Abstract
Objective
To examine the relationships between TNF-α, obesity, and insulin resistance among prepubescent children.
Design
Cross-sectional study.
Setting and subjects
Data were collected from 112 nondiabetic Latino schoolchildren from public schools in three South Florida communities. Of the enrolled participants, 43.8% were obese (BMI 95th percentile) and 51.8% presented with a family history of type 2 diabetes mellitus (T2DM). With one exception, all demonstrated normal glucose tolerance.
Interventions
Plasma TNF-α levels were determined with enzyme-linked immunosorbance assay (ELISA). Homeostasis model assessment (HOMA-IR) was calculated as an index of insulin resistance. Mean levels of TNF-α among obese vs nonobese children were compared with a one-way analysis of variance with two groups, and the association between TNF-α and HOMA-IR was assessed with a Pearson's correlation.
Results
Higher circulating TNF-α levels were revealed among nonobese vs obese children. Nonobese girls demonstrated higher TNF-α levels than obese girls, whereas there were no significant differences for boys. There were no significant differences after stratifying for family history of T2DM. There was a modest relationship between increased TNF-α levels and decreased insulin resistance.
Conclusions
The observed elevated circulating TNF-α concentrations among leaner participants may reflect an inflammatory process that has been associated with higher levels of physical fitness in both adults and prepubescent children. This effect may remain stronger for prepubescent girls, and the mechanism may be attenuated by the hormonal changes that occur with the onset of puberty.
Keywords: obesity, TNF-α, children, insulin resistance
Introduction
Recent data indicate that obesity increased to approximately 20% of all US children between 1973 and 1994, with 24 and 11% of children placing above the 85th and 95th reference percentiles of body mass index (BMI), adjusted for age and sex, respectively, with disproportionate representation among Latino and African-American children (DiMartino-Nardi, 1999; Goran, 2001; Ludwig et al, 2001). However, the increased prevalence of obesity failed to remain confined within the United States, and has reached epidemic proportions worldwide. One significant complication of obesity includes the strong association with insulin resistance, which represents the greatest risk factor for type 2 diabetes mellitus (T2DM).
The cytokine TNF-α appears to play a key role in the pathogenesis of peripheral insulin resistance in obesity. While TNF-α production by adipocytes typically appears to regulate adipocyte function and limit increases in fat mass (Skolnik & Marcusohn, 1996; Prins et al, 1997), overproduction of TNF-α by adipocytes seems to contribute to insulin resistance in obesity and in T2DM by inhibiting insulin receptor signaling and glucose transport in skeletal muscle and other insulin-sensitive tissues (Hotamisligil et al, 1995; Kern et al, 1995; Uysal et al, 1997), at least in part by inhibiting tyrosine kinase activity of the insulin receptor in fat and muscle tissue via stimulation of the p55 TNF receptor (Peraldi et al, 1997). In fact, the ligand TNF-α is overexpressed by adipose tissue in obese rodents and adult humans. Neutralization of TNF-α improves insulin sensitivity of obese-diabetic animals, and the absence of TNF-α significantly improves insulin sensitivity in both diet-induced and genetic (ob/ob) models of obesity (Uysal et al, 1997). These effects appear sex dependent, in that TNF-α seems to contribute to insulin resistance and endothelial dysfunction more for male rather than female obese patients (Pfeiffer et al, 1997; Winkler et al, 1999). However, the molecular mechanisms remain poorly understood.
Although higher TNF-α levels have been associated with increased obesity and insulin resistance in adult studies and animal models of obesity, these relationships remain unknown among prepubescent children. We aimed to examine the relationship between plasma TNF-α concentrations and insulin resistance, and to determine TNF-α levels among obese vs nonobese school-aged children.
Methods
Participants
Letters were sent to parents of all children in grades K–3 (N=2519) who attended three elementary schools in South Florida. The letter described a new health program offered by a mobile clinic that regularly visited the schools. The program offered a physical examination by a pediatrician, a blood test, interviewed parents regarding health habits and history, and provided feedback to parents regarding their child's health. In addition, children received a $20 gift coupon (for toys or movies) for their participation at the conclusion of the assessments. Children were excluded from the study participation if they had a chronic disease (eg, diabetes, cystic fibrosis, renal disease, liver disease), a diagnosed endocrine or hormonal cause for obesity, or medications that could affect glucose tolerance (eg, dilantin, corticosteroids, diuretics, β-blockers). The study protocol was approved by the University of Miami School of Medicine Institutional Review Board, and informed child assent and parental consent was obtained from the child participants and their parents, respectively. Parents of 537 children returned letters, with 415 indicating interest in study participation. Of these, 122 children were randomly selected and subsequently enrolled into the study, at which point we met our previously established recruitment goal of 120 participants, and therefore, discontinued our recruitment procedures. In all, 10 children were not able to complete the blood sampling procedure. As was consistent with the demographics of this geographical region of South Florida, the final sample consisted of 112 prepubertal Latino children in the age range 5–10 y (grades K-3) who completed all study procedures.
Measures
Body composition was assessed with several measures, including height (cm), weight (kg), body mass index (BMI, kg (m2)−1), and waist circumference (cm). Fasting plasma glucose, insulin, lipids, and TNF-α were determined from fasting baseline blood collection from a standardized oral glucose tolerance test (OGTT) administration (Owens et al, 1979). Enzyme-linked immunosorbance assay (ELISA) was used to determine fasting plasma TNF-α concentrations according to recommendations from the manufacturer (Immunotech, Beckman-Coulter, Westbrook, ME, USA), with standards assayed in duplicate. The sensitivity for the cytokine determinations was less than 10 pg ml−1. The Homeostasis Model Assessment (HOMA-IR) method was used to derive an estimate of insulin sensitivity, for example, via the mathematical modeling of fasting plasma glucose and insulin concentrations with the formula: fasting insulin (U ml−1) × fasting glucose (mmol l−1) divided by 22.5 (Bonora et al, 2000). Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity (Bonora et al, 2000). Higher HOMA scores denoted lower insulin sensitivity (greater insulin resistance).
Statistical analysis
All statistical analyses were performed using SPSS software (version 10.5). Data are expressed as mean ± s.e. A one-way analysis of variance with two groups was used to compare the mean levels of TNF-α among obese vs nonobese children, and to evaluate the combined effect of gender and obesity on TNF-α concentrations. The relationship between TNF-α and HOMA-IR, each expressed as continuous data, was assessed with a Pearson's correlation. While the grouped variables were approximately normally distributed, such that no transformations were necessary, the ungrouped TNF-α and HOMA-IR variables were log transformed to correct for non-normality prior to entrance into the univariate analysis.
Results
This study included 57 boys and 55 girls, of whom 43. 8% met criteria for obesity at the 95th percentile (adjusted for age and gender) (Hammer et al, 1991) and 52.7% presented with a family history of T2DM (for both first- and second-degree relatives), as determined by physician interview (Mitchell et al, 1993). The detailed clinical and laboratory characteristics of the obese vs nonobese children have been described in detail elsewhere (Delamater et al, 2001). Briefly, and as expected, obese children demonstrated greater insulin resistance, higher diastolic and systolic blood pressures, higher fasting glucose, triglyceride, and VLDL cholesterol levels, and lower HDL cholesterol levels than nonobese children. With one exception, all children demonstrated glucose tolerance within normal limits.
Surprisingly, the initial ANOVA revealed that nonobese children demonstrated significantly higher circulating TNF-α levels when compared with obese children (mean 80.50 (s.e. 8.83) vs 40.60 (6.02) pg ml−1, P = 0.001). Owing to this unexpected major finding, post hoc 2×2 ANOVAs were conducted to examine potential gender or family history effects regarding obesity and TNF-α levels. Regarding gender, girls demonstrated slightly higher yet statistically insignificant TNF-α levels when compared with boys (69.68 (s.e. 8.64) vs 51.18 (6.18) pg ml−1, P = 0.081). As depicted in Figure 1, when we further stratified for obesity, a significant interaction effect was found, in that nonobese girls demonstrated significantly higher circulating TNF-α levels than obese girls (91.99 (s.e. 9.30) vs 37.80 (11.11), P = 0.000) and nonobese boys (91.99 (s.e. 9.30) vs 58.03 (9.30), P = 0.025). None of the other group comparisons were significant. Regarding family history of T2DM, there were no significant overall effects for family history of T2DM, nor was there a significant obesity × family history interaction. Finally, elevated TNF-α levels demonstrated a modest association with decreased insulin resistance (r=−0.19, P = 0.059).
Figure 1.
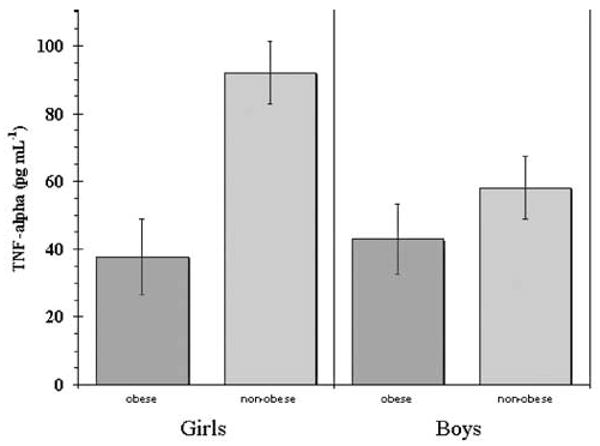
TNF-α in obese vs nonobese girls and boys. Error bars = s.e.
Discussion
Our finding that nonobese vs obese children demonstrated elevated plasma concentrations of TNF-α were striking, in that these results differed from observations by others that showed a relationship between elevated TNF-α levels and increased obesity among human children and adults, as well as rodent models (Chu et al, 2002; Nemet et al, 2003). Potential explanations for this discrepancy include differences in age, body weight, body fat mass, mixed pubertal stages, and mixed ethnic groups among the studies. Our study was unique in that we included younger children who were homogeneous with regard to both pubertal development (eg, all Tanner Stage I) and ethnicity. In fact, to our knowledge, our investigation represents the first study that evaluated TNF-α levels in a relatively homogeneous sample of Latino children, a population that has been identified at high risk for both obesity and T2DM (DiMartino-Nardi, 1999; Nemet et al, 2003).
Importantly, while all participants were younger than 11 y of age and were assessed as prepubertal, the influence that peripubertal hormonal secretions may have had on our results remains undetermined. Our findings could have been influenced, at least in part, by the pulsatile hormonal surges that have been associated with the peripubertal period, which occurs from several months to 1 or 2 y prior to the appearance of secondary sex characteristics, lasts for several years, and during which time different parts of the endocrine system mature (Grumbach & Styne, 1998). Moreover, gonadol steroids, growth hormone (GH), insulin-like growth factor-1 (IGF-I), and insulin levels all increase throughout puberty at the same time as percent body fat increases in girls and decreases in boys (Grumbach & Styne, 1998). As sex steroids, leptin, and IGF-I have been determined to mediate, at least in part, the various physical changes that occur during the pubertal transition (eg, accelerated growth, dimorphic increase in fat, muscle, and bone mass), an analogous complex relationship may also exist between these regulators, the increase in fat and musculoskeletal development, and cytokines such as TNF-α. This effect may partially explain the more pronounced difference between TNF-α concentrations in the nonobese vs obese girls within our study sample. However, the observed lower TNF-α levels in the boys vs the girls remained consistent with observations by others that have revealed significantly lower TNF-α levels in male compared with female nondiabetic adult humans (Pfeiffer et al. 1997). Also, the stronger effect that we found for girls vs boys remains consistent with associations found by others regarding stronger associations between TNF-R1 and leptin levels for girls vs boys (Chu et al, 2002).
It is important to note that TNF-α is a cytokine that is produced primarily by monocytes and macrophages, with a key role in inflammatory responses, in addition to the effects in other tissues, including adipocytes (Hotamisligil et al, 1995; Kern et al, 1995; Bazzoni & Buetler, 1996; Prins et al, 1997). In fact, the proinflammatory cytokines, including TNF-α, have been shown to increase with exercise in both healthy adults and prepubertal children (Scheett et al, 1999; Nemet et al, 2002). These observations, together with our finding that the participants in our study were free from current or recent infection (data not shown), provide further evidence that elevated circulating TNF-α levels do not necessarily implicate pathology. In this regard, one potential yet untested explanation for our observation that the nonobese vs obese participants in our study demonstrated significant elevations in TNF-α may relate to observations by others regarding higher levels of physical fitness, decreased percent body fat, increased muscle mass, and higher circulating levels of TNF-α in both healthy adults and prepubertal children (Nemet et al, 2002, 2003). As such, it is reasonable to speculate that the leaner participants in our study were more physically fit than the more obese children.
Our conclusions remain limited by the lack of developmental normative cytokine values for either human children or ‘pre-adult’ rodent populations. Moreover, we did not investigate potential differences between plasma TNFR60 or TNFR80 levels, or mRNA production of TNF receptors and ligands from adipocytes. Also, given the relatively small sample size and somewhat limited participant rate, the results of this study should be considered preliminary in nature and therefore, interpreted with caution. The results were derived from a population of prepubescent Latino children who resided in South Florida. While the study population remained heterogeneous with regard to gender, obesity, and family history of T2DM, one cannot generalize these research findings to other populations, such as non-Latino children, children who reside elsewhere in the United States, or in other countries, or pubertal or postpubescent children, among others. Nonetheless, we provided important preliminary data regarding TNF-α associations with obesity in prepubescent children. Further research needs to replicate these findings with other pediatric human and animal populations. Moreover, additional investigations are needed to determine if circulating TNF-α levels change during the course of development, or in response to variations physical fitness level or obesity that begins in childhood and persists into adulthood. Finally, additional research is needed to evaluate if this potential effect remains stronger for female than male subjects, as well as potential implications for insulin resistance and T2DM throughout the lifespan.
Acknowledgments
This study was funded in part by a National Research Service Award (NRSA) from the National Institutes of Health (NIH). Dr Denise Dixon held an NIH NRSA postdoctoral fellowship with the University of Miami (#5T32 MH18917-09). We acknowledge Ms Espi Perez, RN, who assisted with the data collection phase of the study.
Sponsorship: This research was supported, in part, by a clinical research grant from the American Diabetes Association, as well as NIMH NRSA # 5T32 MH18917-09.
Footnotes
Guarantor: D Dixon.
Contributors: DD designed the component of the study related to the TNF findings, performed all of the cytokine assays and statistical analyses, interpreted the data, and wrote the manuscript. RG and AD were the primary investigators and designed the main study, obtained funding and ethical approval, supervised the running of the study, managed the data collection, contributed to the writing, and provided final approval of the manuscript. AD supervised the data entry and cleaning. AD, RG, and NS contributed to the interpretation of the data. NS obtained funding for the cytokine assays and contributed to the final approval of the manuscript.
References
- Bazzoni F, Buetler B. The tumor necrosis factor ligand and receptor families. N Engl J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- Chu NF, Shen MH, et al. Plasma TNF-R1 and insulin concentrations in relation to leptin levels among normal and overweight children. Clin Biochem. 2002;35:287–292. doi: 10.1016/s0009-9120(02)00314-4. [DOI] [PubMed] [Google Scholar]
- Delamater A, Brito A, et al. Obesity and family history increase metabolic risk in Hispanic children. Ann Behav Med. 2001;23(Suppl):S130. [Google Scholar]
- DiMartino-Nardi J. Premature adrenarche: findings in pre-pubertal African-American and Caribbean-Hispanic girls. Acta Paediatrica. 1999;433(Suppl):67–72. doi: 10.1111/j.1651-2227.1999.tb14406.x. [DOI] [PubMed] [Google Scholar]
- Goran MI. Metabolic precursors and effects of obesity in children: a decade of progress, 1990–1999. Am J Clin Nutr. 2001;73:158–171. doi: 10.1093/ajcn/73.2.158. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Styne DM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Textbook of Endocrinology. Philadelphia, PA: Saunders; 1998. pp. 1509–1625. [Google Scholar]
- Hammer LD, Kraemer HC, et al. Standardized percentile curves of body-mass index for children and adolescents. Am J Dis Child. 1991;145:259–263. doi: 10.1001/archpedi.1991.02160030027015. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, et al. Increased adipose tissue expression of tumor necrosis factor-[alpha] in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern PA, Saghizadeh M, et al. The expression of tumor necrosis factor in human adipose tissue: regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Peterson KE, et al. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Valdez R, et al. Differences in the prevalence of diabetes and impaired glucose tolerance according to maternal or paternal history of diabetes. Diabetes Care. 1993;16:1262–1267. doi: 10.2337/diacare.16.9.1262. [DOI] [PubMed] [Google Scholar]
- Nemet D, Oh Y, et al. Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics. 2002;110:681–689. doi: 10.1542/peds.110.4.681. [DOI] [PubMed] [Google Scholar]
- Nemet D, Wang P, et al. Adipocytokines, body composition, and fitness in children. Pediatric Res. 2003;53:148–152. doi: 10.1203/00006450-200301000-00025. [DOI] [PubMed] [Google Scholar]
- Owens DR, Wragg KG, et al. Comparison of the metabolic response to a glucose tolerance test and a standardized test meal and the response to serial test meals in normal healthy subjects. Diabetes Care. 1979;2:409–413. doi: 10.2337/diacare.2.5.409. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Xu M, et al. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Investig. 1997;100:1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Janott J, et al. Circulating tumor necrosis factor [alpha] is elevated in male but not in female patients with type II diabetes mellitus. Hormone Metab Res. 1997;29:111–114. doi: 10.1055/s-2007-979001. [DOI] [PubMed] [Google Scholar]
- Prins JB, Niesler CU, et al. Tumor necrosis factor-[alpha] induces apoptosis of human adipose cells. Diabetes. 1997;46:1939–1944. doi: 10.2337/diab.46.12.1939. [DOI] [PubMed] [Google Scholar]
- Scheett TP, Milles PJ, et al. Effect of exercise on cytokines and growth mediators in prepubertal children. Pediatric Res. 1999;46:429–434. doi: 10.1203/00006450-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Skolnik E, Marcusohn J. Inhibition of insulin receptor signaling by TNF: potential role in obesity and non-insulin-dependent diabetes mellitus. Cytokine Growth Factor Rev. 1996;7:161–173. doi: 10.1016/1359-6101(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Uysal K, Wiesbrock S, et al. Protection from obesity-induced insulin resistnace in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Winkler G, Lakatos P, et al. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabetes Med. 1999;16:207–211. doi: 10.1046/j.1464-5491.1999.00052.x. [DOI] [PubMed] [Google Scholar]


