Abstract
Very little manganese is imported into Escherichia coli under routine growth conditions: the import system is weakly expressed, the manganese content is low, and a manganese-dependent enzyme is not correctly metallated. Mutants that lack MntH, the importer, grow at wild-type rates, indicating that manganese plays no critical role. However, MntH supports the growth of iron-deficient cells, suggesting that manganese can substitute for iron in activating at least some metalloenzymes. MntH is also strongly induced when cells are stressed by hydrogen peroxide. This adaptation is essential, as E. coli cannot tolerate peroxide stress if mntH is deleted. Other workers have observed that manganese improves the ability of a variety of microbes to tolerate oxidative stress, and the prevailing hypothesis is that manganese does so by chemically scavenging hydrogen peroxide and/or superoxide. We found that manganese does not protect peroxide-stressed cells by scavenging peroxide. Instead, the beneficial effects of manganese correlate with its ability to metallate mononuclear enzymes. Because iron-loaded enzymes are vulnerable to the Fenton reaction, the substitution of manganese may prevent protein damage. Accordingly, during H2O2 stress, mutants that cannot import manganese and/or are unable to sequester iron suffer high rates of protein oxidation.
Introduction
When organisms dwell in aerobic habitats, molecular oxygen enters cells and adventitiously oxidizes intracellular redox enzymes, thereby producing a continuous stream of intracellular superoxide and hydrogen peroxide. These reactive oxygen species (ROS) are kept at tolerable steady-state concentrations by the actions of superoxide dismutases and reductases and of peroxidases and catalases (Imlay, 2008).
However, microorganisms are perfused with higher levels of H2O2 when they enter environments in which H2O2 has accumulated as a consequence of the chemical oxidation of reduced metals, sulfur species, or organic molecules. Microbes are also exposed to high H2O2 concentrations when other organisms use it as a weapon to poison their competitors. A variety of lactic acid bacteria excrete H2O2 at rates that lead to millimolar concentrations in culture media, while some plants and other bacteria export redox-cycling compounds that trigger ROS formation when they are ingested by cohabitant microbes. Finally, the phagosomal NADPH oxidase sprays captive microbes with very high doses of H2O2.
In these circumstances the basal scavenging systems are likely to be inadequate, and intracellular H2O2 can accumulate to toxic (micromolar) levels (Seaver & Imlay, 2001b). This H2O2 reacts with unincorporated ferrous iron to generate hydroxyl radicals, which then rapidly oxidize nearby biomolecules; when this reaction involves iron that is bound to DNA, mutagenic or lethal lesions can result (Levin et al., 1982, Henle et al., 1999, Imlay et al., 1988, Park et al., 2005). H2O2 also inactivates a family of dehydratases by oxidizing their solvent-exposed iron-sulfur clusters (Jang & Imlay, 2007, Flint et al., 1993). As a consequence, the catabolic and biosynthetic pathways that rely upon these enzymes become non-functional.
To cope with this threat, microbes have evolved inducible defensive regulons that are controlled by H2O2-sensing regulatory proteins—OxyR (Carmel-Harel & Storz, 2000) and PerR (Lee & Helmann, 2006) in bacteria, and Yap-1 (Paget & Buttner, 2003) in yeast. In E. coli, intracellular H2O2 stress activates the OxyR transcription factor, which then directly stimulates synthesis of approximately two dozen proteins (Zheng et al., 2001). The protective roles of about half of these are understood. The AhpCF peroxidase and the KatG catalase directly limit H2O2 accumulation (Seaver & Imlay, 2001a). Dps is a ferritin-like protein that sequesters unincorporated iron and thereby minimizes Fenton chemistry and, consequently, DNA damage (Grant et al., 1998, Ilari et al., 2002, Park et al., 2005). The elevated expression of Fur protein, a repressor of iron-import genes, further limits the amount of intracellular iron (Varghese et al., 2007, Zheng et al., 1999). The suf genes encode an iron-sulfur-cluster assembly complex that replaces the customary Isc machinery (Takahashi & Tokumoto, 2002, Lee et al., 2004), which evidently is also poisoned by H2O2 (Jang and Imlay, in preparation).
The roles of the other OxyR-controlled genes are not yet known. One of these is mntH, which encodes the only dedicated manganese importer in E. coli (Kehres et al., 2000b). Studies in a variety of other microbes have indicated that manganese can help protect cells against oxidative stress. Manganese supplementation allowed Streptococcus pneumoniae (Tseng et al., 2002) and Neisseria gonorrhoeae (Seib et al., 2004) to better tolerate millimolar levels of H2O2 that might otherwise have been lethal. Deinococcus radiodurans, which is exceptionally resistant to ionizing radiation, lost a large component of that resistance when it was cultured in medium that limited the availability of manganese (Daly et al., 2004).
The basis of these protective effects is not clear. Manganese is an essential cofactor of manganese-containing superoxide dismutase, but this enzyme is not co-regulated by OxyR, as one might expect it to be if the intended effect of mntH induction were to charge this enzyme. Instead, many workers have proposed that manganese might mitigate oxidative stress by chemically scavenging superoxide and/or hydrogen peroxide. Manganese complexes exhibit these activities in vitro (Horsburgh et al., 2002b, Gray & Carmichael, 1992, Berlett et al., 1990), although the rate constants depend upon their coordinating ligands and, in the best cases, are still far lower than those of enzymic manganese-dependent superoxide dismutases and Mn-catalases.
The goal of this study was to inspect the role of MntH generally and during oxidative stress in particular. To do so we exploited strains of E. coli that lack catalases and peroxidases (Hpx-). When cultured aerobically, these strains accumulate up to 1 μM of intracellular (and extracellular) H2O2 (Seaver & Imlay, 2001b), a constant stress that moderately exceeds the dose (ca. 0.1 μM) that activates expression of the OxyR regulon (Aslund et al., 1999). We show here that in this situation, a failure to induce MntH results in a profound growth defect. However, our analysis suggests that the requirement for manganese import stems from a need to cofactor enzymes rather than to act as a chemical scavenger of ROS.
Results
Manganese import into unstressed cells is minimal and expendable
E. coli has two cytoplasmic superoxide dismutases (SODs): an iron-dependent isozyme (FeSOD, encoded by sodB) (Yost & Fridovich, 1973) and a manganese-dependent isozyme (MnSOD, encoded by sodA) (Keele et al., 1970). Activity-gel experiments by Pugh et al. indicated that MnSOD activity was elevated by an order of magnitude when manganese was added to standard defined growth medium (Pugh et al., 1984). This result could not be explained by the known mechanisms of sodA regulation, and it led workers to suggest that under routine growth conditions cells may not contain enough manganese to activate the apoenzyme. Using sodB mutants that express only the MnSOD isozyme, we confirmed that MnSOD activity rose from 2 U/mg to 40 U/mg when cells were grown in manganese-supplemented medium. A sodA’-lacZ+ transcriptional fusion was not induced by this treatment, as β-galactosidase activity was 3.2 +/- 0.06 U/mg under routine conditions and 2.9 +/- 0.04 U/mg after manganese supplementation.
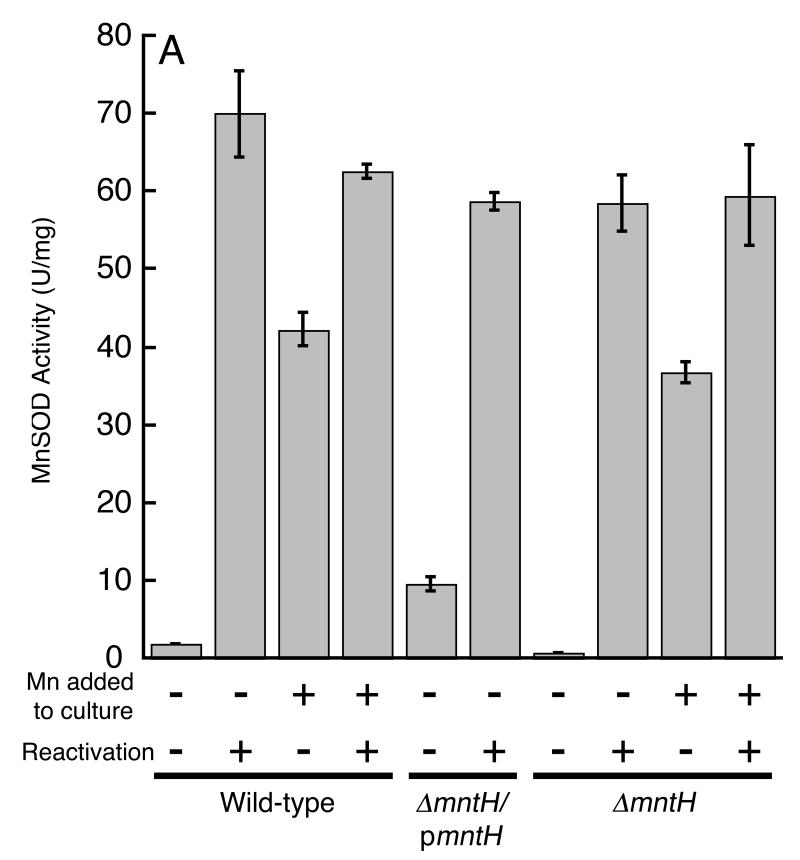
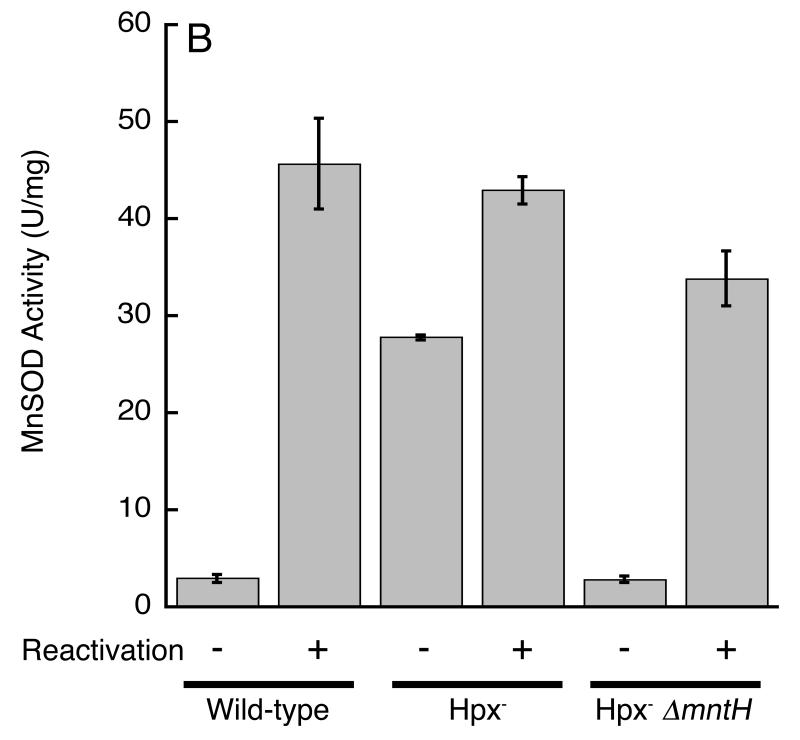
To test the enzyme-activation idea directly, cell extracts were prepared from sodB mutants and assayed immediately in order to quantify MnSOD activity. The extracts were then treated with denaturants, demetallated with the chelators EDTA and 8-hydroxyquinoline-5-sulfonic acid, and then renatured in the presence of manganese in order to fully activate the MnSOD proteins. The denaturation/renaturation protocol boosted the initial extract activity by at least twenty-fold, indicating that < 5% of the enzyme molecules recovered from cells contained manganese in their active sites (Fig. 1A, bars 1 and 2). In contrast, 60% of the MnSOD was already active when it was recovered from manganese-supplemented cells (Fig. 1A, bars 3 and 4). Similar results were obtained from AB1157 and W3110 derivatives of E. coli K-12 (data not shown).
Fig 1.
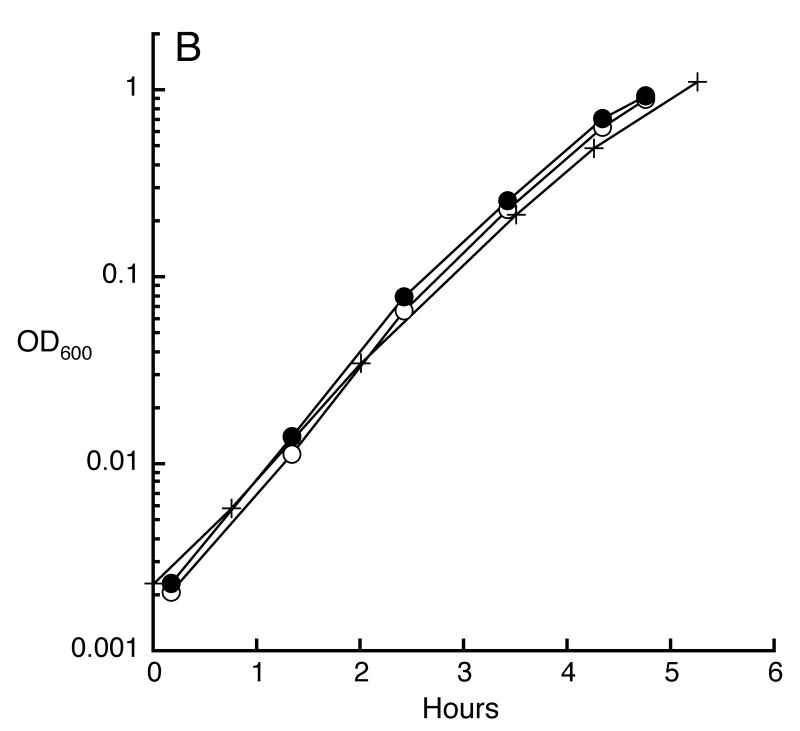
Unstressed cells do not depend upon manganese. A. In standard medium, manganese import is too slight to activate MnSOD. Where indicated, media were unsupplemented or were supplemented with 50 μM manganese, and cell extracts were assayed before and after in vitro demetallation/reactivation with manganese. Each strain lacks sodB, to permit assay of MnSOD. The strains were AA138 (mntH+), AA141 (ΔmntH), and AA141/pAA01 (ΔmntH/pmntH). B. Manganese import is unnecessary for rapid growth. Wild-type MG1655 cultured without (open circles) or with (closed circles) 50 μM manganese supplement, and ΔmntH (AA99, plus sign) without manganese.
MnSOD activity in unsupplemented cells was boosted about six-fold when mntH was provided (behind its own promoter) on a multicopy plasmid (Fig. 1A, bars 5 and 6). Thus the failure to fully metallate MnSOD in vivo derives from inadequate expression of the manganese importer. Several labs have shown that when MnSOD is recovered from iron-rich cells, a significant fraction contains iron and is therefore inactive (Beyer & Fridovich, 1991, Whittaker & Whittaker, 1997). The preceding experiments were conducted at low cell densities in iron-sufficient medium, raising the possibility that inadequate manganese uptake allowed iron to outcompete manganese for binding to the apo-protein. However, follow-up experiments failed to distinguish whether the inactive MnSOD proteins contained iron or lacked metals entirely.
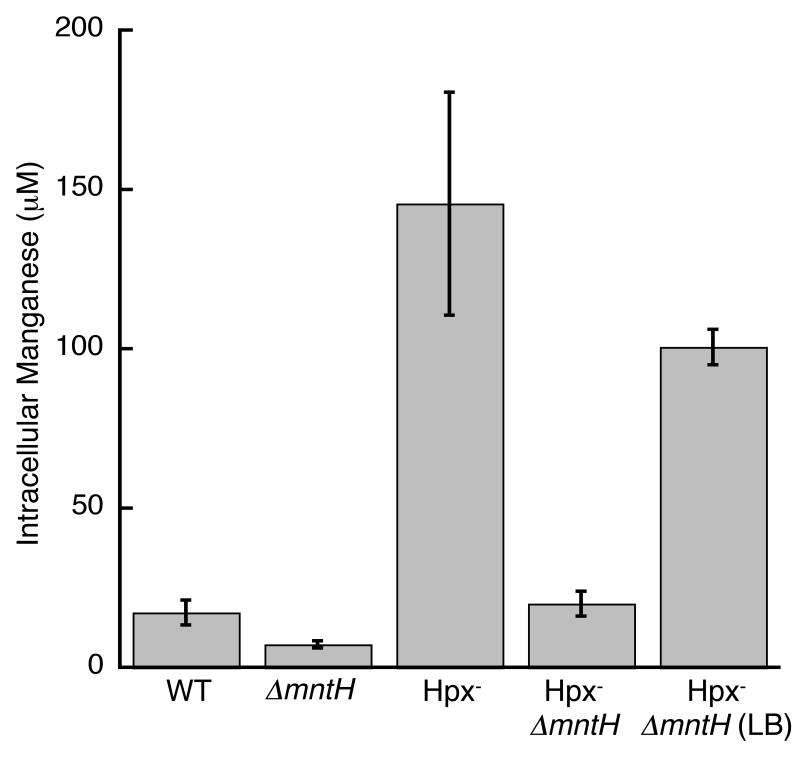
Reciprocal experiments with sodA mutants were performed to test the metallation status of FeSOD. Less than a 10% increase in activity was achieved by demetallation and remetallation with ferrous iron, indicating that the as isolated FeSOD enzymes were fully loaded with iron. Collectively, these data indicate that when wild-type cells were cultured in standard media, they contained sufficient iron to fully metallate newly synthesized apo-FeSOD, while intracellular manganese was too scarce to effectively metallate apo-MnSOD. Indeed, ICP measurements indicated that the total intracellular manganese concentration was only 15 μM (Fig. 2), compared to nearly 1 mM for iron ((Outten & O’Halloran, 2001); data not shown).
Fig 2.
Total manganese concentration of cells, measured by ICP. Cells were grown in defined medium (glucose/amino acids) or, where indicated, LB medium. Data represents means of three independent cultures. The strains used were MG1655 (wild-type), AA99 (ΔmntH), LC106 (Hpx-) and AA30 (Hpx- ΔmntH).
These results also suggest that E. coli can thrive even when it does not import enough manganese to metallate manganese-dependent enzymes. To more rigorously test this conclusion, we deleted the gene that encodes the only specific manganese transporter in E. coli, MntH. The manganese content of these mutants was minimal (Fig. 2), and MnSOD activity was virtually absent (Fig. 1, bars 7 and 8). Nevertheless, growth was as robust as that of wild-type cells (Fig. 1B).
Cells require manganese import during H2O2 stress
These results raise the question: Under what circumstances is manganese import important for cell fitness? The regulatory proteins that control MntH synthesis may provide clues. One of these proteins, Fur, directly senses iron levels in E. coli. When iron is abundant, Fe2+-metallated Fur protein binds the promoter region of mntH and represses its transcription (Kehres et al., 2000b, Patzer & Hantke, 2001). However, in low-iron conditions Fur is predominately in the apo-form, cannot bind DNA, and no longer inhibits mntH transcription. Thus the cell activates manganese import when iron levels are low. Such regulation implies that manganese can compensate for iron deficiency. Indeed, Grass et al. have shown that manganese entry through mntH can stimulate the growth of iron-import mutants (Grass et al., 2005). We replicated these results (data not shown).
A plausible mechanism for this compensation is by the insertion of manganese into enzymes that normally employ iron as a cofactor. Manganese is unlikely to substitute for iron in iron-sulfur clusters or hemes, since the large disparity between its reduction potential and that of iron would interfere with the redox activities of these cofactors. Instead, it is possible that manganese takes the place of mononuclear iron in enzymes that employ divalent metals to bind substrates. In vitro a variety of metals, including manganese, typically suffice to activate these enzymes. The ability of iron to activate such enzymes has not been well explored. However, recent studies show that enzymes such as isocitrate dehydrogenase (Murakami et al., 2006) and transketolase (Sobota and Imlay, unpublished), which are commonly activated by manganese in vitro, are also fully functional if they are charged with ferrous iron. This raises the possibility that iron may be the usual cofactor for some metal-activated enzymes in vivo. If so, then during periods of iron scarcity the induction of MntH may enable manganese to take its place. This substitution might also spare enough iron to charge those enzymes that require heme and iron-sulfur cofactors.
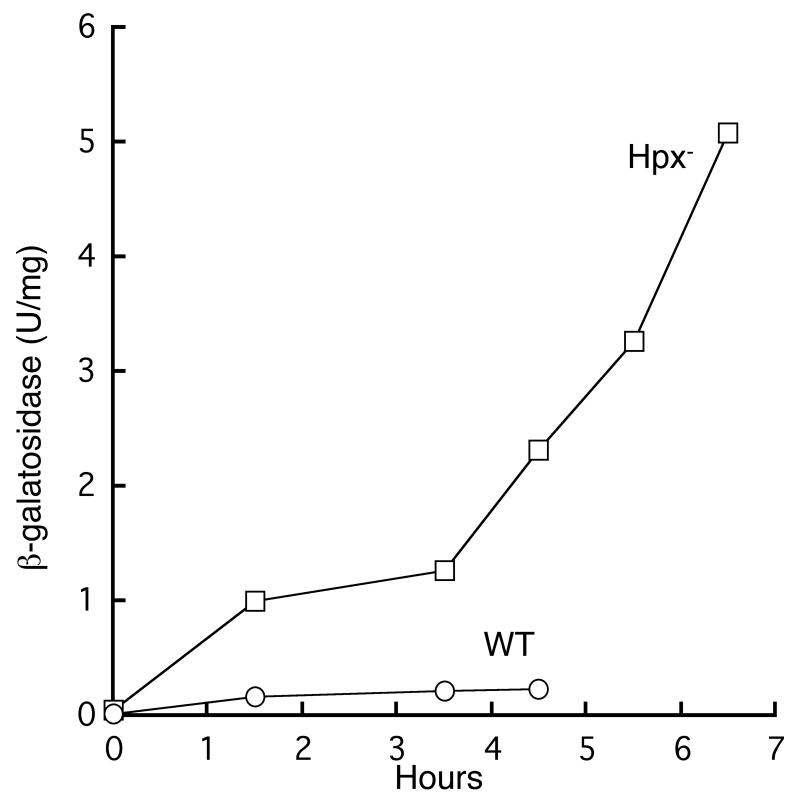
Another transcription factor, OxyR, induces expression of mntH during periods of H2O2 stress (Kehres et al., 2000a, Kehres et al., 2002b). The roles of OxyR-controlled genes have been studied in catalase/peroxidase (Hpx-) mutants. These strains cannot degrade hydrogen peroxide; therefore, when they are cultured in aerobic media, the adventitious oxidation of intracellular redox enzymes leads to the accumulation of up to 1 μM intracellular H2O2 (Seaver & Imlay, 2001b). The OxyR stress response is activated by as little as 0.1 μM H2O2 (Aslund et al., 1999), and we confirmed that an mntH’-lacZ+ fusion was strongly induced in the Hpx- mutant when it was transferred from anaerobic to aerobic media (Fig. 3). In contrast, very little expression occurred in a wild-type background, consistent with the low level of intracellular manganese and the absence of a null-mutant phenotype.
Fig 3.
The gene mntH is strongly induced in aerobically growing Hpx- cells. Cells bearing a mntH’-lacZ fusion were grown in anaerobic defined medium (glucose/amino acids) and aerated at time zero. At intervals β-galactosidase was assayed. This experiment is representative of four independent replicates. AA183 (wild-type, circles) and AA191 (Hpx-, squares).
A deletion mutation of mntH was generated in anaerobic Hpx- mutants, without any apparent effect on anaerobic growth (data not shown). However, when the Hpx- ΔmntH mutants were diluted into aerobic defined medium, they soon stopped growing (Fig. 4a). This phenotype was reproduced in other E. coli K-12 backgrounds and in various types of defined media (data not shown). A plasmid carrying mntH restored normal growth, as did either the katG or ahpCF wild-type alleles. The addition of micromolar concentrations of manganese to the medium also allowed growth—while 100 μM iron, zinc, and copper did not (data not shown)—confirming that the pertinent role of MntH is to import manganese.
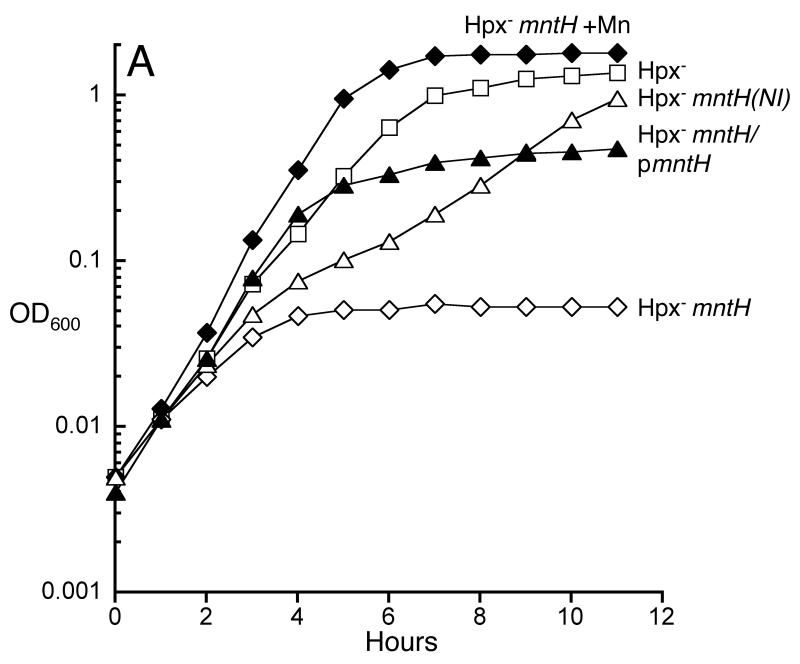
Fig 4.
Manganese import is essential for H2O2-stressed cells. Cells were grown in defined medium (glucose/amino acids). A. Cells were cultured anaerobically and then aerated at time zero. Where indicated, manganese (50 μM) was included in the culture medium. LC106 (Hpx-, open squares), SMV42 (Hpx- ΔmntH, open diamonds), SMV42 + MnCl2 (closed diamonds), SMV42/pAA01 (Hpx- ΔmntH/pmntH, closed triangles) and AA153 (Hpx- mntH(NI), open triangles). B. MnSOD protein is correctly metallated in H2O2-stressed cells, due to increased manganese import. Each strain lacks sodB, to permit assay of MnSOD. SMV32 (wild-type), SMV29 (Hpx-), AA145 (Hpx- ΔmntH).
This result also indicates that there must be a secondary, low-affinity route by which manganese can enter the cell (Fig. 1A, bars 7-10). That route is unidentified but required 50 μM manganese for full growth stimulation, suggesting that its native substrate may be a cation other than manganese. In contrast, MntH imported sufficient manganese from the standard defined medium, which contained only 0.14 +/- 0.01 μM manganese. Interestingly, the Hpx- ΔmntH mutants were able to grow in unsupplemented Luria-Bertani (LB) medium, apparently because they accumulated substantial intracellular manganese (Fig. 2). Luria-Bertani (LB) medium contains more manganese (0.25 +/- 0.01 μM manganese) than does defined medium, and unlike the defined medium it lacks phosphate and citrate, metal chelators that can restrict manganese availability. In the Hpx- mutants the impact of the OxyR-dependent induction of mntH was to elevate the intracellular manganese content to 150 μM, an order of magnitude above that of wild-type cells (Fig. 2). As a consequence, the efficiency of MnSOD activation was much higher (Fig. 4B).
We deleted the OxyR binding site upstream of mntH. This mutation did not diminish basal expression, but it eliminated the OxyR-driven induction of the gene during H2O2 stress (data not shown). The Hpx- mutants containing this non-inducible allele exhibited a growth behavior intermediate between that of the Hpx- ΔmntH strain and its mntH+ parent (Fig. 4A). Thus basal expression of mntH is inadequate for H2O2-stressed cells, presumably because they require supranormal levels of manganese. Indeed, even the Hpx- (mntH+) strain grew best when additional manganese was added to the medium (data not shown).
Manganese import is not required to scavenge reactive oxygen species
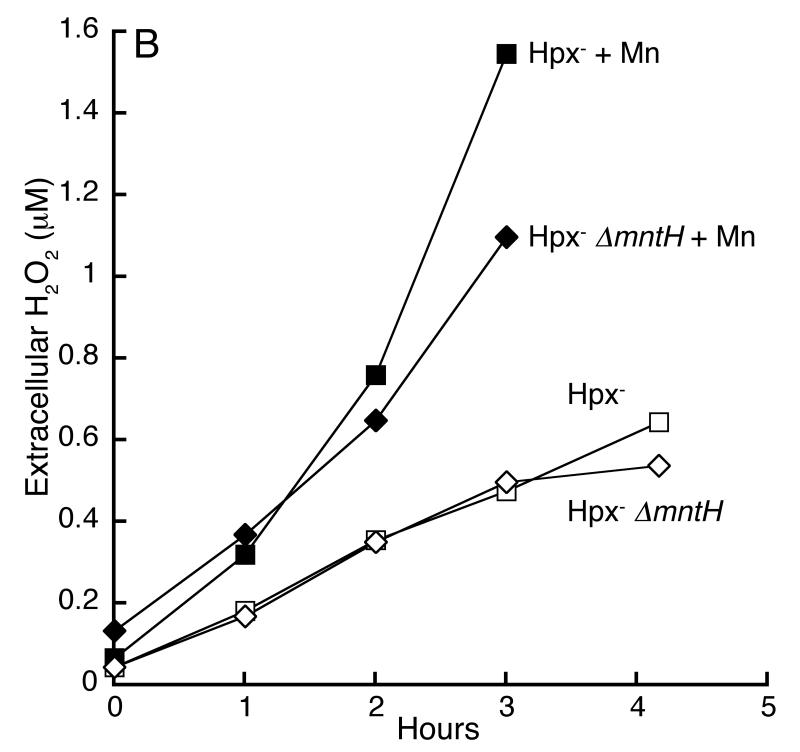
Previous reports speculated that manganese might protect oxidatively stressed cells because it has the chemical ability to scavenge superoxide and H2O2 (Horsburgh et al., 2002b, Gray & Carmichael, 1992, Berlett et al., 1990). The Hpx- strain suffers specifically from H2O2 stress, and so we directly measured the rates at which Hpx- mntH+ and Hpx- ΔmntH cells degraded H2O2 (Fig. 5A). Contrary to the scavenging hypothesis, the MntH+ cells did not degrade H2O2 any more rapidly than did their ΔmntH counterparts. Further, manganese supplements did not provide any activity. More generally, Hpx- mntH+ cells released as much H2O2 as did Hpx- ΔmntH mutants (Fig. 5B). Since the rate of efflux is directly proportional to internal H2O2 concentrations (Seaver & Imlay, 2001b), this result indicates that imported manganese did not diminish intracellular H2O2 levels.
Fig 5.
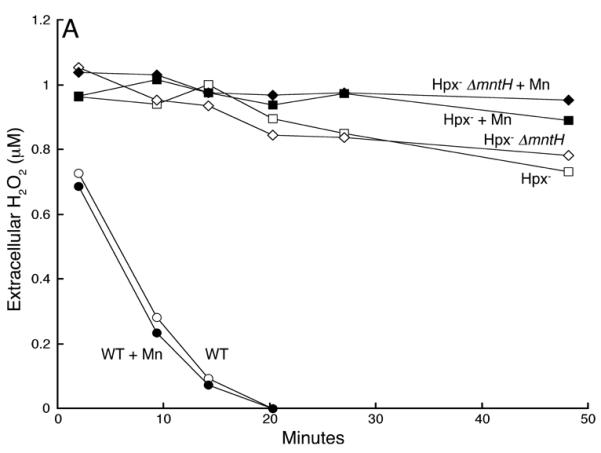
Imported manganese does not scavenge H2O2. A. Manganese did not prevent H2O2 accumulation by Hpx- and Hpx- ΔmntH cells. Cells were grown anaerobically and aerated at time zero. At intervals the medium was assayed for accumulated H2O2. LC106 (Hpx-, squares) and SMV42 (Hpx- ΔmntH, diamonds). Filled symbols: 50 μM manganese was included in the growth medium. B. Manganese-supplemented cells did not exhibit significant H2O2-scavenging activity. H2O2 (1 μM) was added at time zero to cultures of log-phase cells. Filled symbols: 50 μM manganese was included in the growth medium. MG1655 (open circles), LC106 (Hpx-, squares), SMV42 (Hpx- ΔmntH, diamonds).
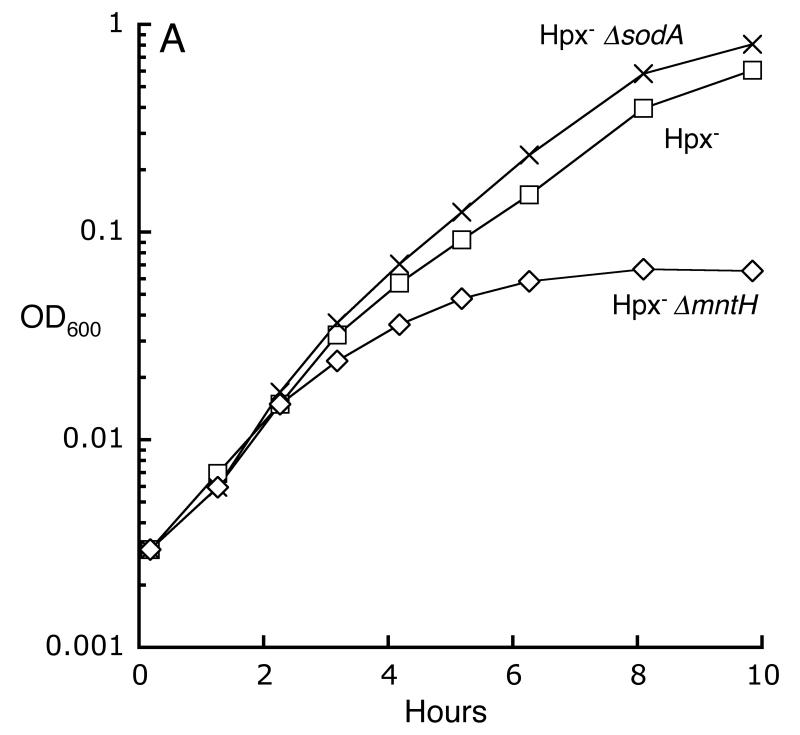
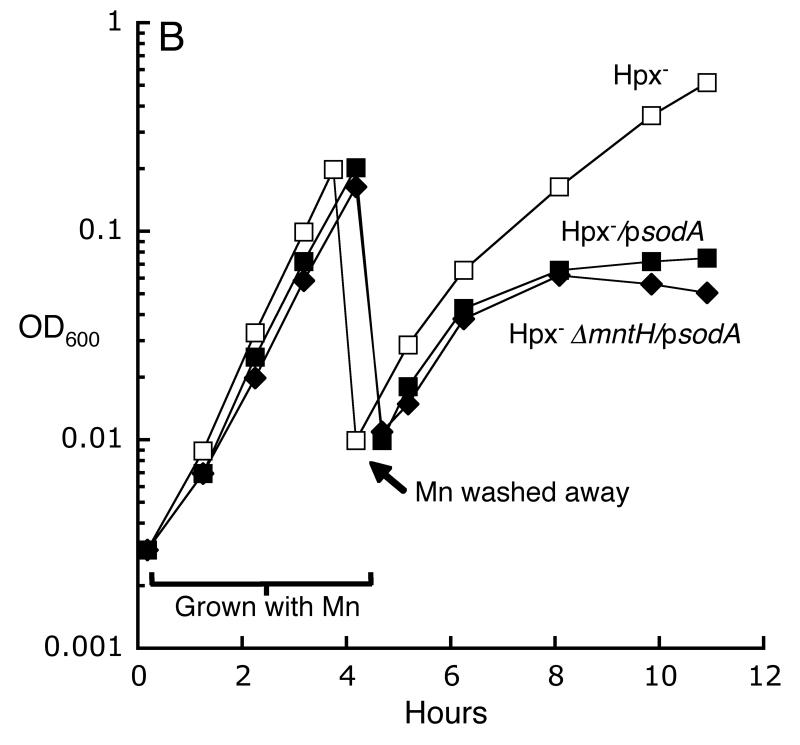
Superoxide degrades the iron-sulfur clusters of dehydratases, and the released iron can catalyze DNA damage by H2O2 (Flint et al., 1993, Liochev & Fridovich, 1994, Keyer & Imlay, 1996, Kuo et al., 1987). This mechanism provides a route by which superoxide can exacerbate the toxicity of H2O2; therefore, we examined the possibility that imported manganese was needed to suppress Fenton chemistry by activating MnSOD. However, a Hpx- ΔsodA mutant grew well in defined medium, indicating both that MnSOD activity was expendable and that imported manganese was critical for some other purpose (Fig. 6A). In a complementary experiment, the sodA gene was provided on a multicopy plasmid to boost the SOD activity of Hpx- ΔmntH cells. Still, the cells were able to grow in aerobic medium only if manganese supplements were provided (Fig. 6B). Indeed, when the cells were washed free of the manganese, growth quickly ceased even though the residual SOD activity substantially exceeded that of a wild-type strain. Most tellingly, the sodA-overexpressing plasmid actually inhibited the aerobic growth of an Hpx- strain that contained the wild-type mntH allele—a strain which otherwise would have grown well—presumably because the elevated level of MnSOD protein sequestered the intracellular manganese so that it could not perform its critical (unknown) function. Finally, the addition of both ΔsodA and ΔsodB alleles to the Hpx- strain did not block its growth in this medium (data not shown). All together, these results indicate that Hpx- growth depends critically upon intracellular manganese rather than upon superoxide scavenging. We conclude that the key role of manganese in these H2O2-stressed cells is something other than the degradation of H2O2 or O2-.
Fig 6.
The primary role of intracellular manganese is not to degrade O2-. A. Metallation of MnSOD is not the primary purpose of manganese import. Cells were precultured in anaerobic defined medium (glucose/amino acids) and then aerated at time zero. LC106 (Hpx-, squares), AA30 (Hpx- ΔmntH, diamonds) and SMV30 (Hpx- ΔsodA, X’s). B. Overproduction of MnSOD debilitates Hpx- ΔmntH cells. Anaerobic cells, with or without the pDT1.5 sodA over-expression plasmid, were aerated at time zero in defined medium (glucose/amino acids) containing 100 μM MnCl2. At the indicated time (arrow), manganese was removed. LC106 (Hpx-, open squares), LC106/pDT1.5 (closed squares) and SMV42/pDT1.5 (Hpx- ΔmntH, closed diamonds).
Manganese does not protect DNA from oxidative damage
A primary target of H2O2 is DNA, through Fenton chemistry catalyzed by DNA-bound iron. We evaluated the possibility that manganese might protect the DNA by displacing iron from its surface. The 150 μM manganese that accumulated inside the Hpx- cells was much less than the DNA nucleotide concentration in growing cells (15-30 mM) (Neidhardt & Umbarger, 1996), indicating that manganese could never shield the DNA from all potential iron binding. Nevertheless, some DNA sequences create strong metal-binding sites and might be particular loci for damage (Rai et al., 2001), and in principle manganese might displace iron from these sites.
However, microscopic examination showed that Hpx- ΔmntH cells did not filament, and despite their poor growth the number of viable cells did not diminish (data not shown). Filamentation and death are two hallmarks of oxidative DNA damage (Park et al., 2005). Further, although ICP analysis confirmed that high-dose manganese supplements raise the manganese content of wild-type cells to about 200 μM, these supplements did not diminish the H2O2 sensitivity of DNA-repair-defective mutants: only 1% of recA mutants survived a five-minute exposure to 2.5 mM H2O2 whether or not the growth medium was supplemented with 100 μM manganese. Finally, manganese supplements did not allow the growth of Hpx- ΔrecA cells (data not shown). These latter experiments indicate that excess manganese does not suppress Fenton-mediated DNA damage. We infer that the growth defect of Hpx- ΔmntH mutants does not arise from DNA oxidation and that it is more likely to reflect a disruption in cell metabolism.
Manganese is required by the cell to protect enzymes
When Hpx- ΔmntH cells were supplemented with manganese, the amount of manganese that was needed to stimulate growth correlated with the amount needed to metallate MnSOD (Fig. 7). Because MnSOD activity per se is not necessary to allow growth, we interpret this correspondance more generally as an indication that H2O2-stressed cells need to import enough manganese to ensure the metallation of one or more key enzymes. Aside from MnSOD, there are few or no enzymes in E. coli that specifically require manganese for function (Kehres & Maguire, 2003). While many enzymes from E. coli can utilize manganese in vitro to satisfy a general requirement for a divalent metal, it is likely that many of these enzymes can use magnesium as an alternative. We knocked out several genes (nrdEF, icd, pgmI, mntR, glpX, maeA) that encode proteins that have been suspected of relying on manganese in vivo, but none of these mutations replicated the ΔmntH growth defect in the Hpx- background (data not shown).
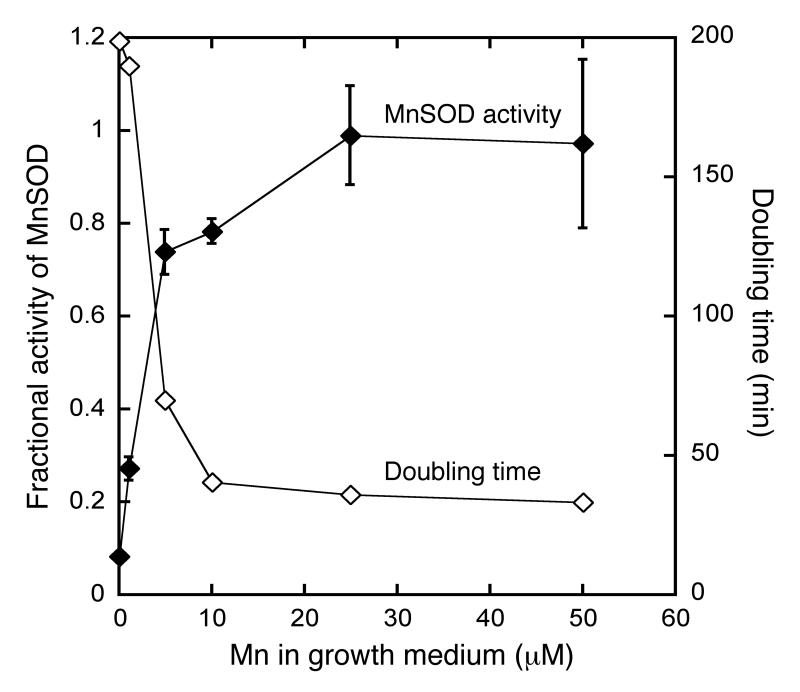
Fig 7.
The amount of manganese needed to protect Hpx- mutants matches the amount needed to metallate enzymes. AA145 (Hpx- ΔmntH) cells were precultured anaerobically in defined medium (glucose/amino acids) and then subcultured at time zero into aerobic defined medium (glucose/amino acids) containing the indicated concentration of manganese. Optical density was monitored continuously, and MnSOD activity was measured when the cultures reached an OD600 of 0.2. The lowest doubling time, and thus the highest growth rate, for each culture is denoted in the figure.
It is notable that magnesium binds more weakly than manganese to transketolase A and isocitrate dehydrogenase, perhaps because magnesium is less tolerant of deviations from its ideal coordination geometry (Kehres & Maguire, 2003). If magnesium is a mediocre metal for such enzymes, and manganese is not imported into unstressed cells, then ferrous iron is a plausible candidate to be the usual activating metal. This logic suggests that manganese might serve to metallate such enzymes only during periods of iron starvation or H2O2 stress, the two conditions that activate synthesis of MntH.
During H2O2 stress the OxyR system also induces Dps, a ferritin-like protein that sequesters loose iron and thereby diminishes the amount of Fenton-based DNA damage (Grant et al., 1998, Ilari et al., 2002, Park et al., 2005). Therefore, one possible rationale for mntH induction during H2O2 stress would be that the action of Dps effectively starves cells for iron, so that without manganese the divalent-metal-requiring enzymes would be left in an apoprotein form. An alternative hypothesis is that ferrous-iron-loaded enzymes would be targets for Fenton reaction, and that the replacement of iron by manganese allows these enzymes to avoid oxidative injury. A testable difference between these two ideas is that the first hypothesis suggests that manganese protects the cell when it has too little unincorporated iron, whereas the second implies that manganese protects the cell when it has too much.
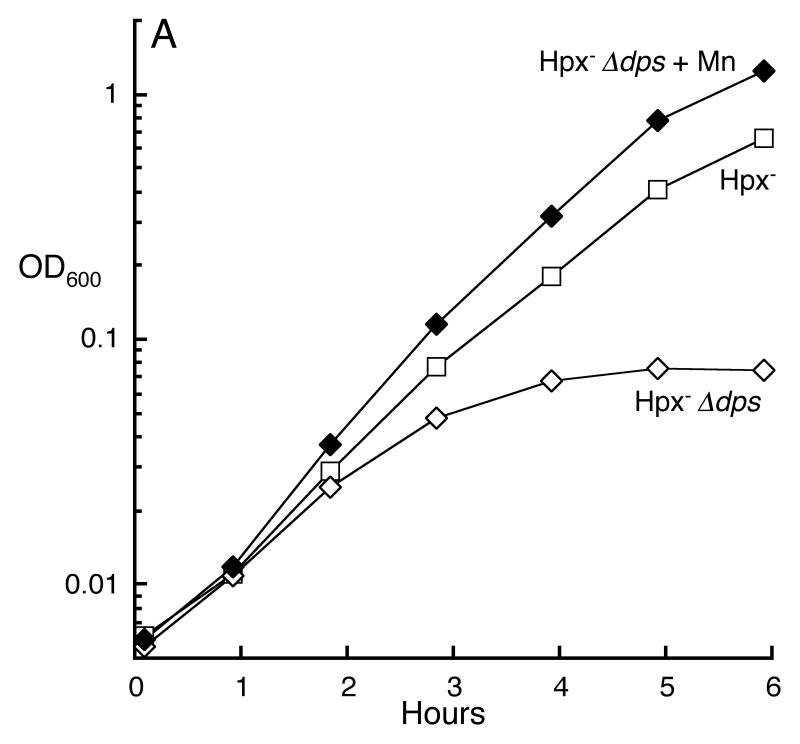
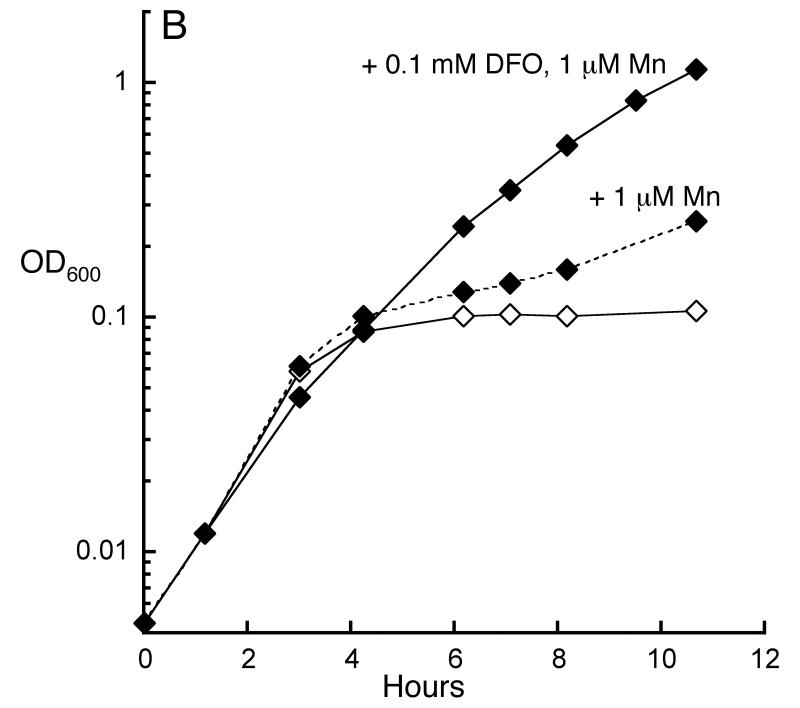
EPR-based measurements indicated that Hpx- mutants contain more unincorporated iron than do wild-type cells (Park et al., 2005). The increase is likely due to the oxidative degradation of iron-sulfur clusters (Jang & Imlay, 2007); in any case, this result is more consistent with the second hypothesis rather than the first. Further, we observed that the profound growth defect of Hpx- Δdps mutants could be suppressed by manganese supplements (Fig. 8A). This result, too, suggests that manganese rescues the cell from iron overload rather than from iron starvation. Finally, iron chelators substantially stimulated the growth of Hpx- ΔmntH cells (Fig. 8B), indicating that the purpose of manganese is to out-compete iron.
Fig 8.
Manganese is required to rescue Hpx- cells from iron overload. A. Manganese supplementation rescues Hpx- Δdps cells. Cells growing in anaerobic defined medium (glucose/amino acids), +/- 50 μM MnCl2, were aerated at time zero. LC106 (Hpx-, open squares), SP66 (Hpx- Δdps, open diamonds), SP66 grown + MnCl2 (closed diamonds). B. The growth defect of Hpx- ΔmntH was suppressed by an iron-specific chelator. Cells (AA30) were grown in aerobic defined medium (glucose/amino acids) without MnCl2 (open diamonds), with 1 μM MnCl2 (dotted line, closed diamonds) or with 1 μM MnCl2 plus 0.1 mM deferoxamine (closed diamonds).
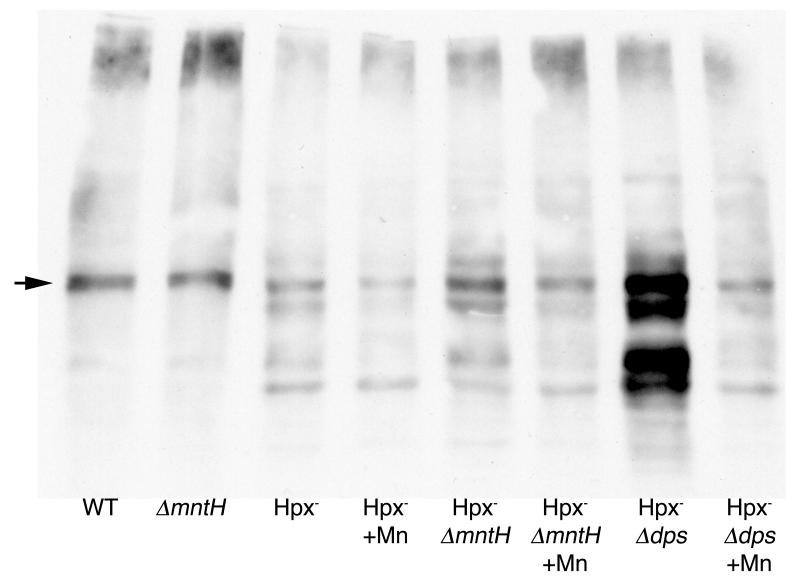
These results supported the notion that manganese incorporation might spare enzymes from iron-driven oxidation reactions. Some fraction of Fenton events within proteins generate carbonyl groups on local amino acid residues. These can be detected on Western blots using antibodies against the DNP-derivatized proteins. Proteins extracted from Hpx- ΔmntH mutants exhibited substantially more carbonyls than did proteins from their MntH+ parents (Fig. 9). The excess carbonylation was suppressed when manganese was supplemented into their growth medium. Further, the Hpx- Δdps mutants exhibited extremely high levels of carbonylation, and this damage was also diminished by manganese. These data confirm that excess intracellular iron accelerates protein oxidation, while manganese import suppresses it.
Fig 9.
Imported manganese suppresses oxidative protein carbonylation. Cells were grown in aerobic defined medium (glucose/amino acids) +/- 50 μM MnCl2, and proteins were harvested at an OD600 of ~ 0.2. Proteins were derivatized and western blotted as described in experimental procedures. The arrow indicates a non-oxidative carbonylation that was detected even when cells were grown and derivatized anaerobically. The strains used were MG1655 (wild-type), AA99 (ΔmntH), LC106 (Hpx-), AA30 (Hpx- ΔmntH) and SP66 (Hpx- Δdps).
Discussion
Are manganese and iron alternative cofactors for mononuclear-metal enzymes?
Life evolved in an anaerobic, iron-rich world. Photosystem II did not begin to generate molecular oxygen until about 2.8 byr ago, and it is likely that during the subsequent billion years the atmosphere still remained essentially anaerobic, as the nascent oxygen was quickly scavenged by reaction with dissolved ferrous iron in the Earth’s seas (Bjerrum & Canfield, 2002). Only later, after the iron had been oxidized and precipitated as ferric minerals, did oxygen accumulate. Thus the fundamental biochemical mechanisms and metabolic pathways that contemporary organisms use were inherited from an environment very unlike our own.
The depletion of bioavailable iron posed a problem for organisms, and they responded by inventing elaborate import systems to satisfy their iron demand. E. coli grown in laboratory cultures contains about 1 mM iron (Outten & O’Halloran, 2001), much of it incorporated as a cofactor in enzymes that rely upon heme or iron-sulfur clusters. However, the fact that manganese import is stimulated upon iron depletion (Kehres et al., 2000b, Patzer & Hantke, 2001), and that it can substantially compensate for an insufficient iron supply (Grass et al., 2005), suggests that a substantial fraction of imported iron might be used as a mononuclear cofactor in non-redox enzymes and that manganese can substitute for it. Indeed, although more than sixty E. coli enzymes can be activated by manganese in vitro (www.ecocyc.org), the robust growth of mntH mutants shows that these enzymes must be able to employ some other metal in vivo. Some of these enzymes are likely to use magnesium, but for others iron is the obvious candidate, since in its ferrous form it shares with manganese(II) a similar size, valence, and coordination geometry. Most manganese-activatable enzymes have not been adequately tested for their ability to use iron, because when such experiments are performed in aerobic buffers, ferrous iron is quickly oxidized to the unusable ferric form. We suspect, then, that some of these proteins evolved with iron as their routine cofactor, and that metallation by manganese is an alternative that is resorted to only when iron levels are low.
The few mononuclear enzymes that have redox functions may be instructive exceptions. For example, although iron and manganese again compete to bind in the protein coordination environments that are presented by superoxide dismutases, the two metals cannot provide equivalent activity, since they differ in their reduction potentials. To cope with this issue, discrete SOD isozymes are synthesized in E. coli and in many other bacteria, one for iron and one for manganese (Yost & Fridovich, 1973, Keele et al., 1970). Under iron-sufficient conditions, the iron-dependent isozyme is synthesized and the manganese-dependent enzyme is substantially repressed (Tardat & Touati, 1993), suggesting that the latter is a back-up enzyme used in circumstances of iron deficiency.
Certain organisms seem to have evolved to not depend on iron for cell chemistry, including Lactobacillus plantarum (Archibald, 1986a), Borrelia burgdorfei (Posey & Gherardini, 2000) and Streptococcus suis (Niven et al., 1999); notably, they have unusually high concentrations of intracellular manganese, which might serve as a cofactor for mononuclear metal enzymes. These bacteria additionally dispense with pathways that depend upon Fe/S- or heme-cofactored enzymes, such as the TCA cycle, and/or employ manganese-based enzymes in their place. For instance, L. plantarum uses a heme-less Mn-catalase to scavenge H2O2 (Kono & Fridovich, 1983, Barynin et al., 2001).
Manganese is not an efficient scavenger of ROS
The ability of manganese to defend microbes against oxidants has been noted in several contexts: imported manganese protects Neisseria gonorrhoeae (Seib et al., 2004, Tseng et al., 2001), Streptococcus pneumoniae (Tseng et al., 2002), and Salmonella typhimurium (Kehres et al., 2000a) against exogenous H2O2, while lactic-acid bacteria, which generate H2O2 as a stoichiometic by-product of central metabolism, are rich in intracellular manganese (Archibald, 1986b). Further, Deinococcus radiodurans loses its characteristic resistance to ionizing radiation if manganese import is limited (Daly et al., 2004). Collectively these results have prompted suggestions that manganese might serve inside the cell as a chemical scavenger of superoxide (Archibald & Fridovich, 1981, Chang & Kosman, 1989, Inaoka et al., 1999, Tseng et al., 2001, Horsburgh et al., 2002a, Al-Maghrebi et al., 2002, Daly et al., 2004, Sanchez et al., 2005), of hydrogen peroxide (Runyen-Janecky et al., 2006), or of both (Kehres et al., 2000b, Horsburgh et al., 2002b, Kehres & Maguire, 2003, Seib et al., 2004). However, the peroxide-efflux experiments reported here directly show that in E. coli, at least, the imported manganese does not effectively degrade H2O2. Why not? Stadtman and colleagues showed that the ability of manganese to rapidly disproportionate H2O2 requires the formation of a manganese complex with three equivalents of HCO -3 (Berlett et al., 1990). Metabolites are likely to interfere with this activity by coordinating the metal; and, even if they did not, the rate constant of the dismutation reaction is too low to enable physiological concentrations of manganese to effectively degrade H2O2. For example, if intracellular bicarbonate were, generously, 28mM and manganese were 150 μM, the half life of H2O2 would be 27 min, exclusive of the activities of Ahp and catalase. When the latter are considered, the half-life would be 1.3 msec (calculated from (Seaver & Imlay, 2001b)). In other words, the scavenging activities of these enzymes exceed the chemical activity of this concentration of manganese by six orders of magnitude. This calculation explains why imported manganese did not alter H2O2 efflux, and it confirms that the role of MntH induction is not to augment the scavenging activity of Ahp and KatG. It also suggests why L. plantarum, which has a high intracellular content of manganese, nevertheless synthesizes a dedicated Mn-catalase.
What about superoxide dismutation? This reaction can be accomplished by manganese that is loosely bound to certain biomolecules, with rate constants that reached 13,200 SOD units/mg Mn(II) in the best case (Archibald & Fridovich, 1982). In a cell containing 150 μM manganese, this ideal complex would provide 108 U/mL of dismutation activity. That amount still pales, however, compared to the 3000 U/mL enzymatic SOD present in unstressed cells (Imlay & Fridovich, 1991) and the 14,000 U/mL in H2O2-stressed E. coli (calculated from this study). For both superoxide and H2O2 dismutation reactions, the kinetic efficiency depends upon the midpoint potential of the Mn(II)/Mn(III) couple and therefore is strongly affected by the coordination environment. The purpose of SOD and catalase proteins is to control that environment. So it seems unlikely that cells would rely on manganese that is loosely deposited on the surfaces of various biomolecules to serve this catalytic role. Interestingly, while there has been great interest in the use of manganese chelates as therapeutic sources of SOD activity, experiments have indicated that these drugs may not work by scavenging superoxide (Munroe et al., 2007). One alternative is that they serve as cell-permeable carriers of manganese, which then acts to metallate and protect mononuclear metal enzymes.
The OxyR regulon is dedicated to the suppression of Fenton chemistry
The OxyR regulon has been calibrated by evolution to be activated by about 0.1 μM intracellular H2O2, suggesting that this dose is physiologically relevant (Aslund et al., 1999). Because the cell membrane is only semi-permeable to hydrogen peroxide, an extracellular concentration of 1 to 5 μM H2O2 is probably what activates this system inside a wild-type (scavenger-proficient) cell (Seaver & Imlay, 2001b); therefore, this is the concentration that one would like to impose in order to study the physiology of oxidative stress. However, it is difficult to establish and maintain this dose in experimental cultures of substantial cell density: wild-type cells rapidly degrade H2O2 that is provided in a single bolus (e.g., Fig. 5A), and it is not easy to arrange continuous H2O2 production in order to replicate the steady-state H2O2 levels that might occur in nature. As an alternative, catalase/peroxidase-deficient (Hpx-) strains permit workers to impose steady, physiological H2O2 stresses for a long enough period of time so that growth defects can become apparent.
To date the debilitating effects of micromolar H2O2 stress have all been tracked to Fenton-type reactions, including the oxidation of DNA (Park et al., 2005), the destruction of dehydratase iron-sulfur clusters (Jang & Imlay, 2007), and the de-regulation of iron homeostasis through the inactivation of Fur protein (Varghese et al., 2007). The rate constant of the Fenton reaction ranges from 103-104 M-1 s-1 for these biological targets. Thus growth defects arise and mutagenesis becomes prominent when intracellular H2O2 concentrations reach 1 μM, and it is fitting that the OxyR protein becomes activated when H2O2 levels approach this value. The proteins whose synthesis it activates include Dps, which suppresses Fenton chemistry by sequestering unincorporated iron; Fur, which restores control of iron import; and the Suf system, which helps to maintain the activities of Fe/S enzymes. The determination that MntH also helps suppress Fenton-based protein damage fits this pattern. However, we have not yet identified the specific metabolic failure that causes either the Hpx- Δdps mutant or the Hpx- ΔmntH mutant to stop growing. The suspicion is that an iron-loaded mononuclear-metal enzyme is oxidized and loses activity, thereby plugging a key metabolic pathway. We are currently working to identify such a bottleneck.
Experimental procedures
Reagents
Desferrioxamine (deferoxamine mesylate), 30% hydrogen peroxide, onitrophenyl-β-D-galactopyranoside (ONPG), acid-hydrolyzed casamino acids (Hy-Case Amino), xanthine, bovine xanthine oxidase, E. coli manganese-containing superoxide dismutase, horseradish peroxidase, E. coli iron-containing superoxide dismutase, horse heart cytochrome c, manganese(II) chloride tetrahydrate, copper(II) sulfate pentahydrate, ferric chloride, ferrous ammonium sulfate hexahydrate, zinc sulfate, and 8-hydroxyquinoline-5-sulfonic acid were from Sigma-Aldrich. Guanidine hydrochloride was obtained from Fisher Scientific. OxyBlot Protein Oxidation Detection Kit (S7150) was from Chemical International. Amplex Red was purchased from Invitrogen.
Bacterial Growth
Luria-Bertani medium (LB) contained (per liter) 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl. Defined medium (glucose/amino acids) consisted of minimal A salts (Miller, 1972) supplemented with 0.2% casamino acids, 0.5 mM tryptophan, and 0.2% glucose. When antibiotic selection was needed, media were supplemented with either 100 μg/mL ampicillin or 20 μg/mL chloramphenicol.
Anaerobic cultures were grown in an anaerobic chamber (Coy Laboratory Products Inc.) under an atmosphere of 85% nitrogen, 10% hydrogen, and 5% carbon dioxide. Aerobic cultures were grown with vigorous shaking at 37°C. To ensure that cells were growing exponentially before they were exposed to oxygen, anaerobic overnight cultures of oxygen-sensitive strains were diluted to OD600 = 0.005 in fresh anaerobic medium and allowed to grow to OD600 ~ 0.15 at 37°C. These cells were then subcultured into fresh aerobic medium to obtain an OD600 of 0.005, with or without manganese(II) chloride, and grown aerobically at 37°C. To remove supplemented manganese, cells were washed once and inoculated in the same medium but without MnCl2.
Strains and strain construction
Strains used in this study are listed in Table 1. All constructions in Hpx- (i.e. katE katG ahpF) backgrounds were performed in an anaerobic chamber to ensure that suppressor mutations were not selected during outgrowth. Null mutations were created using the Red recombinase method (Datsenko & Wanner, 2000). Mutations were introduced into new strains by P1 transduction (Miller, 1972). The resultant mutations were confirmed by PCR analysis and, when possible, by enzyme assays.
Table. 1.
| Strain | Genotype | Reference |
|---|---|---|
| MG1655 | F-wild type | E. coli Genetic Stock Center |
| LC106 | ΔahpF::kan Δ(katG17::Tn10)1 Δ(katE12::Tn10)1 | Seaver and Imlay (2004) |
| CAG18467 | zff-1 ::Tn10 | E. coli Genetic Stock Center |
| MM2115 | mntH9::kan | Kehres and Maguire (2000) |
| SMV41 | mntH9::kan~zff-1::Tn10 | P1(CAG18467) × MM2115 |
| SMV42 | As LC106 plus mntH9::kan~zff-1::Tn10 | P1(SMV41) × LC106 |
| BW25113 | lacI rrnB ΔlacZ hsdK ΔaraBAD ΔrhaBAD | Datsenko and Wanner (2000) |
| AA19 | BW 25113 ΔmntH2::cat | This work |
| AA99 | MG1655 ΔmntH2::cat | P1(AA19) × MG1655 |
| AA30 | LC106 ΔmntH2::cat | P1(AA19) ×LC106 |
| JS141 | CSH7 ΔahpCF1::cat Δ(katG17::Tn10)1 ΔkatE1::kan | Laboratory Stock |
| AA118 | JS141 mntH9::kan~zff-1::Tn10 | P1(SMV41) × JS141 |
| JS148 | AB1157 ΔahpCF1::cat Δ(katG17::Tn10)1 ΔkatE1::kan | Laboratory Stock |
| AA120 | JS148 mntH9::kan~zff-1::Tn10 | P1(SMV41) × JS148 |
| JI370 | MG1655 ΔahpF::kan | Seaver and Imlay (2001) |
| AA116 | JI370 ΔmntH2::cat | P1(AA19) × JI370 |
| JI364 | Δ(katG17::Tn10)1 | Seaver and Imlay (2001) |
| AA142 | JI364 ΔmntH2::cat | P1(AA19) × JI364 |
| DH5a pir+ |
supE44 ΔlacU169 (φ80lacZδM15) hsdR17 recA1 endA1 gyrA96
thi-1 relA1 pir+ |
Jim Slauch |
| SJ99 | MG1655 Δ(lacZ1::cat)1 | Laboratory Stock |
| G169 | F-, aroD shiA proA argE zdg-299::Tn10 | Grogan and Cronan (1984) |
| JI132 | AB1157 (sodA::Mud PR13)25 (sodB::kan)1-Δ2 | Linn and Imlay (1987) |
| KK183 | JI132 zdg-299::Tn10 | P1(G169) × JI132 |
| SMV29 | LC106 (sodB::kan)1-Δ2 | Varghese and Imlay (2007) |
| SMV32 | MG1655(sodB::kan)1-Δ2 | Varghese and Imlay (2007) |
| AA130 | MG 1655 Δ(lacZ1::cat)1 attλ::[pSJ501::sodA’-lacZ+] | This work |
| AA138 | SMV32 Δ(lacZ1::cat)1 attλ::[pSJ501::sodA’-lacZ+] | P1(AA130) × AA136 |
| AA114 | SMV32 ΔmntH2::cat | P1(AA19) × SMV32 |
| AA141 | AA114 Δ(lacZ1::cat)1 attλ::[pSJ501::sodA’-lacZ+] | P1(AA130) × AA137 |
| KCI420 | MG1655 (sodA::Mud PR13)25 | Laboratory Stock |
| AA171 | MG1655 Δ(lacZ1::cat)1 attλ::[pSJ501::mntH’-lacZ+] | This work |
| AA183 | MG1655 Δ(lacZ1::cat)1 attλ::[pSJ501::mntH’-lacZ+] | P1(AA171) × SJ130 |
| SJ108 | LC106 Δ(lacZ1::cat)1 | Laboratory Stock |
| AA191 | SJ108 attλ::[pSJ501::mntH’-lacZ+] | P1(AA171) × SJ108 |
| AA147 | BW25113 mntH1::cat(NI) | This work |
| AA153 | LC106 mntH1::cat(NI) | P1(AA147) × AA153 |
| SMV42/pAA01 | SMV42 with pAA01 | This work |
| AA145 | SMV29 ΔmntH2::cat | P1(AA19) × SMV29 |
| LC106/pDT1.5 | LC106 with pDT1.5 | This work |
| SMV42/pDT1.5 | SMV42 with pDT1.5 | This work |
| Lem17 | MG1655 recA56 srl300::Tn10 | Laboratory Stock |
| XY27 | MG1655 plus recA938::cat ΔahpF::kan Δ(katG17::Tn10)1 Δ(katE12::Tn10)1 |
Park and Imlay (2005) |
| SP66 | LC106 mhpC281::Tn10 lacY1 dps::cat | Park and Imlay (2005) |
| SMV30 | LC106 (sodA::Mud PR13)25 | P1(KK183) × LC106 |
| JEM78 | BW25113 ΔnrdEF1::cat | Julia Martin |
| JEM90 | LC106 ΔnrdEF1::cat | P1(JEM78) × LC106 |
| AA44 | BW25113 Δicd1::cat | This work |
| AA160 | LC106 Δicd1::cat | P1(AA44) × LC106 |
| AA38 | BW25113 ΔpgmI1::cat | This work |
| AA59 | LC106 ΔpgmI1::cat | P1(AA38) × LC106 |
| AA28 | BW25113 ΔmntR1::cat | This work |
| AA33 | LC106 ΔmntR1::cat | P1(AA28) × LC106 |
| AA79 | BW25113 ΔglpX1::cat | This work |
| AA89 | LC106 ΔglpX1::cat | P1(AA89) × LC106 |
| AA94 | BW25113 ΔmaeA1::cat | This work |
| AA110 | LC106 ΔmaeA1::cat | P1(AA94) × LC106 |
| Plasmid | Genotype | Reference |
|---|---|---|
| pAA01 | pBR322 with mntH | This work |
| pSJ501 | pAH125 derivative with cat flanked by flp sites | Soojin Jang |
| pAA02 | pSJ501::sodA’-lacZ+ | This work |
| pAA03 | pSJ501::mntH’ -lacZ+ | This work |
| pDT1.5 | pBR322 with sodA | Carlioz and Touati (1986) |
The chromosomal mntH(NI) allele, in which the OxyR binding site is removed and replaced with a flp scar sequence, was also generated by the Red recombinase method. These mutations were confirmed by PCR analysis and sequencing. Positions -111 to -75 upstream of the transcription start site were removed in the mntH(NI) mutant allele, corresponding to bases 2510803 to 2510839. This deletion removed the OxyR binding site (Kehres et al., 2002a) but did not alter the -10/-35 promoter region or the 5′ UTR of the transcript. The plasmid pAA01 expressing the wild-type mntH allele and its promoter region (base 2509490-base 2510928) was made using the forward primer 5′-ATCTAAAGCTTGCTATGTTGTGTATGGAAGCTGAAAG and reverse primer 5′-GAATCGGATCCCTACAATCCCAGCGCCGACCCCAC with BamHI and HindIII restriction sites. The PCR product was inserted into pBR322 plasmid and confirmed by sequencing.
Single-copy lacZ fusions to sodA and mntH promoter regions were integrated into the lambda attachment site, while the wild-type genes remained at their native positions (Haldimann & Wanner, 2001). The promoter regions were amplified using the forward primer 5′-GAGAGACTAAACGAGCTGTAATACGCC and reverse primer 5′-CATATTCATCTCCAGTATTGTCGGGCG for sodA, and forward primer 5′-ATATCCTGCAGCAACAACGGCAAGTGCCAGTACAAAATG and reverse primer 5′-ATATCGGTACCCATCTTGTGCCTCTAAAACATAGCCTTTG for mntH, both designed with PstI and KpnI restriction sites. CRIM plasmids were modified by replacing the kanamycin-resistance cassette with a chloramphenicol-resistance cassette, to permit antibiotic selection under anaerobic conditions, and engineering FLP sites around the chloramphenicol acetyltransferase gene. The sodA and mntH promoter regions were inserted, and the resultant plasmid constructions were confirmed by sequencing. After chromosomal integration and transduction into recipient strains, the chloramphenicol acetyltransferase gene was removed from mutants, as indicated in table 1, by using the temperature sensitive plasmid pCP20, which was later cured from the strain in question (Datsenko & Wanner, 2000).
Enzyme Assays
Aerobic cultures were grown in defined medium (glucose/amino acids) to an OD600 of 0.2, after which cells were centrifuged, washed, resuspended, and sonicated in 50 mM potassium phosphate buffer (pH 7.8) with 0.1 mM EDTA. We measured β-galactosidase activity by ONPG hydrolysis (Miller, 1972), and determined total protein content by using the Coomassie blue dye-binding assay (Pierce). SOD activity was measured by the xanthine oxidase-cytochrome c method (McCord & Fridovich, 1969). To track the activity of specific SOD isozymes, we measured manganese-containing SOD (MnSOD) in sodB mutants and iron-containing SOD (FeSOD) in sodA mutants. The fraction of each isozyme that was active in cell extracts was determined after extracts were subjected to partial denaturation and renaturation in the presence of manganese/iron to ensure the full activation of MnSOD or FeSOD proteins. MnSOD was denatured at pH 3.8 in the presence of guanidinium chloride, EDTA, and 8-hydroxyquinoline-5-sulfonic acid and then reactivated by dialysis into neutral pH in buffer containing 0.1 mM MnCl2. Details follow the published protocol (Kirby et al., 1980) except that 8-hydroxyquinoline-5-sulfonic acid was used instead of 8-hydroxyquinoline and remetallation was accomplished with two changes of 0.1 mM MnCl2 in 5 mM Tris-HCl buffer at pH 7.8 for 12 h each instead of one. Metals were removed from FeSOD at pH 11 in the presence of EDTA and dithiothreitol, while remetallation was accomplished by dialysis at neutral pH against 0.1 mM ferrous salts and dithiothreitol (Yamakura & Suzuki, 1976), all in an anaerobic chamber (Beyer et al., 1989). For both MnSOD and FeSOD reconstitution, purified MnSOD and FeSOD were used as controls; in both cases, all activity was lost upon demetallation and fully recovered by reconstitution. The periplasmic CuZnSOD is only synthesized in stationary phase, and activity gels confirmed that it did not provide detectable activity under the experimental conditions used here.
Inductively coupled plasma (ICP) measurement of intracellular manganese
One-liter cultures were grown aerobically in defined medium (glucose/amino acids) at 37°C to an OD600 of 0.15-0.2. The cells were then centrifuged, washed twice with 200 mL ice-cold 20 mM Tris-HCl/1mM EDTA (pH 7.4), washed once in the same buffer without EDTA, and then resuspended in 4 mL 20 mM Tris-HCl (pH 7.4). Cells were lysed with a French press, and debris was pelleted by centrifugation at 22,000 × g for 25 min. The metal content of the supernatant was determined with a SCIEX ELAN DRCe (Perkin-Elmer), and protein content was determined using the Coomassie blue dye-binding assay (Pierce). To calculate the manganese concentration of intact cells, the degree of dilution that occurred during lysis was deduced by comparing the protein concentration of the lysate to its known concentration in intact cells (~300 mg/mL, determined previously by isotopic labeling of the cytoplasmic volume, coupled with measurement of cellular protein (Imlay & Fridovich, 1991)).
H2O2 concentration measurement
Cells were grown anaerobically in defined medium (glucose/amino acids) to an OD600 of ~ 0.15. These cells were then centrifuged, suspended in fresh defined medium at an OD600 of 0.005, and grown aerobically at 37°C with vigorous shaking, with or without 50 μM MnCl2. At regular intervals an aliquot was removed and centrifuged, and H2O2 was measured in the supernatant. H2O2 was detected using the Amplex Red/horseradish peroxidase method (Seaver & Imlay, 2001a). Fluorescence was then measured in a Shimadzu RF Mini-150 fluorometer.
The rates of H2O2 scavenging by whole cells were measured in a similar way, except cells were inoculated aerobically to an OD600 of 0.02, and 1 μM H2O2 was added to the culture.
Cell Viability
Air-sensitive strains were grown anaerobically in defined medium (glucose/amino acids) to an OD600 of ~ 0.15. These cells were then subcultured to an OD600 of 0.003 and grown aerobically at 37°C with vigorous shaking. At intervals, aliquots of cells were moved to the anaerobic chamber, diluted in anaerobic LB, and plated on anaerobic LB agar containing 0.2% glucose. Colonies were enumerated after overnight anaerobic incubation at 37°C.
H2O2 killing assay
Cells were grown aerobically in defined medium (glucose/amino acids) to an OD600 of 0.1-0.15 with or without 100 μM MnCl2 supplementation. These cells were subcultured to an OD600 of 0.025, with or without 100 μM MnCl2 supplementation. H2O2 (2.5 mM) was added, and at different times aliquots were diluted with LB containing catalase, and colonies were enumerated on LB plates after overnight incubation at 37°C.
Protein Carbonylation
Cells were grown anaerobically in defined medium (glucose/amino acids) at 37°C to an OD600 of 0.1-0.15. Most strains were diluted to an OD600 of 0.005 in freshly made aerobic defined medium (glucose/amino acids), with or without 50 μM MnCl2 supplementation. Hpx- mntH cells, grown without MnCl2, were subcultured to an OD600 of 0.040, since these cells double in biomass only twice before growth ceases. Cells were harvested at an OD600 of ~ 0.2. These cells were washed twice with ice-cold 50 mM potassium phosphate buffer pH 7.0, after which they were suspended in 500 μL of the same buffer with the addition of 5 mM DETAPAC. DETAPAC prevents further protein oxidation in extracts. Cells were sonicated, and then protein carbonylation was measured using OxyBlot Protein Oxidation Detection Kit (S7150) (Chemicon International). Protein carbonyl groups were derivatized to 2,4-dinitrophenylhydrazone by reaction with 2,4-dinitrophenylhydrazine (DNPH) for 15 min in 3% (w/v) SDS. β-mercaptoethanol (1% v/v) was then added to these derivatized samples, after which the samples were subjected to polyacrylamide denaturing gel electrophoresis (4-15% BioRad). Proteins were then transferred to a nitrocellulose membrane (Amersham Hybond™-ECL™ by GE Healthcare) for 60 min at 100 V. The membrane was then incubated with primary antibody specific to the DNP moiety attached to the derivatized proteins. This step was followed by incubation with a horseradish peroxidase antibody conjugate directed against the primary antibody. The membranes were then treated with chemiluminescent substrate (GE Healthcare, Amersham ECL™ Western Blotting Analysis System) and imaged by exposure to light sensitive films (Amersham Hyperfilm ECL™ chemiluminescence film, GE Healthcare, Buckinghamshire, UK).
Some protein carbonyls were consistently detected in the extracts of cells than had been grown and processed under strictly anaerobic conditions. These carbonyl adducts constitute a background that cannot reflect ROS-mediated oxidative damage and may arise from protein glycation reactions (Adams et al., 2001). This background must be considered when protein carbonylation is used as a proxy for oxidative injury.
Acknowledgements
We thank Rudiger Laufhutte, of the University of Illinois microanalysis laboratory, for help with ICP experiments; Michael E. Maguire (Case Western Reserve University, Cleveland, OH), for strains; and Soojin Jang for pSJ501 construction. This work was supported by GM049640 from the National Institutes of Health.
References
- Adams S, Green P, Claxton R, Simcox S, Williams MV, Walsh K, Leeuwenburgh C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front Biosci. 2001;6:A17–24. doi: 10.2741/adams. [DOI] [PubMed] [Google Scholar]
- Al-Maghrebi M, Fridovich I, Benov L. Manganese supplementation relieves the phenotypic deficits seen in superoxide-dismutase-null Escherichia coli. Arch. Biochem. Biophys. 2002;402:104–109. doi: 10.1016/S0003-9861(02)00065-6. [DOI] [PubMed] [Google Scholar]
- Archibald F. Manganese: its acquisition by and function in the lactic acid bacteria. Crit. Rev. Microbiol. 1986a;13:63–109. doi: 10.3109/10408418609108735. [DOI] [PubMed] [Google Scholar]
- Archibald F. Manganese: its acquisition by and function in the lactic acid bacteria. Crit Rev Microbiol. (1986b;13:63–109. doi: 10.3109/10408418609108735. [DOI] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 1981;145:442–451. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes. 1982. Abstract. [DOI] [PubMed]
- Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcriptional factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc. Natl. Acad. Sci. USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barynin VV, Whittaker MM, Antonyuk SV, Lamzin VS, Harrison PM, Artymiuk PJ, Whittaker JW. Crystal structure of manganese catalase from Lactobacillus plantarum. Structure. 2001;9:725–738. doi: 10.1016/s0969-2126(01)00628-1. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Chock PB, Yim MB, Stadtman ER. Manganese(II) catalyzes the bicarbonate-dependent oxidation of amino acids by hydrogen peroxide and the amino acid-facilitated dismutation of hydrogen peroxide. Proc. Natl. Acad. Sci. USA. 1990;87:389–393. doi: 10.1073/pnas.87.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer WF, Jr., Fridovich I. In vivo competition between iron and manganese for occupancy of the active site region of the manganese-superoxide dismutase of Escherichia coli. J Biol Chem. 1991;266:303–308. [PubMed] [Google Scholar]
- Beyer WF, Jr., Reynolds JA, Fridovich I. Differences between the manganese- and the iron-containing superoxide dismutases of Escherichia coli detected through sedimentation equilibrium, hydrodynamic, and spectroscopic studies. Biochemistry. 1989;28:4403–4409. doi: 10.1021/bi00436a042. [DOI] [PubMed] [Google Scholar]
- Bjerrum CJ, Canfield DE. Ocean productivity before about 1.9 Gyr ago limited by phosphorus adsorption onto iron oxides. Nature. 2002;417:159–162. doi: 10.1038/417159a. [DOI] [PubMed] [Google Scholar]
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- Chang EC, Kosman DJ. Intracellular Mn(II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. JBC. 1989;264:12172–12178. [PubMed] [Google Scholar]
- Daly MJ, Gaidamakova EK, Matrosova VY, Valilenko A, Zhai M, Venkateswaran A, Hess M, Omelchenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gama-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat. Struct. Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J. Bacteriol. 2005;187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B, Carmichael AJ. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electronspin resonance. Biochem. J. 1992;281:795–802. doi: 10.1042/bj2810795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reactions has possible biological implications. J. Biol. Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol. Microbiol. 2002a;44:1269–1286. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Wharton SJ, Karavolos M, Foster SJ. Manganese: elemental defence for a life with oxygen? Trends Microbiol. 2002b;10:496–501. doi: 10.1016/s0966-842x(02)02462-9. [DOI] [PubMed] [Google Scholar]
- Ilari A, Ceci P, Ferrari D, Rossi G, Chiancone E. Iron incorporation into E. coli Dps gives rise to a ferritin-like microcrystalline core. J. Biol. Chem. 2002;277:37619–37623. doi: 10.1074/jbc.M206186200. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Imlay JA, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. JBC. 1991;266:6957–6965. [PubMed] [Google Scholar]
- Inaoka T, Matsumura Y, Tsuchido T. SodA and manganese are essential for resistance to oxidative stress in growing and sporulating cells of Bacillus subtilis. J. Bacteriol. 1999;181:1939–1943. doi: 10.1128/jb.181.6.1939-1943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BB, Jr., McCord JM, Fridovich I. Superoxide dismutase from Escherichia coli B. A new manganese-containing enzyme. J Biol Chem. 1970;245:6176–6181. [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+ J. Bacteriol. 2002a;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME. Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H(2)O(2), Fe(2+), and Mn(2+) J Bacteriol. 2002b;184:3151–3158. doi: 10.1128/JB.184.12.3151-3158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Micro. Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol Microbiol. 2000a;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Zaharik ML, Finlay BB, Maguire ME. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 2000b;36:1085–1100. doi: 10.1046/j.1365-2958.2000.01922.x. [DOI] [PubMed] [Google Scholar]
- Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. PNAS. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T, Blum J, Kahane I, Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980;201:551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- Kono Y, Fridovich I. Isolation and characterization of the pseudocatalase of Lactobacillus plantarum. J Biol Chem. 1983;258:6015–6019. [PubMed] [Google Scholar]
- Kuo CF, Mashino T, Fridovich I. α,β-dihydroxyisovalerate dehydratase: a superoxide-sensitive enzyme. J. Biol. Chem. 1987;262:4724–4727. [PubMed] [Google Scholar]
- Lee JH, Yeo WS, Roe JH. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hdyrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol. Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalyzed histidine oxidation. Nature. 2006;440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- Levin DE, Hollstein M, Christman MF, Schwiers EA, Ames BN. A new Salmonella tester strain (TA102) with A.T base pairs at the site of mutation detects oxidative mutagens. PNAS. 1982;79:7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. The role of superoxide in the production of hydroxyl radical: in vitro and in vivo. Free Rad Biol Med. 1994;16:29–33. doi: 10.1016/0891-5849(94)90239-9. [DOI] [PubMed] [Google Scholar]
- McCord J, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1972. [Google Scholar]
- Munroe W, Kingsley C, Durazo A, Gralla EB, Imlay JA, Srinivasan C, Valentine JS. Only one of a wide assortment of manganese-containing SOD mimicking compounds rescues the slow aerobic growth phenotypes of both Escherichia coli and Saccharomyces cerevisiae strains lacking superoxide dismutase enzymes. J Inorg Biochem. 2007;101:1875–1882. doi: 10.1016/j.jinorgbio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Tsubouchi R, Ogawa MF, Yoshino M. Oxidative inactivation of reduced NADP-generating enzymes in E. coli: iron-dependent inactivation with affinity cleavage of NADP-isocitrate dehydrogenase. Arch. Microbiol. 2006;186:385–392. doi: 10.1007/s00203-006-0153-1. [DOI] [PubMed] [Google Scholar]
- Neidhardt FC, Umbarger HE. Chemical Composition of Escherichia coli. In: Kredich NM, editor. Escherichia coli and Salmonella. ASM Press; Washington D C: 1996. pp. 13–16. [Google Scholar]
- Niven DF, Ekins A, al-Samaurai AA. Effects of iron and manganese availability on growth and production of superoxide dismutase by Streptococcus suis. Can J Microbiol. 1999;45:1027–1032. doi: 10.1139/w99-114. [DOI] [PubMed] [Google Scholar]
- Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Paget MS, Buttner MJ. Thiol-based regulatory switches. Annu Rev Genet. 2003;37:91–121. doi: 10.1146/annurev.genet.37.110801.142538. [DOI] [PubMed] [Google Scholar]
- Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc. Natl. Acad. Sci. USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- Pugh SYR, DiGuiseppi JL, Fridovich I. Induction of superoxide dismutases in Escherichia coli by manganese and iron. JBact. 1984;160:137–142. doi: 10.1128/jb.160.1.137-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P, Cole TD, Wemmer DE, Linn S. Localization of Fe(2+) at an RTGR sequence within a DNA duplex explains preferential cleavage by Fe(2+) and H2O2. J. Mol. Biol. 2001;312:1089–1101. doi: 10.1006/jmbi.2001.5010. [DOI] [PubMed] [Google Scholar]
- Runyen-Janecky L, Dazenski E, Hawkins S, Warner L. Role and regulation of the Shigella flexneri Sit and MntH systems. Infect. Immun. 2006;74:4666–4672. doi: 10.1128/IAI.00562-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RJ, Srinivasan C, Munroe WH, Wallace MA, Martins J, Kao TY, Le K, Gralla EB, Valentine JS. Exogenous manganous ion at millimolar levels rescues all known dioxygen-sensitive phenotypes of yeast lacking CuZnSOD. J. Biol. Inorg. Chem. 2005;10:912–923. doi: 10.1007/s00775-005-0044-y. [DOI] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 2001a;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 2001b;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis. 2004;190:136–147. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J. Biol. Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- Tardat B, Touati D. Iron and oxygen regulation of Escherichia coli MnSOD expression. Competition between the global regulators Fur and ArcA for binding to DNA. Molecular Microbiology. 1993;k9:53–63. doi: 10.1111/j.1365-2958.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Tseng HJ, McEwan AG, Paton JC, Jennings MP. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect Immun. 2002;70:1635–1639. doi: 10.1128/IAI.70.3.1635-1639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 2001;40:1175–1186. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- Varghese S, Wu A, Park S, Imlay KRC, Imlay JA. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol. Microbiol. 2007;64:822–830. doi: 10.1111/j.1365-2958.2007.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker MM, Whittaker JW. Mutagenesis of a proton linkage pathway in Escherichia coli manganese superoxide dismutase. Biochemistry. 1997;36:8923–8931. doi: 10.1021/bi9704212. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Suzuki K. Reconstitution of iron-superoxide dismutase. Biochem Biophys Res Commun. 1976;72:1108–1115. doi: 10.1016/s0006-291x(76)80246-x. [DOI] [PubMed] [Google Scholar]
- Yost FJ, Jr., Fridovich I. An iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1973;248:4905–4908. [PubMed] [Google Scholar]
- Zheng M, Doan B, Schneider TD, Storz G. OxyR and SoxRS regulation of fur. J. Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]