predicted to remove 93 amino acids from the N-terminal region of HLH-2 and introduce six novel amino acids, leaving the bHLH domain intact (Fig. 2B) (see Materials and methods).
Hermaphrodites homozygous for hlh-2(tm1768) had variable and temperature sensitive gonadal defects, which were most severe at 25°C. We therefore characterized the hlh-2 phenotype at 25°C. We scored for presence of DTCs using a transgenic lag-2 transcriptional reporter, lag-2::GFP, to assay DTC specification; we scored for formation of an elongate gonadal arm to assay leader function. In wild-type hermaphrodites, each gonadal arm possessed an hDTC, and gonadal arms were formed normally (Fig. 2C; Table 1). Similarly, most hlh-2(tm1768) hermaphrodites had two hDTCs (97%, n=192), and most had partially elongate gonadal arms, indicative of some leader function (90%, n=192) (Table 1). However, morphogenesis was usually abnormal: arms were short and not reflexed in most gonads (Fig. 2D, data not shown). Thus, hlh-2(tm1768) has little effect on hDTC specification, but a strong effect on normal leader function.
Table 1.
Penetrance of hDTC defects
| Genotypea | hDTC Present (%)b | Leader function present (%)c | nd |
|---|---|---|---|
| wild-type | 100 | 100 | 486 |
| wild-type, control RNAi | >99 | >99 | 262 |
| hlh-2(tm1768)/+ | 96 | 96 | 92 |
| hlh-2(tm1768) | 97 | 90 | 192 |
| 5' hlh-2 RNAi | 76 | 30 | 50 |
| 3' hlh-2 RNAi | 81 | 35 | 48 |
| hlh-2(tm1768+RNAi)e | 2 | 0 | 132 |
All animals carried a GFP marker for the hDTC, qIs57, and were grown at 25°C.
hDTCs scored by GFP and either cell morphology or location on a gonad arm.
Leader function scored by any arm elongation; all elongated arms had an hDTC.
n = number arms scored. Each wild-type gonad has two gonadal arms; hlh-2 defects are often seen in one arm only. Therefore, the number of arms scored (n) is twice the number of animals.
Treated with 3' hlh-2 RNAi. Treatment with 5' hlh-2 RNAi yielded similar results (data not shown).
The hlh-2(tm1768) allele is likely to be a hypomorph and may be either weakly haploinsufficient or dominant negative. This allele exhibited recessive defects in hermaphrodite gonad arm migration (data not shown), and slightly dominant defects in hDTC specification (Table 1). Further RNAi studies support its classification as a hypomorph. On its own, hlh-2 RNAi affected the number of morphogically recognizable hDTCs and their leader function at an intermediate level and more severely than the mutant (Table 1). More importantly, hlh-2(tm1768) was strongly enhanced by hlh-2 RNAi: gonad arm formation was abolished in all hlh-2(tm1768 + RNAi) hermaphrodites (n=132) (Fig. 2E; Table 1). This fully penetrant defect is the same as the most severe defect seen by Karp and Greenwald (2004).
To ask if hDTCs were missing in hlh-2(tm1768+RNAi) animals, we used molecular markers to aid identification and scored for morphologically recognizable hDTCs. Specifically, we used both lag-2::GFP and ceh-22::VENUS, two reporters that are normally expressed brightly in hDTCs but faintly or not detectable in most other somatic gonadal cells. In hlh-2(tm1768+RNAi) L3s and L4s, lag-2::GFP expression was faint and distributed throughout the somatic gonad (Fig. 2E); brightly expressing cells with hDTC morphology were seen only rarely (2%, n=132, Table 1). In about half the gonads, one or two cells expressed the marker at a level that was slightly brighter; these were not likely to be anchor cells (AC), because our reporter lacks enhancer elements required for AC expression (Wilkinson et al., 1994; see Materials and methods), and vulval induction, a readout for AC function, was rarely observed in hlh-2(tm1768+RNAi) animals (data not shown). For ceh-22::VENUS, brightly expressing hDTCs were evident in control L2 and L3 hDTCs (Fig. 2F), and cells with a similar intensity were also found in about half the hlh-2(tm1768+RNAi) gonads. However, in the other half, expression was faint or undetectable (Fig. 2G; Table 2). The most likely explanation is that hDTCs are specified initially in only about half of the hlh-2(tm1768+RNAi) gonads and that those cells express ceh-22::VENUS normally, but express lag-2::GFP at a dramatically reduced level.
Table 2.
ceh-22::VENUS expression in hDTCs
| Bright ceh-22::VENUS | |||
|---|---|---|---|
| Genotypea | Stage | % hDTCsb | nc |
| Wild-type | L2 | 93 | 56 |
| hlh-2(tm1768); 5' hlh-2 RNAi | L2 | 50 | 24 |
| hlh-2(tm1768); 3' hlh-2 RNAi | L2 | 50 | 32 |
| Wild-type | L3 | 72 | 32 |
| hlh-2(tm1768); 3' hlh-2 RNAi | L3 | 49 | 41 |
All strains also included qIs90[ceh-22b::VENUS]; animals grown at 25°C.
Expression scored in the Z1.a or Z4.p cells (i.e. the presumptive hDTC).
Number of presumptive hDTCs scored.
HLH-2 controls lag-2 expression in the hDTC
RNAi directed against hlh-2 caused a partially penetrant loss of hDTCs (see above, Table 1); the remaining hDTCs had reduced lag-2::GFP expression, suggesting that HLH-2 might regulate lag-2 expression. To quantitate this effect, we compared the mean pixel intensity of GFP fluorescence in hDTCs treated with hlh-2 RNAi or a control vector in two different lag-2::GFP lines (Figs. 3A-D), and found that hlh-2 RNAi reduced lag-2::GFP signal intensity in hDTCs by 4- to 11-fold compared to the control (Figs. 3C, 3D). In addition, we asked if HLH-2 overexpression might induce lag-2 expression, using a heatshock-inducible transgene, hs::hlh-2. When L1 hs::hlh-2 larvae were heatshocked, they generated extra hDTCs, as seen before (Karp and Greenwald, 2004), that exhibited a strong lag-2::GFP signal (Figs. 3E, 3F, Table 3). HLH-2 overexpression also induced ectopic intestinal lag-2::GFP expression (Figs. 3E, 3F). Neither effect was seen in controls. The intestine does not normally express HLH-2 (Krause et al., 1997). Therefore, HLH-2 is both critical for lag-2 expression in cells specified as hDTCs, and can also induce lag-2 expression ectopically.
Table 3.
Effect of heat-induced hlh-2 expression on DTC specification
| Genotypea | Heatshock? | hDTC present (%)b | Leader function present (%)c | nd |
|---|---|---|---|---|
| Wild-type | No | 98 | 98 | 124 |
| hs::hlh-2 | No | 98 | 98 | 162 |
| hs::hlh-2e | Yes | 99e | 97 | 150 |
| ceh-22 | Yes | 41 | 41 | 156 |
| ceh-22; hs::hlh-2 | Yes | 41 | 41 | 182 |
| pop-1; hs::hlh-2 | No | 1 | 1 | 158 |
| pop-1 | Yes | 2 | 2 | 186 |
| pop-1; hs::hlh-2 | Yes | 2 | 2 | 434 |
| sys-1 | No | 1 | 1 | 106 |
| sys-1 | Yes | 2 | 2 | 168 |
|
sys-1; hs::hlh-2 |
Yes |
4 |
4 |
74 |
| pop-1 | Yes | 2 | 2 | 128 |
| pop-1; hs::ceh-22f | No | 0 | 0 | 52 |
| pop-1; hs::ceh-22f | Yes | 79 | 79 | 76 |
All animals contained a lag-2::GFP reporter: ceh-22 carried qIs57; pop-1 and sys-1 carried qIs56. Alleles were ceh-22(q632), pop-1(q645) and sys-1(os63). The ceh-22 and ceh-22; hs::hlh-2 animals were maintained at 25°C. All other genotypes were maintained at 20°C.
b-d. As in Table 1.
Animals exhibited 54% extra hDTCs and 28% extra arms.
Heterozygous for qIs131, an integrated hs::ceh-22 transgene.
The lag-2 5’ flanking sequence in the lag-2::GFP reporter contains six predicted E-box sites, of which three are conserved with C. briggsae, C. remanei and C. brenneri (Karp and Greenwald, 2003; Fig. 4A; Fig. S1). To test their function in the hDTC, we constructed lag-2(mutE)::GFP, a transcriptional reporter with the six E-box sites mutated from CANNTG to AANNAG (Fig. 4A, see Materials and methods). Most wild-type lag-2::GFP lines exhibited robust expression in hDTCs, whereas the lag-2(mutE)::GFP lines exhibited much weaker expression in hDTCs (Figs. 4B-4D; data not shown). To quantitate this effect, we measured average pixel intensity of hDTC GFP in wild-type and mutant reporter lines. In hDTCs carrying lag-2(mutE)::GFP, the average pixel intensity was reduced 3.5 fold relative to those carrying wild-type lag-2::GFP (Figs. 4C, 4D). Therefore, E-box sites in the lag-2 promoter are required for robust reporter expression in the hDTC. We suggest that HLH-2 regulates lag-2 transcription via these E-boxes in the lag-2 promoter.
HLH-2 maintains function of the hermaphrodite DTC niche
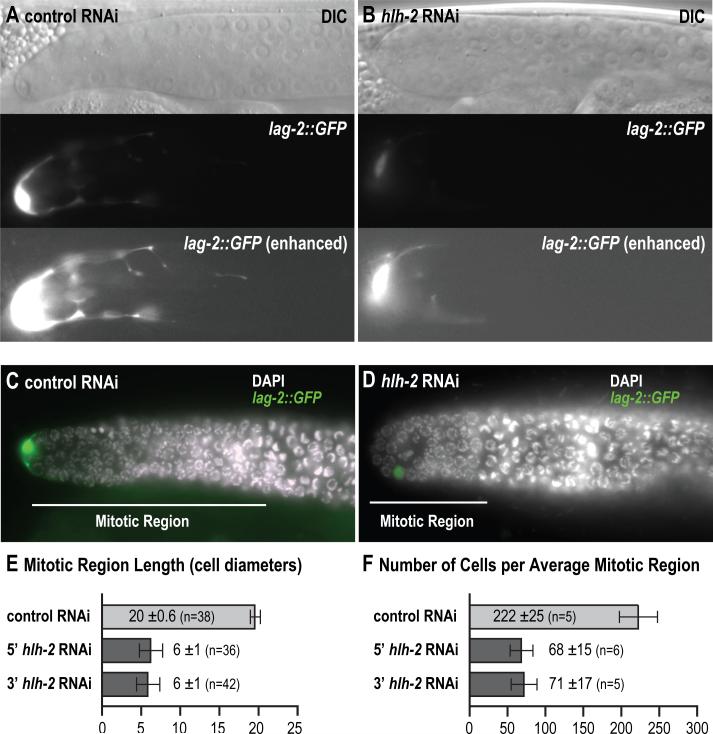
LAG-2/Delta is the hDTC signal for germline self-renewal (Henderson et al., 1994). Given that HLH-2 controls lag-2 expression in hDTCs (see above), we next asked if HLH-2 affects the capacity of hDTCs to control germline self-renewal. To circumvent the HLH-2 role in hDTC specification, we depleted HLH-2 by RNAi from mid-L3, and examined adults for niche defects. This later RNAi was effective: lag-2::GFP expression was lower in hlh-2-depleted adult hDTCs than in controls (Figs. 5A, 5B). To assay niche function, we scored the size of the adult germline mitotic region in DAPI-stained germ lines by standard methods. In hlh-2-depleted adults (24 hr after L4), the mitotic region was much shorter than in control animals (Figs. 5C-E). Indeed, 23% lacked a mitotic region entirely. A similar distribution of mitotic region sizes occurred in adults after an additional 24 hours. To estimate the reduction in germ cell number within the hlh-2 RNAi mitotic regions, we counted those with the average length (6 ± 1 cell diameters), and found a dramatic 3-fold reduction in germ cell number (Fig. 5F). Similar results were observed in hlh-2(tm1768) mutants shifted to 25°C (data not shown).
Fig. 5.
hlh-2 is required for niche function. (A-D) Early adult hermaphrodites, expressing lag-2::GFP (qIs57). (A, B) Top panel, Nomarski image. Middle panel, fluorescence. Bottom panel, fluorescence with brightness and contrast adjustment to enhance visualization of hDTC processes. (A) Control RNAi. The hDTC brightly expresses lag-2::GFP and extends processes proximally. (B) hlh-2 RNAi. The hDTC lag-2::GFP expression is diminished in cell body and processes. (C, D) Adult hermaphrodite germ lines extruded at 24 h past L4, and stained with DAPI. Animals also expressed lag-2::GFP (qIs57). White, DAPI. Green, lag-2::GFP. Feeding RNAi treatment was started late in the L3 stage. White bar, length of mitotic region. (C) Control RNAi. Mitotic region (MR) length extends approximately 20 cell diameters. hDTC is positioned at the distal end and brightly expresses lag-2::GFP. (D) 5’ hlh-2 RNAi. MR length is greatly reduced. The hDTC is slightly proximally displaced and faintly expresses lag-2::GFP. (E) Quantification of MR length (in cell diameters). MR length is dramatically reduced in hlh-2 RNAi-treated hermaphrodites. Control RNAi: 20 ± 0.6 (n=38); 5’ hlh-2 RNAi: 6 ± 1 (n=36); 3’ hlh-2 RNAi: 6 ± 1 (n=42). (F) Quantification of MR cell number from germ lines with the mean MR length. The number of cells in the MR is dramatically reduced in hlh-2 RNAi-treated hermaphrodites. Control RNAi: 222 ± 25 (n=5); 5’ hlh-2 RNAi: 68 ± 15 (n=6); 3’ hlh-2 RNAi: 71 ± 17 (n=5).
To further assess the ability of HLH-2 to promote the hDTC niche function, we asked whether ectopic hDTCs generated by heatshocked hs::hlh-2 animals were able to promote germline mitosis. Indeed, in all cases examined, the ectopic gonad arms generated by heatshocked hs::hlh-2 exhibited mitotic regions that were closely associated with hDTCs (n=19). We conclude that HLH-2 is required for hDTC maintenance and for function of the hDTC as a niche for germline stem cells.
HLH-2 and CEH-22 work in a common process to specify hDTCs
We next explored regulatory relationships of HLH-2 to transcription factors previously implicated in hDTC specification, including POP-1/TCF, SYS-1/β-catenin, CEH-22/Nkx2.5, and NHR-25/SF-1/FtzF1. One possibility might have been that these transcription factors function solely to activate HLH-2 and that HLH-2 is sufficient to specify and maintain the hDTC fate. To test this idea, we used the heat shock inducible hs::hlh-2 transgene to drive ectopic HLH-2 in mutants defective in SYS-1, POP-1 or CEH-22 (Table 3). Successful ectopic HLH-2 production was confirmed by upregulation of intestinal lag-2::GFP in the mutants themselves and by generation of extra hDTCs in their heterozygous siblings. However, ectopic HLH-2 failed to induce hDTCs in any of the mutants (Table 3). [For sys-1(q544), hDTCs were seen after heat shock, but this rescue was not dependent on the transgene.] We conclude that HLH-2 is not sufficient for hDTC specification.
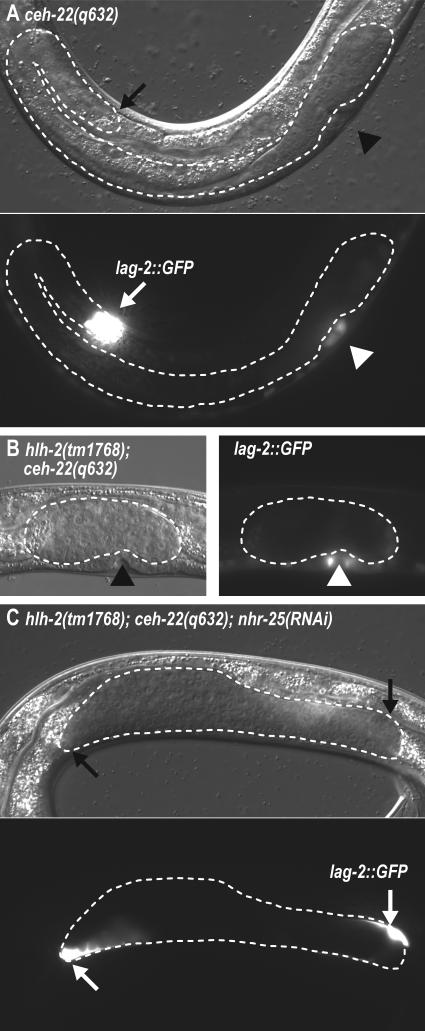
We next focused on the relative roles of HLH-2 and CEH-22 in hDTC specification. CEH-22 acts downstream of POP-1 and SYS-1 to specify the DTC fate (Lam et al., 2006), but its relationship to HLH-2 is unclear. We first examined an hlh-2; ceh-22 double mutant. Whereas hlh-2(tm1768) and ceh-22(q632) single mutants have only rare or partial hDTC loss (hlh-2: 3%; ceh-22: 58%), the vast majority of hlh-2(tm1768); ceh-22(q632) double mutants lacked hDTCs (93%, n=98), and leader function was completely eliminated (100% loss, n=98) (Figs. 2D, 6A, 6B, Table 4). This dramatic genetic enhancement suggests that HLH-2 and CEH-22 function in a common process to promote hDTC specification.
Fig 6.
hlh-2 genetically interacts with ceh-22/Nkx2-5. (A-C) L4 larvae grown at 25°C. All carry the lag-2::GFP reporter qIs57. Exposure times, 20 ms. Other conventions as in Fig. 2 legend. (A) ceh-22(q632). Anterior gonad arm has extended and reflexed normally, posterior gonad arm is missing. lag-2::GFP is bright in the anterior hDTC. (B) hlh-2(tm1768); ceh-22(q632). No gonad arms or hDTCs are observed. lag-2::GFP is faintly expressed in two cells at the posterior end of the somatic gonad. (C) hlh-2(tm1768); ceh-22(q632); nhr-25(RNAi). Partial elongation of posterior arm. Little or no anterior elongation. lag-2::GFP is bright in the hDTCs. (A, B) lag-2::GFP expression also observed in the vulva (arrowhead) and ventral nerve cord.
Table 4.
hlh-2 genetically interacts with ceh-22 in DTC specification
| Genotypea | hDTC present (%)b | Leader function present (%)c | nd |
|---|---|---|---|
| hlh-2 | 97 | 90 | 192 |
| ceh-22 | 42 | 40 | 78 |
| hlh-2; ceh-22 | 7 | 0 | 98 |
| nhr-25(RNAi)e | 100 | 100 | 184 |
| hlh-2; ceh-22; control RNAi | 12 | 6 | 80 |
| hlh-2; ceh-22; nhr-25(RNAi)e | 47 | 24 | 70 |
| ceh-22; control RNAi | 33 | 32 | 598 |
| ceh-22; nhr-25(RNAi)e | 76 | 68 | 256 |
| hlh-2; control RNAi | 98 | 91 | 104 |
| hlh-2; nhr-25(RNAi)e | 98 | 93 | 92 |
All hlh-2 mutants were homozygous for hlh-2(tm1768); other alleles as in Table 3; all strains included qIs57[lag-2::GFP; unc-119(+)]; animals grown at 25°C.
b.-d. As in Table 1.
These genotypes exhibited extra hDTCs: nhr-25(RNAi), 15% extra hDTCs and 10% extra arms. hlh-2; ceh-22; nhr-25(RNAi), 9% extra hDTCs and 0% extra arms. ceh-22; nhr-25(RNAi), 13% extra hDTCs and 6% extra arms. hlh-2; nhr-25(RNAi), 9% extra hDTCs and 0% extra arms.
We also compared the effects of ectopic HLH-2 and ectopic CEH-22 in pop-1 mutants. Whereas HLH-2 failed to drive hDTC production in pop-1 mutants (see above), ectopic CEH-22 produced hDTCs in most pop-1 gonads (79%, n=76) (Table 3), and many animals were fertile. Therefore, CEH-22 is likely to be the regulator missing in the pop-1 mutants with ectopic HLH-2, which reinforces the idea that CEH-22 and HLH-2 act together to drive the hDTC fate. We attempted to ask if ectopic CEH-22 could generate hDTCs in hlh-2(tm1768+RNAi) animals, but the heatshocked hlh-2(tm1768+RNAi); hs::ceh-22b larvae arrested, precluding this experiment.
POP-1 and other early hDTC regulators are not critical for hDTC leader function or lag-2 reporter expression
HLH-2 governs both hDTC specification and later hDTC functions (see above). To test whether POP-1, SYS-1, CEH-22 and NHR-25 similarly control later hDTC functions, we used weak alleles or RNAi to bypass hDTC specification defects. For pop-1(q624), sys-1(RNAi) and ceh-22(q632), some hDTCs were made (29-43%, each n >75), and in each case, hDTCs expressed lag-2::GFP with normal intensity (Fig. S2, data not shown) and most were capable of normal leader function (sys-1 RNAi: 97%, n=29; pop-1(q624): 97%, n=30; ceh-22(q632): 94%, n=33) (Fig. 6A, data not shown).
NHR-25 antagonizes POP-1/SYS-1, and nhr-25 RNAi generates extra hDTCs (Table 4; Asahina et al., 2006). As predicted, nhr-25 RNAi suppressed defects in hDTC specification in both ceh-22 and hlh-2; ceh-22 mutants (Fig. 6C, Table 4); nhr-25 RNAi also generated extra hDTCs in hlh-2 mutants (Table 4). However, nhr-25 RNAi did not affect leader function: hlh-2; nhr-25(RNAi) had hDTC leader defects similar to hlh-2 alone (Fig. 7C, data not shown). Furthermore, the extra hDTCs made in hlh-2; nhr-25(RNAi) mutants lacked leader function (data not shown). Therefore, NHR-25 does not antagonize HLH-2's role in leader function. A simple explanation is that nhr-25 acts to suppress hDTC specification defects in the hlh-2; ceh-22 double mutant largely through its effects on ceh-22, and that genetic interactions between NHR-25 and HLH-2 are indirect. We conclude that HLH-2 stands out among all the other regulators of hDTC specification in controlling later hDTC leader function and lag-2 expression.
Fig. 7.
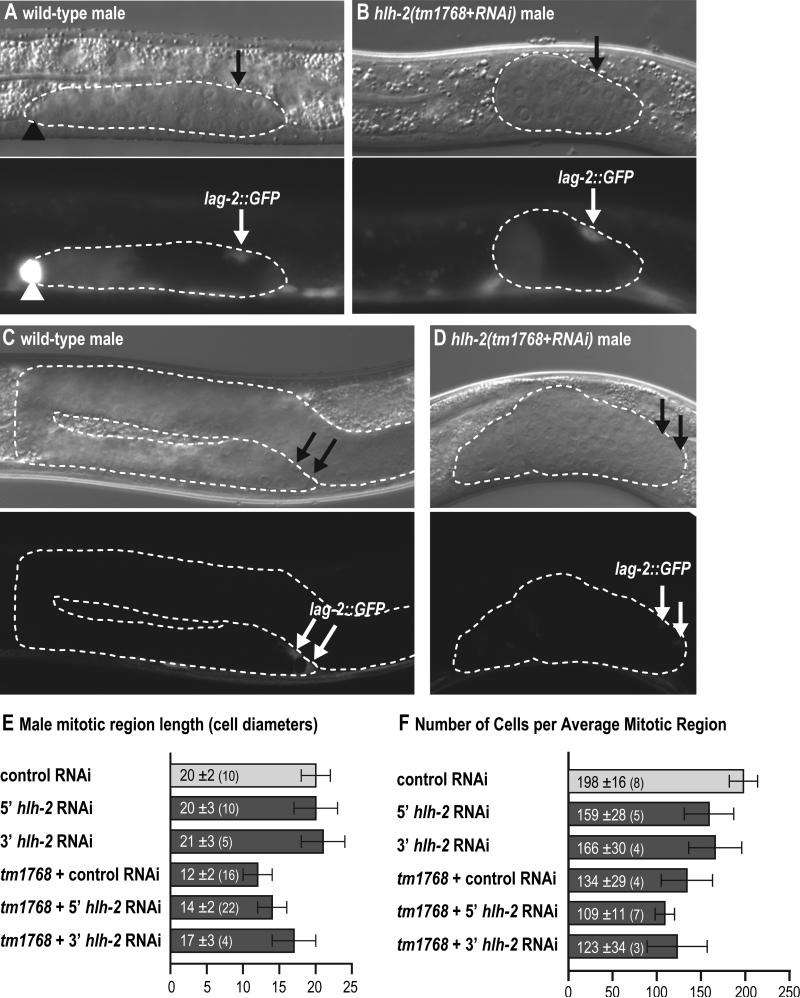
hlh-2 regulates linker cell specification, mDTC maintenance and male niche function. (A-F) Genotype: lag-2::GFP (qIs56); him-5. Conventions as in Fig. 2, except: arrowhead, linker cell. (A-D) L1-feeding RNAi treatment; maintained at 25°C. (E, F) L3-feeding RNAi treatment; maintained at 20°C until late L4 stage, then shifted to 25°C, scored 24 hours after L4. (A, B) Late L2/early L3 males. Exposure times: 20 ms with 30% digital gain. (A) Control-RNAi-treated wild-type male. Linker cell brightly expresses lag-2::GFP and has begun to elongate. mDTC faintly expresses lag-2::GFP. Other DTC not shown (out of the focal plane). (B) hlh-2(tm1768) mutant male treated with hlh-2 RNAi. No linker cell or gonad elongation is observed. mDTC faintly expresses lag-2::GFP. Other mDTC not shown (out of the focal plane). (C, D) Confocal images of early adult males. (C) Control-RNAi-treated wild-type male. Gonad arm is elongated. Two mDTCs present, both expressing lag-2::GFP. (D) hlh-2(tm1768) mutant treated with hlh-2 RNAi. Gonad arm is absent. Two mDTCs present, with reduced lag-2::GFP expression. (E) Quantification of male MR length (in cell diameters). MR length was significantly reduced in hlh-2(tm1768) males relative to control males. (F) Quantification of MR cell number, scored in germ lines with the mean MR length. The number of cells in the MR was significantly reduced in hlh-2(tm1768) males relative to control males.
hlh-2 effects on male gonadal regulatory cells
The experiments described above addressed HLH-2 function in hermaphrodites. We next asked if HLH-2 plays an equivalent role in males. As expected, control males expressed lag-2::GFP brightly in the linker cell (LC), which leads gonadal elongation and controls its shape, and faintly in the two mDTCs (Fig. 7A; Blelloch et al., 1999; Miskowski et al., 2001). By contrast, hlh-2(tm1768+RNAi) males grown at 25°C had no linker cell: the bright GFP-expressing cell was absent and gonads failed to elongate (n=37) (Fig. 7B). Therefore, HLH-2 is required for linker cell specification.
We also scored hlh-2(tm1768+RNAi) males for number of mDTCs and lag-2::GFP in mDTCs. Late L2 or early L3 males possessed two lag-2::GFP expressing mDTCs (96%, n=68 SGPs), as did males of the same stage subjected to control RNAi (100%, n=44 SGPs) (Figs. 7A, 7B). Therefore, we saw no evidence that HLH-2 is involved in mDTC specification. However, this negative result might be explained by a lower feeding RNAi efficacy in males (Timmons et al., 2001). To assess whether HLH-2 is sufficient for mDTC specification, we heatshocked hs::hlh-2 males to test for an increased number of mDTCs. No extra DTCs were found (n=42 SGPs). In these heat shock experiments, extra hDTCs in hermaphrodite siblings served as a control for treatment efficacy, and upregulation of intestinal lag-2::GFP served the same purpose in males (Fig. S3). The simplest interpretation is that HLH-2 is not sufficient for mDTC specification.
We examined the effect of HLH-2 on lag-2::GFP expression in both mid and late larval stage males. In L2s and early L3s, lag-2::GFP was insignificantly reduced in the hlh-2(tm1768+RNAi) mDTCs (Figs. 7A, 7B, S4A). However, by late L4 or early adulthood, lag-2::GFP average pixel intensity was reduced by 51% in hlh-2(tm1768+RNAi) mDTCs relative to control mDTCs (Figs. 7C, 7D, S4B). Indeed, some hlh-2(tm1768+RNAi) mDTCs were absent or undetectable at this stage (hlh-2: 29-42% missing, n=48 SGPs, control: 0-6% missing, n=34 SGPs). Therefore, HLH-2 appears to be required for lag-2::GFP expression and mDTC maintenance.
An hlh-2 transcriptional reporter is expressed in mDTCs
Due to the later onset of mDTC defects observed in hlh-2 depleted males, we generated an hlh-2::GFP transcriptional promoter to ask whether HLH-2 might be expressed in a sexually dimorphic manner. In hermaphrodites, this reporter was expressed soon after the hDTC was born and expression persisted throughout the life of the hDTC, consistent with previous observations (Karp and Greenwald, 2004; Krause et al., 1997). In males, hlh-2::GFP expression was not seen in the SGPs (Z1/Z4), but it was expressed in mDTCs after the first SGP division (Fig. S5A). Moreover, expression persisted throughout development and in adults (Fig. S5B). Therefore, hlh-2 expression is likely not sexually dimorphic.
hlh-2 controls mDTC niche function
The mDTC defects of hlh-2 depleted males and adult mDTC expression of the hlh-2 reporter suggested that HLH-2 might affect male niche function by analogy with its role in hermaphrodite niche function. To test this idea, we scored the size of germline mitotic regions in adult him-5 and hlh-2(tm1768); him-5 males, both fed from L3 with either empty RNAi vector or hlh-2 RNAi, as described above for hermaphrodites. To circumvent problems with gonadal arm migration, all males were maintained at 20°C until late L4 and then shifted to 25°C. The hlh-2(tm1768) mutants had a significantly smaller mitotic region than controls (Figs. 7E, 7F). Unlike in hermaphrodites, hlh-2(RNAi) did not enhance the mutant niche defect. Nonetheless, the mutant shows that HLH-2 plays a role in maintaining a normal-sized male mitotic region, much as it does in hermaphrodites. The simplest explanation is that HLH-2 affects the ability of the mDTC to maintain the mitotic region.
Discussion
HLH-2 is critical for function of the DTC niche
The C. elegans DTCs use Notch signaling to maintain germline stem cells and ensure germline self-renewal (Kimble and Crittenden, 2007). Previous work identified HLH-2/E/Daughterless as a positive regulator of hermaphrodite DTC specification (Karp and Greenwald, 2004). Here we confirm the role of HLH-2 in hDTC specification and find that HLH-2 is also critical in both sexes for maintenance of the DTC niche function. Thus, when HLH-2 is impaired in mid-larval animals, well after DTC specification, germline self-renewal is either reduced or abolished in adults even though the DTC is still present.
Previous studies showed that HLH-2 is expressed in the hDTC from its birth through adulthood (Karp and Greenwald, 2004; Krause et al., 1997). By contrast, in the germ line hlh-2 mRNA is detected by in situ hybridization only in maturing oocytes, which are far removed from the niche. Furthermore, hlh-2 germline expression requires MPK-1 activity (Leacock and Reinke, 2006), and mpk-1 mRNA is repressed in germ cells within the niche (Lee et al., 2007). Therefore, it is most likely that HLH-2 acts in the hDTC to promote germline self-renewal, rather than acting autonomously in the mitotic germ cells. Notably, HLH-2 is also required for robust lag-2 reporter expression in the hDTC. Furthermore, a lag-2 reporter lacking six E-box sites is expressed very weakly, suggesting that the HLH-2 control of lag-2 expression is likely direct. Because LAG-2 controls germline self-renewal (Lambie and Kimble, 1991), we suggest that HLH-2 controls germline self-renewal by its activation of lag-2 expression in hDTCs.
HLH-2 also appears to promote niche function in the male gonad: the mitotic region was significantly smaller in hlh-2(tm1768) males than in controls. In addition, HLH-2 depletion caused diminished lag-2::GFP expression in the late L4 or early adult male DTCs. As unpublished work has implicated LAG-2 in the mDTC niche function (K. Knobel, personal communication), perhaps HLH-2 also regulates lag-2 expression in mDTCs to mediate this effect. However, unlike in hermaphrodites, we did not detect a role for HLH-2 in male DTC specification, using both loss-of-function and overexpression approaches. This may indicate a sexually dimorphic role for HLH-2 in DTC specification. Alternatively, this result may be due in part to reduced efficacy of feeding RNAi in males (Timmons et al., 2001).
The HLH-2 roles in both DTC niche function and lag-2 expression bring together two themes of developmental control. Notch signaling has been implicated in stem cell controls in multiple tissues in addition to the C. elegans germ line (e.g. Androutsellis-Theotokis et al., 2006; Chiba, 2006; Molofsky et al., 2004; Porter and Calvi, 2008), and transcription factors in the E/Daughterless bHLH family are widely conserved transcription activators of DSL ligands (Bertrand et al., 2002). In lateral inhibition, Delta transcription is directly activated by Daughterless in Drosophila neural precursor cells (Heitzler et al., 1996; Hinz et al., 1994; Künisch et al., 1994), and a similar process occurs during mammalian neurogenesis (Bertrand et al., 2002). Our work finds that this positive cassette of bHLH transcription factors regulating DSL expression has been conserved in the C. elegans hDTC, and extends that cassette to stem cell control.
HLH-2 and CEH-22 act in a common process to specify hDTCs
Cell fate specification in multicellular organisms involves complex interplay between different sequence-specific DNA binding transcription factors that form highly interconnected gene regulatory networks (Levine and Davidson, 2005; Loose and Patient, 2004; Swiers et al., 2006). The gene regulatory network state of a given cell is modulated by external inputs from multiple signaling pathways (e.g. Wnt, Notch, TGF-β), as well as by cell history (Levine and Davidson, 2005; Loose and Patient, 2004; Swiers et al., 2006). Organogenesis involves the coordinated development of multiple tissues and hence the coordinated specification of cell fates. One example occurs in the larval C. elegans gonad, where specification of the distal tip cell also controls the concomitant expansion of the germline tissue and specification of the leader cell shapes the expanding gonad (Kimble and White, 1981).
Previous studies identified several regulators of hDTC specification, including POP-1/TCF, SYS-1/β-catenin, CEH-22/Nkx2.5 and NHR-25/SF-1/FtzF1. HLH-2 is expressed later than the others in the hDTC lineage (Asahina et al., 2006; Karp and Greenwald, 2004; Lam et al., 2006; Phillips et al., 2007; Siegfried et al., 2004), but the functional relationship between HLH-2 and these other factors had been unexplored. Thus, HLH-2 might be a downstream target of these transcription factors or it could act with them, perhaps to reinforce the decision. In support of a downstream role, the hlh-2 promoter contains candidate TCF and CEH-22 predicted binding sites (M. Chesney and J. Kimble, unpublished observations). However, HLH-2 overexpression did not rescue pop-1, sys-1, or ceh-22 mutants, which does not exclude the hlh-2 gene as a downstream target, but indicates that it is not the only critical target. Indeed, genetic interactions indicate that HLH-2 and CEH-22 act in a common process to promote hDTC specification. Thus, hypomorphic hlh-2 and ceh-22 alleles strongly enhance each other's hDTC defects.
Our working model has three parts. We suggest that POP-1/TCF and SYS-1/β-catenin activate CEH-22/Nkx2.5 to establish competence for DTC specification, that CEH-22 and HLH-2 work together to specify the hDTC fate in particular, and that HLH-2 continues to function within hDTCs to drive their full differentiation and then maintain their niche and leader functions. Several lines of evidence support this model. First, POP-1, SYS-1 and CEH-22 control the asymmetric SGP division that generates a daughter that is competent for DTC specification; in their absence, the SGP divides symmetrically to produce only proximal cell types, and the distal cell types (e.g. DTCs) are lost (Miskowski et al., 2001; Siegfried and Kimble, 2002; Siegfried et al., 2004). Second, HLH-2 first appears after the asymmetric SGP division soon after the birth of the presumptive hDTC (Karp and Greenwald, 2004). Third, the ceh-22 and hlh-2 genes interact genetically (this work). Fourth, CEH-22 can drive hDTC specification in pop-1 mutants, but HLH-2 cannot (this work). Fifth, SYS-1, POP-1 and CEH-22 are not critical for later lag-2 expression or leader function. However, following hDTC specification, HLH-2 promotes hDTC differentiation by controlling lag-2 and gon-1 expression, thereby promoting both niche and leader functions (this work; Tamai and Nishiwaki, 2007). Further studies will be required to elaborate this model and elucidate the molecular mechanisms underlying hDTC specification and function.
HLH-2 promotes leader cell specification in both sexes
The leader cell is the hDTC in hermaphrodites and the LC in males. In addition to its role in hDTC specification (Karp and Greenwald, 2004; this work), we have found that HLH-2 specifies the male LC. Therefore, HLH-2 is a key regulator of leader cell specification in both sexes. The hDTC and LC have distinct lineage origins (Figs. 1A, 1B) and their other specification regulators are distinct. SYS-1/β-catenin, POP-1/TCF, and CEH-22/Nkx2.5 control hDTC specification, while LIN-12/Notch-mediated lateral signaling controls LC specification (Greenwald et al., 1983; Kimble, 1981). Indeed, the LC is analogous to the hermaphrodite anchor cell (AC) with respect to lineage (Figs. 1A, 1B), specification by LIN-12/Notch-mediated lateral inhibition (Greenwald et al., 1983; Kimble, 1981), specification by HLH-2 (Karp and Greenwald, 2003; Karp and Greenwald, 2004; this work), and function in connecting the gonadal lumen with the animal's exterior (Kimble and Hirsh, 1979).
Thus, HLH-2 acts in different cellular contexts within the developing gonad to promote specification or maintenance of diverse cell types. How HLH-2 achieves these diverse functions is unclear, but one idea is the use of different binding partners. MIG-24, a bHLH transcription factor, can dimerize with HLH-2 to control leader function in both hermaphrodites and males (Tamai and Nishiwaki, 2007). However, MIG-24 has no reported role in hDTC specification, LC specification or control of lag-2 expression (Tamai and Nishiwaki, 2007). Therefore, distinct bHLH partners may be identified for those functions. Identification of HLH-2 partners critical for hDTC specification, DTC niche function and LC specification remain a challenge for the future, and may reveal a combinatorial network of control that modulates the gonadogenesis program.
Supplementary Material
Fig. S1. Conserved E-box sites in the lag-2 5’ flanking sequence from four Caenorhabditis species. (A) Schematic diagram of the lag-2 promoter region extending 3.2 kb upstream from the translational start codon. Horizontal line, lag-2 promoter DNA. Vertical lines, locations of E-box sites. Numbered vertical lines, conserved E-box sites 1, 2, and 3. Scale bar, 500 bp. (B-D) Conserved E-box sites 1, 2, and 3. Alignments of lag-2 promoter sequence between C. elegans (Ce), C. brenneri (Cbn), C. remanei (Cr), and C. briggsae (Cbg). Purple sequence, E-box sites. Gray highlight, nucleotide identity.
Fig. S2. lag-2::GFP expression is not affected in hDTCs specified in sys-1 RNAi or pop-1(q624). (A, B) Quantification of lag-2::GFP pixel intensity in the hDTCs. Normalized mean pixel intensity +/− standard error quantified from fluorescent hDTC images of late L3 larvae or early L4 larvae. Parentheses, number of hDTCs scored. (A) Quantification of lag-2::GFP expression in the hDTCs of control RNAi versus sys-1 RNAi-treated larvae. Animals carried qIs57 and maintained at 20°C. (B) Quantification of lag-2::GFP expression in the hDTCs of wild-type versus pop-1(q624) larvae. Animals carried qIs56 and maintained at 25°C.
Fig. S3. Heatshock induced hs::hlh-2 expression causes lag-2::GFP upregulation in the male intestine. (A-C) him-5 male L3/L4 larvae, carrying the lag-2::GFP reporter qIs56. Maintained at 20°C. (A,B) Also carry arIs63 (hs::hlh-2), a heatshock-inducible hlh-2 transgene. (A) hs::hlh-2 male without heat shock. LC robustly expresses lag-2::GFP (arrow). No fluorescence in the intestine. (B) hs::hlh-2 male after heatshock and then maintained at 20°C. LC robustly expresses lag-2::GFP (arrow). Bright ectopic fluorescence observed in the intestine (arrowheads). (C) Control male after heatshock and then maintained at 20°C. LC robustly expresses lag-2::GFP (arrow). No fluorescence in the intestine.
Fig. S4. hlh-2 regulates lag-2::GFP expression in late L4 or early adult mDTCs. (A, B) Quantification of lag-2::GFP pixel intensity in the mDTCs. Normalized mean pixel intensity +/− standard error quantified from fluorescent mDTCs. Parentheses, number of mDTCs scored. L1-feeding RNAi treatment, maintained at 25°C. Genotypes, qIs56 him-5 control RNAi versus hlh-2(tm1768); qIs56 him-5; 3’ hlh-2 RNAi. (A) Late L2 or early L3 males. (B) Late L4 or early adult males.
Fig. S5. hlh-2::GFP is expressed in the mDTC and linker cell. (A, B) DIC and fluorescent images of qEx730 males, which carry an hlh-2::GFP transcriptional reporter (see Methods). Conventions as in Figure 7. (A) Early L2 male gonad. Left, DIC. Right, fluorescence. hlh-2::GFP is expressed in the somatic gonad, including the LC and mDTCs. One mDTC is out of the focal plane. (B) Extruded adult male gonad. Top, DIC. Bottom, fluorescence. hlh-2::GFP is expressed in the mDTCs.
Acknowledgments
The authors thank Julie Ahringer, Iva Greenwald, Josh Kaplan, Shohei Mitani and the Japanese National BioResource Project for strains and reagents, and Xantha Karp and Karla Knobel for sharing unpublished information. We also thank Peggy Kroll-Conner, Jadwiga Forster and David Rivers for technical assistance, and Anne Helsley-Marchbanks and Laura Vanderploeg for assistance in preparation of the manuscript and figures. B.T.P. was supported by NIH postdoctoral fellowship GM075598, D.E.M. by a NIH training grant in Molecular Biosciences (T32 GM07215) and N.L. by the Damon Runyon Cancer Research Foundation. NIH R01 GM069454 to J.K. also supported this work. J.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–6. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Asahina M, Valenta T, Silhankova M, Korinek V, Jindra M. Crosstalk between a nuclear receptor and β-catenin signaling decides cell fates in the C. elegans somatic gonad. Developmental Cell. 2006;11:203–11. doi: 10.1016/j.devcel.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–30. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–590. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Santa Anna-Arriola S, Gao D, Li Y, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Developmental Biology. 1999;216:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24:2437–47. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Molecular Biology of the Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden SL, Troemel ER, Evans TC, Kimble J. GLP-1 is localized to the mitotic region of the C. elegans germ line. Development. 1994;120:2901–2911. doi: 10.1242/dev.120.10.2901. [DOI] [PubMed] [Google Scholar]
- Greenwald IS, Sternberg PW, Horvitz HR. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell. 1983;34:435–444. doi: 10.1016/0092-8674(83)90377-x. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Bourouis M, Ruel L, Carteret C, Simpson P. Genes of the Enhancer of split and achaete-scute complexes are required for a regulatory loop between Notch and Delta during lateral signaling in Drosophila. Development. 1996;122:161–171. doi: 10.1242/dev.122.1.161. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Gao D, Lambie EJ, Kimble J. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development. 1994;120:2913–2924. doi: 10.1242/dev.120.10.2913. [DOI] [PubMed] [Google Scholar]
- Hinz U, Giebel B, Campos-Ortega JA. The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell. 1994;76:77–87. doi: 10.1016/0092-8674(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. Appendix 1. Genetics. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. pp. 881–1047. [Google Scholar]
- Karp X, Greenwald I. Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans. Genes Dev. 2003;17:3100–11. doi: 10.1101/gad.1160803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp X, Greenwald I. Multiple roles for the E/Daughterless ortholog HLH-2 during C. elegans gonadogenesis. Dev Biol. 2004;272:460–9. doi: 10.1016/j.ydbio.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Kidd AR, III, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A ®-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–772. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Kimble J. Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Developmental Biology. 1981;87:286–300. doi: 10.1016/0012-1606(81)90152-4. [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annual Review of Cell and Developmental Biology. 2007;23:405–433. doi: 10.1146/annurev.cellbio.23.090506.123326. [DOI] [PubMed] [Google Scholar]
- Kimble J, Hirsh D. The postembryonic cell lineages of the hermaphrodite and male gonads in Caenorhabditis elegans. Developmental Biology. 1979;70:396–417. doi: 10.1016/0012-1606(79)90035-6. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Developmental Biology. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Krause M, Park M, Zhang J-M, Yuan J, Harfe B, Xu S-Q, Greenwald I, Cole M, Paterson B, Fire A. A C. elegans E/Daughterless bHLH protein marks neuronal but not striated muscle development. Development. 1997;124:2179–2189. doi: 10.1242/dev.124.11.2179. [DOI] [PubMed] [Google Scholar]
- Künisch M, Haenlin M, Campos-Ortega JA. Lateral inhibition mediated by the Drosophila neurogenic gene Delta is enhanced by proneural proteins. Proc. Natl. Acad. Sci. USA. 1994;91:10139–10143. doi: 10.1073/pnas.91.21.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Current Biology. 2006;16:287–95. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie EJ, Kimble J. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development. 1991;112:231–240. doi: 10.1242/dev.112.1.231. [DOI] [PubMed] [Google Scholar]
- Leacock SW, Reinke V. Expression profiling of MAP kinase-mediated meiotic progression in Caenorhabditis elegans. PLoS Genet. 2006;2:e174. doi: 10.1371/journal.pgen.0020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Hook B, Pan G, Kershner AM, Merritt C, Seydoux G, Thomson JA, Wickens M, Kimble J. Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet. 2007;3:e233. doi: 10.1371/journal.pgen.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M, Davidson EH. Gene regulatory networks for development. Proc Natl Acad Sci U S A. 2005;102:4936–42. doi: 10.1073/pnas.0408031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose M, Patient R. A genetic regulatory network for Xenopus mesendoderm formation. Developmental Biology. 2004;271:467–78. doi: 10.1016/j.ydbio.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Mello C, Fire A. DNA transformation. In: Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press, Inc.; San Diego: 1995. pp. 451–482. [Google Scholar]
- Miskowski J, Li Y, Kimble J. The sys-1 gene and sexual dimorphism during gonadogenesis in Caenorhabditis elegans. Developmental Biology. 2001;230:61–73. doi: 10.1006/dbio.2000.9998. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Morrison SJ. Diverse mechanisms regulate stem cell self-renewal. Curr Opin Cell Biol. 2004;16:700–7. doi: 10.1016/j.ceb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kidd AR, III, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/β-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3231–3236. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RL, Calvi LM. Communications between bone cells and hematopoietic stem cells. Arch Biochem Biophys. 2008;473:193–200. doi: 10.1016/j.abb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman DS, Emmons SW. The basic helix-loop-helix transcription factors LIN-32 and HLH-2 function together in multiple steps of a C. elegans neuronal sublineage. Development. 2000;127:5415–26. doi: 10.1242/dev.127.24.5415. [DOI] [PubMed] [Google Scholar]
- Siegfried K, Kimble J. POP-1 controls axis formation during early gonadogenesis in C. elegans. Development. 2002;129:443–453. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Kidd AR, III, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the C. elegans gonad. Genetics. 2004;166:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiers G, Patient R, Loose M. Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Developmental Biology. 2006;294:525–40. doi: 10.1016/j.ydbio.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Tamai KK, Nishiwaki K. bHLH Transcription factors regulate organ morphogenesis via activation of an ADAMTS protease in C. elegans. Dev Biol. 2007 doi: 10.1016/j.ydbio.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Tax FE, Yeargers JJ, Thomas JH. Sequence of C. elegans lag-2 reveals a cell-signalling domain shared with Delta and Serrate of Drosophila. Nature. 1994;368:150–154. doi: 10.1038/368150a0. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–12. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Voutev R, Hubbard EJ. A “FLP-Out” system for controlled gene expression in Caenorhabditis elegans. Genetics. 2008;180:103–19. doi: 10.1534/genetics.108.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson HA, Fitzgerald K, Greenwald I. Reciprocal changes in expression of the receptor lin-12 and its ligand lag-2 prior to commitment in a C. elegans cell fate decision. Cell. 1994;79:1187–1198. doi: 10.1016/0092-8674(94)90010-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Conserved E-box sites in the lag-2 5’ flanking sequence from four Caenorhabditis species. (A) Schematic diagram of the lag-2 promoter region extending 3.2 kb upstream from the translational start codon. Horizontal line, lag-2 promoter DNA. Vertical lines, locations of E-box sites. Numbered vertical lines, conserved E-box sites 1, 2, and 3. Scale bar, 500 bp. (B-D) Conserved E-box sites 1, 2, and 3. Alignments of lag-2 promoter sequence between C. elegans (Ce), C. brenneri (Cbn), C. remanei (Cr), and C. briggsae (Cbg). Purple sequence, E-box sites. Gray highlight, nucleotide identity.
Fig. S2. lag-2::GFP expression is not affected in hDTCs specified in sys-1 RNAi or pop-1(q624). (A, B) Quantification of lag-2::GFP pixel intensity in the hDTCs. Normalized mean pixel intensity +/− standard error quantified from fluorescent hDTC images of late L3 larvae or early L4 larvae. Parentheses, number of hDTCs scored. (A) Quantification of lag-2::GFP expression in the hDTCs of control RNAi versus sys-1 RNAi-treated larvae. Animals carried qIs57 and maintained at 20°C. (B) Quantification of lag-2::GFP expression in the hDTCs of wild-type versus pop-1(q624) larvae. Animals carried qIs56 and maintained at 25°C.
Fig. S3. Heatshock induced hs::hlh-2 expression causes lag-2::GFP upregulation in the male intestine. (A-C) him-5 male L3/L4 larvae, carrying the lag-2::GFP reporter qIs56. Maintained at 20°C. (A,B) Also carry arIs63 (hs::hlh-2), a heatshock-inducible hlh-2 transgene. (A) hs::hlh-2 male without heat shock. LC robustly expresses lag-2::GFP (arrow). No fluorescence in the intestine. (B) hs::hlh-2 male after heatshock and then maintained at 20°C. LC robustly expresses lag-2::GFP (arrow). Bright ectopic fluorescence observed in the intestine (arrowheads). (C) Control male after heatshock and then maintained at 20°C. LC robustly expresses lag-2::GFP (arrow). No fluorescence in the intestine.
Fig. S4. hlh-2 regulates lag-2::GFP expression in late L4 or early adult mDTCs. (A, B) Quantification of lag-2::GFP pixel intensity in the mDTCs. Normalized mean pixel intensity +/− standard error quantified from fluorescent mDTCs. Parentheses, number of mDTCs scored. L1-feeding RNAi treatment, maintained at 25°C. Genotypes, qIs56 him-5 control RNAi versus hlh-2(tm1768); qIs56 him-5; 3’ hlh-2 RNAi. (A) Late L2 or early L3 males. (B) Late L4 or early adult males.
Fig. S5. hlh-2::GFP is expressed in the mDTC and linker cell. (A, B) DIC and fluorescent images of qEx730 males, which carry an hlh-2::GFP transcriptional reporter (see Methods). Conventions as in Figure 7. (A) Early L2 male gonad. Left, DIC. Right, fluorescence. hlh-2::GFP is expressed in the somatic gonad, including the LC and mDTCs. One mDTC is out of the focal plane. (B) Extruded adult male gonad. Top, DIC. Bottom, fluorescence. hlh-2::GFP is expressed in the mDTCs.