In 1923, Wilson and Finch performed a simple experiment in which ice water was ingested and an ECG was recorded.1 The result led them to describe T wave changes as either primary, arising “from disturbances in the function of fairly large regions of ventricular muscle,” or “secondary to changes in the form of the QRS complex” and persisting as long as the QRS changes were present. Knowledge of primary T wave changes has expanded over the years to incorporate those induced by structural alterations such as hypertrophy, as well as pharmacologic and electrolytic influences and more recently, channelopathies. Examples of secondary T wave changes include those induced by conduction abnormalities, ventricular arrhythmias, and myocardial infarction.
But every story has caveats: one for the categorization of T wave changes as primary or secondary was noted by several groups of individuals and formalized by Mauricio Rosenbaum (Figure 1).2 The caveat was a change in the T wave that appeared at first to be secondary (as it was initiated by a change in the QRS complex) but persisted long after the QRS had normalized, such that it mimicked a primary change. Rosenbaum and associates referred to such T waves as “pseudoprimary,” and – given the property of the persistent T wave to follow the vector of the inciting QRS complex – called the phenomenon “cardiac memory” (Figure 2-1b).2 They performed animal and human experiments on cardiac memory and concluded – although not definitively – that altered electrotonic coupling among cells might provide the mechanism. Studies of Langendorff-perfused rabbit hearts in Michael Franz’ laboratory gave further credence to this idea.3
Figure 1.
Mauricio Rosenbaum, (photograph courtesy of Charlie Antzelevitch).
Figure 2.
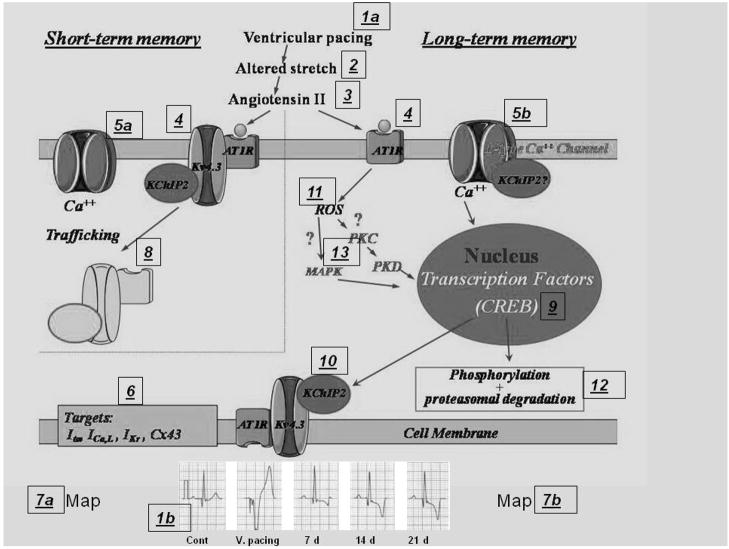
Roadmap of cardiac memory. Numbers in boxes are keyed to text. (Modified after Özgen N and Rosen MR, Cardiac memory: a work in progress: Heart Rhythm, 2009, in press.)
My own interest in T waves evolved over coffees that Mauricio and I used to share at the Hotel Algonquin during his frequent trips from Buenos Aires to New York. There is something about the Algonquin – whose scarred wooden tabletops still carry the imprints of Robert Benchley, Dorothy Parker and the 1920’s New York literati – and there was something about Mauricio – clinician-scientist, friend and raconteur extraordinaire – that made his repeated challenges to me to “look at cardiac memory before you begin losing your own” irresistible. So began my personal voyage into trying to understand the T wave. My guideposts were the Wilson and Finch experiments, the astute observations of a host of investigators who had followed, and Mauricio’s iconoclastic insights.
Because we have recently summarized our own experiments and those of other groups that bear on repolarization and cardiac memory,4 my goal in this paper is to provide a more personal description of the events that have occurred since those coffees with Mauricio. The process has been one of growth and education: I started as a cardiologist who viewed electrophysiology as a be-all and end-all: electricity was sacrosanct…it was the property of cables and batteries and I tried to simplify everything I saw biologically to fit that context. However, exploring the machinations of the T wave has led to collaborations with colleagues in molecular biophysics, molecular biology and signal transduction and we have explored together the mechanisms determining the electrical output (the T wave) of an electrical signal generator (an electronic cardiac pacemaker). While a personal voyage, this has not been a solitary one: the research throughout has been performed in partnership with Peter Danilo and Ira Cohen, and along the way there have been significant contributions by Susan Steinberg, Rich Robinson and Penny Boyden.
Our adventure, coupled with parallel discoveries by colleagues in other labs with interests in remodeling and in channelopathies has led me to marvel at the complexity of events leading to seemingly simple changes in electrical output while I despair of ever learning all the answers. In any event, in querying “why T waves change…” I offer the following summary of what we have learned and how we learned it. Figure 2 provides an annotated roadmap of the experimental results.
Starting off…the T wave change
Ughetta del Balzo and I began with a variant on the Rosenbaum model of memory, pacing dogs from the ventricle for 20–30 min at about 5% faster than their sinus rate, and interspersing this with sinus rhythm or atrial pacing (Figure 2-1a,b).5 Following the Rosenbaum lead, we referred to this as short-term memory and based on the literature we hypothesized that the T wave change resulted from an altered T wave gradient consequent to altered transmural dispersion of Ito (Figure 2-6). To test a potential role for Ito in short-term memory we infused dogs with 4-aminopyridine, to non-selectively block Ito. In the presence of 4-AP memory could not be induced. Subsequently, Christoph Geller created a memory model in isolated, paced ventricular epicardial and endocardial slabs. Here, the “T wave” change (the result of processing the epicardial and endocardial action potentials through a difference amplifier) was prevented by 4-aminopyridine.6
Why did ventricular pacing elicit these changes? Was it the electrical shocks…the altered activation…the resultant alterations in myocardial stretch (Figure 2--2)? Sadoshima and Izumo had shown previously that altering stretch in myocyte-fibroblast cultures results in angiotensin II synthesis and release.7,8 Philippe Ricard hypothesized that if altered myocardial activation in situ resulted in altered stretch and angiotensin II availability then interfering with angiotensin II synthesis or binding to its receptor should prevent memory from evolving (Figure 2-3).9 He was right. He could not produce short-term memory in the presence of an ACE inhibitor, an AT-1 receptor blocker or a tissue chymase inhibitor. That a tissue chymase inhibitor prevented memory induction was consistent with the angiotensin II being synthesized in the heart rather than carried from other sites via the circulation. The same experimental series also demonstrated that nifedipine attenuates the evolution of short-term memory, consistent with a role for Ca2+ in the process (Figure 2-5a).
The next step was to develop a model for long-term memory. Joris de Groot and I performed a series of mapping experiments to document the magnitude and consistency of T wave changes induced by test-pacing at multiple epicardial sites. This provided Alexei Shvilkin with a template for ventricular pacing of chronic dogs that incorporated ease of lead implantation and a T wave change of sufficient magnitude for consistent study. Shvilkin et al then demonstrated that about 3 weeks of pacing could induce long-term memory that persisted for weeks in the absence of coronary flow changes, failure or hypertrophy10 and that evolution of long-term memory was delayed but not prevented by AT-1 receptor blockade or Ca channel blockade (Figure 2-5b).10 As part of these experiments on long-term memory we studied the left ventricular epicardial and endocardial transmembrane action potentials and found an altered transmural gradient, with epicardial potentials lengthening more than endocardial, while the action potential notch diminished.10
How did these changes at the cellular level play out in the heart in situ? Giel Janse, Ruben Coronel and Tobias Opthof joined us to study the T wave changes in short term memory (Figure 2-7a). The experimental plan included long days of mapping and long nights at Peter Luger’s Steakhouse. Under control conditions – during the days – we found a left ventricular apicobasal gradient with the shortest repolarization times antero-basally and the longest repolarization times postero-apically. There was no significant transmural gradient in atrial-paced controls or after 2h of ventricular pacing.11 Because both repolarization time and monophasic action potential durations shortened during induction of short-term memory, we proposed that the deep T wave of short-term memory might be explained by the steeper phase 3 of repolarization. We then studied long-term memory (Figure 2-7b), finding no transmural gradient in the control setting.12 However, a gradient appeared during long term memory, with epicardial repolarization being longer than endocardial.
This contrasts with the finding of David Rosenbaum’s lab that long-term memory is associated with disproportionate and localized action potential prolongation of late-activated myocardial segments, but without changes in transmural action potential duration gradients.13 Their study design was different, however, as it centered on isolated wedge preparations from dogs in long-term memory. Using yet another study, design and species, Yoram Rudy’s group employed electrocardiographic imaging to study patients undergoing radiofrequency ablation for Wolf-Parkinson-White syndrome.14 These patients had long activation-recovery intervals and large apex-to-base dispersion in pre-excited regions of epicardium which required about a month to resolve post-ablation.
To sum up these pharmacologic and electrophysiologic experiments: short-term memory was prevented by interfering with angiotensin II synthesis and binding and by calcium channel blockade. Long-term memory was delayed by these interventions but not prevented. And the key factors appeared to be altered activation, angiotensin II, calcium and Ito.9,10
As for repolarization gradients, these are clearly important in memory. The only study of short-term memory in situ shows the gradient to be apicobasal;11 in long term memory one study showed the change to be establishment of a transmural gradient and two found different results using different techniques.12–14 However, given that the long-term studies were done in two different species using three different methods to induce and record memory, they may not be contradictory. We simply have three pieces of information from what appears to be a complex puzzle.
Is it all a matter of activation?
From early in our studies we questioned whether it was simply a change in activation or changes in myocardial stretch induced by altered activation that was responsible for the increase in angiotensin II, and possibly for gene transcription (the role of which will be detailed below). A wealth of information pointed to the importance of altered wall motion and stretch, including the studies of Printzen and associates on activation and dyssynergy of contraction,15,16 the work of Sadoshima and Izumo showing that stretch in cell culture results in altered angiotensin II synthesis and release,7,8 and the work of Max Lab and associates showing that altered stretch without altered activation increases c-fos and c-jun levels within about 30 minutes,17 consistent with the initiation of transcription.
More recently, David Rosenbaum’s lab13 and that of Sosunov et al18 have indicated that altered stretch is the “missing link” in the path between activation and memory (Figure 2--2). The Rosenbaum lab performed studies in intact dogs, and Sosunov et al studied isolated, perfused rabbit hearts. Both labs reached the same conclusion regarding stretch. Moreover, the latter studies showed that altered activation without altered stretch induced no memory and altered stretch without altered activation still induced memory.
Digging deeper: how do memory, angiotensin II and Ito interrelate?
Yu et al noted that in long-term memory there is a reduction in epicardial Ito density and altered kinetics as well as reduced mRNA for Kv4.3, the genetic determinant of the pore-forming unit of the channel carrying Ito.19 Reinforcing the importance of a gradient for Ito was a study by Plotnikov et al showing that in the absence of Ito and a related repolarization gradient, there is no expression of short-term memory.20 Based on the reduced mRNA for Kv4.3, we opined that the change in Ito for long-term memory is transcriptional. Left open was not only the mechanism for gene transcription but how Ito might be altered in the setting of short-term memory, before transcription has occurred. I shall return to these issues later.
What of angiotensin II? Susan Steinberg had initially suggested that we pursue this signaling pathway aggressively, and Yu et al studied the role of angiotensin II in modulating Ito.21 We isolated epicardial or endocardial ventricular myocytes in test tubes and exposed them for 2–96 hours to angiotensin II, and found that angiotensin II had the same effect on epicardial Ito in the test tube as did pacing to induce long-term memory, a reduction in the current and altered kinetics. This effect was blocked by losartan, confirming the role of the AT-1 receptor in the pathway. Very importantly, mRNA for Kv4.3 was unchanged, arguing against gene transcription playing a role in this setting.
The sum of these studies suggests a link between angiotensin II synthesis and release and expression of Ito that contributes to the occurrence of short-term memory. Whereas long-term memory is associated with altered Ito density and kinetics that appears transcriptional, angiotensin II affects Ito over the short-term, but via a non-transcriptional pathway.
Short term memory: induced by ion channel trafficking?
Doronin and colleagues explored the possibilities of a non-transcriptional pathway for angiotensin II-dependent Ito reduction (Figure 2-4).22 They transfected a cell line with Kv4.3, with KChIP2 (an accessory subunit important to Ito density and kinetics) and with the AT-1 receptor. They found that the three components formed a macromolecular complex within the cell membrane and that angiotensin II binding to the receptor resulted in internalization of the complex and reduction in the current (Figure 2-8). They also found that in myocytes the AT-1 receptor and Kv4.3 co-immunoprecipitate, and that in cell lines the two reside within 100A of one another. Taken together these observations offer an explanation for the non-transcriptional evolution of short term memory induced by pacing the ventricle. This idea of internalization of the macromolecular complex awaits testing in situ.
Long-term memory and gene transcription
The initial suggestion that gene transcription might be important to long term memory came from two sources. One was the work of Kandel and associates on long-term potentiation in aplysia californicus,23 showing that the cyclic AMP response element binding protein (CREB) is important to transcription when electrical shocks are delivered to aplysia. The other insight came from Lab and associates who showed that altering stretch for brief periods without altering activation in pig heart recruited changes in c-fos and c-jun.17
Niels Patberg and Nazira Özgen have studied gene transcription in cardiac memory. Patberg et al initially focused on the molecular mechanisms underlying the loss of the action potential notch and prolongation of epicardial action potential duration in long-term memory. We used a 2 hour pacing protocol to test whether ventricular pacing to induce memory alters CREB production in heart,24 and found that CREB protein is reduced within the 2 hour period (Figure 2-9). The reduction is maximal near the pacing site and is prevented by angiotensin II receptor blockade or calcium channel blockade. We also noted the cyclic AMP response element present in the promoter region of KChIP2 and demonstrated a reduction in KChIP2 protein paralleling that in CREB (Figure 2-10).
Patberg et al next asked whether reduced CREB levels in the absence of pacing might still reduce Ito and alter repolarization, thereby establishing cause and effect between CREB and Ito reduction.25 We injected a CREB antisense virus into a region of the canine LV epicardium. After several days, monophasic action potential recordings in the region showed no phase 1 notch, whereas in control regions the notch was prominent. Myocytes disaggregated from the injected regions had no Ito and Ito was normal in the uninjected sites.
Together, these findings suggested that CREB is a transcription factor for KChIP2 and is involved in Ito regulation in initiating long-term memory. Additional transcription factors are likely involved in memory induction; for example, Özgen et al’s preliminary data suggest AP-1 is important for ERG.26
Özgen et al’s other studies have focused on transduction of events between angiotensin II binding to its receptor and the occurrence of CREB reduction. The preliminary data show that ventricular pacing increases malondialdehyde levels, consistent with occurrence of oxidative stress, implying a role for H2O2 production (Figure 2-11). The outcome appears to be CREB phosphorylation and its proteasomal degradation (Figure 2-12).27 We now are exploring whether reactive oxygen species regulate CREB in cardiac myocytes. Preliminary results suggest that H2O2 downregulates CREB protein content, via MAP kinase and PKC-PKD regulated pathways (Figure 2-13).28
Is it all a matter of Ito?
No, it is much more complicated (Figure 2-6). Plotnikov and colleagues showed that ICa,L kinetics are altered in long-term memory in a way that can explain the prolongation seen in the action potential plateau.29 In trying to understand the determinants of the change in ICa,L Thomsen et al not only reinforced the role of KChIP2 as an accessory protein for Kv4.3, the pore-forming unit of Ito,30 but that it serves the same purpose for the α subunit of ICa,L (Figure 2-5b).31
Yet, ICa,L is not the only other ion channel involved. Obreztchikova et al studied IKr in long-term memory.32 In controls, IKr density and mRNA and protein of its α subunit, ERG, display a transmural gradient such that current density and ERG expression are greater in epicardium than endocardium. In long-term memory, the transmural gradients of current density and ERG mRNA and protein are reversed.
There are also important changes in the connexins that facilitate cell-cell communication. Patel and colleagues showed that in long term memory there is diminished connexin43 density especially near the pacing site.33 In addition there is lateralization of connexin43. These changes jibe well with Mauricio Rosenbaum’s initial idea that altered electrotonus may contribute to memory.2 However, cell-cell conductance has not yet been reported in memory.
Clinical updates
In his initial studies, Mauricio Rosenbaum described the clinical patterns of cardiac memory in detail and also noted the extent to which memory might be confused with ischemic patterns on ECG.2,34 Twenty-five years later, Shvilkin et al described the means for distinguishing the ECG patterns of cardiac memory and ischemic precordial T wave inversion.35 A combination of a positive T wave in aVL, with a positive or isoelectric T in lead I and maximal precordial T waves in lead I>lead III was 92% sensitive and 100% specific for cardiac memory, discriminating it from ischemic precordial T wave inversion. Shvilkin and colleagues also described memory occurring consequent to the QRS widening induced by propafenone toxicity,36 showing a clinical corollary to Plotnikov et al’s earlier work on the interaction of memory and antiarrhythmic drugs in a canine model.37 Finally, Wecke and colleagues have systematically explored the clinical expression of cardiac memory in paced human subjects, detailing which vectorcardiographic measurements most consistently reflect the onset and offset of memory, as well as the time course of accumulation and resolution.38,39
Is cardiac memory really memory, and why bother with it anyhow?
Borys Surawicz painted a provocative image of cardiac memory as a form of forgetting, and he is likely right.40 Whereas the neuronal memory expressed as long-term potentiation is a synthetic process involving the strengthening of protein links23 memory in heart involves two processes: the “forgetting” of a current state of repolarization and the “remembering” of a neonatal repolarization pattern. This was uncovered by Plotnikov et al in a study of neonatal, young and adult dogs.20 The neonate has no Ito, no action potential notch and no pacing-induced cardiac memory. At about 6 weeks of age, Ito, the notch and inducible memory evolve, and with advancing age and a larger Ito, memory is ever more inducible. The memory pattern in the adult is in many ways reminiscent of a neonatal heart with no Ito.
Given the apparent benignity of cardiac memory, is its study much ado about little? I think not. True, we are always impatient to understand the causality of events that are at least discomforting and at worst life-threatening, and memory hardly fits this range of descriptors. Yet, given its associations with angiotensin II and reactive oxygen species, memory may reflect the beginning of a series of changes leading ultimately to hypertrophy, necrosis and worse. If this is true, then we shall come to view these T wave changes as early signs of pathologic processes at a time when their reversal is still possible. We have some inkling of this, already, in the atrium. Herweg et al and Chandra et al studied atrial memory – the changes in the atrial T wave induced by pacing from ectopic sites.41,42 Interestingly, the changes seen with memory were accompanied by an increased propensity to atrial tachyarrhythmias and fibrillation. This sequence and outcome fits well with the Allessie group’s demonstration that atrial fibrillation begets atrial fibrillation.43
A final thought: One thing a career in research affords us, if we are lucky, is the opportunity to study natural phenomena in their own right simply because they are interesting. While this is against the grain of applied research that seeks cures for diseases, the observation of Comroe and Dripps some 30 years ago has to my knowledge never been refuted: that advances in medicine derive foremost from research that asks, “why is the grass greener,” that asks the question simply in its own right because of the curiosity of the investigator.44 While it is undeniable that applied research is essential, at the roots of knowledge has always been the fundamental desire to know for the sake of knowing. In this context, is cardiac memory too “simple” a system, too devoid of pathological meaning to warrant study? I think not: I believe that only by understanding the complexity of that which appears simple can we begin to appreciate the depth of those mysteries that appear to be complex.
Acknowledgments
I express my gratitude to Eileen Franey for her careful attention to the preparation of the manuscript. I also wish to thank all the authors of the studies to which I’ve referred: all have contributed in essential ways, although I’ve taken the liberty of referring only to the first authors. This work was supported by USPHS-NHLBI grant HL-67101.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson FN, Finch R. The effect of drinking iced-water upon the form of the T deflection of the electrocardiogram. Heart. 1923;10:275–278. [Google Scholar]
- 2.Rosenbaum MB, Blanco HH, Elizari MV, Lazzari JO, Davidenko JM. Electrotonic modulation of the T wave and cardiac memory. Am J Cardiol. 1982;50:213–222. doi: 10.1016/0002-9149(82)90169-2. [DOI] [PubMed] [Google Scholar]
- 3.Costard-Jackle A, Goetsch B, Antz M, Franz MR. Slow and long-lasting modulation of myocardial repolarization produced by ectopic activation in isolated rabbit hearts. Evidence for cardiac “memory”. Circulation. 1989;80:1412–1420. doi: 10.1161/01.cir.80.5.1412. [DOI] [PubMed] [Google Scholar]
- 4.Özgen N, Rosen MR. Cardiac memory: A work in progress. Heart Rhythm. 2009 doi: 10.1016/j.hrthm.2009.01.008. In press. [DOI] [PubMed] [Google Scholar]
- 5.del Balzo U, Rosen MR. T wave changes persisting after ventricular pacing in canine heart are altered by 4-aminopyridine but not by lidocaine. Implications with respect to phenomenon of cardiac ‘memory’. Circulation. 1992;85:1464–1472. doi: 10.1161/01.cir.85.4.1464. [DOI] [PubMed] [Google Scholar]
- 6.Geller JC, Rosen MR. Persistent T-wave changes after alteration of the ventricular activation sequence. New insights into cellular mechanisms of ‘cardiac memory’. Circulation. 1993;88:1811–1819. doi: 10.1161/01.cir.88.4.1811. [DOI] [PubMed] [Google Scholar]
- 7.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 9.Ricard P, Danilo P, Jr, Cohen IS, Burkhoff D, Rosen MR. A role for the renin-angiotensin system in the evolution of cardiac memory. J Cardiovasc Electrophysiol. 1999;10:545–551. doi: 10.1111/j.1540-8167.1999.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 10.Shvilkin A, Danilo P, Jr, Wang J, et al. Evolution and resolution of long-term cardiac memory. Circulation. 1998;97:1810–1817. doi: 10.1161/01.cir.97.18.1810. [DOI] [PubMed] [Google Scholar]
- 11.Janse MJ, Sosunov EA, Coronel R, et al. Repolarization gradients in the canine left ventricle before and after induction of short-term cardiac memory. Circulation. 2005;112:1711–1718. doi: 10.1161/CIRCULATIONAHA.104.516583. [DOI] [PubMed] [Google Scholar]
- 12.Coronel R, Opthof T, Plotnikov AN, et al. Long-term cardiac memory in canine heart is associated with the evolution of a transmural repolarization gradient. Cardiovasc Res. 2007;74:416–425. doi: 10.1016/j.cardiores.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Jeyaraj D, Wilson LD, Zhong J, et al. Mechanoelectrical feedback as novel mechanism of cardiac electrical remodeling. Circulation. 2007;115:3145–3155. doi: 10.1161/CIRCULATIONAHA.107.688317. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Rhee EK, Avari JN, Woodard PK, Rudy Y. Cardiac memory in patients with Wolff-Parkinson-White syndrome: noninvasive imaging of activation and repolarization before and after catheter ablation. Circulation. 2008;118:907–915. doi: 10.1161/CIRCULATIONAHA.108.781658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McVeigh ER, Prinzen FW, Wyman BT, Tsitlik JE, Halperin HR, Hunter WC. Imaging asynchronous mechanical activation of the paced heart with tagged MRI. Magn Reson Med. 1998;39:507–513. doi: 10.1002/mrm.1910390402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meghji P, Nazir SA, Dick DJ, Bailey ME, Johnson KJ, Lab MJ. Regional workload induced changes in electrophysiology and immediate early gene expression in intact in situ porcine heart. J Mol Cell Cardiol. 1997;29:3147–3155. doi: 10.1006/jmcc.1997.0545. [DOI] [PubMed] [Google Scholar]
- 18.Sosunov EA, Anyukhovsky EP, Rosen MR. Altered ventricular stretch contributes to initiation of cardiac memory. Heart Rhythm. 2008;5:106–113. doi: 10.1016/j.hrthm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, McKinnon D, Dixon JE, et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation. 1999;99:1898–1905. doi: 10.1161/01.cir.99.14.1898. [DOI] [PubMed] [Google Scholar]
- 20.Plotnikov AN, Sosunov EA, Patberg KW, et al. Cardiac memory evolves with age in association with development of the transient outward current. Circulation. 2004;110:489–495. doi: 10.1161/01.CIR.0000137823.64947.52. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Gao J, Wang H, et al. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 22.Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem. 2004;279:48231–48237. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 23.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 24.Patberg KW, Plotnikov AN, Quamina A, et al. Cardiac memory is associated with decreased levels of the transcriptional factor CREB modulated by angiotensin II and calcium. Circ Res. 2003;93:472–478. doi: 10.1161/01.RES.0000088785.24381.2F. [DOI] [PubMed] [Google Scholar]
- 25.Patberg KW, Obreztchikova MN, Giardina SF, et al. The cAMP response element binding protein modulates expression of the transient outward current: implications for cardiac memory. Cardiovasc Res. 2005;68:259–267. doi: 10.1016/j.cardiores.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Özgen N, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Rosen MR. Altered transmural expression of IKr in cardiac memory is regulated by the transcription factor, Activator Protein-1. Circulation. 2007;116:II–280. [Google Scholar]
- 27.Özgen N, Lau DH, Shlapakova IN, Sherman W, Danilo P, Jr, Rosen MR. The decreased CREB level determining K channel transcription in cardiac memory results from its ubiquitination and subsequent proteosomal degradation. Heart Rhythm. 2008;5:S358. [Google Scholar]
- 28.Özgen N, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Steinberg SF, Rosen MR. A reactive oxygen species-mediated PKC-ERK-RSK pathway decreases the cyclic AMP response element binding protein and may initiate transcriptional changes in cardiac memory. Circulation. 2007;116:II–88. [Google Scholar]
- 29.Plotnikov AN, Yu H, Geller JC, et al. Role of L-type calcium channels in pacing-induced short-term and long-term cardiac memory in canine heart. Circulation. 2003;107:2844–2849. doi: 10.1161/01.CIR.0000068376.88600.41. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen MB, Sosunov EA, Anyukhovsky EP, Özgen N, Boyden PA, Rosen MR. Deleting the accessory subunit KChIP2 results in loss of Ito,f and increased IK,slow that maintains normal action potential configuration. Heart Rhythm. 2009;6:370–377. doi: 10.1016/j.hrthm.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen MB, Sosunov EA, Anyukhovsky EP, Pitt GS, Rosen MR. Deficiency of the accessory ion channel subunit KChIP2 leads to decreased L-type calcium current density in mice. Heart Rhythm. 2008;5:S106. [Google Scholar]
- 32.Obreztchikova MN, Patberg KW, Plotnikov AN, et al. IKr contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71:88–96. doi: 10.1016/j.cardiores.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Patel PM, Plotnikov A, Kanagaratnam P, et al. Altering ventricular activation remodels gap junction distribution in canine heart. J Cardiovasc Electrophysiol. 2001;12:570–577. doi: 10.1046/j.1540-8167.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum MB, Blanco HH, Elizari MV. Electrocardiographic characteristics and main causes of pseudoprimary T wave changes; Significance of concordant and discordant T waves in human and other animal species. Ann NY Acad Sci. 1990;601:36–50. doi: 10.1111/j.1749-6632.1990.tb37290.x. [DOI] [PubMed] [Google Scholar]
- 35.Shvilkin A, Ho KKL, Rosen MR, Josephson ME. T-Vector direction differentiates postpacing from ischemic T-wave inversion in precordial leads. Circulation. 2005;111:969–974. doi: 10.1161/01.CIR.0000156463.51021.07. [DOI] [PubMed] [Google Scholar]
- 36.Wylie JV, Jr, Zimetbaum P, Josephson ME, Shvilkin A. Cardiac memory induced by QRS widening due to propafenone toxicity. Pacing Clin Electrophysiol. 2007;30:1161–1164. doi: 10.1111/j.1540-8159.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 37.Plotnikov AN, Shvilkin A, Xiong W, et al. Interactions between antiarrhythmic drugs and cardiac memory. Cardiovasc Res. 2001;50:335–344. doi: 10.1016/s0008-6363(01)00233-4. [DOI] [PubMed] [Google Scholar]
- 38.Wecke L, Gadler F, Linde C, Lundahl G, Rosen MR, Bergfeldt L. Temporal characteristics of cardiac memory in humans: Vectorcardiographic quantification in a model of cardiac pacing. Heart Rhythm. 2005;2:28–34. doi: 10.1016/j.hrthm.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Wecke L, Rubulis A, Lundahl G, Rosen MR, Bergfeldt L. Right ventricular pacing-induced electrophysiological remodeling in the human heart and its relationship to cardiac memory. Heart Rhythm. 2007;4:1477–1486. doi: 10.1016/j.hrthm.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Surawicz B. Letter to the Editor. J Cardiovasc Electrophysiol. 2001;12:390. doi: 10.1046/j.1540-8167.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- 41.Herweg B, Chang F, Chandra P, Danilo P, Jr, Rosen MR. Cardiac memory in canine atrium. Identification and Implications. Circulation. 2001;103:455–461. doi: 10.1161/01.cir.103.3.455. [DOI] [PubMed] [Google Scholar]
- 42.Chandra P, Rosen TS, Herweg B, Danilo P, Jr, Rosen MR. Left atrial pacing induces memory and is associated with atrial tachyarrhythmias. Cardiovasc Res. 2003;60:307–314. doi: 10.1016/s0008-6363(03)00536-4. [DOI] [PubMed] [Google Scholar]
- 43.Wijffels MCEF, Kirchof CJHJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 44.Comroe JH, Jr, Dripps RD. Scientific basis for the support of biomedical science. Science. 1976;192:105–111. doi: 10.1126/science.769161. [DOI] [PubMed] [Google Scholar]