Summary
Antibody-based therapies, such as rituximab and alemtuzumab, have contributed significantly to the treatment of Chronic Lymphocytic leukemia (CLL). The CD40 antigen is expressed predominantly on B-cells and represents a potential target for immune-based therapies. SGN-40 is a humanized IgG1 monoclonal antibody currently in Phase I/II clinical trials for indolent lymphomas, diffuse large B cell lymphomas and Multiple Myeloma. Its biological effect on CLL cells has not been studied. The present study demonstrated that SGN-40 mediated modest apoptosis in a subset of patients with secondary cross-linking but did not mediate complement-dependent cytotoxicity. SGN-40 also mediated antibody-dependent cellular cytotoxicity (ADCC) predominantly through natural killer (NK) cells. Previous studies by our group and others have demonstrated that lenalidomide up regulates CD40 expression on primary B CLL cells and activates NK-cells. We therefore examined for the combinatorial effect of lenalidomide and SGN-40 and demonstrated that both enhanced direct apoptosis and ADCC against primary CLL B cells. These data together provide justification for clinical trials of SGN-40 and lenalidomide in combination for CLL therapy.
Keywords: SGN-40, monoclonal antibodies, CD40 activation, chronic lymphocytic leukemia, Lenalidomide
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common types of adult leukemia. Treatment of symptomatic CLL has evolved significantly over the past decade, with a number of randomized trials demonstrating higher overall response (OR) and complete response (CR) rates with fludarabine compared with alkylator-based chemotherapy regimens (Johnson, et al 1996, Leporrier, et al 2001, Rai, et al 2000, Zhu, et al 2004). Recently, two randomized phase III trials demonstrated that fludarabine/alkylator based therapy was superior to monotherapy with fludarabine (Byrd, et al 2003, Eichhorst, et al 2006). Furthermore, the introduction of monoclonal antibodies targeting CD20 (rituximab) and CD52 (alemtuzumab) into initial or salvage regimens for CLL has generated promising results. (Byrd, et al 2005, Hainsworth, et al 2003, Hillmen, et al 2007, Keating, et al 2005, Schulz, et al 2002). Nonetheless, virtually all CLL patients eventually relapse and become refractory to conventional therapy for CLL. The development of new therapies for CLL is therefore a priority.
CD40 is a member of the Tumour Necrosis Factor (TNF) receptor super family expressed as a type-I transmembrane protein (40kDa) on both hematopoietic and non-hematopoietic tissues. CD40 is expressed on normal B-cells and at relatively high levels in most B cell malignancies including CLL (Banchereau, et al 1994, Pellat-Deceunynck, et al 1994, Wang, et al 1997). These findings together provide a rationale for targeting this antigen with therapeutic antibodies. Two therapeutic antibodies that target the CD40 antigen, SGN-40 and HCD122, are under clinical development. SGN-40 has previously been reported to be a partial agonist antibody (Law, et al 2005) whereas HCD122 blocks CD40 mediated signaling (Luqman, et al 2008). SGN-40 has been shown to promote growth arrest, apoptosis, and natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC) against large cell lymphoma and multiple myeloma cell lines (Law, et al 2005, Tai, et al 2004, Tai, et al 2005). Additionally, one pre-clinical study showed that the immune modulating agent lenalidomide augmented SGN-40 mediated ADCC against multiple myeloma cells (Tai, et al 2005). We have recently demonstrated lenalidomide to upregulate CD40 antigen expression in primary B cells from CLL patients (Andristos et. al. 2008). Further, we have also showed lenalidomide to mediate NK cell activation and subsequent enhanced ADCC function against target CLL B cells using clinically relevant therapeutic antibodies. (Lapalombella et al 2008). Given the well defined expression of CD40 antigen on CLL B cells (Uckun, et al 1990) and its ability to serve as a potential target for antibody therapy in large cell lymphoma and multiple myeloma cell lines (Law, et al 2005, Tai, et al 2004, Tai, et al 2005), this study examined the pre-clinical activity of SGN-40 and its combination with lenalidomide in primary B cells from CLL patients. Modest apoptosis and ADCC mediated by SGN-40 in CLL B cells was found to be significantly enhanced by lenalidomide, an agent that upregulates CD40 antigen in CLL B cells.
Methods
Reagents and antibodies
SGN-40 was provided by Seattle Genetics, Seattle, WA. Phycoerythrin (PE)-labeled isotype control mouse IgG1, fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) were purchased from BD Pharmingen, (San Diego, CA). Alemtuzumab (anti-CD52), Rituximab (anti-CD20) and trastuzumab (anti-Her2) were provided by the Ohio State University pharmacy. Goat anti-human IgG antibody (Fc gamma fragment-specific, αFc) was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Lenalidomide was obtained as previously described (Andritsos, et al 2008).
Patient sample processing and cell culture
Blood was obtained from patients with informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of Ohio State University (Columbus, OH). All patients examined in this series had immunophenotypically defined CLL, as outlined by the modified 1996 National Cancer Institute criteria (Cheson et al 1996). All of the B-CLL patients had been without prior therapy for a minimum of two months. CLL B cells were isolated from freshly donated blood using ficoll density gradient centrifugation (Ficoll-Paque Plus, Amersham Biosciences, Piscataway, NJ). Enriched CLL fractions were prepared by using the “Rosette-Sep” kit from Stem Cell Technologies (Vancouver, British Columbia, Canada) according to the manufacturer’s instructions. Isolated cells were incubated in RPMI 1640 media supplemented with 10% heat-inactivated human serum (HS, Valley Biomedical, Winchester, VA), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), and 100 U/mL penicillin/100 ug/mL streptomycin (Sigma-Aldrich, St. Louis) at 37°C in an atmosphere of 5% CO2. The purity of enriched populations was always greater than 95% of the total yield, as detected by CD19 and CD3 staining. For ADCC experiments CD14+, CD56+ and CD19+ cells were negatively purified from whole blood obtained from either healthy volunteers or CLL patients by Magnetic Activated Cell Sorting (MACS™) according to the manufacturer's recommendations (MiniMACS; Miltenyi Biotec, Bergisch-Gladbach, Germany).
Assessment of apoptosis by flow cytometry
Cells (1×106 cells/mL) were treated with SGN-40, rituximab, alemtuzumab or trastuzumab at a concentration of 10 µg/mL. The cross-linker, goat anti-human IgG (Fc specific; Jackson ImmunoReasearch Laboratories, West Grove, PA), was added to the cell suspension 5 min after adding the primary antibodies at a concentration of 50 µg/mL. In addition, a group of samples with no treatment was collected as media control. The apoptosis of cells at 24 and 48 h post-treatment was measured using annexin V-FITC/ PI staining followed by fluorescent-activated cell sorting (FACS) analysis according to the supplier's instructions (BD Biosciences, San Jose, CA). Results were expressed as percentage of total positive cells over untreated control [% positive cells = (% annexin+ and/or PI+ cells of treatment group) – (% annexin+ and/or PI+ cells of untreated control)]. FACS analysis was performed using EPICS XL cytometer (Beckman-Coulter, Miami, FL). ExpoADC32 software package (Beckman-Coulter) was applied to analyse the data. Ten thousand events were collected from each sample and data was acquired in list mode.
Analysis of antibody-dependent cellular cytotoxicity
ADCC activity was determined by standard 4-h 51Cr-release assay. 51Cr-labeled target cells (1 × 104 B-CLL cells) were incubated with media alone or in the presence of various antibodies (10µg/ml) at 37 °C for 30 min. Unbound antibody was washed off and the cells plated at 1 × 104 cells/well. Effector cells (peripheral blood mononuclear cells (PBMC) or negatively isolated NK cells from healthy donors) were then added to the plates at indicated effector to target (E:T) ratios. After a 4-h incubation, supernatants were removed and the radioactivity was counted in a gamma counter. The percentage of specific cell lysis was determined by: % lysis = 100 × (ER-SR)/ (MR-SR). ER, SR, and MR represent experimental, spontaneous and maximum 51Cr release. Data were normalized to the media control.
Monocyte Antibody-Dependent Cellular Cytotoxicity
The Monocytes were cultured in RPMI containing 10% fetal bovine serum and 2 mM L-glutamine (Invitrogen Antibiotics, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 µg/ml; Sigma-Aldrich, St. Louis) at 37°C in an atmosphere of 5% CO2 supplemented with either interferon γ (IFNγ; 20 µg/ml) or media alone for 18 h and standard ADCC assay as described above was performed with Cr labeled target cells (primary B CLL cells) in the presence of indicated antibodies.
Analysis of complement-dependent cytototoxicity (CDC)
B-CLL cells at 106/mL were suspended in RPMI media alone, media with 30% autologous serum from the patient blood samples or media with 30% heat-inactivated (56°C, 30 min) serum. Cells were then treated with SGN-40, alemtuzumab, rituximab or trastuzumab at 10 µg/ml. After incubation at 37°C for 1 h, cytotoxicity was measured as previously reported (Zhao, et al 2007).
Statistical analyses
All analyses were performed by the Center for Biostatistics, the Ohio State University. Mixed effects models were fitted to the cytotoxicity data. Random effects associated with the interactions were always included in the models to ensure that error was not underestimated. The Statistical Package for the Social Ssciences (SPSS) software (version 13.0, SPSS Inc., Chicago, IL) was used for the statistical analysis. Data were analyzed by a paired t-test. Differences were considered significant at a P value of <0.05. Non-parametric paired Wilcoxon signed ranked tests were performed to examine both the proportion and mean fluorescence intensity change of cells expressing specific antigens following treatment with lenalidomide or media for 48 h.
Results
SGN-40 mediates modest apoptosis and no complement dependent cytotoxicicity against CLL cells
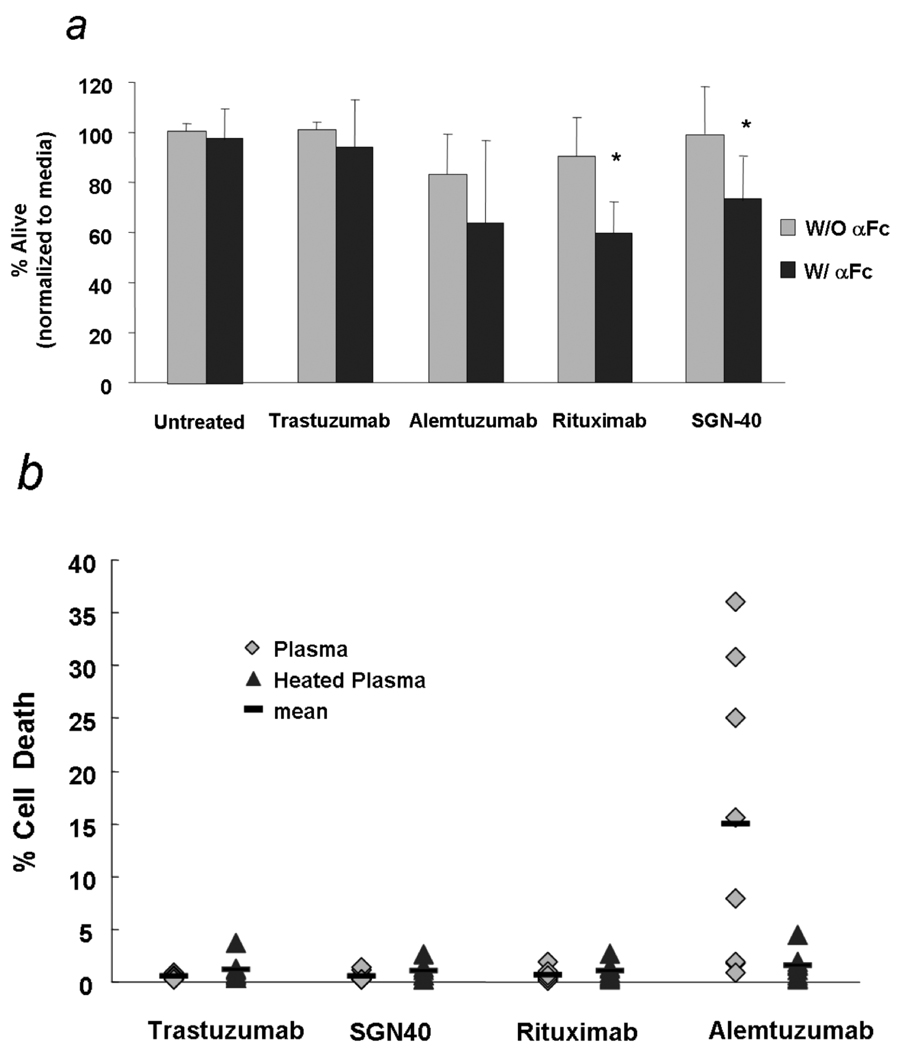
Given the observation that CD40 is expressed on virtually all CLL cells, we first sought to determine if SGN-40 could activate CLL cells or mediate direct cell death and complement mediated cytotoxicity in B CLL cells. No increase in CD80, CD86, CD38 or DR5 expression was observed when CLL cells were treated with SGN-40 alone or in presence of a crosslinker whereas very modest increase in HLA-DR expression (mena fluorescence intensity) was noted (data not shown). Fig 1a demonstrates the results from 8 patient samples treated with the SGN-40 at 10µg/ml concentration and goat anti-human IgG (Fc specific) as a cross linker or without the cross linker. Commercially available antibodies, such as Rituximab and Alemtuzumab, were used as positive controls, while transtuzumab and cross linker alone were used as negative controls at similar concentration. The direct cytotoxic effect was examined at 24 h by Annexin and PI staining followed by flow cytometry. SGN-40 induced modest apoptosis in presence of a crosslinker (72% ± 14%, p<0.0002) . The level of apoptosis was similar to that induced by rituximab (Fig 1a). The capability of SGN-40 to fix complement and induce CDC was evaluated in these same 8 patient samples. While alemtuzumab mediated CDC that was abrogated upon heat inactivation of the complement, SGN-40 failed to fix complement and induce CDC against primary CLL cells (Fig 1b).
Fig 1.
(a) SGN-40 mediated modest direct cytotoxicity in primary CD19+ CLL cells: CD19+ CLL cells were incubated with 10 µg/mL of indicated antibodies with (W/) or without (W/O) 50µg/mL cross-linking goat anti-human Fc antibody (αFc). The percentage of apoptosis was determined by Annexin V/propidium iodide staining after 24h. (N=8) Annexin V−/PI− cells normalized with the media control are shown as a percentage of live cells.
(b) SGN-40 does not mediate complement-mediated cytotoxicity: CD19+ CLL cells were incubated with 10 µg/mL of SGN-40, rituximab, alemtuzumab or trastuzumab in the presence of 30% of either active or heat-inactivated human serum as a source of complement. Relative cytotoxicity was determined after 1h of incubation at 37°C by Annexin V/propidium iodide staining (N=8)
SGN-40 Mediates Antibody Dependent Cellular Cytotoxicity of B CLL Cells
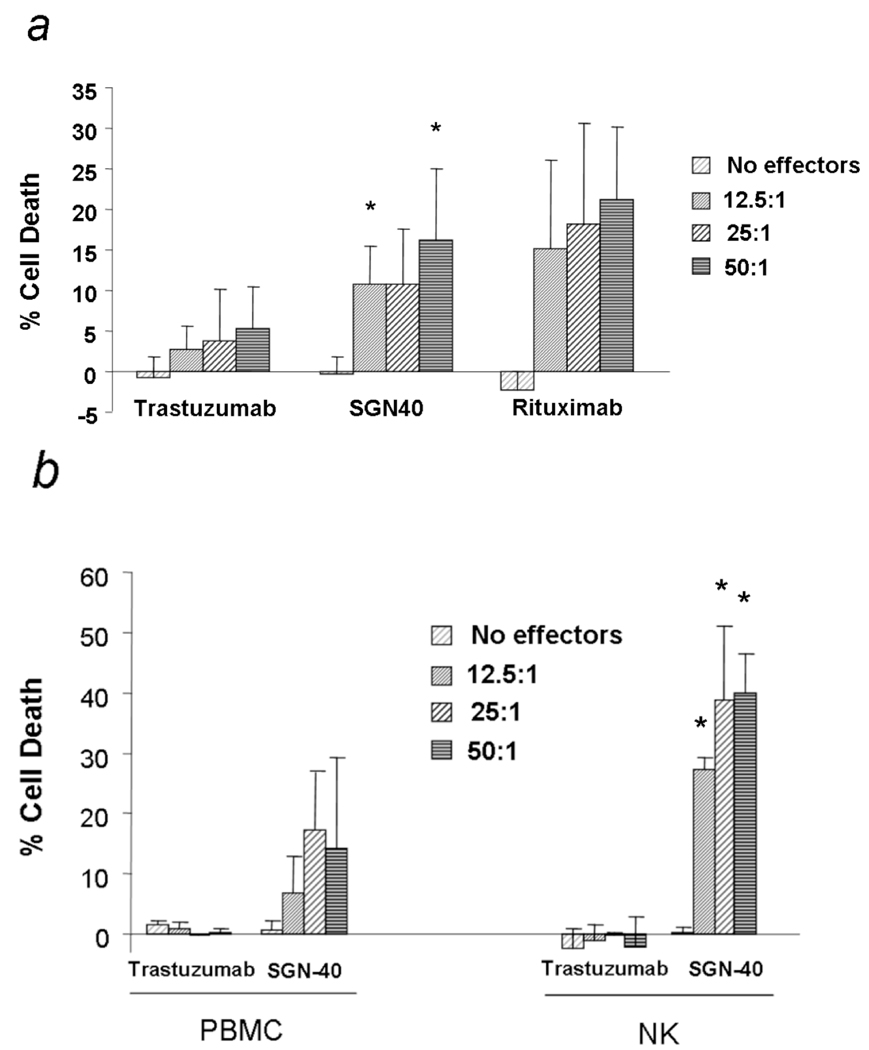
ADCC represents a major mechanism by which therapeutic antibodies mediate their effect in vivo. We therefore investigated the ability of SGN-40 to mediate ADCC against primary CLL cells as previously shown to occur with lymphoma and multiple myeloma cells (Hayashi, et al 2003) (Law, et al 2005). SGN-40 mediated ADCC in 10 patient samples using freshly isolated human PBMC from healthy donors as effectors, when compared to the negative control trastuzumab (n=10 p= 0.009 for 12.5:1; p=0.001 for 50:1; overall cytotoxicty (averaging over all of the effector:target ratios between the SGN-40 and trastuzumab conditions) p<0.003). The SGN-40 mediated ADCC observed against primary CLL cells was not statistically significantly different to that of Rituximab, an antibody directed against CD20 that is commonly utilized for the treatment of CLL.
Given the demonstration of effective ADCC against primary CLL cells by SGN-40 with PBMC effectors, we next sought to determine the relevant effector cell population(s) involved in mediating this cytotoxic function. As ADCC effector cells in the above described experiments were derived from Ficoll-isolated mononculear cells, we focused predominately on NK cells and monocytes. Fig 2b demonstrates that autologous natural killer cell derived from CLL patients (3 patients) had significantly increased ADCC against primary CLL cells when compared to whole PBMC (p<0.001 for 12.5:1, p<0.05 for 25:1, p<0.005 for 50:1 effector to target ratio) (N=3). This suggested that SGN-40 can mediate ADCC even when KIR mismatching does not exist in an autologous effector:target system and that NK cells are important mediator of the ADCC observed with SGN-40. In contrast, neither resting (Fig. 2c) nor IFNγ activated monocytes (Fig. 2d) were able to mediate ADCC with SGN-40 at 4h . These findings suggest that efforts to enhance ADCC of SGN-40 should be directed at enhancing NK cell activity.
Fig 2.
(a) SGN-40 mediates ADCC. ADCC was measured using PBMC from normal volunteers and B-CLL cells at 12.5:1, 25:1 and 50:1 effector:target (E:T) ratio in the presence or absence of 10 µg/mL SGN-40, trastuzumab or rituximab. Columns represent the average of triplicate wells and are representative of three independent experiments; bars, SD. The differences observed are significant for the 12.5:1 (p=0.009) and the 50:1 (p=0.01) effectors to target ratios The overall SGN-40 versus trastuzumab mediated ADCC was significantly higher for SGN-40 (n=10 p<0.003). (b, c, d) ADCC mediated by SGN-40 is predominately mediated by NK. (b) ADCC was measured using PBMC, purified NK and purified B cells from CLL patients at 12.5:1 , 25:1 and 50:1 E:T ratio in presence or absence of 10 µg/mL SGN-40 or trastuzumab. Columns, average of triplicate wells and were representative of three independent experiments; bars, standard deviation. (p<0.001 for 12.5:1, p<0.05 for 25:1, p<0.005 for 50:1 effector to target ratio) (N=3). Autologous Natural killer cell derived from CLL patients had significantly increased ADCC against primary CLL when compared to whole PBMC. (c) Resting or (d) IFNγ-activated monocytes were not able to mediate ADCC with SGN-40.
Lenalidomide Enhances Direct and NK Cell Killing of SGN-40 Against Primary CLL Cells
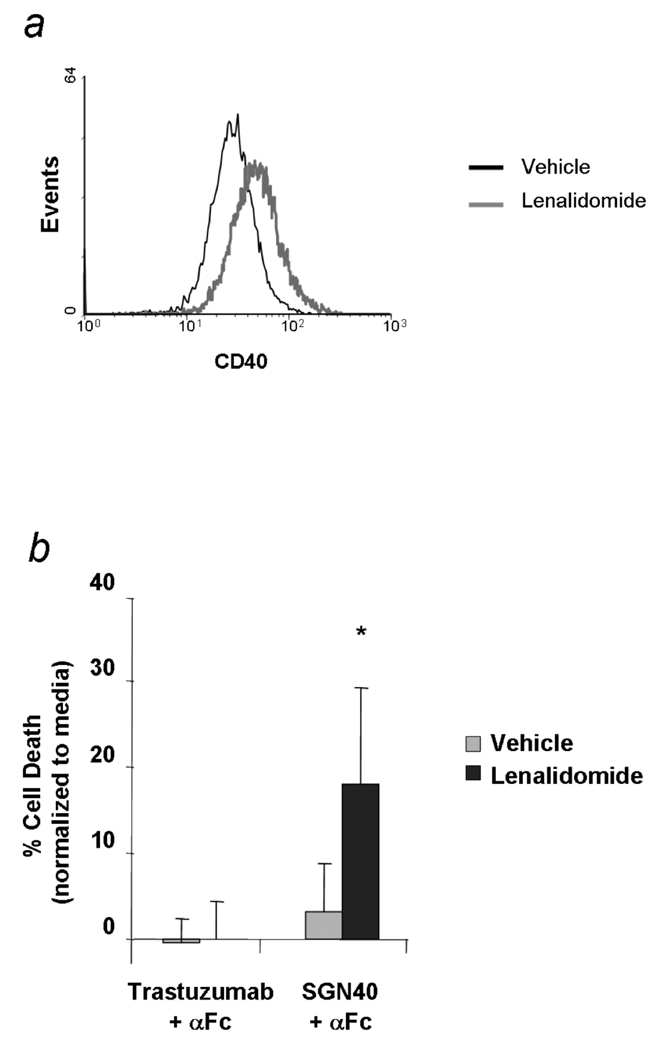
Lenalidomide is an immune modulatory agent demonstrated to enhance ADCC against multiple myeloma tumour cell targets. Lenalidomide was recently reported to up-regulate CD40 in vitro in CLL patients through a direct mechanism of action (Andristos et al 2008) and a representative case is illustrated in Fig 3a. We therefore sought to determine if concentrations of lenalidomide that upregulate CD40 surface expression would enhance SGN-40 mediated direct cytotoxicity. Treatment of CLL cells for 48 and 72 h with lenalidomide resulted in enhanced direct cytotoxicity by SGN-40 compared to lenalidomide untreated cells (n=6, p<0.05) (Fig 3b). Lenalidomide was recently reported to mediate NK cell activation and subsequent enhanced ADCC function against target CLL B cells with clinically relevant therapeutic antibodies (Lapalombella et al 2008). In order to determine if lenalidomide treatment potentiates NK cell-mediated SGN-40-ADCC function, effector and/or target CLL B cells were treated with lenalidomide and evaluated SGN-40-ADCC. While NK cells mediated moderate SGN-40-ADCC function in lenalidomide untreated samples, SGN-40-mediated ADCC was also enhanced by lenalidomide treatment of target B cells (n=3; p=0.008 for 50:1; p=0.007 for 25:1; p=0.005 for 12.5:1. Overall SGN-40 cytotoxicity (averaging over all of the effector:target ratios) between the vehicle and lenalidomide conditions p<0.0003) (Fig 3c). Further, treatment of NK cells with lenalidomide also enhanced the SGN-40 mediated ADCC function against untreated target CLL B cells. (data not shown). Importantly, as both target CLL cells and NK cells would be subject to lenalidomide effects in vivo, we tested the effect of co-treatment of target CLL B cells and autologous NK cells on SGN-40-mediated ADCC function. Peripheral blood mononuclear cells (PBMC) from CLL patients were incubated for 72 h with a predetermined concentration of lenalidomide (0.5µM) or vehicle control. Following this, B and NK cell fractions were purified and compared in an ADCC assay in the presence of SGN-40. Compared to NK-only controls, the co-treatment of autologous NK cells and primary CLL cells with lenalidomide resulted in increased SGN-40-mediated ADCC (n=3, p<0.02 for both 12.5 and 25:1 ratios; p<0.005 for the overall SGN-40 cytotoxicity between the vehicle and lenalidomide conditions) (Fig 3d). These data provide support for combination strategies of lenalidomide treatment with SGN-40.
Fig 3.
(a) Lenalidomide induces up regulation of CD40 in CLL B cells: Results for CD40 surface expression from a representative experiment: CD19+ cells were incubated with Lenalidomide (0.5µM) or vehicle control. CD40 surface expression was analyzed by flow after 48h of treatment. Grey line denotes lenalidomide-treated cells and dark line denotes vehicle-treated cells. (b,c,d) Lenalidomide treatment at 48h significantly increased SGN-40 mediated direct and NK dependent cytotoxicity. (b) Direct cytotoxicity: CD19+ CLL cells were incubated with 10 µg/mL of SGN-40 or trastuzumab with or without 50µg/mL cross-linking goat anti-human Fc antibody (αFc). The percentage of apoptosis was determined by Annexin V/propidium iodide staining after 24h. (N=6, p<0.05 for SGN-40+ Lenalidomide vs SGN-40 + vehicle; p<0.00005 for SGN-40+lenalidomide and trastuzumab+lenalidomide). (c) ADCC was measured using freshly isolated NK cells from normal volunteers and lenalidomide (0.5µM) or vehicle control-treated B-CLL cells at 12.5:1 ,25:1 and 50:1 E:T ratio in presence or absence of 10 µg/mL SGN-40 or trastuzumab. Columns represent the average of triplicate wells and are representative of five independent experiments; bars, SD. The differences observed for all E:T ratios were statistically significant (P=0.008 for 50:1; p=0.007 for 25:1; p=0.005 for 12.5:1. The overall Lenalidomide versus vehicle SGN-40 mediated ADCC was significantly higher for lenalidomide (N=3, p<0.0003) (d) ADCC was measured using lenalidomide- or vehicle control-treated NK and B cells from CLL patients at 12.5:1 and 25:1 E:T ratio in the presence or absence of 10 µg/mL SGN-40, rituximab or trastuzumab. Columns represent the average of triplicate wells and are representative of two independent experiments; bars, SD. (N=2, p<0.02 for both the effector to target ratios).
Discussion
SGN-40, a humanized monoclonal antibody to CD40, has shown significant preclinical activity in non-Hodgkin lymphoma and multiple myeloma (Cheson, et al 1996, Tai, et al 2004) (Hayashi, et al 2003). These results have been pursued in clinical trials of both these diseases, where modest clinical activity was observed in patients with relapsed diffuse large cell lymphoma (Advani, et al 2006) but not in multiple myeloma (Hussein, et al 2006). Herein, we report SGN-40 has modest pre-clinical activity in CLL cells. Specifically, SGN-40 has the dual property of mediating modest apoptosis when cross linked and also mediating cytotoxic effect through ADCC. From our studies of evaluating effector subtypes involved in the SGN-40 ADCC, resting or IFNγ-activated monocytes were found to have a minimal role while NK cells were found to be involved predominantly. However, the difference between PBMC- and NK-mediated SGN-40-ADCC was modest. This may be attributed to variability in the levels of the surface CD40 target antigen on the target cells used, Fcγ receptor levels on NK cells and FcγR polymorphisms that were shown to contribute to variability in ADCC functions by NK effector cells. This was consistent with reports in carcinomas where antigen density on the target cells has been shown to influence the degree of ADCC observed with IgG1 antibodies (Velders, et al 1998). Similarly, differences in the ADCC function by antibodies has been attributed to differences in the FcγR polymorphism on different NK cells (Hatjiharissi, et al 2007) (Ghielmini, et al 2005) (Cartron, et al 2002) (Weng and Levy 2003) (Khan, et al 2006)). In line with previous studies in multiple myeloma, we demonstrated that, in CLL, lenalidomide enhances ADCC when combined with SGN-40(Tai, et al 2005). Together, these studies provide justification for further studies of SGN-40 and its combination with lenalidomide in CLL.
To date, clinical studies with CD40-directed antibodies in CLL have not been reported in CLL. While SGN-40 has been previously reported to be a weak agonist against normal B-cells (Law, et al 2005), our studies did not show significant up-regulation of co-stimulatory molecules CD80 or CD86 whereas there was a very modest activation of CLL cells by SGN-40 as evidenced by induction of HLA-DR. Additionally, in contrast to CD40 ligand signaling that has been shown by several groups to promote survival, the present study demonstrated that SGN-40 mediated very modest cytotoxicity against primary CLL cells when cross-linked with a secondary antibody. The significance of apoptosis induced with a therapeutic antibody and cross-linking is uncertain, but it is likely that similar engagement of the Fc region of the antibody and the FcγR of the effector cell occurs. Nonetheless, our data do not provide concern that SGN-40 might significantly activate CLL cells to promote tumor flare or early disease acceleration that has been in observed in a subset of CLL patients. Furthermore, in the clinical trial of SGN-40 reported for diffuse large cell lymphoma, no clinical evidence of early tumor cell activation was observed (Advani, et al 2006).
Given the very modest apoptosis observed with cross-linking, ADCC, and lack of complement activation, where should SGN-40 be pursued in the treatment of CLL? Of clear importance to antibody-based therapy is having sufficient target antigen present on the CLL cell. Previously, we demonstrated that the immune modulating agent lenalidomide increased CD40 antigen expression on CLL cells in vitro and also enhances CLL NK-cell activity toward antibody laden tumor targets (Andritsos, et al 2008). Lenalidomide has been shown to have promising clinical activity in CLL (Chanan-Khan, et al 2006, Chen 2007, Ferrajoli, et al 2008) and is approved for use in several other hematological malignancies. Consideration of a clinical trial of SGN-40 in combination with lenalidomide, known to up-regulate the target CD40 antigen as well as the enhancement of NK effector functions, presents a paradigm shift in combination therapies with biological and chemotherapeutic agents. Pursuing such combination strategies offers the greatest opportunities to see benefit of antibodies that have modest single agent activity and yet very modest toxicity.
Acknowledgments
This work was supported by the T32 Grant (T-32-5CA009338), National Cancer Institute (P01 CA95426; CLL Research Consortium P01 CA81534-02), The Leukemia and Lymphoma Society and The D. Warren Brown Foundation.
Bibliography
- Advani R, Forero-Torres A, Furman RR, Rosenblatt JD, Younes A, Shankles B, Harrop K, Drachman JG. SGN-40 (Anti-huCD40 mAb) Monotherapy Induces Durable Objective Responses in Patients with Relapsed Aggressive Non-Hodgkin's Lymphoma: Evidence of Antitumor Activity from a Phase I Study. Blood (ASH Annual Meeting Abstracts) 2006;108:695. [Google Scholar]
- Andritsos L, Johnson AJ, Lozanski G, Blum W, Kefauver C, Awan F, Smith LL, Lapalombella R, May SE, Raymond CA, Wang DS, Knight RD, Ruppert AS, Lehman A, Jarjoura D, Chen CS, Byrd JC. Higher Doses of Lenalidomide Are Associated With Unacceptable Toxicity Including Life Threatening Tumor Flare in Patients With Chronic Lymphocytic Leukemia. J Clin Oncol. 2008;26:2519–2525. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Peterson BL, Morrison VA, Park K, Jacobson R, Hoke E, Vardiman JW, Rai K, Schiffer CA, Larson RA. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Rai K, Peterson BL, Appelbaum FR, Morrison VA, Kolitz JE, Shepherd L, Hines JD, Schiffer CA, Larson RA. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- Chen CI, Paul Harminder, Mariela Pantoja, Brandwein Joseph, Kukreti Vishal, Trudel Suzanne, Wei Ellen, Tong Jieffei, Moran Mike. A Phase II Study of Lenalidomide in Previously Untreated, Symptomatic Chronic Lymphocytic Leukemia (CLL) Blood. 2007;110:2042. [Google Scholar]
- Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, Rai KR. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C, Siehl S, Jager U, Bergmann M, Stilgenbauer S, Schweighofer C, Wendtner CM, Dohner H, Brittinger G, Emmerich B, Hallek M. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Leger-Falandry C, Cogliatti S, Fey M, Martinelli G, Stahel R, Lohri A, Ketterer N, Wernli M, Cerny T, Schmitz SF. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2005;16:1675–1682. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- Hainsworth JD, Litchy S, Barton JH, Houston GA, Hermann RC, Bradof JE, Greco FA. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, Modica M, Cao Y, Manning RJ, Leleu X, Dimmock EA, Kortsaris A, Mitsiades C, Anderson KC, Fox EA, Treon SP. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood. 2007;110:2561–2564. doi: 10.1182/blood-2007-01-070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Treon SP, Hideshima T, Tai YT, Akiyama M, Richardson P, Chauhan D, Grewal IS, Anderson KC. Recombinant humanized anti-CD40 monoclonal antibody triggers autologous antibody-dependent cell-mediated cytotoxicity against multiple myeloma cells. Br J Haematol. 2003;121:592–596. doi: 10.1046/j.1365-2141.2003.04322.x. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J, Sirard C, Mayer J. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- Hussein MA, Berenson JR, Niesvizky R, Munshi NC, Matous J, Harrop K, Drachman JG. Results of a Phase I Trial of SGN-40 (Anti-huCD40 mAb) in Patients with Relapsed Multiple Myeloma. Blood (ASH Annual Meeting Abstracts) 2006;108:3576. [Google Scholar]
- Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B, Wyld PJ, Hiddemann W. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996;347:1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- Keating MJ, O'Brien S, Albitar M, Lerner S, Plunkett W, Giles F, Andreeff M, Cortes J, Faderl S, Thomas D, Koller C, Wierda W, Detry MA, Lynn A, Kantarjian H. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Khan KD, Emmanouilides C, Benson DM, Jr., Hurst D, Garcia P, Michelson G, Milan S, Ferketich AK, Piro L, Leonard JP, Porcu P, Eisenbeis CF, Banks AL, Chen L, Byrd JC, Caligiuri MA. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin's lymphoma. Clin Cancer Res. 2006;12:7046–7053. doi: 10.1158/1078-0432.CCR-06-1571. [DOI] [PubMed] [Google Scholar]
- Law CL, Gordon KA, Collier J, Klussman K, McEarchern JA, Cerveny CG, Mixan BJ, Lee WP, Lin Z, Valdez P, Wahl AF, Grewal IS. Preclinical antilymphoma activity of a humanized anti-CD40 monoclonal antibody, SGN-40. Cancer Res. 2005;65:8331–8338. doi: 10.1158/0008-5472.CAN-05-0095. [DOI] [PubMed] [Google Scholar]
- Lapalombella R, Yu B, Triantafillou G, Liu Q, Butchar JP, Lozanski G, Ramanunni A, Smith LL, Blum W, Andritsos L, Wang DS, Lehman A, Chen CS, Johnson AJ, Marcucci G, Lee RJ, Lee LJ, Tridandapani S, Muthusamy N, Byrd JC. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood. 2008 Sep 4; doi: 10.1182/blood-2008-01-133108. [Epub ahead of print] PMID:18772452. doi:10.1182/blood-2008-01-133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporrier M, Chevret S, Cazin B, Boudjerra N, Feugier P, Desablens B, Rapp MJ, Jaubert J, Autrand C, Divine M, Dreyfus B, Maloum K, Travade P, Dighiero G, Binet JL, Chastang C. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–2325. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J, Goldbeck C, Xu X, Kadel EE, 3rd, Lee SH, Aukerman SL, Jallal B, Aziz N, Weng WK, Wierda W, O'Brien S, Younes A. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood. 2008;112:711–720. doi: 10.1182/blood-2007-04-084756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Juge-Morineau N, Wijdenes J, Amiot M. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994;84:2597–2603. [PubMed] [Google Scholar]
- Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, Hines J, Threatte GA, Larson RA, Cheson BD, Schiffer CA. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- Schulz H, Klein SK, Rehwald U, Reiser M, Hinke A, Knauf WU, Aulitzky WE, Hensel M, Herold M, Huhn D, Hallek M, Diehl V, Engert A. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100:3115–3120. doi: 10.1182/blood-2002-03-0972. [DOI] [PubMed] [Google Scholar]
- Tai YT, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, Hideshima T, Chauhan D, Hamasaki M, Ishitsuka K, Richardson P, Treon SP, Munshi NC, Anderson KC. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004;64:2846–2852. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, Schlossman R, Richardson P, Treon SP, Grewal IS, Munshi NC, Anderson KC. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Gajl-Peczalska K, Myers DE, Jaszcz W, Haissig S, Ledbetter JA. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin's lymphoma cells. Blood. 1990;76:2449–2456. [PubMed] [Google Scholar]
- Velders MP, van Rhijn CM, Oskam E, Fleuren GJ, Warnaar SO, Litvinov SV. The impact of antigen density and antibody affinity on antibody-dependent cellular cytotoxicity: relevance for immunotherapy of carcinomas. Br J Cancer. 1998;78:478–483. doi: 10.1038/bjc.1998.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Freeman GJ, Levine H, Ritz J, Robertson MJ. Role of the CD40 and CD95 (APO-1/Fas) antigens in the apoptosis of human B-cell malignancies. Br J Haematol. 1997;97:409–417. doi: 10.1046/j.1365-2141.1997.422688.x. [DOI] [PubMed] [Google Scholar]
- Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, Baum PR, Lin TS, Jarjoura D, Lehman A, Kussewitt D, Lee RJ, Caligiuri MA, Tridandapani S, Muthusamy N, Byrd JC. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Tan DC, Samuel M, Chan ES, Linn YC. Fludarabine in comparison to alkylator-based regimen as induction therapy for chronic lymphocytic leukemia: a systematic review and meta-analysis. Leuk Lymphoma. 2004;45:2239–2245. doi: 10.1080/10428190412331283260. [DOI] [PubMed] [Google Scholar]