Abstract
Ag-specific CD8+ T cells immunized in the absence of CD4+ T cell help, so-called “unhelped” CD8+ T cells, are defective in function and survival. We investigated the role of the proapoptotic molecule TRAIL in this defect. We first demonstrate that TRAIL does not contribute to the CD8+ T cell response to Listeria monocytogenes strain expressing OVA (LmOVA) in the presence of CD4+ T cells. Secondly, we generated mice doubly deficient in CD4+ T cells and TRAIL and analyzed their CD8+ T cell response to LmOVA. Memory CD8+ T cells in double-deficient mice waned over time and were not protective against rechallenge, similar to their TRAIL-sufficient unhelped counterparts. To avoid the effects of CD4+ T cell deficiency during memory maintenance, and to address whether TRAIL plays a role in the early programming of the CD8+ T cell response, we performed experiments using heterologous prime and early boost immunizations. We did not observe activation-induced cell death of unhelped CD8+ T cells when mice were infected with followed vaccinia virus expressing OVA 9 days later by LmOVA infection. Furthermore, primary immunization of CD4+ T cell-deficient mice with cell-associated Ag followed by LmOVA infection did not reveal a role for TRAIL-mediated activation-induced cell death. Overall, our results suggest that CD4+ T cell help for the CD8+ T cell response is not contingent on the silencing of TRAIL expression and prevention of TRAIL-mediated apoptosis.
The factors that shape the CD8+ T cell response to acute bacterial or viral infection are numerous and complex. Following infection, Ag-specific CD8+ T cells progress through three distinct phases: during the expansion phase, naive CD8+ T cells undergo extensive proliferation, differentiating into effector cells capable of cytokine production and lytic activity; following pathogen clearance, a contraction phase occurs in which 90 –95% of the CD8+ effector T cells die via programmed cell death; and in the final phase, the remaining cells differentiate to constitute the long-lived memory pool, poised to mount a rapid, protective recall response upon pathogen reencounter (1, 2). Each of these phases is influenced by a myriad of signals from stromal cells and other immune cells, including APC and CD4+ T cells.
The generation and maintenance of long-lived CD8+ T cell memory is critical to life-long protection against a variety of infections. Indeed the long-term survival and fitness of this pool represent an active process, controlled by numerous factors, including (but not limited to) cytokines and signals from CD4+ T cells (3–5). With regard to the requirement for CD4+ T cell help for the CD8+ T cell immune response, it has been shown that the primary CD8+ T cell response to many infections, including clonal expansion and differentiation of Ag-specific naive cells into effector cells able to clear the pathogen, is CD4+ T cell-independent. Recently, it was established that while CD4+ T cell help is dispensable for the primary response against infectious agents, it is absolutely required for functional memory. Memory CD8+ T cells generated and maintained in a CD4+ T cell-deficient environment decrease in number over time, and they lose their ability to expand, to exert effector functions, including cytokine production and cytotoxicity, and to ultimately protect following rechallenge (3, 6–10). Additional experiments have demonstrated that the presence of CD4+ T cells is required during the memory maintenance stage of the CD8+ T cell response (4), suggesting that CD4+ T cells either directly or indirectly contribute to the overall quality and functionality of the CD8+ memory T cell pool.
The primary response to noninfectious stimuli, such as Ag-loaded, transformed, or allogeneic cells, is thought to be dependent on CD4+ T cell help (5). Recent experiments involving the use of noninflammatory immunogens have proposed that, although CD4+ T cell help appears to be required for the primary response, this is actually a reflection of CD4+ T cell help during the first few days of the immune response to allow development of a secondary response (11, 12). The data suggest that the early presence of CD4+ T cells “programs” the ensuing CD8+T cell memory response such that CD8+ T cells that did not receive CD4+ T cell help during priming (termed “unhelped” cells) commit to a genetic program in which they undergo activation-induced cell death (AICD)3 upon restimulation, thus explaining why the effects of priming in the absence of CD4+ T cell help are only evident following rechallenge (13). Using a cellular immunogen requiring cross-priming or lymphocytic choriomeningitis virus (LCMV) infection, it was demonstrated that mRNA for TRAIL was selectively up-regulated in unhelped CD8+ T cells following in vitro restimulation and that TRAIL blockade rescued the expansion of restimulated CD8+ T cells (13). It was thus proposed that unhelped CD8+ T cells commit TRAIL-mediated AICD upon Ag reencounter. These data suggest that TRAIL could play a significant role in the defective phenotype observed when CD8+ T cells are primed and maintained in the absence of CD4+ T cell help. They also support a model in which signals delivered by CD4+ T cells during initial CD8+ T cell priming inform the subsequent function and fate of CD8+ memory T cells.
TRAIL (also known as Apo2L) is a type II transmembrane protein belonging to the TNF superfamily (14). It has been implicated in numerous experimentally induced autoimmune diseases, including murine models of lupus and airway hyperresponsiveness (15, 16). One functional receptor has been identified in mice, death receptor 5 (also known as TRAIL-receptor 2 (TRAIL-R2)) (17). Both TRAIL−/− and TRAIL-R2−/− mice have been generated, and neither displays any obvious phenotype (18–20). Earlier reports focused on TRAIL as a negative regulator of the innate arm of the immune system, including one report showing that activated TRAIL-R2-deficient macrophages and dendritic cells exhibit enhanced cytokine production (19). Few studies to date have focused on the contribution of TRAIL to the adaptive immune system following acute infection. One such study investigated a role for TRAIL during LCMV infection in the presence and absence of CD4+ T cells (3). It was found that up to 60 days after infection TRAIL deficiency was indeed able to rescue the impaired function and recall ability of unhelped CD8+ memory T cells, but by day 90 after primary infection, the CD8+ T cells in CD4+ T cell-depleted TRAIL-deficient mice were just as dysfunctional as their TRAIL-sufficient counterparts. It was thus concluded that TRAIL-dependent mechanisms may be involved in the early altered differentiation of CD8+ T cells in the absence of CD4+ T cell help, but that TRAIL-independent mechanisms may also be at play, which are responsible for the long-term loss of function of unhelped CD8+ T cells.
Prior studies have led to somewhat differing conclusions as to the contribution of TRAIL to the unhelped CD8+ T cell phenotype. Depending on the nature of the primary immunization, that is, either noninfectious (13) or infectious (3), the absence of TRAIL expression is or is not able to fully rescue the defective priming of CD8+ T cells observed in the absence of CD4+ T cell help. Additionally, the ability of TRAIL deficiency to reverse the unhelped phenotype appears to be dependent on the timing of the secondary challenge, in that the earlier the restimulation, the more TRAIL expression seems to be playing a role. In this study we set out to reconcile some of these differences. We examined the role of TRAIL in the CD8+ T cell response to acute bacterial infection or cellular immunogens in CD4+ T cell-sufficient and CD4+ T cell-deficient (MHC class II−/−) mice. We found that TRAIL does not significantly contribute to the primary, memory phase or recall CD8+ T cell response to Listeria monocytogenes strain expressing OVA (LmOVA) infection, consistent with a prior report that used viral infection (3). Interestingly, in the absence of CD4+ T cell help, we also demonstrated no significant role for TRAIL in the unhelped phenotype in response to either an infectious or noninfectious priming that was followed by early LmOVA secondary infection 9 –10 days later. Overall, the data presented here do not support a role for TRAIL expression in the defective CD8+ T cell memory response observed in the absence of CD4+ T cell help.
Materials and Methods
Mice
C57BL/6 (B6) and B6-Abβ−/− mice were obtained from Taconic Farms at 6 – 8 wk of age. TRAIL−/− mice (backcrossed 10 times to B6) were obtained from Amgen and bred in the animal facilities at the University of Washington (Seattle, WA) under specific pathogen-free conditions. Double knockout (TRAIL−/− × MHC class II−/−) mice were bred and maintained in the same facilities. Act-mOVA (β-actin promoter driven membrane-bound Ova) transgenic mice (21) were obtained from The Jackson Laboratory and bred and maintained in the same animal facilities at the University of Washington. All experiments began when mice were 6 –12 wk of age and were done in accordance with the Institutional Animal Care and Use Committee guidelines.
Immunizations and infections
Erythromycin-resistant LmOVA was kindly provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) (22). Frozen stocks of LmOVA were grown in brain-heart infusion broth. At mid-log growth phase, culture samples were measured by OD (A600) and diluted in PBS for injection. Bacterial counts were verified by plating on brain-heart infusion plates supplemented with 5 mg/ml erythromycin. For primary infections, mice were injected i.v. via tail vein with 2000 CFU LmOVA. For rechallenge experiments mice were injected i.v. with 2–5 × 105 CFU LmOVA and analyzed 3 days later. Bacterial counts in the spleen and liver were determined by making suspensions of each organ in PBS, which were then serially diluted in PBS with 0.1% Nonidet P-40 (Sigma-Aldrich). Serial dilutions were spread on brain-heart infusion plates supplemented with 5 mg/ml erythromycin, incubated overnight at 37°C, and counted. For heterologous prime/boost experiments, mice were rechallenged with 2– 4 × 104 CFU LmOVA. Recombinant vaccinia virus expressing OVA (Vacc-OVA), kindly provided by J. Yewdell (National Institute of Allergy and Infectious Diseases, Bethesda, MD), was grown and titered as previously reported (23). Mice were infected with 2 × 106 PFU Vacc-OVA injected i.p. For cellular immunizations, splenocytes from Act-mOVA mice were harvested in PBS supplemented with 0.5% mouse serum albumin, RBC were lysed with a hypotonic buffer, and cells were irradiated with 1500 rad from a cesium source. Cells were washed twice in PBS and 107 cells were injected s.c.
Flow cytometry and intracellular cytokine staining
H-2Kb tetramers bound to the OVA-derived SIINFEKL peptide were generated as described (24). Single-cell suspensions were made from the spleen by grinding the tissue and lysing RBC in a hypotonic buffer. Lymphocytes were obtained from perfused livers as previously described (25) and peripheral blood was obtained via retroorbital bleeding, followed by RBC lysis in hypotonic buffer. Cells were resuspended in FACS buffer (PBS + 2% FCS + 0.1% sodium azide) and stained on ice for 1–1.5 h with Abs (CD8-APC-Cy7 and CD44-FITC from BD Pharmingen; CD4-PE-Cy7, CD62L-PE-Cy7, and CD127-APC from eBioscience) and tetramer. Flow cytometry was performed on a FACSCanto (BD Biosciences), and data were analyzed using FlowJo software (Tree Star). For intracellular cytokine staining (ICS), lymphocytes from the spleen, blood, or liver were resuspended in RPMI 1640 supplemented with 10% FCS, 2 mM L-glutamine, 10 mM HEPES, 0.5 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were stimulated in a 96-well round-bottom plate plus 1 μg/ml brefeldin A (GolgiPlug, Cytofix/Cytoperm kit; BD Pharmingen) with or without OVA peptide (SIINFEKL) at a concentration of 0.1 μg/ml for 4 h at 37°C. Following incubation, cells were surface stained with CD8-PerCP (BD Pharmingen), permeabilized, and stained for intracellular cytokine expression (IFN-γ-APC, IL2-PE, and TNF-α-FITC from eBioscience) according to the manufacturer’s instructions in the Cytofix/Cytoperm kit. Cells were subsequently analyzed by flow cytometry.
Results
The CD8+ T cell response to LmOVA infection is TRAIL-independent
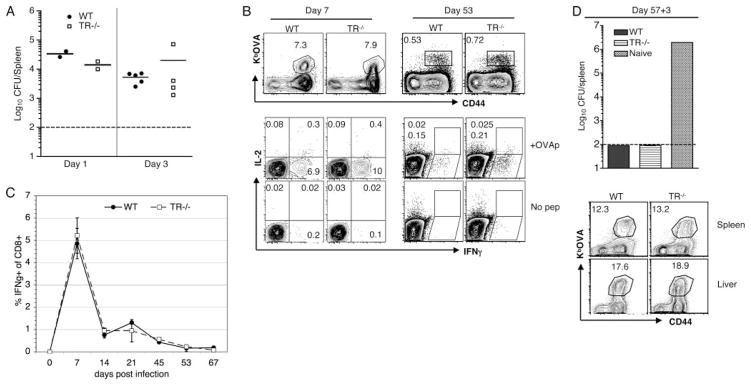
Prior reports conflict as to whether TRAIL plays a role in immunity against the intracellular bacterium L. monocytogenes, with reports varying based on the knockout used or the genetic background of the mice (19, 26). We first assessed the role of TRAIL in the global innate response to LmOVA by infecting wild-type (WT) B6 and TRAIL-deficient mice with 2000 CFU LmOVA. The bacterial burden was not significantly different in the spleen (Fig. 1A) or liver (data not shown) 1 or 3 days after infection, suggesting that any TRAIL-dependent function during this early phase is not significant with this dose of LmOVA on the B6 background. We next quantified the OVA-specific CD8+ T cells at the peak of the response, that is, day 7 after infection. There was no difference in the magnitude of the response (Fig. 1B) or in the surface phenotype of the OVA-specific cells (data not shown). Furthermore, the TRAIL-deficient CD8+ T cells exhibited a similar cytokine profile as did their TRAIL-sufficient counterparts, with a small proportion producing IL-2 (Fig. 1B, lower left) and a similar frequency of IFN-γ-producing CD8+ T cells also producing TNF-α (e.g., an average of 35% in WT mice and 40% in TRAIL−/− mice in one representative experiment; data not shown). To assess whether TRAIL affects the development of CD8+ T cell memory, we analyzed the proportion of OVA-specific CD8+ T cells 53 days postinfection by both tetramer staining and the ability to produce cytokines following brief in vitro restimulation, and again we found no significant differences between the memory CD8+ T cells in the TRAIL-sufficient and TRAIL-deficient animals (Fig. 1B). Additionally, the overall kinetics of the response (i.e., expansion, contraction, and memory maintenance) were similar between the two groups (Fig. 1C). Lastly, to assess the fitness of memory cells generated in the absence of TRAIL and also whether TRAIL contributed to the secondary CD8+ T cell response, previously immunized mice were rechallenged with a lethal dose of LmOVA (5 × 105 CFU). The TRAIL-deficient CD8+ T cells rapidly expanded and controlled this dose of infection in both the spleen and liver, similar to their WT counterparts (Fig. 1D). These results led to the conclusion that TRAIL is not necessary for the primary or secondary CD8+ T cell response to acute bacterial infection.
FIGURE 1.
TRAIL deficiency does not affect the kinetics or magnitude of the primary and secondary CD8+T cell responses to LmOVA. WT and TRAIL−/− (TR−/−) mice were infected with 2000 CFU LmOVA. A, The bacterial burden in the spleen was determined at the indicated time points postinfection. Each point is an individual mouse. The limit of detection was 100 CFU as indicated by the dotted line. B, At the indicated time points after infection, the OVA-specific response in the blood (day 7) and spleen (day 53) was quantified by tetramer staining and ICS following 4 h in vitro peptide stimulation (OVAp). FACS plots are gated on CD8+ T cells with at least two mice per genotype. The frequencies of cytokine producers indicated at the day 53 time point are the percentages of cells in each gate minus the background of the no peptide control (No pep). C, Each point indicates the average percentage of IFN-γ+CD8+ T cells ± SEM in the blood or spleen at the indicated time points postinfection. Data are representative of at least 2–3 mice per time point. D, Fifty-seven days after primary infection, WT and TR−/− mice were infected with 5 × 105 CFU LmOVA. Three days after infection the bacterial burden in the spleen and the OVA-specific response in the spleen and liver were quantified. A control mouse that had not been immunized before was infected with the same high dose (Naive). The FACS plots are gated on CD8+ T cells and are representative of 2–3 mice per genotype.
Defective maintenance and quality of CD8+ memory T cells generated in the absence of CD4+ T cell help is TRAIL-independent
Having established that the primary response to LmOVA is TRAIL-independent in the presence of CD4+ T cell help, we next addressed a role for TRAIL in the absence of CD4+ T cells. We generated double knockout (Dbl KO) mice (TRAIL−/− × MHC class II−/−), infected them with 2000 CFU LmOVA, and analyzed the OVA-specific response at days 7 and 67 after primary infection. At the peak of the CD8+ T cell response, the levels of OVA-specific cells were similar in the presence and absence of CD4+ T cells, regardless of TRAIL expression (Fig. 2A), consistent with previous reports (3, 13). Furthermore, the cytokine profile of the effector cells in all groups was similar, with the same small fraction of IFN-γ-producing effectors producing IL-2 (Fig. 2A, lower) and at least one-third also producing TNF-α (data not shown). Additionally, the level of memory cell precursors, as defined based on expression of IL-7Rα, was equivalent in all groups (Fig. 2B). It has been previously shown that following LmOVA infection in MHC class II−/− mice the number of Ag-specific memory CD8+ T cells declines with time, as does the fitness of these cells (4, 8, 9). When we analyzed the size of the memory pool 67 days after primary infection, we observed this characteristic decrease in the frequency (Fig. 2C) and absolute numbers (Fig. 3B) of memory cells in Dbl KO mice, despite their concomitant lack of TRAIL expression. Additionally, the functionality of the memory cells in Dbl KO mice was also compromised; that is, whereas more than one-half and almost one-third of memory cells in WT mice produced TNF-α and IL-2, respectively, only one-third and less than one-fifth of the unhelped cells produced these corresponding cytokines, regardless of whether TRAIL was expressed (Fig. 2D). These data indicate that TRAIL deficiency does not overcome the defective maintenance of CD8+ T cell memory numbers or fitness observed in the absence of CD4+ T cell help.
FIGURE 2.
Despite TRAIL deficiency, CD8+ memory T cell numbers and function decline in the absence of CD4+ T cells. WT, MHC class II−/− (MHCII−/−), and double knockout TR−/− × MHCII−/− (Dbl KO) mice were infected with 2000 CFU LmOVA. A, Seven days postinfection the level of OVA-specific cells in the blood was determined by tetramer staining and ICS. All FACS plots are gated on CD8+ T cells. Data are representative of at least 3 mice per genotype. B, Expression of IL-7Rα by tetramer-positive cells was determined at day 7. Histograms are gated on KbOVA+CD8+ T cells. C, Sixty-seven days postinfection, OVA-specific memory levels were assessed in the spleen. The FACS plots are gated on CD8+ T cells. Blood from an unimmunized WT mouse was used as a tetramer staining control. D, Cytokine expression by the memory cells. The FACS plots are gated on CD8+ T cells, and the lower histograms depict the percentage of IL-2+ cells of IFN-γ-producing CD8+ T cells, with the gray lines indicating expression by CD8+ T cells from the same animals that were not restimulated with peptide (No pep). Data are representative of 2–3 mice per genotype.
FIGURE 3.
TRAIL deficiency does not restore impaired recall response of CD8+ T cells observed in the absence of CD4+ T cell help. Seventy-one days following primary infection with LmOVA WT, MHCII−/− and Dbl KO mice were infected with 2 × 105 CFU LmOVA. The bacterial burden and OVA-specific response were assessed 3 days later. Mice of each genotype that had not been immunized before were infected as controls (Naive). A, The OVA-specific recall response in the spleen was determined by tetramer staining and ICS. All FACS plots are gated on CD8+ T cells. B, The absolute number of KbOVA+CD8+ T cells was quantified in the spleen of mice that were not rechallenged (day 67 post primary infection) and in other mice that were rechallenged (3 days postrecall at 71 days after primary infection). The numbers on the graph indicate the fold expansion. C, Three days after rechallenge, the bacterial burden in the spleen was determined. The dotted line indicates the limit of detection. Data depict the average CFU/spleen of 2–3 mice per group ± SEM.
The protective ability of CD8+ memory T cells is compromised in the absence of CD4+ T cell help and TRAIL expression
To definitively assess the effectiveness of memory cells generated in the absence of both CD4+ T cells and TRAIL expression, we rechallenged mice 71 days after primary infection with a lethal dose of LmOVA (2 × 105 CFU). It has been reported that the characteristic defective secondary CD8+ T cell response observed in the absence of CD4+ T cell help can be attributed to TRAIL-mediated AICD (13). If this were the case, one would expect CD8+ memory T cells in Dbl KO mice to expand and protect against rechallenge, because they should not be subject to TRAIL-mediated AICD. Despite the absence of TRAIL expression, memory CD8+ T cells exhibited very poor expansion upon in vivo restimulation in Dbl KO mice (Fig. 3, A, top and B). Furthermore, the ability of the small frequency of responding cells to produce effector cytokines was also compromised in the MHC class II−/− and Dbl KO mice (Fig. 3A, bottom). The ultimate consequence of the observed defective secondary responses was the inability of the memory CD8+ T cells in the CD4+ T cell-deficient environments to control this dose of LmOva (Fig. 3C). Notably, the equivalent dose in the CD4+ T cell-sufficient environment, regardless of TRAIL expression, was controlled (Fig. 3C and data not shown). These data argue against TRAIL-mediated AICD as the reason for defective secondary CD8+ T cell responses in the absence of CD4+ T cell help in the context of acute bacterial priming and rechallenge.
TRAIL-mediated AICD is not observed following early secondary challenge in the absence of CD4+ T cells
The preceding experiments examined the role of TRAIL expression when CD4+ T cells were lacking during both the “programming” phase (days 1–7 postinfection) as well as the memory maintenance phase (at least day 30 postinfection). To examine whether TRAIL-mediated AICD might occur following restimulation early after primary infection, we used a heterologous prime-boost system in which mice were primed by infection with Vacc-OVA and challenged 9 days later with LmOVA. Using this regimen, in which strong inflammation is present both during the primary and the secondary responses, we observed no role for CD4+ T cells, nor a role for TRAIL (Fig. 4). The primary response to Vacc-OVA assayed on day 7 was of equal magnitude in all the groups (Fig. 4A). Furthermore, the OVA-specific CD8+ T cells in all groups robustly expanded following LmOVA infection (Fig. 4B). Thus, in this setting neither CD4+ T cells nor TRAIL expression strongly affected the expansion of effector CD8+ T cells either in the primary or in an early secondary challenge.
FIGURE 4.

Early secondary challenge reveals no role for CD4+ T cell help or TRAIL. Mice of the indicated genotypes were infected with 2 × 106 PFU Vacc-OVA i.p. A, The OVA-specific response was analyzed 7 days later by tetramer staining in the blood. FACS plots are gated on CD8+ T cells and show the average ± SEM of 2– 4 mice per group. An uninfected mouse is shown as a tetramer staining control (No Vacc-OVA). B, Nine days after primary infection, mice were infected with 2 × 104 CFU LmOVA i.v. The OVA-specific response was analyzed 5 days later in the spleen (day 14) by tetramer staining. FACS plots are gated on CD8+ T cells and are representative of 2– 4 mice per genotype. A WT mouse infected only with LmOVA 5 days before is shown (No Vacc-OVA).
It has been argued that inflammatory cytokines and other inflammation-associated signals alter the programming of cells that differentiate in the absence of CD4+ T cell help, potentially masking the unhelped phenotype (13). To further examine this possibility, we used a primary immunization known to be helper-dependent followed by an early rechallenge with pathogen. Mice were immunized with irradiated splenocytes from Act-mOVA mice (in which membrane-bound OVA expression is driven by the β-actin promoter) and infected with LmOVA 10 days later. The primary response to this cellular immunogen measured at day 14 was compromised in the absence of CD4+ T cells, irrespective of TRAIL expression (Fig. 5A). Thus, WT and TRAIL−/− mice showed ~0.3% of CD8+ cells specifically making IFN-γ following OVA peptide stimulation, while MHC class II−/− and Dbl KO mice had only background levels of OVA-specific cells. Following LmOva infection at day 10, the CD8+ T cells in mice containing CD4+ T cells expanded dramatically to account for up to 30% of total CD8+ T cells in the spleens 4 days later. In contrast, animals lacking CD4+ T cells, whether TRAIL-sufficient or TRAIL-deficient, showed a much weaker secondary response (Fig. 5B). Although it was to a much reduced level than in CD4+ T cell-sufficient mice, OVA-specific CD8+ T cells in the CD4+ T cell-deficient groups (MHC class II−/− and Dbl KO mice) were primed and underwent further expansion, as determined by comparing the absolute number of responding cells in the Act-mOva-primed/ LmOVA-boosted groups to that from unprimed mice infected with LmOVA 4 days before assay (Fig. 5C). Notably, TRAIL-sufficient and TRAIL-deficient unhelped cells expanded to the same extent following rechallenge, again arguing against any role for TRAIL-mediated AICD in the impaired secondary CD8+ T cell response observed in the absence of CD4+ T cells.
FIGURE 5.
Absence of TRAIL does not rescue cells primed by a helper-dependent immunogen. Mice of the indicated genotypes were immunized with 107 irradiated Act-mOVA splenocytes s.c. and 10 days later were infected (B) or not (A) with 4 × 104 CFU LmOVA i.v. The OVA-specific response was analyzed 4 days later (day 14 after cellular immunization) by ICS in the spleen. FACS plots are gated on CD8+ T cells. Numbers indicate the frequency of cytokine-producing CD8+ T cells in each gate. C, The absolute number of IFN-γ-producing cells was quantified in the spleen. Frequencies were determined by subtracting the staining from the no peptide control. Naive WT mice infected with LmOVA 4 days before are shown as a control. Data represent the average of at least two mice per genotype ± SEM.
Discussion
In this report we analyzed the role of TRAIL during the CD8+ T cell response in the presence and absence of CD4+ T cell help. We found no substantial contribution of TRAIL to the immune response to LmOVA. TRAIL-deficient and WT mice cleared this infection equivalently and generated equally protective long-lived CD8+ T cell memory pools. A prior study documented that TRAIL−/− mice on the BALB/c genetic background were less susceptible to L. monocytogenes-induced hepatocyte death, which correlated with a decreased bacterial burden in the liver up to 7 days postinfection (26). BALB/c mice are more susceptible to L. monocytogenes infection than are B6 mice, and this and other factors could account for the fact that we do not see any contribution of TRAIL. Our results are consistent with a prior study in which TRAIL receptor-deficient mice on the B6 background were infected with a lethal dose of L. monocytogenes, in which case high bacterial counts were detected in both the spleens and livers of WT and knockout mice (19). This previous study did not examine the adaptive immune response. Herein, using a priming dose of LmOVA, our data show no role for TRAIL signaling during the early innate phase or the primary or secondary CD8+ T cell response in B6 mice.
We also report that in the concomitant absence of CD4+ T cells and TRAIL expression, LmOva-primed CD8+ memory T cells faded over time, similar to the situation when only CD4+ T cells are lacking. Memory cells generated and maintained in Dbl KO mice were also functionally impaired; that is, they expanded poorly and displayed defective production of effector cytokines upon rechallenge, again similar to the situation in MHC class II−/− mice. Therefore, the data presented herein support a model in which CD4+ T cells provide crucial factors during the memory phase to maintain both the size and fitness of the CD8+ memory T cell pool. It is possible that lifelong absence of TRAIL and MHC class II in the Dbl KO mice might lead to new compensatory mechanisms called into play to prevent robust recall responses. However, TRAIL deficiency does not affect the CD8+ T cell response in single knockout mice, and, if such an alternative exists, it must operate as efficiently as TRAIL itself.
CD4+ T cells may also impact the generation of this population early in their development, but our data indicate that this influence is not dependent on TRAIL. Other studies have demonstrated differential changes imprinted early in “unhelped” vs “helped” CD8+ T cells during their differentiation. Specifically, the presence of CD4+ T cell help affects CD8+ T cells at the epigenetic level. CD8+ T cells differentiating from naive to effector T cells in CD4−/− mice displayed increased DNA methylation at the IL-2 promoter and decreased histone 3 acetylation at the IFN-γ promoter and enhancer compared with those developing in WT mice (7). Additionally, proper delivery of IL-2 signals is critical during the initial priming of CD8+ T cells to ensure their functionality following secondary challenge. It was demonstrated that CD8+ T cells lacking the high-affinity IL-2 receptor mounted equivalent primary responses as did WT CD8+ T cells, but they then divided and died upon secondary stimulation with Ag (27, 28), reminiscent of the unhelped phenotype following priming with noninfectious Ag (13). As such, it is possible that TRAIL-mediated AICD could play a role in the defective recall response of memory cells generated in the absence of IL-2 signals, and future studies are needed to address this possibility. Although CD4+ T cells produce IL-2, the distinct phenotypes manifest by CD8+ T cells generated in the absence of CD4+ T cells and those generated in the absence of IL-2 signals indicate that IL-2 is not the main factor provided by CD4+ T cells for functional CD8+ T cell memory generation following pathogen immunization.
We also found that CD8+ T cells primed by Vacc-OVA in the absence of CD4+ T cell help mounted extremely robust secondary responses upon infection with LmOVA 9 days later, independently of TRAIL expression. These data imply that the potent inflammatory conditions generated by this regimen are sufficient to obscure the characteristic unhelped defects at this early time point. In contrast, analysis of CD8+ T cell priming by a cell-associated Ag in the absence of CD4+ T cells revealed a crucial role for CD4+ T cell help during the primary response, again independent of TRAIL expression. These experiments are consistent with other reports that assayed the Ag-specific primary response to a noninflammatory immunogen directly ex vivo, in the absence of any in vitro restimulation (29 –31), which also showed the requirement for CD4+ T cell help to generate a robust primary CD8+ T cell response. By quantifying the OVA-specific response via tetramer staining after cellular immunization and again after early secondary challenge with LmOVA, we observed decreased priming followed by moderate expansion in the absence of CD4+ T cell help. This poor expansion of unhelped cells was not ameliorated by the absence of TRAIL expression, arguing that TRAIL-mediated AICD is not the principal cause for defective CD8+ T cell responses to noninflammatory Ags in the absence of CD4+ T cells.
Other reports have documented roles for TRAIL during the memory CD8+ T cell response. It was shown that memory-like CD8+ T cells generated by homeostatic proliferation in CD4+ T cell-deficient lymphopenic mice were not protective against LmOVA, but lack of TRAIL expression reversed this failing (32). The reason for the impaired protection was unclear, as the memory-like CD8+ T cells, which were not able to protect, were able to expand and gain effector functions in response to infection. It is thus difficult to posit how these data correlate with our observation that TRAIL deficiency is insufficient to rescue the ability of endogenous memory CD8+ T cells to protect against secondary infection in MHC class II−/− mice, as we did observe impaired expansion and functionality. Another study demonstrated that LCMV memory CD8+ T cells in TRAIL−/− mice continuously depleted of CD4+ T cells via weekly Ab administration maintained their protective function for up to 60 days after primary viral infection, in contrast to their TRAIL-sufficient CD4+ T cell-depleted counterparts, but by 90 days this rescued fitness waned (3). The differences in the outcomes of these studies might be partially attributed to the use of different pathogens and models of CD4+ T cell deficiency. Additionally, by 60 days postinfection we already detected phenotypic and functional impairment of CD8+ T cells in TRAIL-deficient MHC class II−/− mice. Although the timing of the loss of function differs between the prior study and ours, both studies conclude that TRAIL deficiency is insufficient to overcome the defective functionality of the CD8+ memory T cell pool generated and maintained in the absence of CD4+ T cells. Interestingly, it has recently been reported that unhelped CD8+ T cells express higher levels of the transcription factor T-bet than do their helped counterparts (6). It is thus possible that signals provided by CD4+ T cells lead to extinguished expression of T-bet to allow the complete differentiation of fully functional memory CD8+ T cells. Further studies are needed to address the mechanism by which CD4+ T cells regulate the expression of T-box family transcription factors in Ag-specific CD8+ T cells.
The long-term maintenance of CD8+ T cell memory is crucial to combating many viral and intracellular bacterial infections. The continued preservation of this pool’s overall quantity and quality is a complex process. Additionally, it is becoming increasingly clear that this pool is composed of a heterogeneous mixture of memory populations characterized by distinct surface marker expression and functional properties (2). Our data further document the critical contribution played by CD4+ T cells to ensure long-term CD8+ T cell-mediated protection against bacterial infection. In this study we addressed the role of one particular proapoptotic cascade in modulating the development of CD8+ memory T cells. We observe that following LmOVA infection, TRAIL-deficient memory CD8+ T cell numbers decline, as does their functionality in the absence of CD4+ T cell help, similar to their TRAIL-sufficient counterparts. Furthermore, analyzing the secondary response 9 –10 days after the initial priming also did not reveal a role for TRAIL early during the generation of CD8+ T cell memory. By using several immunization strategies, our data also help to reconcile published differences emphasizing a role for TRAIL in mitigating the defective phenotype of memory CD8+ T cells generated in the absence of CD4+ T cell help. These data underscore the complex nature of CD4+ T cell help for the CD8+ T cell response in that removal of one protein, namely TRAIL, was insufficient to ensure the functionality of unhelped CD8+ memory T cells.
Footnotes
This work was supported by a grant from the National Institutes of Health (AI19335) and the Howard Hughes Medical Institute (to M.J.B.) and a Cancer Research Institute Predoctoral Training Grant in Tumor Immunology (to J.A.S.).
Abbreviations used in this paper: AICD, activation-induced cell death; B6, C57BL/6; Dbl KO, double knockout; ICS, intracellular cytokine staining; LCMV, lymphocytic choriomeningitis virus; LmOVA, Listeria monocytogenes strain expressing OVA; Vacc-OVA, recombinant vaccinia virus expressing OVA; WT, wild type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation, and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badovinac VP, Messingham KA, Griffith TS, Harty JT. TRAIL deficiency delays, but does not prevent, erosion in the quality of “helpless” memory CD8 T cells. J Immunol. 2006;177:999–1006. doi: 10.4049/jimmunol.177.2.999. [DOI] [PubMed] [Google Scholar]
- 4.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 6.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 8.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 9.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. doi: 10.4049/jimmunol.172.5.2834. [DOI] [PubMed] [Google Scholar]
- 11.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 12.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 13.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 14.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 15.Rus V, Nguyen V, Puliaev R, Puliaeva I, Zernetkina V, Luzina I, Papadimitriou JC, Via CS. T cell TRAIL promotes murine lupus by sustaining effector CD4 Th cell numbers and by inhibiting CD8 CTL activity. J Immunol. 2007;178:3962–3972. doi: 10.4049/jimmunol.178.6.3962. [DOI] [PubMed] [Google Scholar]
- 16.Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PA, Smyth MJ, Yagita H, Matthaei KI, Hansbro N, Whitehead B, et al. Critical link between TRAIL and CCL20 for the activation of T(H)2 cells and the expression of allergic airway disease. Nat Med. 2007;13:1308–1315. doi: 10.1038/nm1660. [DOI] [PubMed] [Google Scholar]
- 17.Wu GS, Burns TF, Zhan Y, Alnemri ES, El-Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 18.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 19.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 20.Sedger LM, Glaccum MB, Schuh JC, Kanaly ST, Williamson E, Kayagaki N, Yun T, Smolak P, Le T, Goodwin R, Gliniak B. Characterization of the in vivo function of TNF-α-related apoptosis-inducing ligand, TRAIL/Apo2L, using TRAIL/Apo2L gene-deficient mice. Eur J Immunol. 2002;32:2246–2254. doi: 10.1002/1521-4141(200208)32:8<2246::AID-IMMU2246>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 23.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 24.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 25.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58–64. doi: 10.1172/JCI200419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 28.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews KE, Qin JS, Yang J, Hermans IF, Palmowski MJ, Cerundolo V, Ronchese F. Increasing the survival of dendritic cells in vivo does not replace the requirement for CD4+ T cell help during primary CD8+ T cell responses. J Immunol. 2007;179:5738–5747. doi: 10.4049/jimmunol.179.9.5738. [DOI] [PubMed] [Google Scholar]
- 30.Tyznik AJ, Bevan MJ. The surprising kinetics of the T cell response to live antigenic cells. J Immunol. 2007;179:4988–4995. doi: 10.4049/jimmunol.179.8.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339–6343. doi: 10.4049/jimmunol.171.12.6339. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]