Abstract
Prostate cancer is a major cause of cancer-related death in American men, for which finding new therapeutic strategies remains a challenge. Early growth response-1 (EGR1) is a transcription factor involved in cell proliferation and in the regulation of apoptosis. Although it has long been considered a tumor suppressor, a wealth of new evidence shows that EGR1 promotes the progression of prostate cancer. This review addresses the paradoxes of EGR1 function. While EGR1 mediates apoptosis in response to stress and DNA damage by regulating a tumor suppressor network, it also promotes the proliferation of prostate cancer cells by a mechanism that is not fully understood. Thus, EGR1 might be targeted for prostate cancer therapy either by ectopic expression in combination with radiotherapy or chemotherapy, or by direct inhibition for systemic treatment. Possible strategies to antagonize EGR1 function in a therapeutic setting are discussed.
Keywords: EGR1, oncogene, prostate cancer, transgenic mouse mode, tumor suppressor
Prostate cancer is the most commonly diagnosed cancer in men and the second highest cause of cancer-related death among men in the USA. In 2007, there were an estimated 218,890 new cases of prostate cancer and an estimated 27,050 deaths [1]. Routine use of prostate-specific antigen (PSA) screening enables a better diagnosis, but is still deficient in two ways. First, the correlation between PSA levels and the presence of cancer is indirect, so the presence of cancer must be confirmed by biopsy. Second, current biopsy methodology is diagnostic but not prognostic. Consequently, prostate cancer patients with minimally invasive forms of cancer needlessly undergo an aggressive surgical procedure.
The progression of the disease follows multiple steps, from benign hyperplasia to hormone-independent metastatic disease. Unfortunately, approximately 90% of patients with advanced disease will develop bone metastases, which are associated with severe pain, loss of mobility and spinal cord compression. Other affected organs may include the liver, lungs and brain [2]. Despite extensive research efforts, little hope exists for patients with hormone-refractory prostate cancer that is resistant to hormone therapy, radiation and conventional chemotherapy. Discovery of new treatments for advanced prostate cancer and identification of patients at risk of progression can only be achieved through a better understanding of the molecular mechanisms underlying the progression of the disease.
Recent studies have highlighted the involvement of the transcription factor early growth response-1 (EGR1) in prostate cancer. This is paradoxical, since EGR1 had been demonstrated to act as a tumor supressor in other types of cancer (reviewed in [3]). This review offers an overview of the evidence of EGR1 contribution to prostate cancer and its molecular mechanism of action. We briefly discuss potential strategies to target EGR1 for cancer therapy, providing that the uncertainties regarding its function are eliminated.
EGR1
EGR1 is a nuclear phosphoprotein that was first identified based on its early induction by mitogens and differentiation factors [4-8]. It is also known as NGFI-A, Zif268, T1S8 and krox-24. The unstimulated EGR1 level of expression is low in most tissues, except in brain where high expression is observed [9]. EGR1 contains a highly conserved DNA-binding domain composed of three zinc fingers that bind to the prototype target GC-rich consensus sequence GCG (G/T) GGGCG [3,10]. In addition, EGR1 binds to the regulatory proteins NAB-1 and NAB-2 (NGF-I A-binding proteins) that repress its transcriptional activity [11-13].
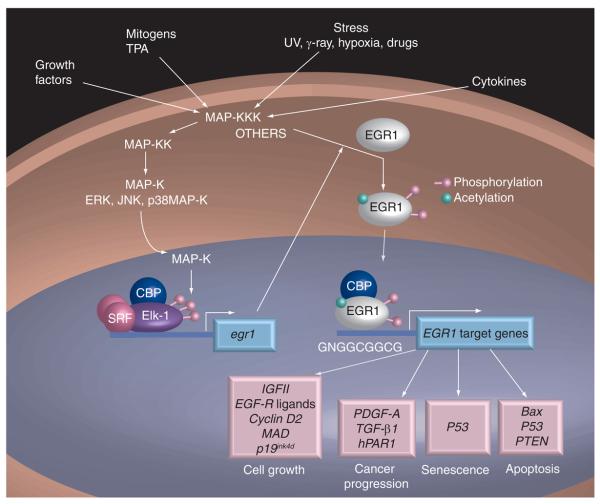
EGR1 is induced by growth factors, cytokines and stress signals such as radiation, injury or mechanical stress [3,10,14]. Cloning of the EGR1 promoter has revealed the presence of response elements for various transcription factors. Specifically, the promoter contains several serum response elements (SREs), an AP-1 binding site, several cAMP regulatory elements (CREs) and Sp1 consensus sequences [15-18]. Most often, increased transcription of EGR1 is mediated by the MAP-K signaling pathway (Figure 1). Three MAP-K families, known as ERK1/2, JNK and p38MAP-K, are most commonly involved in EGR1 activation. While ERK1/2 mediates EGR1 expression in response to growth factors [19-22], a combination of ERK1/2, p38MAP-K and/or JNK is required to induce EGR1 in response to stress [23,24]. The activated MAP-K translocates to the nucleus, phosphorylates and activates transcription factors of the Elk-1/SAP-1/2 family. Elk-1 then associates with the ternary complex factor consisting of CREB-binding protein (CBP) and serum response factor (SRF), and binds to and transactivates the EGR1 promoter (reviewed in [25,26]). An alternative pathway for EGR1 activation was recently discovered, in which transcription factor NF-κB mediates EGR1 transcription in response to UV exposure of human skin. This study identified a canonical NF-κB binding site and demonstrated the direct binding of p65 (RelA) to the EGR1 promoter [27]. In another study, two functional nonconsensus binding sites for the tumor suppressor p53 were identified. Binding of p53 to the EGR1 promoter in response to DNA damage leads to sustained expression of EGR1 and efficient apoptosis [28]. Finally, EGR1 binds to its own promoter and represses transcription, thereby initiating a negative feedback loop soon after activation [29].
Figure 1.
EGR1 mechanism of activation.
EGR1 activity and stability are regulated by post-translational modifications. Acetylation, mediated by the transcriptional co-activator p300/CBP, stabilizes EGR1 and may promote survival, whereas phosphorylation in response to stress may favor cell death [30]. Sumoylation is mediated by tumor suppressor p19ARF and directs EGR1 to the nucleus [31]. The short-lived EGR1 is then ubiquitinated on multiple sites and degraded by the proteasome [32]. We suspect that the full spectrum of post-translational modifications regulating EGR1 activity is far from being fully deciphered.
EGR1 knockout mice
EGR1 is involved in the development and response to stress of various organs, and consequently emerges in a number of patho logical conditions. However, suppression of EGR1 in mice produces few defects. Two strains of EGR1−/− mice have been generated that exhibit somewhat distinct phenotypes. The EGR1−/− mice generated by Milbrandt's team show a fairly normal phenotype, except for infertility in the female [33,34]. By contrast, the mice developed by Charnay's team, which are also infertile (both male and female), have reduced body size and weight resulting from the altered development of the pituitary gland. Female EGR1−/− mice in this study had atrophied reproductive organs with a smaller uterus and ovaries, whereas the EGR1−/− males had smaller testes, seminal vesicles and, notably, a smaller prostate [35].
Mouse embryo fibroblasts (MEFs) derived from Charnay's animals display a reduced rate of proliferation compared with EGR1+/+ MEFs [35]. Importantly, EGR1−/− MEFs derived from Milbrandt's animals bypass senescence and display the growth properties of immortalized cells. EGR1 regulation of senescence is mediated by p53, as indicated by the observation that forced expression of EGR1 restores senescence in EGR1−/− cells, but not in p53−/− cells [36].
EGR1-null mice do not spontaneously develop tumors, although an accelerated development of tumors was observed in a two-step carcinogenesis model of skin cancer, arguing in favor of a tumor suppressor role in response to DNA damage in vivo [37]. Many studies have used these animals to examine the role of EGR1 in a variety of pathological situations, which is beyond the scope of this review. Findings that are the most relevant to prostate cancer come from the breeding of EGR1 knockout mice with transgenic mouse models of prostate cancer, such as the cryptdin-2-T-antigen model (CR2-TAg) and the transgenic adenocarcinoma mouse prostate (TRAMP). CR2-TAg mice develop prostate carcinoma from neuro endocrine prostate cells due to the targeted expression of SV40 large T antigen (SV40-TAg) [38]. Strikingly, CR2-TAg/EGR1−/− mice survive appreciably longer than CR2-TAg/EGR1+/− or CR2-TAg/EGR1+/+ mice [39]. Increased tumor latency is observed in CR2-TAg/EGR1−/− mice that is not caused by a decreased rate of initiation or by a decreased rate of tumor growth, but is due to delayed progression from prostatic intraepithelial neoplasia lesions to adenocarcinoma. A similar delay in prostate cancer progression was observed in TRAMP mice [39], which develop tumors from the luminal epithelial cells of the prostate [40]. These observations strongly establish EGR1 as a key player in the progression of prostate cancer.
Role of EGR1 in prostate cancer progression
Many other observations support the notion that EGR1 contributes to prostate cancer progression (previously reviewed in [41,42]). In prostate adenocarcinoma, the mRNA encoding EGR1 is expressed at higher levels compared with normal tissues [43,44]. The observation that levels of mRNA and protein expression correlate with Gleason scores and inversely correlate with the degree of differentiation of carcinoma cells also suggests that EGR1 is involved in cancer progression [43]. EGR1 expression in the primary tumor correlates with radiation response in terms of complete control of the local tumor. In the postirradiated biopsies it correlates with residual tumor and treatment failure [45]. NAB2, the regu latory protein that represses the transcrip tional activity of EGR1, is downregulated in human primary prostate carcinomas [46]. Interestingly, EGR1 is also overexpressed in the tumors of CR2-TAg mice, whereas NAB2 expression is decreased [39]. Thus, both the upregulation of EGR1 and loss of its repressor, NAB2, participate in determining the high level of EGR1 activity in human prostate cancer.
There are only a few documented examples of EGR1 overexpression in cancer. EGR1 is elevated in Wilm's tumors and ectopic expression of EGR1 increases the proliferation of kidney cells. In addition, it was observed that EGR1 antagonizes the effect of the tumor suppressor Wilm's tumor-1 (WT-1) [47]. In melanoma, EGR1 overexpression is associated with mutation of B-RAF [48]. Approximately 60% of human melanomas contain a mutation in the B-RAF gene [49]. As RAF acts upstream of the ERK pathway, activating mutations of B-RAF result in constitutive ERK1/2 activity. EGR1 overexpression requires both the presence of mutant B-RAF and the activity of ERK1/2 [48].
To understand the molecular mechanism leading to EGR1 upregulation in prostate cancer, we used a collection of transformed, immortalized or primary prostate cell lines. A stringent correlation between EGR1 and p53 expression was observed [Sauer L et al.: Egr-1 initiates a feedback loop that involves the EGF-receptor/ERK1/2 pathway and is regulated by p53 in prostate cancer cells. Manuscript submitted]. Among EGR1 expressing cells, the DU145 cell line contains a p53 mutant [50]. Other cell lines in which EGR1 was overexpressed exhibit high p53 protein expression, owing to its stabilization by SV40-TAg used for immortalization. EGR1 expression was high in all cells with the p53 mutant or SV40-TAg, whether they were transformed or not, and its expression was abrogated by pharmacological inhibition or silencing of p53. Conversely, EGR1 expression was low in cancer and nontransformed cells containing low or undetectable p53 expression.
It is interesting to note, in this context, that EGR1 overexpression was observed in CR2-TAg mice that also express SV40-TAg [39], suggesting that it is a common mechanism with in vivo significance. EGR1 deficiency results in delayed cancer progression in these mice or in SV40-Tag-expressing TRAMP mice. One can wonder what would be the effect of EGR1 deficiency in other models of cancer that do not rely on SV40-TAg.
Whether EGR1 is involved in the etiology of prostate cancer is yet to be established. One would contend that EGR1 is not essential for tumor initiation because tumors do develop in EGR1−/− transgenic mouse models of prostate cancer [39]. On the other hand, transgenic expression of SV40-TAg in prostate cells is powerfully tumorigenic and overrides the need for an endogenous initiating signal. To our knowledge, forced expression of EGR1 in nontransformed prostate cells has not been achieved, with one arguable exception. Stable transfection of EGR1 in 267B nontransformed prostate cells results in increased colony formation and increased growth in soft agar, two hallmarks of transformation in vitro [51]. However, the 267B cell line originated from a fetal prostate epithelium and was immortalized using SV40-Tag [52], which is now known to interfere with EGR1 function [Sauer L et al.: Egr-1 initiates a feedback loop that involves the EGF-receptor/ERK1/2 pathway and is regulated by p53 in prostate cancer cells. Manuscript submitted]. Therefore, the possibility is real that similar results may not be observed in nontransformed cells that do not contain SV40-TAg. The fact that increased transformation was observed in 267B clones overexpressing EGR1 suggests that the oncogenic action of EGR1 may be proportional to its level of expression.
The particular role of EGR1 in prostate cancer could be due, at least in part, to a crosstalk with the androgen receptor (AR). Specifically, EGR1 physically interacts with the AR in hormone-sensitive prostate cancer cells. Moreover, the EGR1–AR complex binds to the promoter of PSA, an endogenous target of AR [53]. Overexpression of an activated mutant of EGR1 in AR-positive prostate cancer cells leads to translocation of AR to the nucleus and contributes to the regulation of PSA transcription [53]. As a consequence of this crosstalk, over expression of a constitutively active mutant of EGR1 in hormone-sensitive LNCaP cancer cells leads to increased proliferation in vitro. In a xenograft model, it enhances tumor growth in castrated animals [54]. Since castration mimics hormone therapy in human patients, these results suggest that EGR1 may be involved in the acquisition of resistance to hormone therapy.
Several new targets of EGR1 were identified in a microarray analysis of mouse prostate cancer cells, some of which are involved in cell-cycle regulation [55]. Three targets with cell-cycle function were formally validated: cyclin D2, MAD and p19ink4d. It was demonstrated that MAD and p19ink4d, which are growth inhibitors, were downregulated by EGR1, whereas cyclin D2, which contributes to cell-cycle progression through G1, was upregulated. Through altered transcription of cell-cycle regulators, EGR1 promotes the growth of prostate cancer cells, as evidenced by the fact that EGR1 silencing inhibits cell proliferation, colony formation and growth in soft agar [55,56].
EGR1 also stimulates the production of many growth factors and cytokines. A microarray analysis performed in LAPC4 prostate cancer cells, in which EGR1 overexpression was driven by adenovirus-mediated transfection, identified IGF-II, PDGF-A and TGF-β1 as EGR1 targets [57]. Interestingly, several genes identified in this array are associated with neuroendocrine differentiation, which is known to occur frequently in prostate cancer. In vivo, the amount of both TGF-β1 and PDGF-A produced in prostate tumors was decreased in CR2-TAg/EGR1−/− mice compared with CR2-TAg/EGR1+/+ mice [39]. Accordingly, human tumor-derived DU145 cells that display high EGR1 expression secrete TGF-β1 and PDGF-A [Sauer L et al.: Egr-1 initiates a feedback loop that involves the EGF-receptor/ERK1/2 pathway and is regulated by p53 in prostate cancer cells. Manuscript submitted]. In addition, three ligands of EGF-R (epiregulin, HB-EGF and amphiregulin) are induced by EGR1 in DU145 cells, resulting in autocrine activation of the EGF-R and activation of survival and proliferative signals.
Thus, the increased transcription of growth factors and cytokines leads to autocrine cell growth and to alteration of the tumor environment, and is likely to directly contribute to cancer progression. Indeed, PDGF-A promotes angiogenesis (reviewed in [58]), whereas TGF-β1 is associated with advanced prostate cancer and promotes alteration of the microenvironment and metastasis, as well as immune suppression and angiogenesis [59-61].
Finally, a role for EGR1 in metastasis has been suggested. Salah et al. demonstrated that EGR1 regulates the transcription of human protease-activated receptor 1 (hPAR1), which is implicated in epithelial malignancies [62]. hPAR1 expression is regulated by androgen, explaining its high and low levels in prostate cancer tissues before and after hormone ablation therapy, respectively. However, high levels of hPAR1 are found in hormone-resistant prostate carcinoma [63]. EGR1 binds the hPAR1 promoter in vivo and in vitro. Bombesin, a neuro endocrine peptide known to activate EGR1 [64], induces hPAR1 expression and cell invasion in an androgen-independent manner [62]. A direct correlation was observed between the expression of EGR1 and PAR1, and the degree of progression in human prostate tumors [62]. Another study found that heparanase, which degrades heparan sulfate and has been implicated in invasion and metastasis, is regulated by EGR1 in prostate cancer. High heparanase expression in tumors appears to be associated with increased EGR1 expression, coupled with the hypomethylation of the heparanase promoter in an EGR1 target sequence [65]. Finally, EGR1 regulates the transcription of HYAL-1, an enzyme that degrades hyaluronic acid and is involved in tumor growth and metastasis. Interestingly, the EGR1 binding site in the HYAL-1 promoter overlaps with an SP1 binding site. Whereas SP1 binds to the methylated promoter to repress transcription, EGR1 binds to the nonmethylated promoter and activates transcription. Levels of HYAL-1 expression are proportional to EGR1 expression in prostate cancer cell lines [66]. Taken together, these studies indicate that EGR1 regulates the expression of various extracellular proteins that function in the remodeling of the extracellular matrix and are known to participate in metastasis.
Role of EGR1 in stress-induced apoptosis
A paradoxical role of EGR1 in cancer lies in its capacity to promote apoptosis in response to stress and DNA damage, potentially acting as a tumor suppressor. In fact, in a number of other types of cancer, EGR1 has a tumor suppressive function (reviewed in [67]). EGR1 transcription is increased following DNA damage, stress or mitogen exposure. Strong evidence for a pro-apoptotic function is provided by the observation that EGR1−/− MEFs are resistant to apoptosis induced by ionizing radiation [68].
EGR1 belongs to a network of tumor suppressor genes that function together in response to stress. For example, EGR1 activates the transcription of the tumor suppressor PTEN in vitro and in vivo. EGR1-deficient cells that are resistant to apoptosis also lack PTEN induction upon stress [69]. In a recent study, EGR1 and PTEN were identified as mediators of p14ARF function [31]. EGR1-dependent expression of PTEN is controlled by p14ARF through ARF-mediated sumoylation of EGR1. This requires the phosphorylation of EGR1 by the protein kinase AKT, which promotes the association of EGR1 with p14ARF. EGR1 is not sumoylated in ARF−/− fibroblasts or ARF−/− mouse tissues, and this correlates with defective PTEN expression. Both tumor suppressors, PTEN and p14ARF, are often downregulated in human cancer, or their function impaired owing to genetic mutation [31].
There are numerous instances of EGR1 activity in response to stress in prostate cancer cells. For example, EGR1 is activated by hypoxia in DU145 prostate cancer cells and binds to the promoter of HIF-1α, increasing its transcription [70]. In prostate cancer cells lacking p53, apoptosis caused by ionizing irradiation is mediated by EGR1-induced secretion of TNF-α, a cytokine with known pro-apoptotic properties [71]. In cells that do contain it, p53 is a target of EGR1 and a major mediator of EGR1 apoptotic function [36,37,72]. Another study has demonstrated that EGR1 increases the expression of Rb, which prevents Mdm2-mediated degradation of p53, and thus also upregulates p53 [68]. In addition to p53, EGR1 also induces the expression of p73 upon stress. p73 is an isoform of p53 that also functions as a transcription factor and is involved in stress-induced apoptosis. The three proteins EGR1, p73 and p53 are required for the full apoptotic response to take place. In addition, both p73 and p53 induce EGR1 expression in a feedback loop that promotes sustained expression of the three proteins and persistence of the apoptotic signal [28].
A recent study from Ahmed's laboratory shows that sensitivity of prostate cancer cells to irradiation-induced apoptosis is increased upon forced expression of EGR1, but is decreased when EGR1 is knocked down [73]. In response to DNA damage, EGR1 directly interacts with the YAP-1 protein, and the EGR1–YAP-1 complex induces the transcription of the pro-apoptotic protein Bax. Overall, these results indicate that direct transactivation of Bax by EGR1–YAP-1 sensitizes the cells to irradiation-induced apoptosis [73].
Arora et al. found that EGF-R in M12 prostate cancer cells (that contain SV40-TAg) mediates UV-induced apoptosis through activation of EGR1 [74]. The fact that UV exposure activates the EGF-R has been known for some time [75]. In this recent study, UV irradiation activated the EGF-R, induced EGR1 expression and led to apoptosis. Novel targets of EGR1 were identified by ChIP-on-chip analysis, 24 of which belong to the EGF-R signaling pathway. The validated targets include FasL, MAX and RRAS2, which are involved in apoptosis or growth arrest [74].
How do we resolve the paradox of an oncogenic function of EGR1 (when overexpressed in prostate cancer) with its tumor suppressor function? We have previously proposed that in prostate cancer, the tumor suppressor function of EGR1 becomes deficient in many ways [76]. For example, two major mediators of EGR1 apoptotic function are inactivated in the majority of prostate tumors. The first, p53, is inactivated in 25–50% of prostate cancer cases [77]. The second, PTEN, is not normally regulated by EGR1 in prostate cancer cells that contain wild-type PTEN ([56] and [Baron VT et al., The Vaccine Research Institute of San Diego. Unpublished data]). One hypothesis is that inactivation of the PTEN gene owing to hypermethylation, which occurs in approximately 50% of prostate cancer cases [78], may prevent the binding of EGR1 to the promoter. In addition, PTEN is the most commonly altered gene in prostate cancer [79]. Lack of PTEN correlates with high Gleason score and advanced stage cancer [80]. Up to 60–80% of prostate tumors contain a hemizygous deletion of PTEN, and haplo-insufficiency is enough for the progression of prostate cancer [79,81,82].
Another tumor suppressor that is regulated by EGR1 in prostate cancer is TGF-β1 [55,57,83]. Forced expression of EGR1 in HT1080 fibrosarcoma suppresses transformation, owing, in part, to upregulation of TGF-β1, which inhibits growth by an autocrine mechanism [83]. However, many prostate cancer cells become resistant to TGF-β1 [59,84]. In addition, a switch of function occurs, since TGF-β1 also promotes cancer progression in vivo by acting as an angiogenesis factor and by suppressing the anti-tumor immune response [60,61].
Targeting EGR1 for prostate cancer therapy
Despite the role that EGR1 plays in prostate cancer, very little has been done towards its validation as a target for therapy. This is likely to be owing to the fact that EGR1 function in cancer remains paradoxical and is not fully understood. These paradoxes must be addressed before we can target EGR1 for cancer therapy. In particular, the observation that EGR1 can be a tumor suppressor warns us of the possible danger of long-term inhibition. EGR1-null mice do not spontaneously develop cancer, which argues against the hypothesis that long-term treatment with EGR1 inhibitors will cause the formation of tumors. On the other hand, whereas loss of EGR1 delays prostate cancer progression in transgenic mouse models [39], it accelerates tumor growth in a two-step skin carcinogenesis model [37]. This suggests that systemic inhibition of EGR1 could increase the incidence of DNA damage-induced cancers caused by, for example, smoking or excessive sun exposure. This may be addressed by using local delivery (such as intratumoral injection), or through molecular targeting of the drug to the tumor.
Strategies to antagonize EGR1 function
RNA interference (RNAi; antisense oligonucleotides, vector-based small interfering RNA or DNAzyme) is currently the best available strategy to specifically inhibit EGR1. Discussing the limitations of the RNAi approach for clinical use is not within the scope of this review. However, the lack of other effective and specific small-molecule EGR1 inhibitors does limit our ability to investigate EGR1 function and pursue other therapeutic options.
Antisense oligonucleotides that block EGR1 expression have demonstrated efficacy in a mouse model of prostate cancer. In a test experiment performed in TRAMP mice, a dose of 25 mg/kg of EGR1 antisense oligonucleotide nearly completely inhibited EGR1 expression in the prostate tumor [51]. Male TRAMP mice were treated with saline, a three-base mismatch control oligonucleotide or EGR1 antisense oligonucleotide. The mice were 22 weeks old at the start of treatment and received intraperitoneal injections for 10 weeks [56]. The incidence of tumor was lower in antisense-treated mice, and inhibition of EGR1 significantly delayed tumor growth [56]. Importantly, at a dose of antisense oligonucleotide that is commonly used in mice, no apparent toxicity was observed. Thus, inhibition of EGR1 delays the development of prostate cancer and does not seem to be associated with significant short-term side effects.
Overexpression of the EGR1 repressor NAB2 has been examined as a way to decrease EGR1 activity. Forced NAB2 expression in vascular cells decreases the transcription of EGR1 target genes, and inhibits angiogenesis in vitro [85]. To our knowledge, this approach has not been used in cancer cells. Potential problems may arise from the fact that NAB2 binds to all EGR family members. In addition, a co-activator function of NAB2 has been recently discovered. Thus, NAB2 represses or activates EGR1-mediated transcription depending on each target gene [86], adding a complexity that may preclude its use for therapeutic purposes.
The use of natural compounds that have no toxic side effects, such as curcumin, may be an interesting alternative. Curcumin, found in turmeric, exhibits antioxidant, anti-inflammatory and anticarcinogenic effects [87]. Curcumin inhibits the activity of EGR1 in monocytes and colon cancer cells through inhibition of ERK-1/2 and Elk-1 phosphorylation [88,89]. It prevents EGR1 transcription in response to IL-1β in lung epithelial cells, which decreases the expression of the inflammation enzyme mPGES-1 [90]. On the other hand, curcumin activates EGR1 in human glioma cells, leading to EGR1-mediated transcription of cell-cycle inhibitor p21 and inhibition of cell proliferation [91]. It should be noted that curcumin exerts its anticancer effects through a variety of signaling molecules, and is by no means specific to EGR1 [92].
Other strategies that may be developed include single or double-stranded DNA mole cules that mimic the EGR1 cognate DNA-binding sequence. These small DNA analogs could compete with cellular DNA for EGR1 binding and would capture EGR1 and prevent its binding on target genes. Such an approach has been used, for example, against the STAT1 and STAT3 transcription factors [93,94]. Finally, a high-throughput platform could be used to screen chemical libraries in order to isolate small-molecule drugs that decrease EGR1 transcriptional activity. A small-molecule drug may have the advantage of solubility and good pharmacological properties.
Ectopic expression of EGR1
Another strategy to target EGR1 for the therapy of prostate cancer could take advantage of its proapoptotic function in response to DNA damage. Indeed, a very recent study has demonstrated that forcing EGR1 expression in PC3 prostate cancer cells using an adenovirus vector (adEGR1), combined with irradiation, decreased the growth of xenograft tumors in nude mice more efficiently than irradiation alone [73]. This result is especially relevant to the fact that prostate cancer is often treated with radiotherapy, and indicates that forced expression of EGR1 can increase the sensitivity of cancer cells to DNA damage. Prostate tumors are notoriously resistant to chemotherapy, and therefore it would be interesting to determine whether the combination with ectopic expression of EGR1 would also improve the efficacy of current chemotherapies. Systemic treatment, rather than targeted treatment, may be considered, since ectopic expression of EGR1 in endothelial cells was found have a potent anti-angiogenic function and to inhibit cell invasion in a xenograft model; it also inhibited tumor growth in a mouse fibrosarcoma model [95]. It remains to be determined if the anti-angiogenic function of EGR1 would be maintained in combination with radio- or chemo-therapy. Finally, the potential danger of increasing EGR1 oncogenic function will have to be carefully evaluated.
Other therapeutic uses of EGR1
A different approach has been developed that does not directly target EGR1, but takes advantage of its responsiveness to DNA damage and stress, such as that caused by irradiation. In this approach, the EGR1 promoter is placed upstream of the TNF-α gene into a replication-deficient adenovirus, termed Ad.Egr.TNF. This vector is currently delivered by intratumoral injection and shows little toxicity. In principle, local radiation therapy allows for temporal (and spatial) control of TNF-α release, which induces apoptosis of the tumor cells. In vivo studies have demonstrated that injection of tumors with Ad.Egr.TNF potentiates the cytotoxic effect of irradiation. Ad.Egr.TNF is currently under clinical investigation for the treatment of various solid tumors (reviewed in [96]). A new alternative for patients who are intolerant to radiation comes from resveratrol, a natural compound that induces the expression of EGR1 [97]. A recent study demonstrated that resveratrol activated Ad.Egr.TNF in tumor xenografts, and increased the anti-tumor response in both human and rat models. Thus, the activation of TNF-α expression by resveratrol may extend the use of Ad.Egr.TNF [98].
Conclusion & future perspective
The observation that EGR1 is overexpressed in human prostate tumors and promotes prostate cancer progression undeniably challenged the view that EGR1 is purely a tumor suppressor. Our knowledge of EGR1 function in prostate cancer has improved considerably since these discoveries were made. However, given its paradoxical function in cancer, further elucidation of its mechanism of action remains essential and many uncertainties must be resolved before EGR1 can safely be considered a target for prostate cancer therapy.
The paucity of EGR1 antagonists greatly restricts our ability to study its function, especially in vivo. The availability of a cell permeable, small-molecule inhibitor of EGR1 DNA binding or transcriptional activity would definitely advance the field. In addition, more research is required to delineate the best approach to treatment. Larger preclinical studies are needed to evaluate various approaches to block EGR1 function and to study the combination of ectopic expression with radiotherapy or chemotherapy in various animal models. Finally, further understanding of EGR1-related signaling networks in human tumors may eventually allow the definition of populations of patients who would be the most likely to benefit from EGR1-targeted therapies.
Executive summary.
EGR1
■ Early growth response-1 (EGR1) is an early response gene involved in cellular responses to stress and growth factors.
■ EGR1 regulated mainly at the transcriptional level, following the activation of transcription factors of the Elk family by the MAP-kinase pathway.
Knockout mice
■ Two laboratories have generated EGR1−/− mice with somewhat distinct phenotypes.
■ Breeding of EGR1−/− mice with transgenic prostate cancer mouse models has indicated that a lack of EGR1 delays the progression of prostate cancer.
Role in prostate cancer progression
■ EGR1 is overexpressed in human prostate tumors. However, its involvement in the etiology of prostate cancer has not been formally demonstrated.
■ EGR1 may play a specific role in prostate cancer owing to its interaction with the androgen receptor.
■ Microarray analyses and other studies have identified EGR1-responsive genes in prostate cancer cells. These include various growth factors, cell-cycle regulators and proteins with a role in metastasis.
Role in stress-induced apoptosis
■ EGR1 induces the transcription of several tumor suppressor genes in response to stress and belongs to a tumor suppressor network.
■ These tumor suppressors are often altered in human cancer, either genetically or epigenetically.
Targeting EGR1 for prostate cancer therapy
■ Available strategies to antagonize EGR1 function are still very limited. Silencing of EGR1 using antisense oligonucleotides delays the growth of tumors in vivo, validating this transcription factor as a potential target for prostate cancer therapy.
■ On the other hand, ectopic expression of EGR1 through gene therapy may increase cancer cell sensitivity to chemotherapy or irradiation.
■ Another therapeutic use takes advantage of EGR1 responsiveness to stress and DNA damage. A gene therapy vector was engineered with the EGR1 promoter controlling the cytotoxic TNF-α cytokine.
Acknowledgements
The authors apologize to colleagues whose contribution was not cited due to space limitations. The authors thank Gaelle Rondeau and Shane Atwell for critical reading of the manuscript.
This work was supported by a grant from NIH/NCI-RO1 CA102688 (V Baron).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Delphine Gitenay, The Vaccine Research Institute of San Diego, 10835 Road to the Cure, San Diego, CA 92121, USA Tel.: +1 858 581 3960 Fax: +1 858 581 3970 delphinegitenay@yahoo.fr.
Véronique T Baron, The Vaccine Research Institute of San Diego, 10835 Road to the Cure, San Diego, CA 92121, USA Tel.: +1 858 581 3960 Fax: +1 858 581 3970 vbaron@sdibr.org.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1589 patients. Hum. Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Rangnekar VM, Adamson E, Mercola D. Suppression of growth and transformation and induction of apoptosis by EGR1. Cancer Gene Ther. 1998;5:3–28. [PubMed] [Google Scholar]
- 4.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- 5.Lim RW, Varnum BC, Herschman HR. Cloning of tetradecanoyl phorbol ester-induced ‘primary response’ sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1:263–270. [PubMed] [Google Scholar]
- 6.Christy BA, Lau LF, Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with ‘zinc finger’ sequences. Proc. Natl Acad. Sci. USA. 1988;85:7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemaire P, Revelant O, Bravo R, Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc. Natl Acad. Sci. USA. 1988;85:4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukhatme VP, Cao XM, Chang LC, et al. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988;53:37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- 9.Beckmann AM, Wilce PA. Egr transcription factors in the nervous system. Neurochem. Int. 1997;31:477–510. doi: 10.1016/s0197-0186(96)00136-2. discussion 517–476. [DOI] [PubMed] [Google Scholar]
- 10.O'Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- 11.Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor EGR1. Mol. Cell. Biol. 1993;13:4556–4571. doi: 10.1128/mcb.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo MW, Matheny C, Milbrandt J. Transcriptional activity of the zinc finger protein NGFI-A is influenced by its interaction with a cellular factor. Mol. Cell. Biol. 1993;13:6858–6865. doi: 10.1128/mcb.13.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svaren J, Sevetson BR, Apel ED, et al. NAB2, a corepressor of NGFI-A (EGR1) and Krox20, is induced by proliferative and differentiative stimuli. Mol. Cell. Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gashler A, Sukhatme VP. Early growth response protein 1 (EGR1): prototype of a zinc-finger family of transcription factors. Prog. Nucleic Acid Res. Mol. Biol. 1995;50:191–224. doi: 10.1016/s0079-6603(08)60815-6. [DOI] [PubMed] [Google Scholar]
- 15.Christy B, Nathans D. Functional serum response elements upstream of the growth factor-inducible gene zif268. Mol. Cell. Biol. 1989;9:4889–4895. doi: 10.1128/mcb.9.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakamoto KM, Bardeleben C, Yates KE, et al. 5′ upstream sequence and genomic structure of the human primary response gene, EGR1/TIS8. Oncogene. 1991;6:867–871. [PubMed] [Google Scholar]
- 17.Tsai-Morris CH, Cao XM, Sukhatme VP. 5′ flanking sequence and genomic structure of EGR1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 1988;16:8835–8846. doi: 10.1093/nar/16.18.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwachtgen JL, Campbell CJ, Braddock M. Full promoter sequence of human early growth response factor-1 (EGR1): demonstration of a fifth functional serum response element. DNA Seq. 2000;10:429–432. doi: 10.3109/10425170009015615. [DOI] [PubMed] [Google Scholar]
- 19.Cohen DM, Gullans SR, Chin WW. Urea inducibility of Egr-1 in murine inner medullary collecting duct cells is mediated by the serum response element and adjacent Ets motifs. J. Biol. Chem. 1996;271:12903–12908. doi: 10.1074/jbc.271.22.12903. [DOI] [PubMed] [Google Scholar]
- 20.Harada S, Smith RM, Smith JA, White MF, Jarett L. Insulin-induced Egr-1 and c-fos expression in 32D cells requires insulin receptor, Shc, and mitogen-activated protein kinase, but not insulin receptor substrate-1 and phosphatidylinositol 3-kinase activation. J. Biol. Chem. 1996;271:30222–30226. doi: 10.1074/jbc.271.47.30222. [DOI] [PubMed] [Google Scholar]
- 21.Hipskind RA, Buscher D, Nordheim A, Baccarini M. Ras/MAP kinase-dependent and -independent signaling pathways target distinct ternary complex factors. Genes Dev. 1994;8:1803–1816. doi: 10.1101/gad.8.15.1803. [DOI] [PubMed] [Google Scholar]
- 22.Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of Elk-1 and expression of c-fos, Egr-1, and JunB through activation of extracellular signal-regulated kinases 1 and 2. J. Biol. Chem. 1998;273:31327–31336. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]
- 23.Lim CP, Jain N, Cao X. Stress-induced immediate-early gene, Egr-1, involves activation of p38/JNK1. Oncogene. 1998;16:2915–2926. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- 24.Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J. Biol. Chem. 1999;274:19559–19564. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- 25.Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr. Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- 26.Silverman ES, Collins T. Pathways of EGR1-mediated gene transcription in vascular biology. Am. J. Pathol. 1999;154:665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thyss R, Virolle V, Imbert V, et al. NF-κB/EGR1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. Embo. J. 2005;24:128–137. doi: 10.1038/sj.emboj.7600501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Baron V, Mercola D, Mustelin T, Adamson ED. A network of p73, p53 and Egr1 is required for efficient apoptosis in tumor cells. Cell Death Differ. 2007;14:436–446. doi: 10.1038/sj.cdd.4402029. [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Mahendran R, Guy GR, Tan YH. Detection and characterization of cellular EGR1 binding to its recognition site. J. Biol. Chem. 1993;268:16949–16957. [PubMed] [Google Scholar]
- 30■.Yu J, de Belle I, Liang H, Adamson ED. Coactivating factors p300 and CBP are transcriptionally crossregulated by Egr1 in prostate cells, leading to divergent responses. Mol. Cell. 2004;15:83–94. doi: 10.1016/j.molcel.2004.06.030. Provides a molecular mechanism for a distinct EGR1 response to stress or to growth factors. [DOI] [PubMed] [Google Scholar]
- 31■.Yu J, Zhang SS, Saito K, et al. PTEN regulation by Akt-EGR1-ARF-PTEN axis. Embo. J. 2009;28:21–33. doi: 10.1038/emboj.2008.238. Describes a network of tumor suppressors. This is the first evidence of EGR1 regulation by p19ARF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae MH, Jeong CH, Kim SH, et al. Regulation of EGR1 by association with the proteasome component C8. Biochim. Biophys. Acta. 2002;1592:163–167. doi: 10.1016/s0167-4889(02)00310-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J. Biol. Chem. 1995;270:9971–9977. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- 34■.Lee SL, Wang Y, Milbrandt J. Unimpaired macrophage differentiation and activation in mice lacking the zinc finger transplantation factor NGFI-A (EGR1) Mol. Cell. Biol. 1996;16:4566–4572. doi: 10.1128/mcb.16.8.4566. Describes the generation of EGR1 knockout mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35■.Topilko P, Schneider-Maunoury S, Levi G. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, EGR1)-targeted mice. Mol. Endocrinol. 1998;12:107–122. doi: 10.1210/mend.12.1.0049. Describes the generation of EGR1 knockout mice. [DOI] [PubMed] [Google Scholar]
- 36.Krones-Herzig A, Adamson E, Mercola D. Early growth response 1 protein, an upstream gatekeeper of the p53 tumor suppressor, controls replicative senescence. Proc. Natl Acad. Sci. USA. 2003;100:3233–3238. doi: 10.1073/pnas.2628034100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krones-Herzig A, Mittal S, Yule K, et al. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res. 2005;65:5133–5143. doi: 10.1158/0008-5472.CAN-04-3742. [DOI] [PubMed] [Google Scholar]
- 38.Garabedian EM, Humphrey PA, Gordon JI. A transgenic mouse model of metastatic prostate cancer originating from neuroendocrine cells. Proc. Natl Acad. Sci. USA. 1998;95:15382–15387. doi: 10.1073/pnas.95.26.15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39■■.Abdulkadir SA, Qu Z, Garabedian E, et al. Impaired prostate tumorigenesis in Egr1-deficient mice. Nat. Med. 2001;7:101–107. doi: 10.1038/83231. First formal evidence of EGR1 contribution to prostate cancer. This seminal study demonstrates that lack of EGR1 delays cancer progression in transgenic mouse models of prostate cancer. [DOI] [PubMed] [Google Scholar]
- 40.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc. Natl Acad. Sci. USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulkadir SA. Mechanisms of prostate tumorigenesis: roles for transcription factors Nkx3.1 and Egr1. Ann. NY Acad. Sci. 2005;1059:33–40. doi: 10.1196/annals.1339.018. [DOI] [PubMed] [Google Scholar]
- 42.Adamson ED, Mercola D. Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumour Biol. 2002;23:93–102. doi: 10.1159/000059711. [DOI] [PubMed] [Google Scholar]
- 43.Eid MA, Kumar MV, Iczkowski KA, Bostwick DG, Tindall DJ. Expression of early growth response genes in human prostate cancer. Cancer Res. 1998;58:2461–2468. [PubMed] [Google Scholar]
- 44.Thigpen AE, Cala KM, Guileyardo JM, et al. Increased expression of early growth response-1 messenger ribonucleic acid in prostatic adenocarcinoma. J. Urol. 1996;155:975–981. [PubMed] [Google Scholar]
- 45.Ahmed MM, Chendil D, Lele S, et al. Early growth response-1 gene: potential radiation response gene marker in prostate cancer. Am. J. Clin. Oncol. 2001;24:500–505. doi: 10.1097/00000421-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 46.Abdulkadir SA, Carbone JM, Naughton CK, et al. Frequent and early loss of the EGR1 corepressor NAB2 in human prostate carcinoma. Hum. Pathol. 2001;32:935–939. doi: 10.1053/hupa.2001.27102. [DOI] [PubMed] [Google Scholar]
- 47.Scharnhorst V, Menke AL, Attema J, et al. EGR1 enhances tumor growth and modulates the effect of the Wilms' tumor 1 gene products on tumorigenicity. Oncogene. 2000;19:791–800. doi: 10.1038/sj.onc.1203390. [DOI] [PubMed] [Google Scholar]
- 48.Gaggioli C, Robert G, Bertolotto C, et al. Tumor-derived fibronectin is involved in melanoma cell invasion and regulated by V600E B-Raf signaling pathway. J. Invest. Dermatol. 2007;127:400–410. doi: 10.1038/sj.jid.5700524. [DOI] [PubMed] [Google Scholar]
- 49.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 50.Isaacs WB, Carter BS, Ewing CM. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991;51:4716–4720. [PubMed] [Google Scholar]
- 51.Baron V, Duss S, Rhim J, Mercola D. Antisense to the early growth response-1 gene (EGR1) inhibits prostate tumor development in TRAMP mice. Ann. NY Acad. Sci. 2003;1002:197–216. doi: 10.1196/annals.1281.024. [DOI] [PubMed] [Google Scholar]
- 52.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest. Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 53■.Yang SZ, Abdulkadir SA. Early growth response gene 1 modulates androgen receptor signaling in prostate carcinoma cells. J. Biol. Chem. 2003;278:39906–39911. doi: 10.1074/jbc.M307250200. A possible explanation for the prostate-specific effects of EGR1. [DOI] [PubMed] [Google Scholar]
- 54.Yang SZ, Eltoum IA, Abdulkadir SA. Enhanced EGR1 activity promotes the growth of prostate cancer cells in an androgen-depleted environment. J. Cell. Biochem. 2006;97:1292–1299. doi: 10.1002/jcb.20736. [DOI] [PubMed] [Google Scholar]
- 55.Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D. Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J. Biol. Chem. 2003;278:11802–11810. doi: 10.1074/jbc.M210279200. [DOI] [PubMed] [Google Scholar]
- 56.Baron V, De Gregorio G, Krones-Herzig A, et al. Inhibition of EGR1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22:4194–4204. doi: 10.1038/sj.onc.1206560. [DOI] [PubMed] [Google Scholar]
- 57.Svaren J, Ehrig T, Abdulkadir SA, et al. EGR1 target genes in prostate carcinoma cells identified by microarray analysis. J. Biol. Chem. 2000;275:38524–38531. doi: 10.1074/jbc.M005220200. [DOI] [PubMed] [Google Scholar]
- 58.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biswas S, Criswell TL, Wang SE, Arteaga CL. Inhibition of transforming growth factor-β signaling in human cancer: targeting a tumor suppressor network as a therapeutic strategy. Clin. Cancer Res. 2006;12:4142–4146. doi: 10.1158/1078-0432.CCR-06-0952. [DOI] [PubMed] [Google Scholar]
- 60.Teicher BA. Transforming growth factor-β and the immune response to malignant disease. Clin. Cancer Res. 2007;13:6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- 61.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-β and the immune response: implications for anticancer therapy. Clin. Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 62.Salah Z, Maoz M, Pizov G, Bar-Shavit R. Transcriptional regulation of human protease-activated receptor 1, a role for the early growth response-1 protein in prostate cancer. Cancer Res. 2007;67:9835–9843. doi: 10.1158/0008-5472.CAN-07-1886. [DOI] [PubMed] [Google Scholar]
- 63.Salah Z, Maoz M, Cohen I, et al. Identification of a novel functional androgen response element within hPar1 promoter: implications to prostate cancer progression. FASEB J. 2005;19:62–72. doi: 10.1096/fj.04-2386com. [DOI] [PubMed] [Google Scholar]
- 64.Xiao D, Chinnappan D, Pestell R, Albanese C, Weber HC. Bombesin regulates cyclin D1 expression through the early growth response protein EGR1 in prostate cancer cells. Cancer Res. 2005;65:9934–9942. doi: 10.1158/0008-5472.CAN-05-1830. [DOI] [PubMed] [Google Scholar]
- 65.Ogishima T, Shiina H, Breault JE, et al. Increased heparanase expression is caused by promoter hypomethylation and up-regulation of transcriptional factor early growth response-1 in human prostate cancer. Clin. Cancer Res. 2005;11:1028–1036. [PubMed] [Google Scholar]
- 66.Lokeshwar VB, Gomez P, Kramer M, et al. Epigenetic regulation of HYAL-1 hyaluronidase expression. Identification of HYAL-1 promoter. J. Biol. Chem. 2008;283:29215–29227. doi: 10.1074/jbc.M801101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu C, Calogero A, Ragona G, Adamson E, Mercola D. EGR1, the reluctant suppression factor: EGR1 is known to function in the regulation of growth, differentiation, and also has significant tumor suppressor activity and a mechanism involving the induction of TGF-β1 is postulated to account for this suppressor activity. Crit. Rev. Oncog. 1996;7:101–125. [PubMed] [Google Scholar]
- 68■.Das A, Chendil D, Dey S, et al. Ionizing radiation down-regulates p53 protein in primary EGR1−/− mouse embryonic fibroblast cells causing enhanced resistance to apoptosis. J. Biol. Chem. 2001;276:3279–3286. doi: 10.1074/jbc.M008454200. Demonstrates that EGR1−/− fibroblasts are resistant to apoptosis. [DOI] [PubMed] [Google Scholar]
- 69■.Virolle T, Adamson ED, Baron V, et al. The EGR1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat. Cell Biol. 2001;3:1124–1128. doi: 10.1038/ncb1201-1124. First study describing the regulation of PTEN transcription in response to DNA damage. [DOI] [PubMed] [Google Scholar]
- 70.Sperandio S, Fortin J, Sasik R, Robitaille L, Corbeil J, de Belle I. The transcription factor Egr1 regulates the HIF-1α gene during hypoxia. Mol. Carcinog. 2008;48:38–44. doi: 10.1002/mc.20454. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed MM, Sells SF, Venkatasubbarao K, et al. Ionizing radiation-inducible apoptosis in the absence of p53 linked to transcription factor EGR1. J. Biol. Chem. 1997;272:33056–33061. doi: 10.1074/jbc.272.52.33056. [DOI] [PubMed] [Google Scholar]
- 72.Nair P, Muthukkumar S, Sells SF, Han SS, Sukhatme VP, Rangnekar VM. Early growth response-1-dependent apoptosis is mediated by p53. J. Biol. Chem. 1997;272:20131–20138. doi: 10.1074/jbc.272.32.20131. [DOI] [PubMed] [Google Scholar]
- 73■.Zagurovskaya M, Shareef MM, Das A, et al. EGR1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene. 2009;28:1121–1131. doi: 10.1038/onc.2008.461. A new mechanism for EGR1-mediated apoptosis is described. Results presented in this article suggest that ectopic expression of EGR1 may sensitize prostate cancer cells to irradiation in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 74.Arora S, Wang Y, Jia Z, et al. Egr1 regulates the coordinated expression of numerous EGF receptor target genes as identified by ChIP-on-chip. Genome Biol. 2008;9:R166. doi: 10.1186/gb-2008-9-11-r166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang RP, Wu JX, Fan Y, Adamson ED. UV activates growth factor receptors via reactive oxygen intermediates. J. Cell. Biol. 1996;133:211–220. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baron V, Adamson ED, Calogero A, Ragona G, Mercola D. The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFβ1, PTEN, p53, and fibronectin. Cancer Gene Ther. 2006;13:115–124. doi: 10.1038/sj.cgt.7700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Downing SR, Russell PJ, Jackson P. Alterations of p53 are common in early stage prostate cancer. Can. J. Urol. 2003;10:1924–1933. [PubMed] [Google Scholar]
- 78.Whang YE, Wu X, Suzuki H, et al. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc. Natl Acad. Sci. USA. 1998;95:5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong JT. Chromosomal deletions and tumor suppressor genes in prostate cancer. Cancer Metastasis Rev. 2001;20:173–193. doi: 10.1023/a:1015575125780. [DOI] [PubMed] [Google Scholar]
- 80.McMenamin ME, Soung P, Perera S, et al. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999;59:4291–4296. [PubMed] [Google Scholar]
- 81.Kwabi-Addo B, Giri D, Schmidt K, et al. Haploinsufficiency of the PTEN tumor suppressor gene promotes prostate cancer progression. Proc. Natl Acad. Sci. USA. 2001;98:11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 83.Liu C, Adamson E, Mercola D. Transcription factor EGR1 suppresses the growth and transformation of human HT-1080 fibrosarcoma cells by induction of transforming growth factor β1. Proc. Natl Acad. Sci. USA. 1996;93:11831–11836. doi: 10.1073/pnas.93.21.11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trapani JA. The dual adverse effects of TGF-β secretion on tumor progression. Cancer Cell. 2005;8:349–350. doi: 10.1016/j.ccr.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 85.Houston P, Campbell CJ, Svaren J, Milbrandt J, Braddock M. The transcriptional corepressor NAB2 blocks EGR1-mediated growth factor activation and angiogenesis. Biochem. Biophys. Res. Commun. 2001;283:480–486. doi: 10.1006/bbrc.2001.4810. [DOI] [PubMed] [Google Scholar]
- 86.Sevetson BR, Svaren J, Milbrandt J. A novel activation function for NAB proteins in EGR-dependent transcription of the luteinizing hormone β gene. J. Biol. Chem. 2000;275:9749–9757. doi: 10.1074/jbc.275.13.9749. [DOI] [PubMed] [Google Scholar]
- 87.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann. NY Acad. Sci. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 88.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor EGR1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 89.Giri RK, Rajagopal V, Kalra VK. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J. Neurochem. 2004;91:1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 90.Moon Y, Glasgow WC, Eling TE. Curcumin suppresses interleukin 1β-mediated microsomal prostaglandin E synthase 1 by altering early growth response gene 1 and other signaling pathways. J. Pharmacol. Exp. Ther. 2005;315:788–795. doi: 10.1124/jpet.105.084434. [DOI] [PubMed] [Google Scholar]
- 91.Choi BH, Kim CG, Bae YS, Lim Y, Lee YH, Shin SY. p21 Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: role of early growth response-1 expression. Cancer Res. 2008;68:1369–1377. doi: 10.1158/0008-5472.CAN-07-5222. [DOI] [PubMed] [Google Scholar]
- 92.Shishodia S, Singh T, Chaturvedi MM. Modulation of transcription factors by curcumin. Adv. Exp. Med. Biol. 2007;595:127–148. doi: 10.1007/978-0-387-46401-5_4. [DOI] [PubMed] [Google Scholar]
- 93.Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol. Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- 94.Battle TE, Frank DA. STAT1 mediates differentiation of chronic lymphocytic leukemia cells in response to Bryostatin 1. Blood. 2003;102:3016–3024. doi: 10.1182/blood-2002-09-2972. [DOI] [PubMed] [Google Scholar]
- 95.Lucerna M, Pomyje J, Mechtcheriakova D, et al. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res. 2006;66:6708–6713. doi: 10.1158/0008-5472.CAN-05-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mezhir JJ, Smith KD, Posner MC, et al. Ionizing radiation: a genetic switch for cancer therapy. Cancer Gene Ther. 2006;13:1–6. doi: 10.1038/sj.cgt.7700879. [DOI] [PubMed] [Google Scholar]
- 97.Quinones A, Dobberstein KU, Rainov NG. The Egr-1 gene is induced by DNA-damaging agents and non-genotoxic drugs in both normal and neoplastic human cells. Life Sci. 2003;72:2975–2992. doi: 10.1016/s0024-3205(03)00230-3. [DOI] [PubMed] [Google Scholar]
- 98.Bickenbach KA, Veerapong J, Shao MY, et al. Resveratrol is an effective inducer of CArG-driven TNF-α gene therapy. Cancer Gene Ther. 2008;15:133–139. doi: 10.1038/sj.cgt.7701103. [DOI] [PubMed] [Google Scholar]