Abstract
Objective
Nicotinic cholinergic stimulation has known beneficial effects in attention-deficit/hyperactivity disorder (ADHD). Mecamylamine is a non-competitive nicotinic antagonist which is reported in several animal studies to have paradoxical positive effects on cognition at ultra-low doses. Comparable studies in humans have not been conducted. The aim of this study was to determine the effects of acute ultra-low doses of mecamylamine on cognition in adult ADHD.
Methods
Fifteen (6 female) non-smokers with ADHD completed this acute, within subjects, double blind study of 0.2, 0.5, 1.0 mg of oral mecamylamine and placebo. Behavioral inhibition, recognition memory, and delay aversion were assessed at each dose.
Results
The 0.5 mg dose of mecamylamine significantly improved recognition memory and reduced tolerance for delay. Mecamylamine increased participant rated irritability and investigator rated restlessness. There were no effects on vital signs or physical side effects.
Conclusion
This is the first study to find measurable effects of ultra-low doses of mecamylamine in humans. Mecamylamine did not improve core ADHD cognitive symptoms, but significantly improved recognition memory. These effects may represent mixed receptor activity (activation and blockade) at the doses tested. The finding of beneficial effects on memory processes has important clinical implications and further exploration of this effect is warranted.
Keywords: mecamylamine, ADHD, cognition, nicotinic, impulsivity, cholinergic
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common childhood psychological disorders, occurring in as many as 3–5% of children (APA, 1994). While ADHD is a disorder that appears in childhood, up to 80% of children diagnosed with ADHD show symptoms that persist into adolescence (Barkley et al., 1991) and adulthood (Biederman et al., 2000; Hervey et al., 2004)
Studies have found beneficial effects of nicotine (Conners et al., 1996; Levin et al., 2001; Shytle et al., 2002) and a novel nicotinic agonist (Wilens et al., 1999) on ADHD symptoms. In addition, acute nicotine administration has positive effects on cognition including behavioral inhibition and recognition memory in adolescents and young adults with ADHD (Potter and Newhouse, 2004, 2008). Thus, it has been proposed that the cholinergic system may be important in understanding the cognitive symptoms of ADHD (Levin et al., 2001; Potter et al., 2006).
Current literature on the cognitive deficits associated with ADHD, emphasize impairments in behavioral inhibition, delay aversion, sustained attention, and executive function (Nigg, 2005; Sergeant, 2005). Behavioral inhibition refers to the ability to inhibit a pre-potent response due to environmental cues. Deficient behavioral inhibition is believed to underlie impulsive behavior and executive function deficits in ADHD (Barkley, 1997). Deficits in behavioral inhibition are among the most well-documented cognitive deficits in ADHD. A recent meta-analysis found that the strongest and most consistent group differences between ADHD and control participants were on the stop signal reaction time (SSRT) measure of the Stop Signal Task (Willcutt et al., 2005). Delay aversion is the expression of a motivationalstate inwhichpeoplewithADHD chooseto avoid delay (Sonuga-Barke, 2002). Delay aversion has been experimentally examined using the choice delay task (CDT). Children with ADHD show significantly greater aversion to experimental delay than controls (Solanto et al., 2001; Sonuga-Barke et al., 2003). Deficits in behavioral inhibition and delay aversion are both hypothesized to result in impulsive behavior in ADHD, and these deficits have been shown to contribute independently toADHDsymptoms (Solantoetal.,2001; Sonuga-Barke et al., 2003). Sustained attention is also consistently impaired in children with ADHD (Willcutt et al., 2005 for review) although these deficits may be secondary to deficits in inhibition (Nigg, 2001).
Abundant research demonstrates that the cognitive deficits that characterize ADHD in childhood are present in adolescents and adults with the disorder (Gualtieri and Johnson, 2006; Seidman, 2006). A recent review found that studies comparing ADHD subjects to control subjects found clear deficits in executive function associated with ADHD across all ages examined (Seidman, 2006). A large cross-sectional study found deficits in set shifting and Stroop performance in ADHD subjects compared to controls (ages 10–29; Gualtieri and Johnson, 2006). This study found an interesting developmental shift in performance with age. Normal adults had both faster reaction times and greater accuracy than normal adolescents. In contrast, adults with ADHD had greater accuracy but not faster reaction times compared to ADHD adolescents (Gualtieri and Johnson, 2006). This along with the deficits in set-shifting in all age subjects with ADHD was interpreted as demonstrating that young adults with ADHD have difficulty with the allocation of attentional resources (Gualtieri and Johnson, 2006). The finding that ADHD is associated with persistent deficits in the efficient allocation of attention supports the notion that regulation of the cholinergic system may improve these cognitive deficits in ADHD. It has been suggested that the effects of nicotine are most pronounced on tasks that demand effortful processing (Rusted and Warburton, 1994). In addition, a recent theory proposes that the cholinergic system allocates additional attentional resources during tasks that are demanding (Sarter and Bruno, 1997). Thus it may be that in ADHD, cholinergic systems are under-responsive or under-developed and thus stimulation of nicotinic receptors via nicotine results in improved cognitive performance particularly on tests requiring effortful processing (i.e., Levin et al., 2001; Potter and Newhouse, 2004; Potter et al., 2006).
The finding that nicotine improves cognition, especially attention, is well documented throughout the literature (for reviews see Newhouse et al., 2004; Levin et al., 1998; Sacco et al., 2004). However, there are several issues that make the therapeutic use of nicotine itself unlikely to be successful. The first is the relatively small therapeutic window of nicotine. In non-smokers nicotine produces side effects at doses that are similar to the doses that produce beneficial cognitive effects. In addition, it is unknown if the cognitive benefits of nicotine are maintained with chronic nicotine administration. While chronic nicotine does not cause down-regulation of receptors, and in fact is associated with an increase in the number of receptors (Schwartz and Kellar, 1983, 1985), there may be downstream accommodations in neural systems that receive nicotinic innervation, which would result in tolerance to the cognitive effects of nicotine. An alternative strategy to the chronic administration of nicotine is the development of pharmacologic strategies to stimulate nicotinic receptors using novel agonists. Several of these compounds have been tested in human disease states including Alzheimer’s disease, MCI, and ADHD and have been found to produce relevant cognitive benefits (i.e., Wilens et al., 1999; Shytle et al., 2002; Potter and Newhouse, 2004; Wilens et al., 2006). To date, there are no FDA approved nicotinic therapeutic agents available.
Mecamylamine is a non-competitive nicotinic antagonist and has been used by researchers at high doses to block nicotinic receptors (Newhouse et al., 1988; Dumas et al., 2008; Dumas et al., 2009). While mecamylamine clearly produces cognitive impairment at high doses (>5 mg), there is evidence to suggest a paradoxical effect of cognitive enhancement at low doses. Studies in primates and rodents have found measurable cognitive improvement following low-dose mecamylamine administration on tasks such as match-to-sample and the Morris water maze, which both require focused attention (Terry et al., 1999; Driscoll, 1976). The precise mechanism of action for the beneficial effects of ultra-low dose mecamylamine have yet to be determined, but may include paradoxical activation of nicotinic receptors at these low doses. Studies examining human cognitive performance after low doses of mecamylamine (<5 mg.) have not been published. Thus this study was conducted to investigate if ultra-low dose mecamylamine produces improvements on cognitive domains shown to be sensitive to nicotinic stimulation in non-smoking young adults with ADHD.
We hypothesized that specific cognitive operations known to be deficit in ADHD, including behavioral inhibition as measured by the stop signal task, delay aversion as measured by the CDT, recognition memory as measured by the high–low imagery task, and cognitive interference control as measured by the Stroop task would be improved by acute administration of ultra-low doses of mecamylamine.
MATERIALS AND METHOD
Design overview
This study was an acute, single dose, within subjects, double blind study with the following drug conditions: (1) 0.2 mg oral mecamylamine, (2) 0.5 mg oral mecamylamine, (3) 1.0 mg oral mecamylamine, and (4) placebo. Each drug was administered on a separate study day (randomly assigned) at the General Clinical Research Center (GCRC) of the University of Vermont. Study days were scheduled at least 48 h and no more than 10 days apart. This study was approved by the Institutional Review Board at the University of Vermont. Primary outcome measures were tests of cognition including behavioral inhibition, recognition memory, and delay aversion which have demonstrated sensitivity in prior studies of acute cholinergic system manipulation in populations including adult ADHD, Alzheimer’s disease, and normal aging (Potter et al., 1999; Potter and Newhouse, 2004, 2008; Dumas et al., 2008).
Subjects
Fifteen (9 male and 6 female) non-smoking young adults (age 18–24) diagnosed with DSM-IV ADHD-combined type (ADHD-C) and weighing at least 45.5 kg (100 lbs.) provided informed consent and participated in this study. No participant met criteria for any other current Axis-I disorder as assessed by the SCID (First et al., 2002) modified to include the K-SADS-PL structured interview behavior disorder supplement (Kaufman et al., 1997). Participants with a past history of oppositional defiant disorder and/or conduct disorder were allowed entrance into the study. Participants were not allowed to have a history of any other Axis-I disorder. The Wender–Utah rating scale was used to further characterize childhood ADHD symptoms. Participants were screened for cognitive function using the Wechsler abbreviated scales of intelligence (WASI) and were required to have a full scale intelligence quotient (FSIQ) greater than 80. Specific deficits in behavioral inhibition were also measured at screening using the SSRT measure of the stop signal task. Participants were required to perform at least 1.5 standard deviations below the mean for controls in the reference age range on this measure. Norms for this task were taken from published data (Williams et al., 1999) conducted on a random sample of volunteers. A summary of the demographic characteristics of the participants is presented in Table 1.
Table 1.
Demographic characteristics of participants (n = 15)
| Mean (stdev) | Min | Max | |
|---|---|---|---|
| Age | 20.0 (1.7) | 18.0 | 24.1 |
| WASI (FSIQ) | 117.64 (13.19) | 97 | 136 |
| Wender–Utah total score | 46.3 (15.3) | 21 | 63 |
| SSRT at screening (m sec) | 347.3 (61.8) | 313.0 | 524.5 |
FSIQ = full scale intelligence quotient, SSRT = stop signal reaction time.
The status of current (past 3 months) medication treatment for ADHD symptoms was collected during the clinical interview at screening. Five participants were not taking any medications for ADHD, five participants were prescribed stimulants but reported using them less than twice a week, three participants were on stable daily doses of stimulant medication, and two participants were on stable daily doses of non-stimulant treatment for ADHD. All participants who were taking medication treatment for ADHD abstained from treatment for at least three half-lives before each study day. This was verified by participant’s report of their last dose of medication at the beginning of each study day.
Following a screening visit, each participant completed a computer training session to minimize learning effects on the cognitive tasks used in this study.
Study day procedures
Participants abstained from eating and/or drinking anything but water after midnight the night before a study day. Participants were admitted to the outpatient facility at the GCRC at approximately 8:00 am. Confirmation of non-smoking status was obtained by an expired carbon monoxide level of <10 ppm. Female participants were required to have a negative urine pregnancy test prior to drug administration each morning.
Drug was administered double-blind in gel-capsules containing either mecamylmine or placebo. Mecamylamine and matching placebo was manufactured and provided by Targacept, Inc. Cognitive testing began 150 min following oral drug administration. At the conclusion of the cognitive testing session participants were served lunch, and were assessed for discharge 60 min later. Vital signs (blood pressure and pulse) were monitored at 30-min intervals throughout the study session.
Cognitive and behavioral battery
Cognitive outcome measures
Stop signal task: This is a computer-administered test of behavioral inhibition. Participants are asked to respond to two equally probable “go” signals (the letters X and O) by pressing corresponding keys on a computer keyboard. Participants are instructed not to respond to the target stimulus if an auditory signal (the stop signal) is present. The version of this task used here begins using a 250 ms stop signal delay (the interval between the onset of the go signal and the stop signal). This delay is adjusted after every trial according to the participant’s performance to achieve a 50 per cent inhibition success rate. With the probability of inhibition known, the SSRT (a measure of the speed of inhibiting) is calculated (Williams et al., 1999). Other dependent variables include “go” reaction time and accuracy.
The Stroop task: This task measures reaction time during three conditions, word reading, color naming, and conflict. In the word reading condition, participants respond on a keyboard to 100 words presented one at a time on the monitor. The words are all color names: red, green, or blue. In the color naming condition, participants respond to 100 blocks of color presented on the screen (red, green, or blue). In the interference condition, participants are instructed to respond to the color of the “ink” for words that are color names (i.e., the word blue printed in red where the correct response is red). The Stroop effect is the expected longer reaction times in the interference condition compared to the color naming condition. Dependent measures for this task include reaction times for the different stimulus conditions (word reading, color naming, and conflict).
The CDT: In this task, participants are presented with a circle and a square on a computer monitor. They are instructed to choose (using a mouse click) either the circle or the square to earn points. Choosing the circle always gives five points following a 5 s delay. Choosing the square gives 15 points following a variable delay. The delay begins at 15 s and increases 2 s for each consecutive choice, but the delay for the 15 points decreases by 2 s for each choice of the circle. Participants complete 40 choices in this task. In this study, points were not exchanged for money or any other tangible reward.
Recognition memory task
This is a computer-administered recognition memory test in which participants are presented (on a computer screen) 14 target words. Seven of the words are high imagery (“cat”) and seven are low imagery (“idea”) (Snodgrass and Corwin, 1988). Next, participants are tested on 28 words, the 14 original plus 14 distractors (7 high imagery, and 7 low imagery). Participants are asked to indicate which words are old (from the original 14) and which words are new. Two learning trials and a delayed trial are completed. Dependent variables from this task include number of hits (correct yes responses), and false alarms (incorrect yes responses). From these variables hit rates and false alarm rates are calculated as intermediate variables used in the calculation of discrimination (Pr) and response bias (Br) according to two-high threshold theory. Discrimination is the probability of correctly recognizing a target word, and is related to the accuracy of performance of the participant adjusting for the subjects rate of false alarms. Response bias provides information about the participant’s performance when they are uncertain. Respondents with a yea-saying or liberal bias tend to endorse a high number of both target and distracter words. Respondents with a nay-saying or conservative bias tend to under-respond to both target and distracter words.
Behavioral assessments
Clinical global impression (NIMH, 1985): This is a paper-and-pencil form which is completed by the investigator. This form measures several global domains of functioning.
Profile of mood states (POMS) (McNair et al., 1971): The POMS is a self-report measure of mood and/or physical well-being. Participants are presented with a list of adjectives, and asked to indicate the severity of each item for that day only. Dependent variables from the POMS include the total score, and cluster scores for vigor, tension, depression, anger, fatigue, and difficulty concentrating.
Visual analog battery (subject rating): This is a paper-and-pencil measure in which participants are presented with a series of 100 mm lines, each representing a dimension of functioning. Descriptive anchors are provided (i.e., sleepiness ranges from alert to about to fall asleep). Participants are asked to indicate (by placing a mark on the line) how they feel on each domain using “this morning” as the reference time range. This scale measures: anxiety, mood, alertness, physical comfort, fear, irritability, hunger, and sense of interest. This scale has been used in many studies and is sensitive to acute drug manipulations in ADHD (i.e., Potter and Newhouse, 2004, 2008).
Visual analog battery (observer rating): This is a paper-and-pencil measure in which the blind investigator indicates on a series of 100 mm lines, each representing a dimension of functioning, how the participant is functioning on different domains. This items measured are: drowsy, motoric restlessness, disoriented, impaired speech, euphoria, irritability, sweating, incoordination, fatigue, depression, anxiety, and alertness. This scale has been used in many studies and is sensitive to acute drug manipulations in ADHD (i.e., Potter and Newhouse, 2004, 2008).
Data collection and storage
Computer generated data were downloaded into Microsoft Excel workbooks. Scores of paper-and-pencil tests were calculated and entered into Excel by study personnel. All data were double entered (by two different individuals) into spreadsheets which were then compared via computer. All data entry discrepancies were resolved using source data. All study data were archived on the computer information system of the University of Vermont GCRC.
Data analysis
The basic approach to data analysis was to perform repeated measures mixed model ANOVAs to determine differences related to the doses of mecamylamine on the dependent variables. For tasks that included blocks of trials (the stop signal task) or different trial types (immediate and delayed recognition memory trials), a series of mixed model ANOVA’s with drug condition and trial type as within subject factors were run to determine the combined effects of drug treatment and task condition on performance.
RESULTS
Cognitive measures
Stop signal task
There were no significant effects of mecamylamine on SSRT (a measure of inhibition) or accuracy on this task. There was a significant [F(3,42) = 2.96, p <.05] main effect of drug on reaction time to the go signal (GO-RT) with the 1.0 mg dose of mecamylamine associated with significantly (p <.05) slower reaction time and the 0.2 mg dose associated with a trend (p <.10) for slower reaction time than placebo.
The Stroop task
There was a significant [F(3,42) = 4.77, p <.05] main effect of drug on median reaction time with all three doses of mecamylamine associated with slower reaction times than placebo on this task. All three doses of mecamylamine were approximately 50 ms slower than placebo. We found the expected effects of task condition with the conflict condition associated with significantly slower reaction time than the word reading or color naming conditions. The drug related slowing was independent of reaction time differences due to task condition. Analysis of the Stroop effect examining the slowing due to stimulus conflict (compared to color naming) found no significant drug related difference.
High–low imagery task
Dependent measures (measures of hits, false alarms, discrimination, and response bias) were analyzed for all words as well as separately for high and low imagery condition. In addition, data were analyzed collapsed over all trials as well as by trial (two learning and one delayed).
Discrimination
There were no significant drug related effects on discrimination for total or high imagery words. There was a significant [F(3,42) = 3.19, p <.05] main effect of drug on discrimination for low imagery words (Figure 1). Discrimination was improved following the 0.5 mg dose of mecamylamine comparedtoplacebo (p <.05). Therewas nodoseX trial (two immediate and one delayed) interaction.
Figure 1.
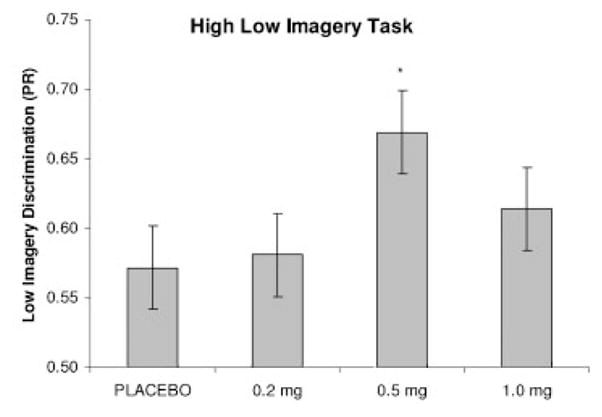
High low imagery task: discrimination for low imagery words. *=p <.05 different from placebo by Bonferonni corrected post hoc t-test
Examination of the total number of hits (words correctly identified as old; maximum of 14) on this task revealed a significant [F(6,14) = 4.71 p <.05] dose X trial interaction. Post hoc analyses showed significantly more hits associated with the 0.5 mg dose of mecamylamine than placebo during the delayed condition.
Response bias (BR)
There was a trend [F(3,40) = 2.52, p = .07] for a main effect of drug treatment on response bias for low imagery words, with the 0.5 mg dose of mecamylamine associated with a more liberal response bias (increased tendency to say “yes” when uncertain). There were no significant drug x trial interactions, nor any significant drug related differences on response bias for high imagery or total words.
Additional measures
Analysis of the change in discrimination and response bias between trials 1 and 2 (representing learning) and trials 2 and 3 (representing forgetting) was completed. None of the doses of mecamylamine were significantly different from placebo on measures of discrimination or response bias on these derived variables.
Choice delay task
Due to a computer error, data from the first five participants on this task were unavailable for analysis. Data from the remaining 10 participants were analyzed. There was a significant [F(3,24) = 6.26, p <.05) effect of drug on the number of delayed choices made during this task (Figure 2). Participants made significantly fewer delayed reward choices following administration of the 0.5 mg dose of mecamylamine than during placebo.
Figure 2.
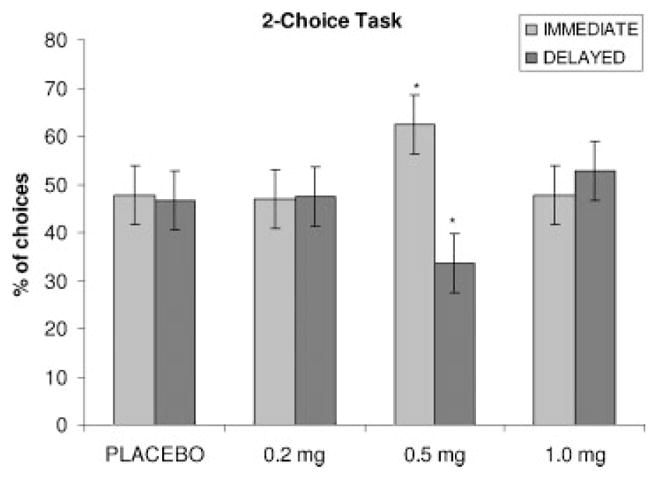
Effect of mecamylamine on the two-choice task. * indicates p <.05 different from placebo by Bonferonni corrected t-test
Behavioral measures
Subjective visual analog scale
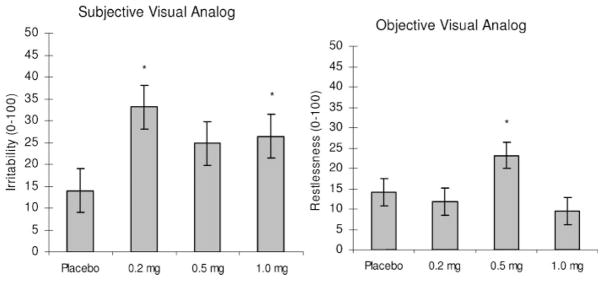
There was a significant [F(3,14) = 3.73 p <.05] effect of mecamylamine on self-rated irritability (Figure 3, left panel). Participants rated themselves significantly more irritable following the 1.0 and 0.2 mg doses of mecamylamine than placebo. There was a trend (p = .08) for the 0.5 mg dose of mecamylamine to be different from placebo.
Figure 3.
Behavioral effects. Left panel: subjective visual analog scale irritability rating, right panel: objective visual analog scale restlessness. * = p <.05 different from placebo by Bonferonni corrected t-test
Observer visual analog battery
There was a significant [F(3,14) = 3.61, p <.05] effect of drug on clinician rated restlessness (Figure 3, right panel). Post hoc comparisons revealed that the 0.5 mg dose of mecamylamine was associated with significantly greater restlessness than placebo.
Profile of mood states (POMS)
There were no significant effects of mecamylamine on either the total mood disturbance of any of the six sub-scales (tension, vigor, anger, confusion, depression, anxiety) on this measure.
Clinical global impressions scale
Mecamylamine did not significantly affect clinician’s ratings of illness severity, change in symptoms, or efficacy of drug.
Vital signs
Vital sign data (systolic and diastolic blood pressure and pulse) were analyzed using mixed-model repeated measures ANOVAs comparing the two drug conditions across the nine time points. Secondary analyses examined changes in vital signs from pre-drug baseline (each day) to 150-min post drug (estimated time for maximal physiological effects). There were no significant drug-related changes in vital signs in any of these analyses.
DISCUSSION
This is the first study to demonstrate measurable cognitive and behavioral effects of ultra-low doses of mecamylamine in humans. Within the ultra-low doses tested, there appeared to be an inverted U-shaped dose–response curve, with larger effects seen at the 0.5 mg dose compared to placebo, 0.2 and 1.0 mg.
Mecamylamine showed beneficial effects on recognition memory with the 0.5 mg dose associated with improved discrimination for low imagery words. This was evident across all trials with the largest improvement during the delay trial compared to placebo. This effect is consistent with the effects of acute nicotine on recognition memory in adolescents and young adults with ADHD (Potter and Newhouse, 2004, 2008).
There was no significant effect of ultra-low dose mecamylamine on SSRTon the stop signal task. The 0.2 and 1.0 mg doses of mecamylamine were associated with general slowing indicated by slower go reaction times. In our studies with nicotine, we have shown the opposite pattern of results with nicotine improving behavioral inhibition (SSRT) without affecting go-reaction time (Potter and Newhouse, 2004, 2008).
The 0.5 mg dose of mecamylamine was associated with fewer delayed choices on the CDT representing a reduced tolerance for delay. Reduced tolerance for delay is believed to represent impulsive decision-making and may underlie other impulsive behaviors in ADHD (Sonuga-Barke, 2002). This is consistent with the trend for the 0.5 mg dose of mecamylamine to be associated with a more liberal response bias which may reflect impulsive decision making. These findings are in contrast to prior work demonstrating that acute nicotine increases tolerance for delay (Potter et al., 2006; Potter and Newhouse, 2008). These data together with the findings that behaviorally mecamylamine was associated with increased subject-rated irritability and investigator-rated restlessness may limit the clinical utility of this dose of mecamylamine as a treatment for impulsivity in ADHD. While these effects would be consistent with nicotinic antagonism, the lack of significant effects on vital signs or rating of physical side effects suggests that the doses given in this study did not cause significant blockade of peripheral nicotinic receptor.
As this pilot study is the first to examine the effects of these doses of mecamylamine in humans it may be that we had insufficient power to detect the full range of effects of ultra-low doses of mecamylamine in this study. However, this is unlikely as the sample size was selected based on our prior work with nicotine in this population (Potter and Newhouse, 2004, 2008), and there are significant effects on recognition memory as hypothesized. Another possible interpretation of the finding that mecamylamine had a beneficial effect on recognition memory but not on behavioral inhibition is that the dose–response curve for behavioral inhibition may be different than the one for recognition memory and that an effect on behavioral inhibition would be seen at a different dose of mecamylamine. However, in our previous studies a low dose of nicotine (7 mg) for 45 min produced positive effects on recognition memory, delay aversion and behavioral inhibition at the single dose tested (Potter and Newhouse, 2004, 2008) arguing against low power, ineffective dosing, and the timing of our assessments as explanations for the lack of effect of mecamylamine on behavioral inhibition.
In acute drug challenge studies, it is important to consider the effect of repeated cognitive testing during the study. To minimize practice effects in this study, participants completed a training session prior to the drug challenge days. In addition, the order of drug administration was randomized and counterbalanced to further minimize the effects of repeated testing on the pattern of results seen in this study. Finally, the data from all of the cognitive tasks were examined to determine if the data indicated that there were ceiling effects, but this was not the case (see Table 2).
Table 2.
Results of cognitive tasks
| Placebo mean (SE) | 0.2 mg mean (SE) | 0.5 mg mean (SE) | 1.0 mg mzean (SE) | ANOVA (main effect of dose) | |
|---|---|---|---|---|---|
| Stop signal task | |||||
| Go reaction time | 553.65 (18.76) | 572.38 (18.76) | 553.78 (18.80) | 578.8 (18.79) | F(3,42) = 2.96 p <.05 |
| Stop signal reaction time | 233.63 (12.) | 241.00 (12.0) | 238.96 (12.0) | 252.85 (12.0) | F(3,42) = 1.12 p = NS |
| Accuracy | 97.7 (.89) | 97.0 (.89) | 98.6 (.90) | 98.6 (.90) | F(3,42) = 0.78 p = NS |
| Recognition memory | |||||
| Total PR | 0.70 (.02) | 0.69 (.02) | 0.74 (.02) | 0.73 (.02) | F(3,42) = 2.19 p = NS |
| High PR | 0.74 (.02) | 0.71 (.02) | 0.73 (.02) | 0.76 (.02) | F(3,42) = 1.06 p = NS |
| Low PR | 0.57 (.03) | 0.58 (.03) | 0.67 (.03) | 0.61 (.03) | F(3,420) = 3.19 p <.05 |
| Total BR | 0.27 (.03) | 0.28 (.03) | 0.30 (.03) | 0.27 (.03) | F(3,42) = 0.30 p = NS |
| High BR | 0.37 (.03) | 0.36 (.03) | 0.36 (.03) | 0.37 (.03) | F(3,42) = 0.18 p = NS |
| Low BR | 0.30 (.04) | 0.33 (.04) | 0.37 (.04) | 0.29 (.04) | F(3,42) = 2.07 p = NS |
| Two-choice task | |||||
| # delayed choices | 18.00 (2.4) | 20.00 (2.4) | 14.22 (2.4) | 21.11 (2.4) | F[3,24) = 6.84 p <.01 |
A final caveat to be considered is that participants in this study had different histories of taking stimulant medication. The effect of this on the cognitive results in our study cannot be determined. In trying to understand the effect of medication history on cognition and neural function, Rubia et al. (2005) examined brain activation during behavioral inhibition in adolescents with ADHD who were treatment naïve. They found patterns of brain activation that were consistent with prior findings in adolescents who had a history of medication use. The authors concluded that the abnormal brain activation was related to the disorder since it is found in ADHD adolescents both with and without a history of stimulant medication usage (Rubia et al., 2005). However a study by Pliszka et al. (2006) found volumetric differences in the anterior cingulate cortex (ACC) related to stimulant medication history in children with ADHD, with smaller right ACC in medication naïve children. However this study did not look at performance differences related to these volumetric differences, and did not report the dosages of medication in the treatment group. It is also unknown if these volumetric differences change over time and development and specifically if they persist into adulthood.
Taken together this study demonstrates that there are measurable cognitive and behavioral effects of ultra-low doses of mecamylamine with beneficial effects on memory, no effect on behavioral inhibition, and reduced tolerance for delay. These results are intriguing as we have found an opposite effect of nicotine on delay aversion in ADHD (Potter and Newhouse, 2004, 2008). In addition, our prior studies found that nicotine improved both behavioral inhibition and recognition memory in adult ADHD (Potter and Newhouse, 2004, 2008). The finding of improved recognition memory but decreased delay tolerance suggests that ultra-low doses of mecamylamine are not having a paradoxical “agonist” effect at NACH receptors or we would expect to see results that parallel our previous nicotine findings. These effects are not fully understood and may represent mixed receptor activity (activation and blockade) of nicotinic receptors at the doses tested. Further study is needed to determine the mechanism of action as well as the time-course of cognitive effects associated with ultra-low doses of mecamylamine.
Acknowledgments
This work was supported by: GCRC M01–00109 and Targacept Inc. The authors thank Dr Gordon Logan and Dr Donald Dougherty for providing cognitive testing software that was used in this study.
References
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4. U.S. Government Printing Office; 1994. [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psych Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Anastopoulos AD, Guevremont DC, Fletcher KE. Adolescents with ADHD: patterns of behavioral adjustment, academic functioning, and treatment utilization. J Am Acad Child Adolesc Psychiatry. 1991;30:752–761. doi: 10.1016/s0890-8567(10)80010-3. [DOI] [PubMed] [Google Scholar]
- Biederman J, Mick E, Faraone SV. Age-dependant decline of symptoms of attention deficit hyperactivity disorder: Impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- Conners CK, Levin ED, Sparrow E, et al. Nicotine and attention in adult ADHD. Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- Driscoll P. Nicotine-like behavioural effects after small dose of mecamylamine in Roman high-avoidance rats. Psychopharmacologia. 1976;46:119–121. doi: 10.1007/BF00421559. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen interacts with the cholinergic system to affect verbal memory in post-menopausal women: evidence for the critical period hypothesis. Horm Behav. 2008;53:159–169. doi: 10.1016/j.yhbeh.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas J, Saykin A, McAllister T, McDonald B, Hynes M, Newhouse P. Nicotinic versus muscarinic blockade alters verbal working memory performance and brain activation patterns in older women. Am J Geriatr Psychiatry. 2009 doi: 10.1097/JGP.0b013e3181602a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. (SCID-I/P) [Google Scholar]
- Gualtieri CT, Johnson LG. Efficient allocation of attentional resources in patients with ADHD: maturational changes from age 10 to 29. J Atten Disord. 2006;9(3):534–542. doi: 10.1177/1087054705283758. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein J, Curry JF. The neuropsychology of adults with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2004;18(3):485–503. doi: 10.1037/0894-4105.18.3.485. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D. Schedule for affective disorders and schizophrenia for school-age children-president and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, et al. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Canu W, March J. Effects of chronic nicotine and methylphenidate in adults with ADHD. Exp Clin Psychopharmacol. 2001;9:83–90. doi: 10.1037/1064-1297.9.1.83. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Manual for the Profile of Mood States Educational and Industrial Testing Service. San Diego, CA: 1971. [Google Scholar]
- National Institute of Mental Health. CGI (Clinical Global Impression) scale. Psychopharmacol Bull. 1985;21:839–843. [Google Scholar]
- Newhouse PA, Potter AS, Singh S. Effects of nicotinic stimulation on cognitie performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, et al. Intravenous nicotine in Alzheimer’s disease: a pilot study. Psychopharmacology. 1988;95:171–175. doi: 10.1007/BF00174504. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychological theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57(11):1424–1435. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Lancaster J, Liotti M, Semrud-Clikeman M. Volumetric MRI differences in treatment-naïve vs chronically treated children with ADHD. Neurology. 2006;67:1023–1037. doi: 10.1212/01.wnl.0000237385.84037.3c. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse P. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer’s disease. Psychopharmacology. 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Potter A, Newhouse P, Bucci D. Central nicotinic cholinergic systems: a role in the cognitive dysfunction in attention-deficit/hyper-activity disorder? Behav Brain Res. 2006;175(2):201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Potter A, Newhouse P. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacol, Biochem Behav. 2008;88(4):407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detectionin mediation-naïve adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rusted JM, Warburton DM. Facilitation of memory by post-trial administration of nicotine: evidence for an attentional explanation. Psychopharmacology. 1994;108(4):452–455. doi: 10.1007/BF02247420. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms in normal states and neuropsychiatric disease. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: towards a unifying hypothesis. Brain Res Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic binding sites in the brain: in vivo regulation. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. In vivo regulation of [3H]-acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem. 1985;45:427–433. doi: 10.1111/j.1471-4159.1985.tb04005.x. [DOI] [PubMed] [Google Scholar]
- Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Shytle DR, Silver AA, Wilkinson BJ, Sanberg PR. A pilot controlled trial of transdermal nicotine in the treatment of Attention Deficit Hyperactivity Disorder. World J Biol Psychiatry. 2002;3(3):150–155. doi: 10.3109/15622970209150616. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Abikoff HB, Sonuga-Barke E, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A Supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol. 2001;29:215–223. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Dalen L, Remington B. Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psychiatry. 2003;42:1335–1338. doi: 10.1097/01.chi.0000087564.34977.21. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E. Psychological heterogeneity in AD/HD - a dual pathway model of behanviour and cognition. Behav Brain Res. 2002;130:29–36. doi: 10.1016/s0166-4328(01)00432-6. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Prendergast MA. Dose-specific improvements in memory-related performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Dev Res. 1999;47:127–136. [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wilens T, Verlinden M, Adler L, Wozniak P, West S. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults. Results of a Pilot Study. Biological Psychiatry. 2006;59(11):1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AD, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams B, Ponesse J, Schachar R, Logan G, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]