Convergence of excitatory and inhibitory afferents is common in the central nervous system. Single-unit recordings of non-primary sensory neurons often reflect the topography of this convergence in the existence of overlapping or contiguous receptive fields of opposite sign. For example, it has been observed that single-unit excitatory response areas are accompanied by inhibitory surrounds in a variety of sensory systems. While inhibitory sidebands have been observed at several levels in the auditory system 2,6,7,11, their presence in the lateral superior olive (LSO) has not been demonstrated. The principal cells of the LSO are described as being excited by ipsi- and inhibited by contralateral ear stimulation 1,4,9,13. This description is based on studies in anesthetized preparations, in which LSO neurons exhibit little or no spontaneous activity. Inhibition can only be demonstrated with extracellular recording techniques by the suppression of driven or spontaneous activity with test stimuli. Our results show that LSO neurons in decerebrate cats can display a robust spontaneous discharge 3, permitting a demonstration of inhibitory sidebands in response to ipsilateral ear stimulation. The decerebrate preparation has also permitted an investigation into the effects of anesthetics on LSO neuronal discharge patterns.
Cats were rendered decerebrate under halothane anesthesia – either by ligation of the carotids and rostral basilar artery or by pre-collicular transection. Anesthetic was discontinued following decerebration. Surgical procedures for exposing the ventral brain stem, the recording electrodes used, and histological procedures for electrode tract reconstruction have been previously described2,7,16. Beyer DT-48 dynamic speakers were used to produce stimuli. The envelopes of tone burst stimuli had 10 msec rise and fall times. Stimulus control, data collection and on- and off-line analysis procedures have also been described previously2,5,16.
This report will deal only with those units histologically identified within the confines of the LSO. Of 42 LSO units, 38 were explored for their binaural response properties. Eight units were encountered that responded only to ipsilateral ear stimulation, and two were observed that responded only to contralateral ear stimulation, Two units, excited by stimulation of the ipsilateral ear, were facilitated during binaural stimulation while contralateral stimulation alone failed to elicit a response.
The remaining 26 units were binaural in the sense that they were affected by stimulation of either ear alone. All 26 displayed spontaneous activity. Seven units, located either along the LSO border or in the lateral limb, were excited by stimulation of either ear (EE responses). Nineteen units were excited by best frequency tones presented to the ipsilateral ear, while contralateral stimulation was inhibitory at all frequencies, Ipsi-excitatory, contra-inhibitory (EI) responses are thought to reflect the physiology of the principal cell type of the LSO in all but the low frequency region of the lateral limb13. The following results deal only with EI units.
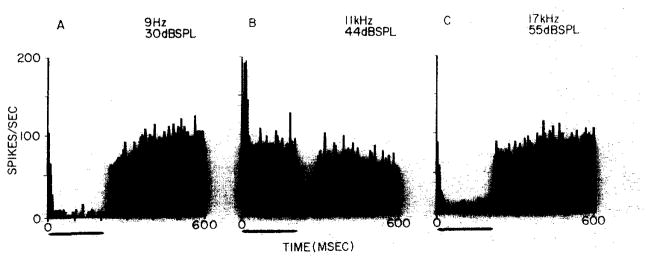
Ipsilateral ear stimulation at frequencies away from best frequency was found to inhibit the background discharge in 9 EI units. In only one unit were we unable to observe inhibitory sidebands for ipsilateral ear stimulation. Insufficient data were collected to determine their presence for the remaining 9 units. Fig. 1 demonstrates inhibitory responses collected while stimulating the ipsilateral ear within the lower (Fig. 1A) and upper (Fig. 1C) inhibitory sidebands of an EI unit. The suggestion of weak excitation during the first bin of Fig. 1A is not seen at lower frequencies.
Fig. 1.
Post-stimulus time histograms collected from an EI unit in the LSO of a decerebrate unanesthetized cat. Ipsilateral ear stimulation, with 200 msec tone bursts, 50 repetitions at 1/sec; frequency and intensity are given to the upper right of each histogram. Histogram binwidth is 6 msec. A: averaged response to tone bursts in lower inhibitory sideband. B: averaged response to tone burst near best frequency. C: averaged response to tone bursts in upper inhibitory sideband. This unit was selected because its high spontaneous discharge (which varied between 75–90 spikes/sec) accentuated the appearance of inhibitory responses.
Firing rate was plotted against sound intensity at best frequency for 16 units. The rate-intensity functions for 9 of these were non-monotonic 6,7 for ipsilateral ear stimulation. The remainder were monotonic or nearly so. All displayed a monotonically decreasing function for contralateral ear stimulation. Binaural rate-intensity functions (determined for constant interaural intensity differences) were all non- monotonic.
Fig. 1B demonstrates the excitation observed for a moderately intense, best frequency, ipsilateral tone. The shape of the post-stimulus time histogram (PST) is characteristic for those units that showed non-monotonic rate-intensity functions at best frequency. The excitatory responses of all EI units were also analyzed using PST histograms with fine bin widths (0.5 msec/bin). For example, the left PST in Fig. 2 shows the first 50 msec of the response displayed in Fig. 1B (without anesthesia), An onset response occurs with a high instantaneous discharge rate, and then the discharge declines to a steady rate above spontaneous.
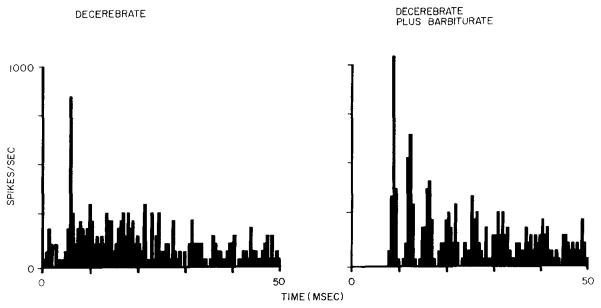
Fig. 2.
Fine binwidth (0.5 msec) post-stimulus time histograms for identical stimulus conditions presented before (left) and after (right) administration of anesthetic dose of sodium pentobarbital. Histogram processed from the first 50 msec of 200 msec tone burst. Same unit and stimulus conditions as Fig. 1B. Chopper responses with interpeak intervals greater than 2 msec (right histogram) were not observed in LSO EI units collected from unanesthetized decerebrate animals.
In two cats, the last EI unit to be studied was re-examined after an anesthetic dose of sodium pentobarbital (37 mg/kg) was administered. In both cases the response characteristics changed dramatically. The post-barbiturate properties of both units were consistent with previous descriptions of EI units in the LSO for all the response parameters measured. Spontaneous activity vanished, as did any indication of ipsilateral inhibitory sidebands. Monaural rate-intensity functions changed from non- monotonic to monotonic for one unit and remained monotonic for the other. In both cases the interspike interval distributions became more symmetric (15 out of 16 EI units in unanesthetized preparations generated interspike interval distributions that were asymmetric about the mode). Fig. 2 demonstrates one of the more dramatic effects of barbiturate anesthesia. After barbiturate, the response displayed peaks of activity that were time-locked to stimulus onset with an interpeak interval of 3.5 msec. PST histograms of this type were first observed in the cochlear nucleus complex12 and labeled chopper responses10. Previous recordings from EI units in the LSO of barbiturate anesthetized animals demonstrated chopper responses with interpeak intervals of greater than 2 msec1,8,9,12.
The presence of spontaneous activity in the unanesthetized LSO is consistent with previous observations that barbiturate decreases spontaneous activity in auditory nuclei both above and below the LSO15,16. It might also be argued that spontaneous activity in the decerebrate animals results from the release of cortical inhibition. This possibility cannot be excluded and it will require chronic electrode studies in awake animals to resolve this.
The principal excitatory input to the LSO has long been thought to arise from spherical cells in the rostral anterior ventral cochlear nucleus (AVCN) (for a review of the anatomy and physiology of the LSO see Brugge and Geisler4). The anatomical substrate for ipsilateral inhibition in the LSO is not known. There are several possible sources of the monaural ipsilateral sidebands: (1) they may exist in the ipsitateral input from the rostral AVCN. Their presence in presumptive spherical cell response has not been demonstrated, but an exhaustive study of these cells in the decerebrate animal must be carried out to eliminate this as a possible source: (2) if there is a non-spherical cell projection from the rostral AVCN to the principal cells of the LSO, it may be responsible for the inhibition: and (3) short axon inhibitory interneurons could exist within the superior olivary complex, but short axon interneurons have not been shown anatomically to terminate on the principal cells of the LSO. One candidate for such an interneuron might be those monaural LSO units that are excited only by ipsilateral ear stimulation. The input that produces inhibitory sidebands may also contribute to the non-monotonic rate–intensity functions observed for some of these units in response to monaural ipsilateral stimulation.
Augmentation of chopper responses with post-barbiturate interpeak intervals of greater than 2 msec is most intriguing. It is possible that a high level of spontaneous activity competes with a clear demonstration of this chopper response in the unanesthetized case. This possibility is weakened by the fact that we have observed chopper responses with interpeak intervals of less than 2 msec from spontaneously active units recorded in the LSO as well as in the posteroventral cochlear nucleus of decerebrate cats (Ritz and Brownell, unpublished observations). Webster has also reported their presence in the cochlear nucleus complex of awake cats 15.
Our investigations of EI units in the LSO of unanesthetized decerebrate cats do not alter previous descriptions of tonotopic organization or the effects of ipsilateral and contralateral input at best frequency. They do however reveal response characteristics of greater richness. The presence of spontaneous activity permits inhibitory processes to modify spike train activity. The total frequency response area is greater when inhibitory sidebands are considered. The fine temporal aspects of the neuronal response are modified. While the exact mechanism has yet to be determined, chopper responses in the LSO seem to be augmented by barbiturate anesthetic.
Acknowledgments
We are grateful to Mr. Darrell J. Duffy for computer programming, Ms. M. Ann Smith for histological assistance, Mr. James W. Fleshman for participating in some of the experiments, and Dr. C. J. Vierck for his helpful criticism.
Supported by USPHS Grants NS12209 and MH10320.
References
- 1.Boudreau JC, Tsuchitani C. Superior olive S-segment cell discharge to tonal stimulation. In: Neff WD, editor. Contributions to Sensory Physiology. Vol. 4. Academic Press; New York: 1970. pp. 144–213. [DOI] [PubMed] [Google Scholar]
- 2.Brownell WE. Organization of the cat trapezoid body and the discharge characteristics of its fibers. Brain Research. 1975;94:413–433. doi: 10.1016/0006-8993(75)90226-7. [DOI] [PubMed] [Google Scholar]
- 3.Brownell WE, Manis PB, Ritz LA, Fleshman JW. Response characteristics of superior olivary complex neurons in the decerebrate cat. Neurosci Abstr. 1978;4:4. [Google Scholar]
- 4.Brugge JF, Geisler CG. Auditory mechanisms of the lower brainstem. Ann Rev Neurosci. 1978;1:363–394. doi: 10.1146/annurev.ne.01.030178.002051. [DOI] [PubMed] [Google Scholar]
- 5.Duffy DJ, Manis PB, Brownell WE. A program for acquisition of primary and ancillary neurophysiological data. Brain Theory Newsletter. 1978;3:139–141. [Google Scholar]
- 6.Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg JM, Brownell WE. Discharge characteristics of neurons in anteroventral and dorsal cochlear nuclei of cat. Brain Research. 1973;64:35–54. doi: 10.1016/0006-8993(73)90169-8. [DOI] [PubMed] [Google Scholar]
- 8.Guinan JJ, Jr, Guinan SS, Norris BE. Single auditory units in superior olivary complex. I: Responses to sounds and classifications based on physiological properties. Int J Neurosci. 1972;4:101–120. [Google Scholar]
- 9.Guinan JJ, Jr, Norris BE, Guinan SS. Single auditory units in the superior olivary complex. II: Locations of unit categories and tonotopic organization. Int J Neurosci. 1972;4:147–166. [Google Scholar]
- 10.Kiang NYS, Pfeiffer RR, Warr WB, Backus ASN. Stimulus coding in the cochlear nucleus. Ann Otol (St Louis) 1965;74:463–485. doi: 10.1177/000348946507400216. [DOI] [PubMed] [Google Scholar]
- 11.Moushegian G, Rupert A, Whitcomb MA. Brain-stem neuronal response patterns to monaural and binaural tones. J Neurophysiol. 1964;27:1174–1191. doi: 10.1152/jn.1964.27.6.1174. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer RR. Some response characteristics of single units in the cochlear nucleus to tone-burst stimulation. Research Laboratory of Electronics, M.I.T; 1962. pp. 306–315. Quart. Progr. Report No. 66. [Google Scholar]
- 13.Tsuchitani C. Functional organization of lateral cell groups of cat superior olivary complex. J Neurophysiol. 1977;40:296–318. doi: 10.1152/jn.1977.40.2.296. [DOI] [PubMed] [Google Scholar]
- 14.Webster WR. Chopper units recorded in the cochlear nucleus of the awake cat. Neurosci Lett. 1977;7:261–265. doi: 10.1016/0304-3940(78)90211-2. [DOI] [PubMed] [Google Scholar]
- 15.Webster WR, Aitkin LM. Central auditory processing. In: Gazzaniga MS, Blake-more C, editors. Handbook of Psychobiology. Academic Press; New York: 1975. pp. 325–364. [Google Scholar]
- 16.Young ED, Brownell WE. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol. 1976;39:282–300. doi: 10.1152/jn.1976.39.2.282. [DOI] [PubMed] [Google Scholar]